Sol–Gel Synthesis of Carbon-Containing Na3V2(PO4)3: Influence of the NASICON Crystal Structure on Cathode Material Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Physico-Chemical Methods
3. Results and Discussion
3.1. Characterization of Obtained Materials
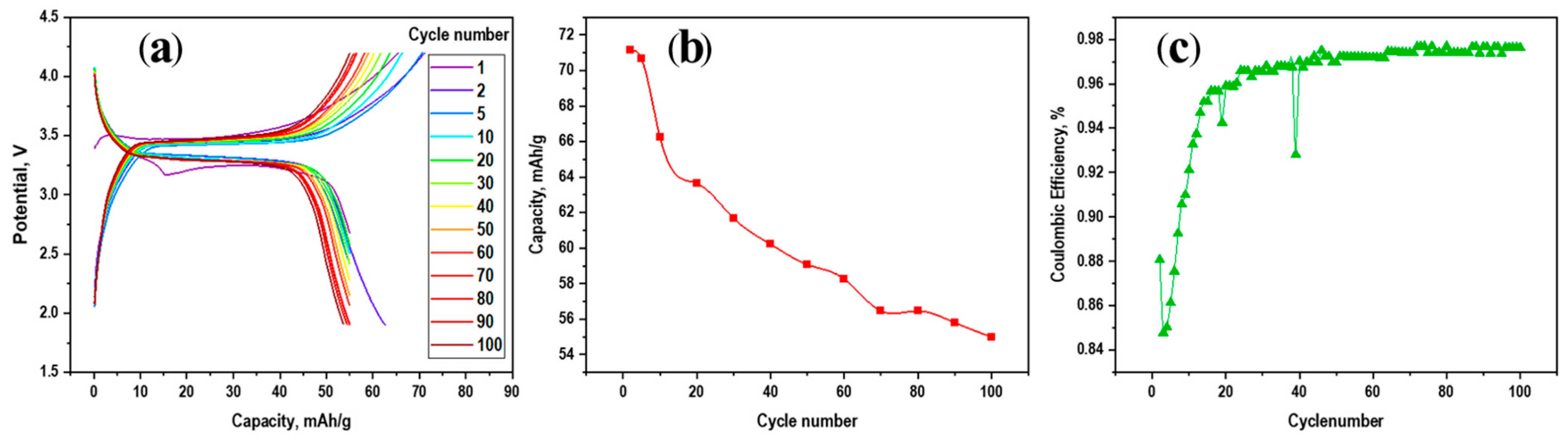
3.2. Electrochemical Performance of the Investigated Materials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Xie, B.; Hu, Q.; Zhao, R.; Zheng, Q.; Huang, X.; Deng, S.; Huo, Y.; Zhao, J.; Xu, B.; et al. Regulating the helmholtz plane by trace polarity additive for long-life Zn ion batteries. Energy Storage Mater. 2024, 66, 103202. [Google Scholar] [CrossRef]
- Che, H.; Yang, X.; Yu, Y.; Pan, C.; Wang, H.; Deng, Y.; Li, L.; Ma, Z.-F. Engineering optimization approach of nonaqueous electrolyte for sodium ion battery with long cycle life and safety. Green Energy Environ. 2021, 6, 212–219. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, C.; Chen, Y.; Wang, Y.; Guo, L. Construction of simultaneous modified Na3V2(PO4)3/C cathode with K/Zr substitution and carbon nanotubes enwrapping for high performance sodium ion battery. Ceram. Int. 2022, 48, 397–406. [Google Scholar] [CrossRef]
- Cui, G.; Dong, Q.; Wang, Z.; Liao, X.-Z.; Yuan, S.; Jiang, M.; Shen, Y.; Wang, H.; Che, H.; He, Y.-S.; et al. Achieving highly reversible and fast sodium storage of Na4VMn(PO4)3/C-rGO composite with low-fraction rGO via spray-drying technique. Nano Energy 2021, 89, 106462. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Dai, Y.; Jin, H. Homogeneous Na+ transfer dynamic at Na/Na3Zr2Si2PO12 interface for all solid-state sodium metal batteries. Nano Energy 2021, 88, 106293. [Google Scholar] [CrossRef]
- Liu, Y.; Ullah, M.M.; Gao, X.; Liu, P.; Li, Y.; Wang, W. Hierarchical fragmented Na3V2(PO4)3@reduced graphene composites with enhanced sodium-ion storage performance. J. Power Sources 2025, 631, 236230. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Wu, Q.; Li, L.; Mou, P.; Chen, Z.; Yu, H.; Yan, L.; Shu, J.; Zhang, L. Tough and temperature-resistant mask-based separator realized by K-β″-Al2O3 protective layer for stabilizing NaK liquid metal batteries. Chem. Eng. J. 2024, 499, 156336. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; He, S.; Zhang, B.; Guo, L. A new high-voltage plateau of Na3V2(PO4)3 for sodium ion batteries: A promising cathode with high energy density. Ceram. Int. 2021, 47, 26579–26583. [Google Scholar] [CrossRef]
- Yao, G.; Zhang, X.; Yan, Y.; Zhang, J.; Song, K.; Shi, J.; Mi, L.; Zheng, J.; Feng, X.; Chen, W. Facile synthesis of hierarchical Na2Fe(SO4)2@rGO/C as high-voltage cathode for energy density-enhanced sodium-ion batteries. J. Energy Chem. 2020, 50, 387–394. [Google Scholar] [CrossRef]
- Jiang, C.; Yu, Q.; Ding, Y.L. Enabling high working voltage and rate capability of NASICON cathode via moderately regulating coordination environment. J. Energy Storage 2025, 119, 116398. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Sun, X.; Zhang, B.; He, S.; Li, L.; Wang, C. Preventing structural degradation from Na3V2(PO4)3 to V2(PO4)3: F-doped Na3V2(PO4)3/C cathode composite with stable lifetime for sodium ion batteries. J. Power Sources 2018, 378, 423–432. [Google Scholar] [CrossRef]
- Lin, B.; Wu, X.; He, X.; Ye, J.; Song, Y.; Jiang, P.; Bai, L.; Liao, X.; Li, Y.; Huo, Y.; et al. High-entropy doping in NASICON cathodes: Activating the V4+/V5+ redox couple and inducing a reversible single solid-solution phase reaction for advanced sodium ion batteries. J. Colloid Interface Sci. 2025, 690, 137299. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lou, C.; Lu, Y.; He, L.; Tang, M.; Wu, X.-L.; Lu, F. A cationic-ordering NASICON-type cathode with high-capacity and long-life span for sodium-ion batteries. Chem. Eng. J. 2025, 512, 162741. [Google Scholar] [CrossRef]
- Shi, H.; Chen, Y.; Li, J.; Guo, L. Outstanding long cycle stability provide by bismuth doped Na3V2(PO4)3 enwrapped with carbon nanotubes cathode for sodium-ion batteries. J. Colloid Interface Sci. 2023, 652, 195–207. [Google Scholar] [CrossRef]
- Yang, X.; Li, W.; Duan, J.; Xin, Y.; Han, H.; Wang, D. Synthesis and properties optimization of Na3(VOPO4)2F cathode material for sodium-ion batteries by co-precipitation method. Int. J. Electrochem. Sci. 2024, 19, 100704. [Google Scholar] [CrossRef]
- Ding, X.; Huang, X.; Zhou, S.; Xiao, A.; Chen, Y.; Zuo, C.; Jin, J. Synthesis of Na3V2 (PO4)3/C composites as high-performance cathode materials for sodium ion batteries. Int. J. Electrochem. Sci. 2019, 14, 2815–2821. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, Q.; Zhang, S.; Liu, C.; Wang, H.; Liu, X. Enhanced Na+/electron transport of NASICON by multiple regulations for sodium ion battery with high rate capability and ultralong lifespan. J. Alloys Compd. 2024, 983, 173858. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Q.; Guo, P.; Liu, X. Rational design of two dimensional single crystalline Na3V2(PO4)2F3 nanosheets for boosting Na+ migration and mitigating grain pulverization. Chem. Eng. J. 2022, 439, 135533. [Google Scholar] [CrossRef]
- Zhu, P.; Peng, W.; Guo, H.; Li, X.; Wang, Z.; Wang, D.; Duan, J.; Wang, J.; Yan, G. Toward high-performance sodium storage cathode: Construction and purification of carbon-coated Na3V2(PO4)2F3 materials. J. Power Sources 2022, 546, 231986. [Google Scholar] [CrossRef]
- Deng, L.; Yu, F.-D.; Xia, Y.; Jiang, Y.-S.; Sui, X.-L.; Zhao, L.; Meng, X.-H.; Que, L.-F.; Wang, Z.-B. Stabilizing fluorine to achieve high-voltage and ultra-stable Na3V2(PO4)2F3 cathode for sodium ion batteries. Nano Energy 2021, 82, 2020. [Google Scholar] [CrossRef]
- Iordache, M.; Oubraham, A.; Bazga, M.; Ungureanu, G.E.; Borta, S.E.; Marinoiu, A. Assessing the Efficacy of Seawater Batteries Using NASICON Solid Electrolyte. Appl. Sci. 2025, 15, 3469. [Google Scholar] [CrossRef]
- Fan, D.; Wang, Y.; Zhao, X.; Jin, J.; Shen, Q.; Li, Z.; Qu, X.; Jiao, L.; Liu, Y.; Guo, Z. A novel NASICON-Na3.4MnV0.2Cr0.2Ti0.6(PO4)3 cathode with ultrahigh energy density and remarkable cycling stability toward practical Na-ion batteries. Mater. Today 2025, 86, 63–73. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, H.; Bao, Y.; Li, S.; Chen, Y. Investigating the effect of calcination temperature on the electrochemical properties of Na4MnV(PO4)3/NC@CNTs cathode materials for sodium ion batteries. J. Energy Storage 2024, 90, 111910. [Google Scholar] [CrossRef]
- Ragupathi, M.; Selvan, R.K. K-ion intercalation behavior of carbon-coated K3V2(PO4)3 nanostructures prepared by the sol-gel method. Ceram. Int. 2024, 50, 14490–14496. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Iorio, F.B.R.D.; Liberatore, A.M.A.; Koh, I.H.J.; Otani, C.; Camilo, F.F. Ozonated Mineral Oil: Preparation, Characterization and Evaluation of the Microbicidal Activity. Ozone Sci. Eng. 2016, 38, 253–260. [Google Scholar] [CrossRef]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J. Electron. Spectros. Relat. Phenom. 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Ma, B.; Liang, Y.; Ma, X.; Wang, W.; Miao, C.; Yu, G.; Wang, Q.; Xu, C.; Cui, X. High-entropy doping for high-performance Na3V2(PO4)3@C cathode materials in sodium-ion and hybrid lithium/sodium-ion batteries. J. Colloid Interface Sci. 2025, 696, 137824. [Google Scholar] [CrossRef]
- Liao, X.; Wu, X.; Xie, M.; Li, X.; Li, Y.; Fu, Z.; Su, G.; Fang, C.; Zhang, H.; Zheng, Q.; et al. Leveraging high-entropy substitution to achieve V4+/V5+ redox couple and superior Na+ storage in Na3V2(PO4)3-based cathodes for sodium-ion battery. Energy Storage Mater. 2025, 77, 104166. [Google Scholar] [CrossRef]
- Lu, F.; Lu, Y.; Zhao, L. Dual carbon decorated Na3.5Mn0.5V1.5(PO4)3 cathode with high-density and long-cycling span-life for sodium-ion batteries. J. Power Sources 2025, 645, 237194. [Google Scholar] [CrossRef]
- Boutelle, E.; Chen, A.; Gopal, R.; Bai, P. One-Pot Aqueous Synthesis of Hierarchical Na3V2(PO4)3 Particles for High-Performance Sodium Batteries. J. Electrochem. Soc. 2025, 172, 060532. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Q.; Shen, X.; Sun, X.; Li, X.; Li, G.; Chen, D.; Li, Q.; Yuan, X.; Liu, Y. Space-mediated confinement engineering of NaTi2(PO4)3 inside hollow carbon nanofibers via coaxial electrospinning: Enabling ultra-robust and highly-efficient faradic capacitive deionization. Sep. Purif. Technol. 2025, 362, 131978. [Google Scholar] [CrossRef]
- Zhao, J.; Zou, B.; Yan, W.; Li, S.; Wu, W.; Wang, W.; Li, S.; Bai, Y. Accelerating Electrochemical Responses of Na4VMn(PO4)3 via Bulk-Defects and Architecture Engineering for High-Performance Sodium-Ion Batteries. Adv. Sci. 2025, 12, e2415331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, W.; Bai, J.; Wang, Q.; Ding, B.; Gong, H. Potassium-pillared Na4FeV(PO4)3@C cathode for high-performance sodium-ion batteries. Electrochem. Commun. 2025, 173, 107883. [Google Scholar] [CrossRef]
- Zhan, H.; Gao, L.; Li, J.; Zhang, C.; Wu, Y.; Tan, M.; Wang, Q.; Lv, F.; Tao, L.; Cao, M. Potassium regulated Na4MnV(PO4)3 microspheres cathode towards robust sodium ion batteries. Vacuum 2024, 233, 113947. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Zhang, C.; Zhang, X.; Zhang, Z.; Wang, Y.; Zhou, B.; Shen, J. Seed strategy synthesis of porous NASICON structured NaTi2(PO4)3/carbon aerogel nanocomposites for high-performance hybrid capacitive deionization. Sep. Purif. Technol. 2025, 354, 129092. [Google Scholar] [CrossRef]
- Li, T.; Yang, D.-X.; Xu, G.-R.; Tang, A.-P.; Zheng, J.-C.; Zhang, X.-H.; Tang, L.-B.; Huang, Y.-D.; Chen, H.-Z. A novel NASICON-typed Na3V1.96Cr0.03Mn0.01(PO4)2F3 cathode for high-performance Na-ion batteries. J. Energy Storage 2024, 104, 114596. [Google Scholar] [CrossRef]
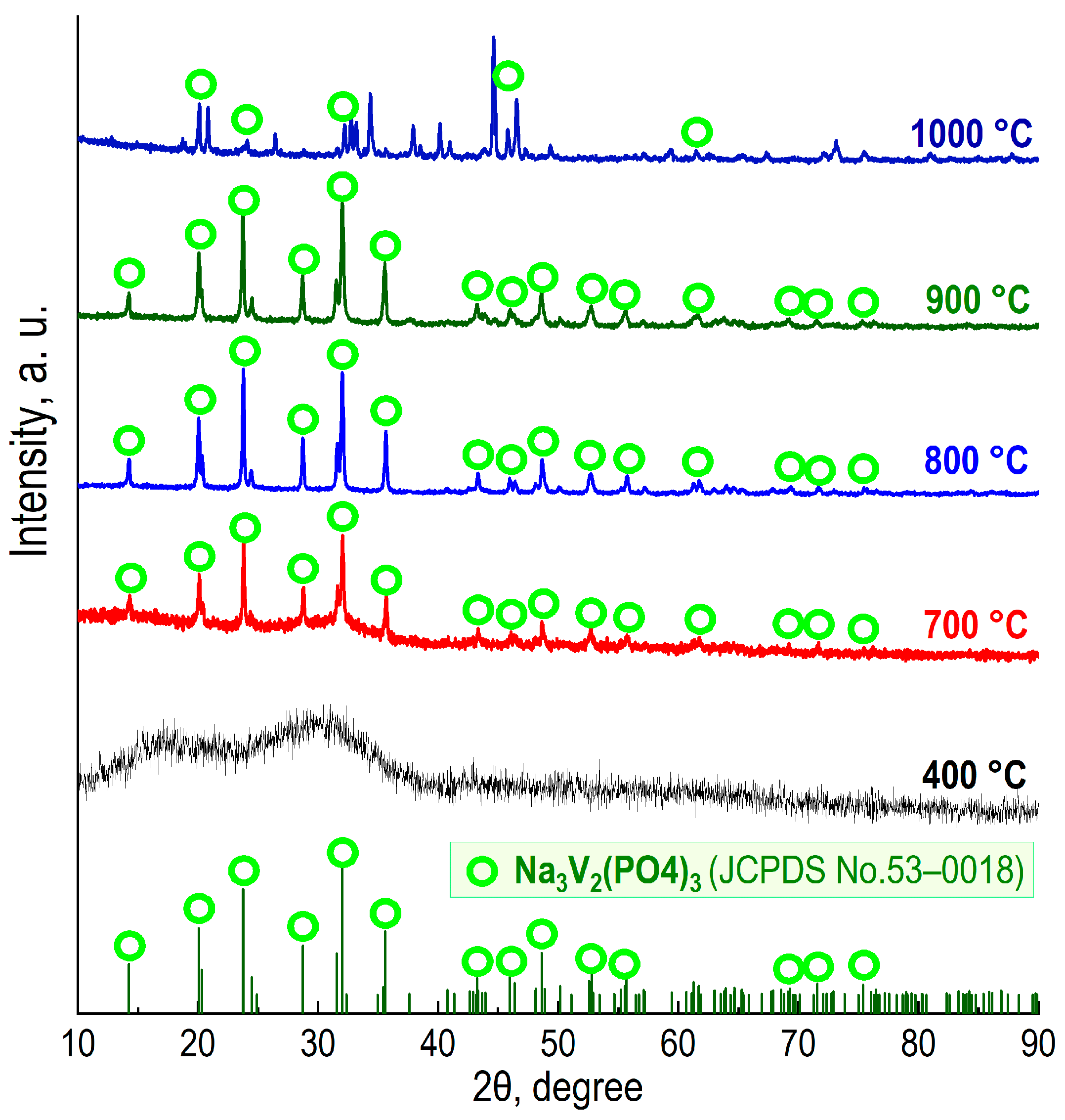

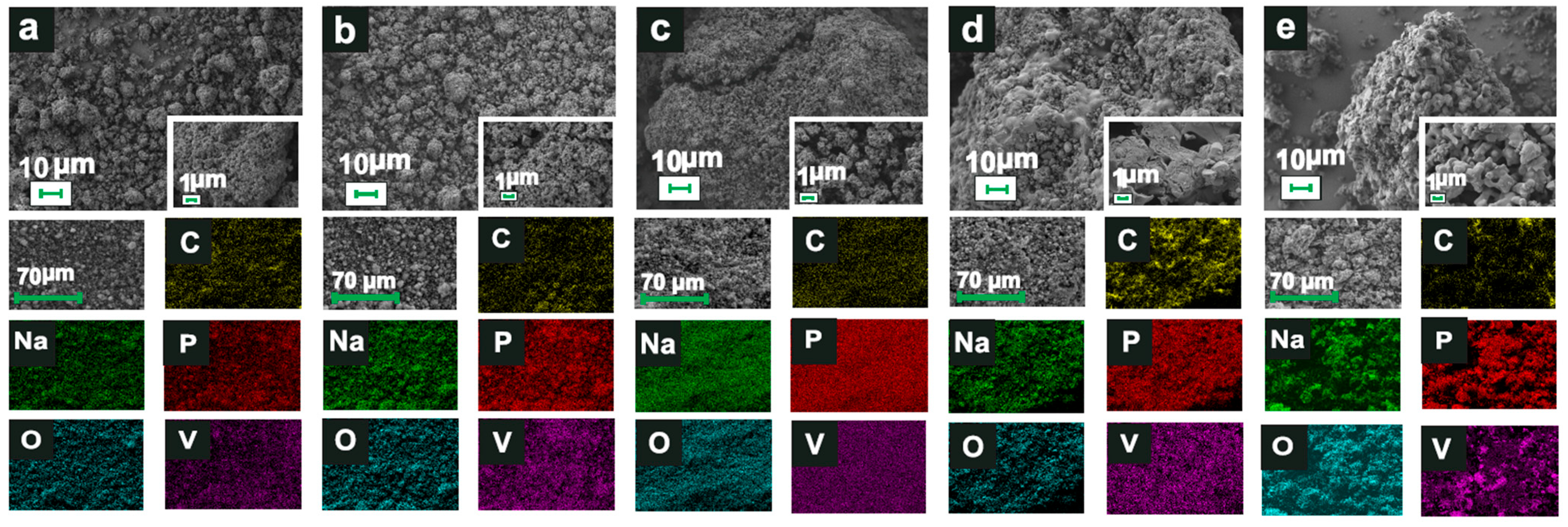




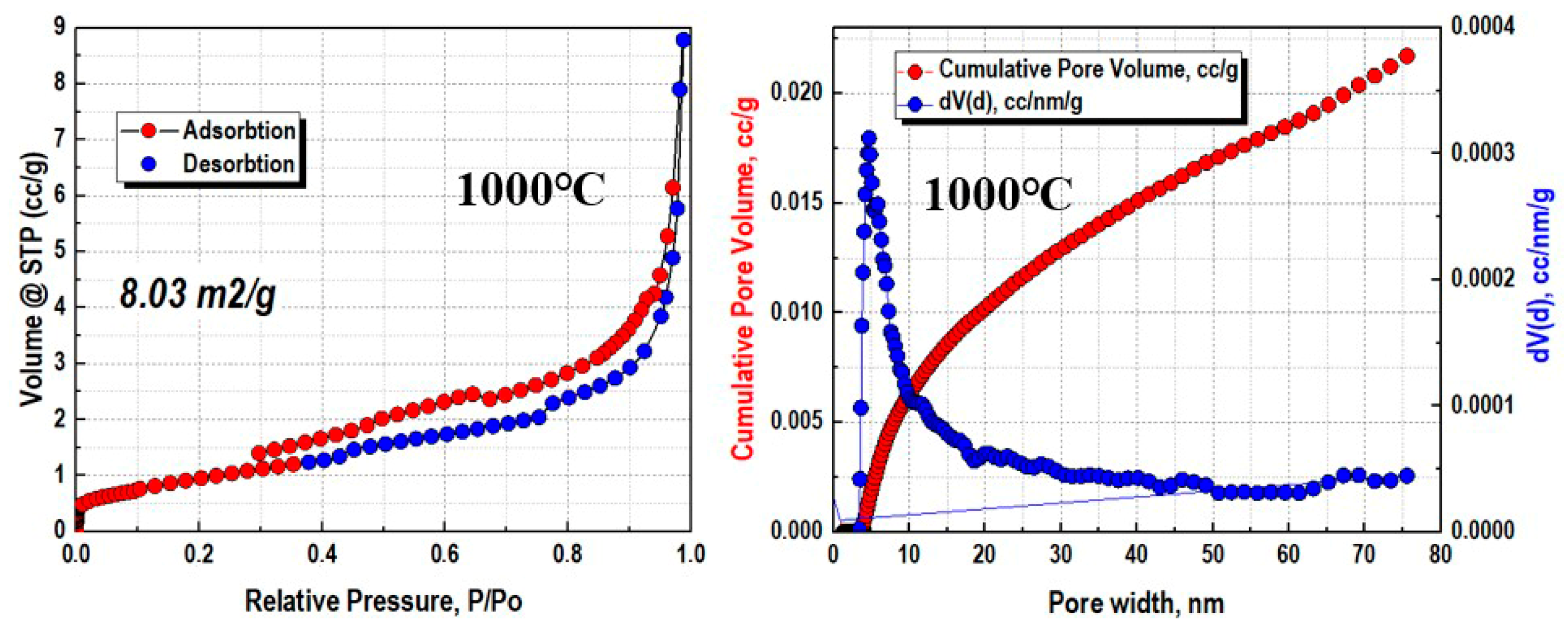
| Sample | Identified Phase | Unit Cell Parameters, Å | Calculated Density, g/cm3 | V, Å3 | Crystallite Size, nm | Rw, % | |
|---|---|---|---|---|---|---|---|
| 900 °C | Na3V2(PO4)3 Rc (№ 167) | a = 8.73907 | c = 21.79022 | 3.150 | 1441.196 | 81.6 | 5.537 |
| 800 °C | Na3V2(PO4)3 Rc (№ 167) | a = 8.72064 | c = 21.84907 | 3.155 | 1439.000 | 84.8 | 5.444 |
| 700 °C | Na3V2(PO4)3 Rc (№ 167) | a = 8.71631 | c = 21.82557 | 3.162 | 1436.023 | 86.2 | 4.835 |
| Na3V2(PO4)3 at Different Temperatures | Spin–Orbit Splitting, eV | |
|---|---|---|
| V3+ 2p(3/2-1/2) | V4+ 2p(3/2-1/2) | |
| 400 °C | 6.9 | — |
| 700 °C | 6.8 | — |
| 800 °C | 7.0 | — |
| 900 °C | 6.7 | — |
| 1000 °C | 7.1 | 6.6 |
| Sample | Synthesis Method | Precursor | Specific Surface Area, m2 g−1 | Average Pore Size, nm | Capacity, mA h g−1 | Capacity Retention, % | Reference |
|---|---|---|---|---|---|---|---|
| Na3V2(PO4)3 with (Ti4+, Mn2+, Fe2+, Zr4+ и Mo6+) doping | High-entropy sol–gel | Ethylene glycol | 37.99 | 2–5 | 104.8 | 97.15 | [30] |
| Na3.75Fe0.75V1.25(PO4)3 | Sol–gel with subsequent calcination | Citric acid | 65.1 | 3.91–5.79 | 111.4 | 88.7 | [13] |
| Na3.5Mn0.5V1.5 (PO4)3 | Sol–gel | Citric acid | 31.7 | 3.8 | 112.9 | 86.3 | [32] |
| Na3V1.45(Fe,Al,Cr,Mn,Ni)0.5Mo0.02Zr0.03(PO4)3 | Solid-state reaction | - | 157.99 | - | 112.2 | 93.5 | [31] |
| Na3V2(PO4)3 | all milling with spray drying followed by calcination | - | 39.55 | 10–30 | 118 | 88.7 | [33] |
| NaTi2(PO4)3 | Hydrothermal synthesis | Ethylene glycol | 74.4 | - | 158.2 | 93.8 | [34] |
| Na3.4MnV0.2Cr0.2Ti0.6(PO4)3 | Sol–gel | - | 117.6 | 7.98 | 176.7 | 92.4 | [22] |
| Na4VMn (PO4)3 | - | - | 27.4 | 120.1 | 94.5 | [35] | |
| Na3.9K0.1FeV (PO4)3@C | Sol–gel method with subsequent high-temperature calcination | Citric acid | 54.2 | 3.70 | 83.85 | 91.7 | [36] |
| Na4MnV(PO4)3 | Sol–gel with subsequent spray drying | 35.9 | 47.6 | 49.5 | [37] | ||
| Na4K0.1MnV (PO4)3 | Sol–gel with subsequent spray drying | 33.2 | 78.0 | 79.8 | [37] | ||
| NaTi2(PO4)3/carbon aerogel | Sol–gel polycondensation | Hexadecyltrimethylammonium bromide | 420 | 6.4 | [38] | ||
| NaTi2(PO4)3 | Sol–gel polycondensation | Hexadecyltrimethylammonium bromide | 8 | 16.9 | [38] | ||
| Na3V1.96Cr0.03 Mn0.01(PO4)2F3 | Sol–gel | Citric acid | 37.13 | 10 | 109.8 | 80 | [39] |
| Na3V2(PO4)2F3 | Sol–gel | Citric acid | 31.00 | 8 | 91.8 | 65.8 | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shichalin, O.O.; Priimak, Z.E.; Seroshtan, A.; Marmaza, P.A.; Ivanov, N.P.; Shurygin, A.V.; Tsygankov, D.K.; Korneikov, R.I.; Efremov, V.V.; Ognev, A.V.; et al. Sol–Gel Synthesis of Carbon-Containing Na3V2(PO4)3: Influence of the NASICON Crystal Structure on Cathode Material Properties. J. Compos. Sci. 2025, 9, 543. https://doi.org/10.3390/jcs9100543
Shichalin OO, Priimak ZE, Seroshtan A, Marmaza PA, Ivanov NP, Shurygin AV, Tsygankov DK, Korneikov RI, Efremov VV, Ognev AV, et al. Sol–Gel Synthesis of Carbon-Containing Na3V2(PO4)3: Influence of the NASICON Crystal Structure on Cathode Material Properties. Journal of Composites Science. 2025; 9(10):543. https://doi.org/10.3390/jcs9100543
Chicago/Turabian StyleShichalin, Oleg O., Zlata E. Priimak, Alina Seroshtan, Polina A. Marmaza, Nikita P. Ivanov, Anton V. Shurygin, Danil K. Tsygankov, Roman I. Korneikov, Vadim V. Efremov, Alexey V. Ognev, and et al. 2025. "Sol–Gel Synthesis of Carbon-Containing Na3V2(PO4)3: Influence of the NASICON Crystal Structure on Cathode Material Properties" Journal of Composites Science 9, no. 10: 543. https://doi.org/10.3390/jcs9100543
APA StyleShichalin, O. O., Priimak, Z. E., Seroshtan, A., Marmaza, P. A., Ivanov, N. P., Shurygin, A. V., Tsygankov, D. K., Korneikov, R. I., Efremov, V. V., Ognev, A. V., & Papynov, E. K. (2025). Sol–Gel Synthesis of Carbon-Containing Na3V2(PO4)3: Influence of the NASICON Crystal Structure on Cathode Material Properties. Journal of Composites Science, 9(10), 543. https://doi.org/10.3390/jcs9100543








