1. Introduction
In the field of restorative dentistry, achieving a reliable bond between prosthetic materials and dental adhesives is critical for longevity and restoration’s success [
1]. Among the various materials used in dental prosthetics, base metal alloys are commonly selected due to their strength, durability, and cost-effectiveness [
2]. However, one challenge that persists is enhancing the bond strength between these alloys and dental adhesives. Modern dental adhesive systems have introduced self-adhesive resin cements, which combine the simplicity of conventional luting agents with the excellent mechanical strength and adhesion of resin-based systems. They can bond directly to both tooth structures and restorative materials without any surface pretreatment, which makes the clinical procedure easier and faster. Their bonding efficiency primarily depends on their chemical composition, particularly the presence of functional acidic monomers such as 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP) and glycerol phosphate dimethacrylate (GPDM). These monomers chemically interact with metallic oxides, forming stable phosphate–metal complexes that enhance adhesion to oxide-containing substrates like base metal alloys and zirconia, thereby improving the durability and longevity of the bond [
3,
4]. Moreover, the application of a metal primer or bonding agent prior to cementation with self-adhesive resin cement has been shown to enhance bond strength. This improvement is attributed to the presence of functional monomers such as 10-MDP, which chemically interact with the oxide layer on metal surfaces to form stable phosphate–metal complexes. Additionally, primers improve surface wettability, reduce interfacial energy, and facilitate better resin infiltration into the micro-irregularities of roughened alloy surfaces, resulting in a stronger and more durable adhesive interface [
5,
6].
Surface treatment plays a crucial role in improving the bonding ability between base metal alloys and resin materials [
5,
6]. One widely studied and clinically accepted method is airborne-particle abrasion, or sandblasting, where aluminum oxide (Al
2O
3) particles are directed at the alloy surface to create a roughened texture, thereby improving the surface area and allowing the resin cement to penetrate more effectively into surface irregularities, causing micromechanical retention upon polymerization [
7,
8]. Additionally, the high-velocity abrasion removes loosely bound oxides, contaminants, and surface irregularities, which creates a cleaner and more reactive surface for bonding [
9]. Apart from its mechanical effects, airborne-particle abrasion may also influence the surface energy and wettability of the alloy, promoting better flow and adaptation of the resin cement [
10].
The timing of bonding after surface treatment is another important factor, as it is thought that the alloy’s surface may undergo changes over time, which can influence the adhesive bond [
11]. Previous studies have shown that immediate bonding after airborne-particle abrasion generally yields good bond strengths, but the effects of delayed bonding have not been fully elucidated [
12].
One critical factor contributing to reduced bond strength over time is the formation of an oxide layer on the metal surface during storage. This passive oxide film, which naturally develops in the presence of oxygen and moisture, may inhibit effective interactions between the metal substrate and adhesive components [
13]. Self-adhesive resin cements rely on chemical adhesion between their functional monomers and metallic oxides to establish excellent bonds. However, excessively thick or unstable oxide layers may impair this interaction by masking available metal ions and reducing the surface’s reactivity [
14,
15]
In situations where the dental clinic does not have access to an airborne-particle abrasion device, this procedure is often delegated to the dental laboratory. As a result, the airborne-particle abrasion of the restoration may be performed several hours or even days prior to cementation. Such a delay between surface treatment and final bonding procedures may alter the surface characteristics of the base metal alloy. The role of storage time between surface treatment and cementation, particularly at intervals such as 1 day, 7 days, and 14 days, remains an area of interest. These timeframes may allow for the formation of an oxide layer or other alterations on the base metal alloys surface, potentially affecting its bonding ability to the resin cement. However, sealing the airborne-particle-abraded restoration in a vacuum bag may prevent the progressive formation of oxide layers on the base metal alloy surface during storage. This approach could help to preserve the surface reactivity and bonding potential. Nevertheless, scientific evidence regarding this aspect remains limited and warrants further investigation.
The purpose of this research was to investigate the effect of delay time (1 day, 7 days and 14 days) and storage after airborne-particle abrasion on the shear bond strength between base metal alloys and self-adhesive resin cement. Besides evaluating the effects of time delay, this study also examined the impact of storing the airborne-particle-abraded specimens under vacuum-sealed conditions for 14 days. A re-airborne-particle abrasion protocol was also included to determine its effectiveness. These additional groups aim to simulate realistic clinical scenarios where delay is unavoidable and surface retreatment may be necessary. The null hypothesis was that the delay time and storage after airborne-particle abrasion would have no effect on the shear bond strength of base metal alloys and self-adhesive resin cement. An additional hypothesis was that vacuum sealing and re-airborne-particle abrasion would have no significant effect on bond strength compared to the untreated delayed groups.
2. Materials and Methods
2.1. Specimens’ Preparation
The required sample size was calculated using the G*Power 3.1 software, with the significance level (α) set at 0.05 and statistical power set at 0.95 to ensure adequate sensitivity for detecting significant differences among experimental groups.
In this investigation, sixty base metal alloys (BMAs, Prep Lab, Bangkok, Thailand; 60–80 wt% nickel, 12–27 wt% chromium, 3–6 wt% molybdenum, 2–6 wt% aluminum, 2 wt% beryllium and <3 wt% ferrum, silicon and carbon) were tested. Sixty BMAs (diameter was 6.0 mm, thickness was 6.0 mm) were positioned in a polyvinyl chloride (PVC) tube with dental stone type IV (
Figure 1). All BMAs were polished using 600-grit silicon carbide paper (Baisdy sandpaper, Shenzhen Baisidi Technology Co., Ltd., Shenzhen, China), followed by 15 min of cleaning in distilled water with an ultrasonic cleaner (ACE Ultimate Co., Ltd., Bang Bua Thong, Nonthaburi, Thailand) and then 10 s of oil-free air drying with a triple syringe.
Table 1 indicates a comprehensive summary of all materials employed in this study, including the BMAs, resin cement, and resin composite used during experimental procedures. Detailed information, such as brand names, manufacturers, and material compositions, is also presented to ensure reproducibility and to facilitate a better understanding of the experimental context.
2.2. Airborne-Particle Abrasion Technique
Each specimen was airborne-particle-abraded with a grain size of 50 microns, at a pressure of 2.5 bars. The sandblasting procedure was conducted by positioning the nozzle (BPS-2 Twin-Pen, Wuhan Jinguang Medical Technology Co., Ltd., Wuhan, China) approximately 10 mm from the substrate surface, maintaining a perpendicular orientation relative to the specimen, and applying the treatment for approximately 10 s per designated area [
16]. To ensure a consistent 90° incidence angle, each specimen was positioned on a stabilized horizontal holder, while the nozzle was mounted on a fixed support to maintain perpendicular alignment throughout the abrasion process. The operator verified the vertical orientation visually before each treatment to minimize angular deviation. The specimens were properly cleansed under flowing water to remove any residual abrasive particles and surface debris. The specimens underwent ultrasonic cleaning using an ultrasonic cleaner in distilled water for 20 min, followed by drying with a triple syringe.
The airborne-particle-abraded specimens were prepared using six distinct protocols (n = 10 per group), as outlined in
Table 2. Each protocol involved specific parameters such as duration and storage method, which were systematically varied to evaluate their effects on the surface characteristics and subsequent bonding performance. These protocols were designed to simulate different laboratory conditions, thereby providing a comprehensive comparison of the outcomes associated with each treatment approach.
2.3. Resin Cement Application
An 80 µm-thick single-sided adhesive tape was prepared by cutting it into a 10 × 10 mm2 and punching a 2 mm diameter aperture to define the bonding area. A slit was made from the edge to the hole to facilitate tape removal after bonding. The tape was then affixed to the surface of BMAs. Self-adhesive resin cement (RelyX Universal, 3M, Seefeld, Germany) was used following the manufacturer’s recommendations. The cement was subsequently introduced into the pre-defined hole in the tape to form the bond.
2.4. Bond to Resin Composite
A resin composite rod (Harmonize A2D, Kerr Corporation, Orange, CA, USA) was fabricated using a silicone mold measuring 3 mm in both height and diameter. The mold was pushed with the composite material and cured using a light-curing unit for 40 s, with the light applied perpendicularly and positioned as close as possible to the mold. The cured rod was then inserted into an opening within one-sided tape, which had been pre-filled with self-adhesive resin cement. A constant force of 1000 g was applied to the setup, and the assembly was light-cured again for 40 s in a perpendicular orientation. The samples were left at room temperature for 10 min. Subsequently, all bonded specimens were incubated in distilled water at 37 °C for one day in an incubator (CLN/CLW series, 15–1000 L, Bell Technology Ltd., Auckland, New Zealand) before testing.
2.5. Shear Bond Test and Failure Mode Determination
Shear bond strength testing was conducted using an AGS-X 500N universal testing machine (Shimadzu Corporation, Kyoto, Japan) with a constant crosshead displacement rate of 0.5 mm/min until specimen failure occurred (
Figure 2). Bond strength values in megapascals (MPa) were calculated by dividing the maximum failure load by the bonded surface area.
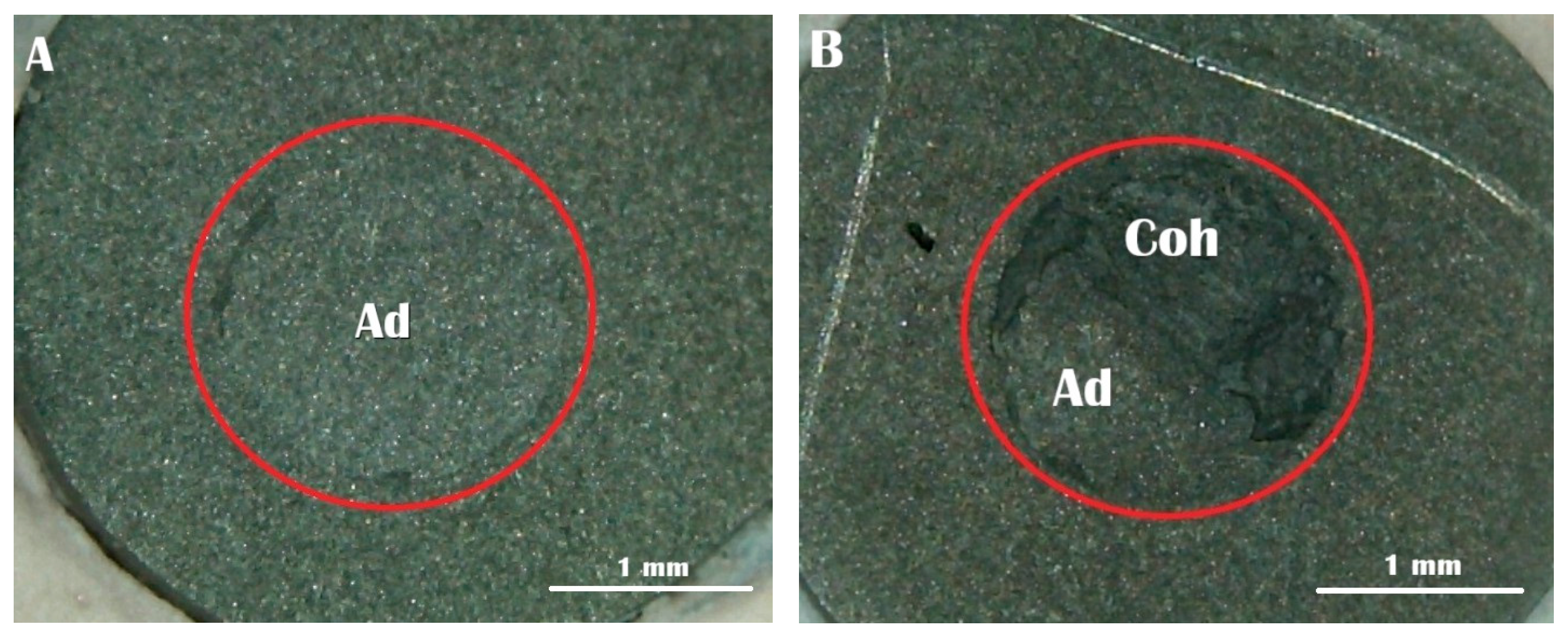
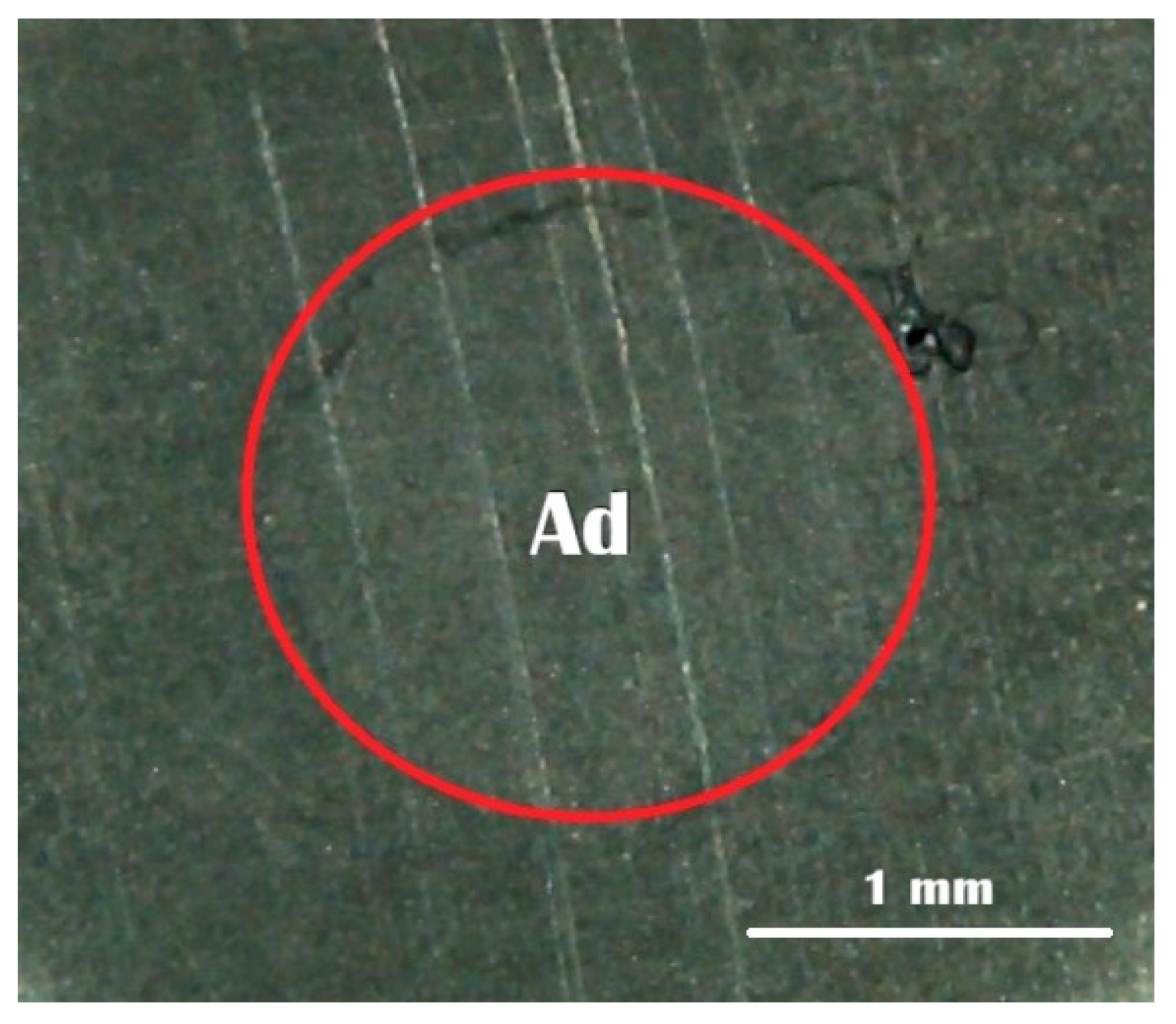
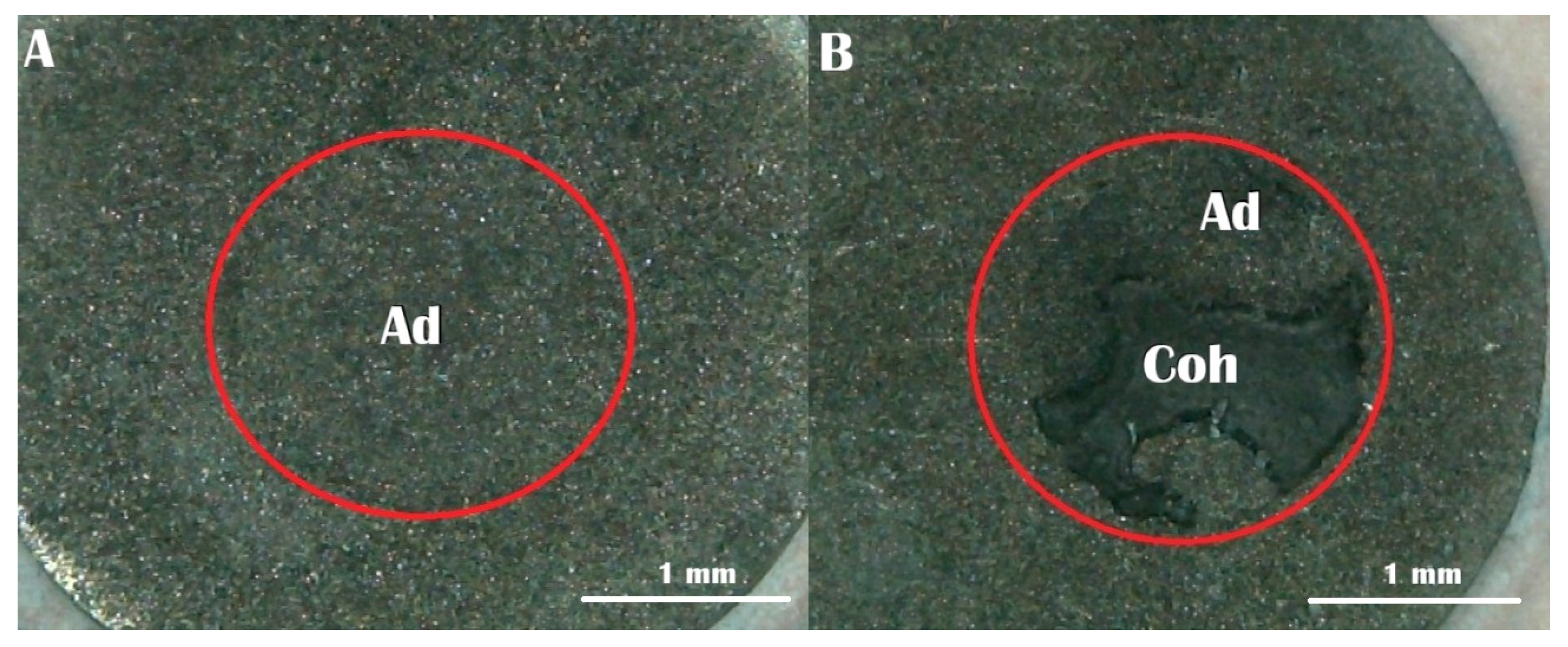
Following fracture, the failure patterns on BMA specimen surfaces were inspected using a stereomicroscope (Meiji RZ Research Zoom, Meiji Techno Co., Ltd., Saitama, Japan) at 40× magnification. The observed failures were categorized into three distinct types according to established criteria [
17,
18]: Type A represented adhesive failure at the interface between the BMA surface and self-adhesive resin cement; Type B indicated mixed failure, characterized by both interfacial adhesive failure and cohesive failure within the cement material; and Type C denoted cohesive failure, occurring exclusively within the self-adhesive resin cement matrix.
2.6. Statistical Analysis
The statistical analysis revealed that the data satisfied the assumption of normality, as confirmed by the Kolmogorov–Smirnov test, and demonstrated the homogeneity of variances according to Levene’s test.
Data analysis was performed using one-way analysis of variance to compare mean shear bond strength measurements between experimental groups. Post hoc analysis using Tukey’s test was applied when ANOVA revealed statistically significant differences (p < 0.05) to identify specific group pairs that differed significantly. A probability value of less than 0.05 was considered statistically significant for all analyses.
3. Results
The averages and standard deviations of the shear bond strength (SBS) values are indicated in
Figure 3. The highest SBS value was observed in Group 1 (immediate bonding) at 26.50 ± 2.74 MPa, which showed a statistically significant difference (
p < 0.05) when compared to the delayed bonding groups. Group 2 (1 day delay) exhibited a significantly lower SBS (21.19 ± 4.94 MPa), followed by Group 4 (14 days delay) and Group 3 (7 days delay), which demonstrated the lowest SBS values at 16.01 ± 4.69 MPa and 15.20 ± 4.52 MPa, respectively. No statistically significant difference was noted between Group 3 and Group 4 (
p > 0.05).
Interestingly, Group 5 (seal for 14 days before bonding) exhibited a high SBS value of 25.92 ± 3.94 MPa, which was statistically comparable to the immediate bonding group (Group 1) (p > 0.05). This finding suggests that surface sealing may preserve the treated surface and maintain bonding potential over time. Notably, re-airborne-particle abrasion after 14 days (Group 6) effectively restored the bond strength to 20.66 ± 3.70 MPa, which was significantly lower than the strength of Groups 1 and 5 (p < 0.05), but comparable to that of Group 2 (p > 0.05).
Regarding failure mode (
Figure 4), the majority of specimens across all groups exhibited adhesive failure. Groups 3 (7 days delay) and 4 (14 days delay) showed 100% adhesive failure, indicating weak interfacial bonding after prolonged exposure. In contrast, Groups 1, 2, 5 and 6 each demonstrated 80% adhesive and 20% mixed failure. No cohesive failure was observed in any group. These findings suggest that higher bond strength tends to result in more mixed failures, which indicates better bonding between the BMA surface and the resin cement.
The experimental groups are shown in stereomicroscopic pictures in
Figure 5,
Figure 6,
Figure 7,
Figure 8,
Figure 9 and
Figure 10. A stereomicroscope was used to take these pictures, enabling the close examination of the specimens’ surface appearance and structural features.
4. Discussion
The present study aimed to investigate the effect of delay time (1 day, 7 days and 14 days) and storage after airborne-particle abrasion on the shear bond strength between base metal alloys and self-adhesive resin cement. The results demonstrated a significant decrease in bond strength with increasing delay time, particularly at 7 and 14 days, but the bond strength of the seal in the 14-day group was statistically similar to that of the immediate group. Therefore, the null hypothesis was denied. These findings suggest that the timing of bonding post-surface treatment plays a role in achieving optimal adhesion.
Showing bonding immediately after airborne-particle abrasion, Group 1 exhibited the highest SBS value (26.50 ± 2.74 MPa), indicating that freshly treated surfaces of base metal alloys provide optimal conditions for bonding. This superior performance can be attributed to the combination of increased surface roughness and high surface energy generated by the airborne-particle abrasion process, which enhances both mechanical retention and chemical affinity with self-adhesive resin cement. Moreover, the oxide films immediately after airborne-particle abrasion ensure intimate contact between the substrate and the resin material [
19], allowing the phosphate functional monomers in self-adhesive resin cement, such as 10-MDP, to form stable chemical interactions with the metallic oxides on the substrate surface [
4,
6]. These synergistic effects collectively contribute to the establishment of a durable and reliable adhesive interface. However, over time, spontaneous re-passivation and oxide formation occur on the surface of the BMAs, particularly due to the high content of nickel (Ni), chromium (Cr), and molybdenum (Mo), all of which are known to form stable oxide films under ambient conditions [
20].
The progressive formation of the oxide layer, especially within the 7–14 day timeframe, may prevent resin infiltration into micro-retentive areas and reduce the availability of reactive metallic sites essential for chemical bonding. These modifications can result in decreased wettability, altered surface energy, and diminished mechanical retention, leading to a significant drop in SBS values, as seen in Group 3 (15.20 ± 4.52 MPa) and Group 4 (16.01 ± 4.69 MPa). The SBS in Group 4 tends to be higher than in Group 3. It might imply the oxide growth kinetics and composition are not strictly linear with time [
21]; subtle differences in oxide porosity or chemical composition between the 7-day and 14-day surfaces may have transiently enhanced resin infiltration or chemical reactivity in a subset of specimens. However, the fact that no statistically significant difference was indicated between these two groups suggests that oxide layer formation may reach a stable level after one week, with minimal further impact on bond strength beyond this point.
Group 5, in which the specimens were sealed for 14 days before bonding, exhibited a high SBS value (25.92 ± 3.94 MPa), comparable to that of the immediate bonding group (Group 1). This result suggests that surface sealing may help preserve the integrity of the treated surface by preventing oxidation during the storage period, thereby maintaining the bonding potential over time. Interestingly, the SBS was effectively restored in Group 6 (20.66 ± 3.70 MPa) after re-airborne-particle abrasion the specimens that had been aged for 14 days prior to bonding. This finding supports the hypothesis that re-airborne-particle abrasion can mechanically remove the superficial oxide layer formed during storage, thereby re-exposing a fresh, high-energy surface favorable for chemical and micromechanical interaction with the resin cement. Nevertheless, the obtained bond strength did not fully recover to the level of the freshly airborne-particle-abraded specimens in Group 1, suggesting that prolonged storage may induce subtle, irreversible surface alterations or contamination that cannot be completely eliminated by re-airborne-particle abrasion alone. This partial recovery may also be attributed to changes in the chemical composition of the alloy surface, which could reduce the availability of reactive metal oxides essential for chemical bonding with functional monomers such as 10-MDP. Since 10-MDP interacts with metal oxides through phosphate–metal chelation, the reduced density or altered nature of these oxides after aging might limit the formation of stable chemical bonds. Therefore, while re-airborne-particle abrasion can effectively restore the micromechanical retention, its ability to completely re-establish optimal chemical interaction remains limited, highlighting the importance of minimizing the delay between surface treatment and cementation in clinical practice. It also implies that the degradation in bonding performance over time is predominantly surface-related and reversible through appropriate re-treatment [
22,
23]. These findings align with the previous literature, indicating that surface oxidation interferes with bonding mechanisms both mechanically and chemically. Moreover, studies have shown that oxide layer thickness on Ni-Cr alloys increases progressively within the first few days of exposure and can reach up to 30–50 nm, depending on humidity, temperature, and alloy composition [
24,
25]. Such a layer can act as a barrier to the functional monomers of resin cement, such as 10-MDP, which require direct contact with metal ions for effective chemical adhesion [
13,
26].
The failure mode analysis further supports the bond strength findings. Groups with prolonged delays (7 and 14 days) exhibited 100% adhesive failure, indicating weak bonding at the interface between the BMA surface and the resin cement, likely due to surface oxidation. In contrast, groups with higher SBS values such as immediate bonding, sealing, and re-sandblasting showed a combination of adhesive and mixed failures, with 20% of specimens in each group exhibiting mixed failure. This pattern suggests that as bond strength increases, the mode of failure shifts from purely adhesive to mixed failure. The presence of mixed failure indicates that the interfacial bond is sufficiently strong [
6,
27,
28,
29], causing stress during testing to be partially absorbed by the resin cement itself. Therefore, mixed failure may be considered a sign of more effective and durable adhesion between BMAs and resin cement.
From a clinical perspective, and following clinical guidelines, these results suggest that bonding procedures should be performed as soon as possible after airborne-particle abrasion. Alternatively, sealing the airborne-particle-abraded restorations in a vacuum bag immediately after surface treatment can serve as a practical method to preserve the surface integrity. This approach allows the maintenance of surface reactivity without requiring immediate re-airborne-particle abrasion, thus saving chairside time and avoiding unnecessary surface damage. Consequently, the surface retains its high reactivity and readiness for adhesive bonding even after delayed cementation. If simple storage in ambient air lasts for a long time, re-airborne-particle abrasion of the surface before cementation is still the best way to restore surface reactivity. This guideline is particularly relevant for laboratory-to-clinic transfers or multi-stage prosthodontic workflows where time delays are unavoidable.
Limitations and recommendations for further study are as follows: This study was conducted in a controlled laboratory condition, which may not fully replicate clinical situations. Additionally, we only evaluated only one type of base metal alloy and self-adhesive resin cement. The specimens of this study were stored for only 24 h before testing; these factors limited clinical significance. Future research should encompass a broader range of alloy compositions and resin cement types, while also incorporating intraoral simulation conditions such as thermal cycling, moisture exposure, and long-term aging to enhance clinical relevance. Moreover, detailed surface characterization is recommended through advanced analytical techniques, including scanning electron microscopy (SEM) and complementary spectroscopic analyses, to quantitatively assess both morphological and chemical alterations of the treated surfaces.