Development of 3D-Printed Carbon Capture Adsorbents by Zeolites Derived from Coal Fly Ash
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Zeolite Powders from Coal Fly Ash
2.2. Preparation of Inks for 3D-Printing of Adsorbents Based on CFAZ
2.3. 3D Printing by Extrusion of Adsorbent Structures
2.4. Characterization of Powder and 3D-Printed Adsorbents
2.5. CO2 Adsorption Tests
3. Results and Discussions
3.1. 3D Structuring of Adsorbent Elements
3.1.1. Designing 3D Models
3.1.2. Preparing a 3D Model for Printing
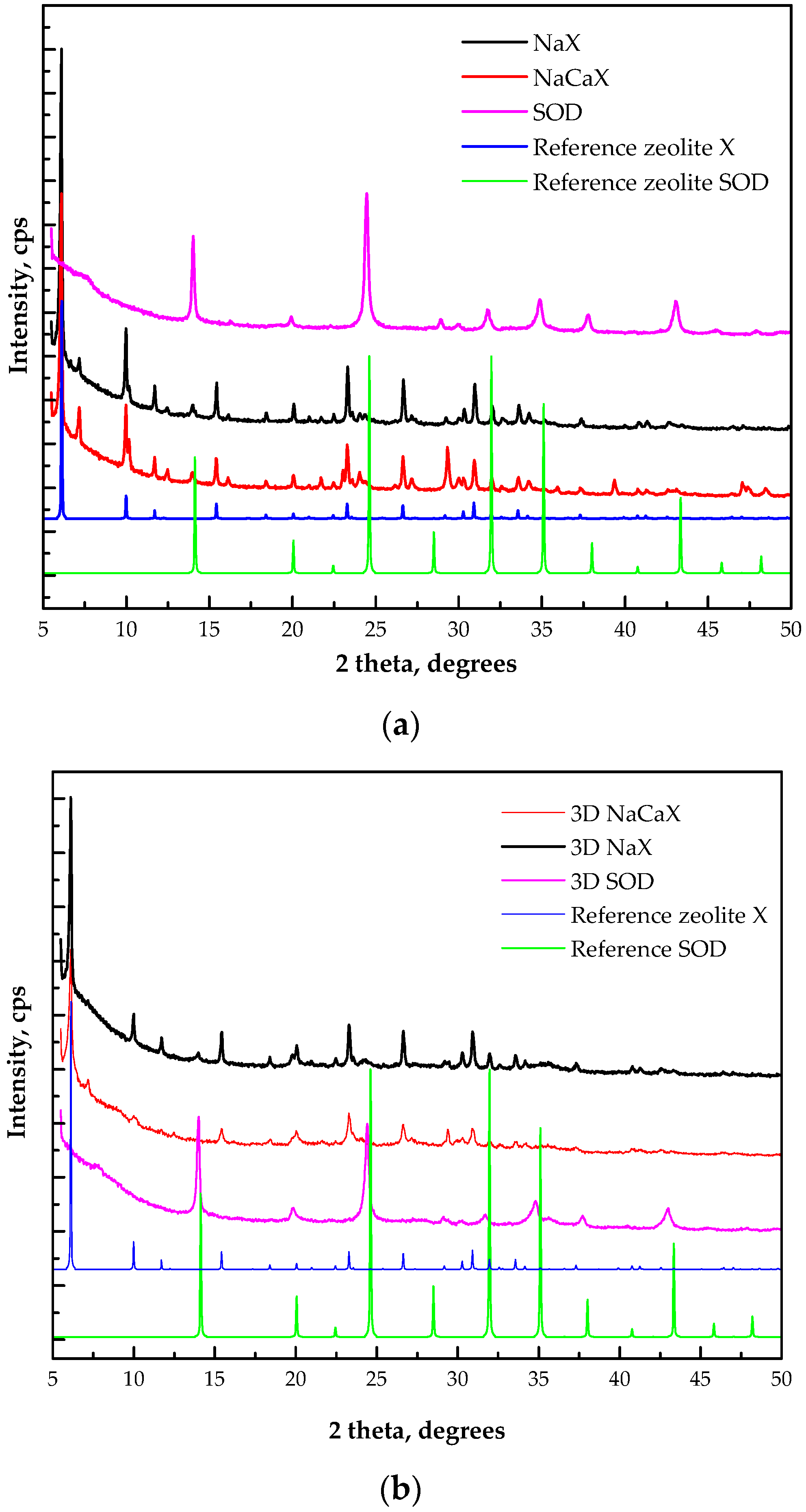
3.2. Phase Characterization of Powder and 3D Printed CFAZ Adsorbents
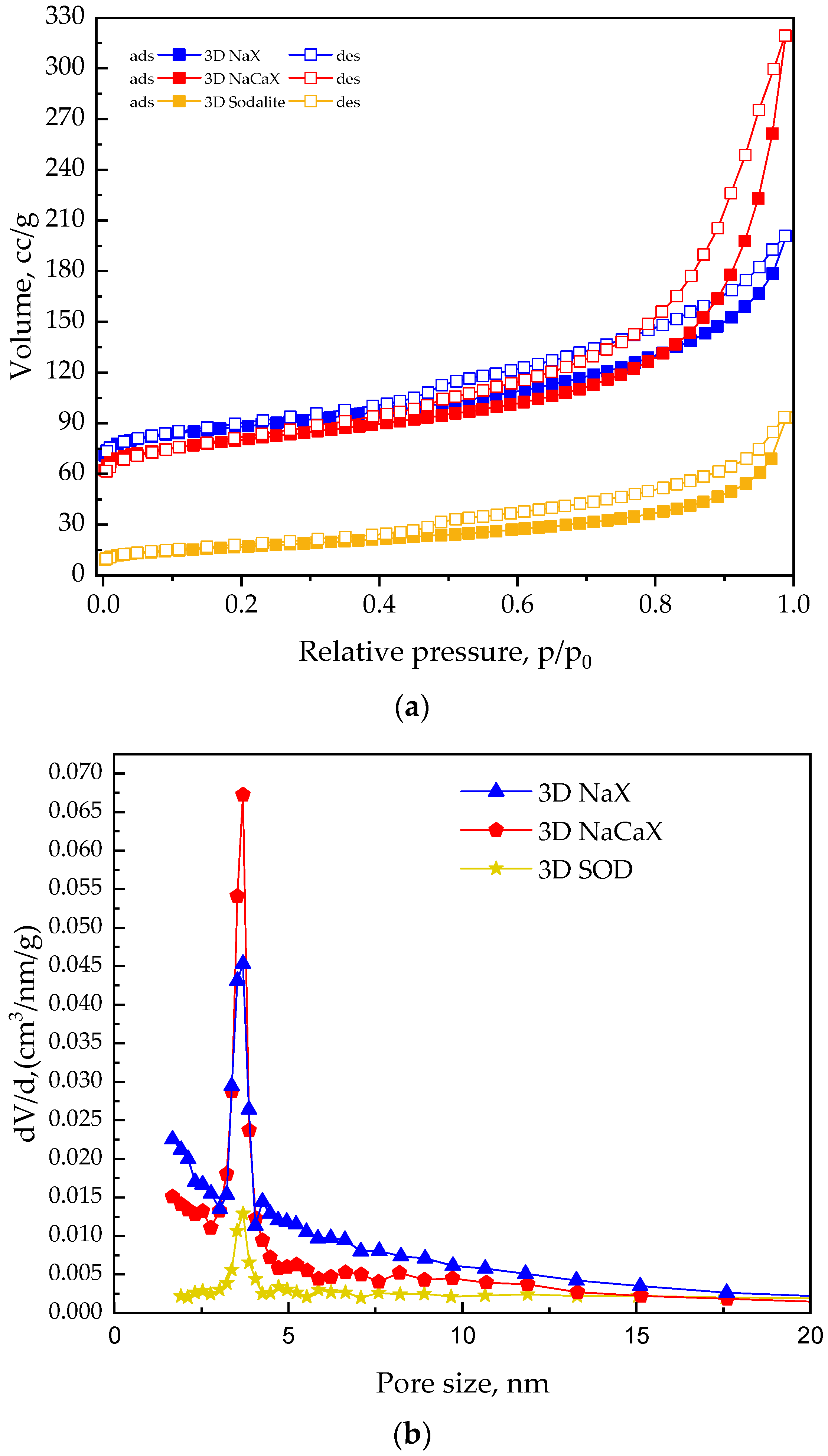
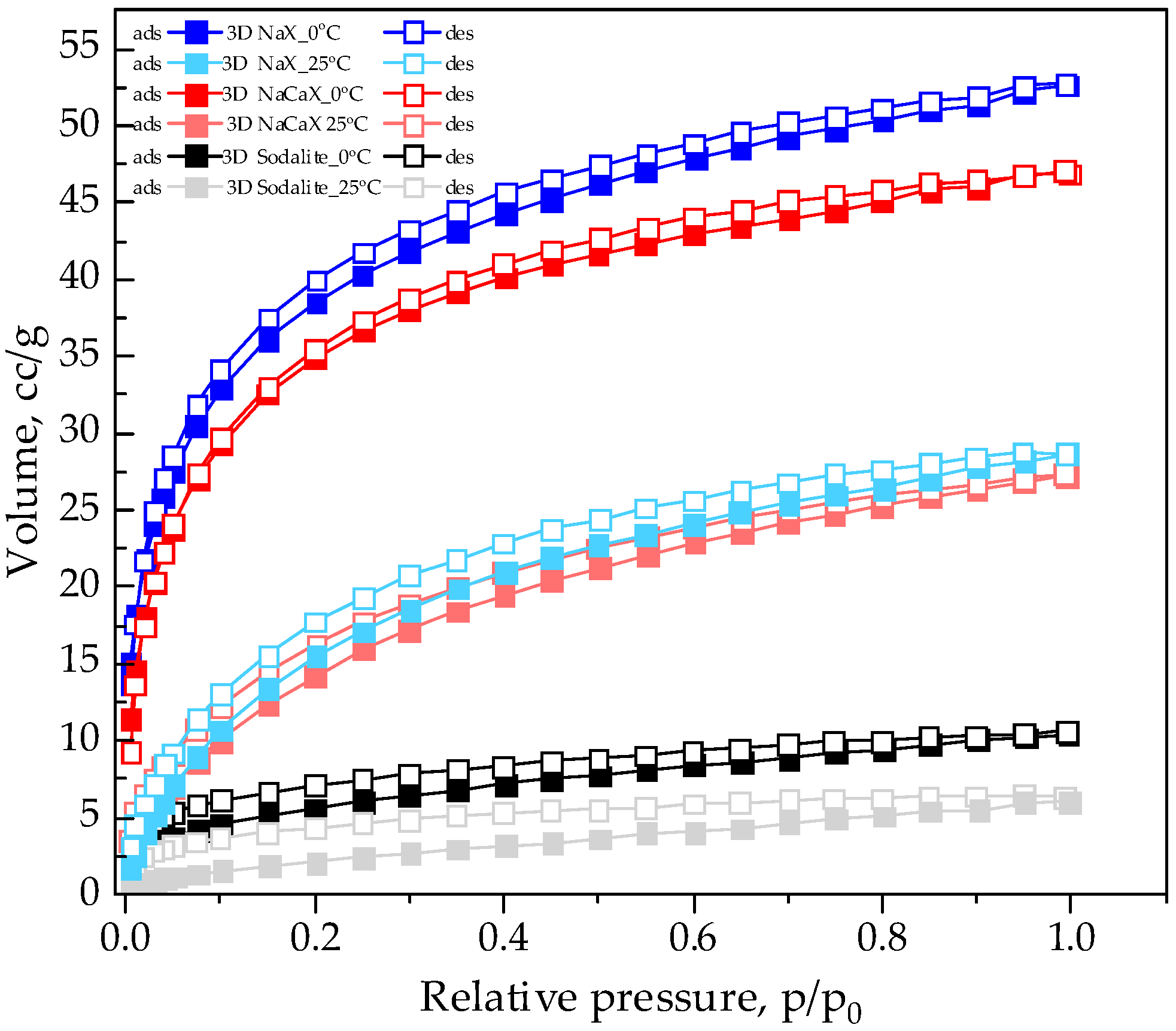
3.3. Surface Characterization of Powder CFAZ Adsorbents
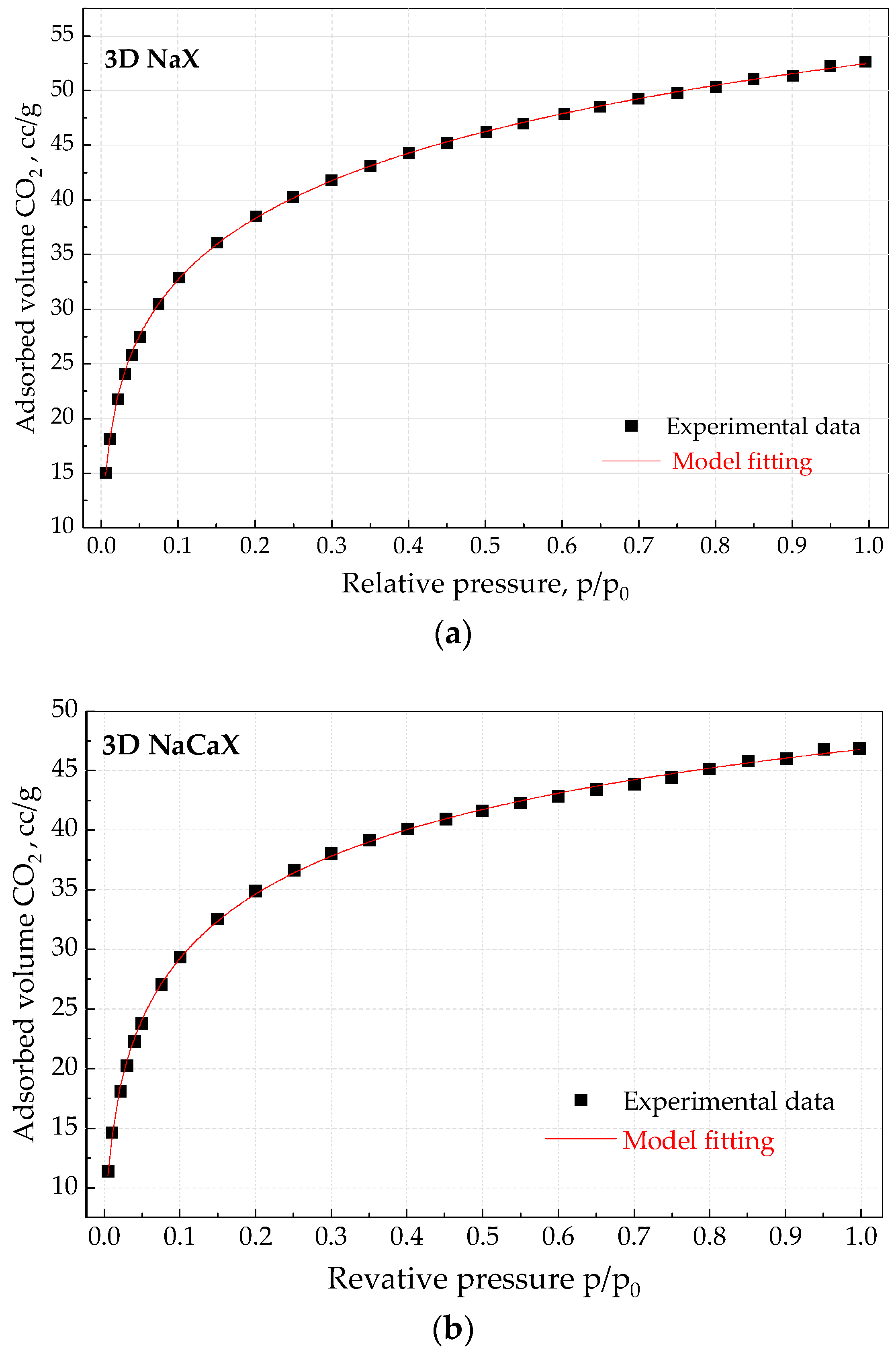
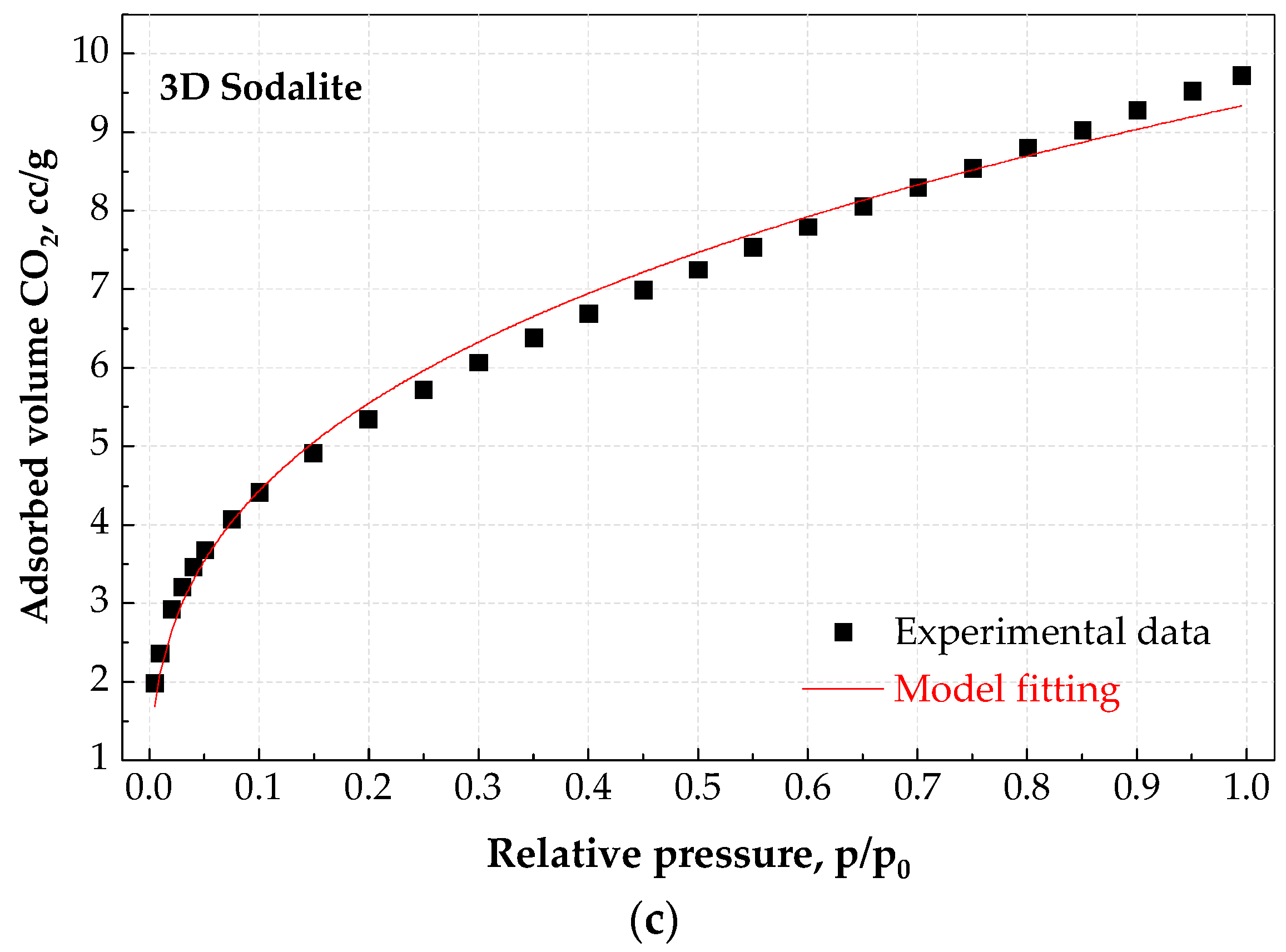
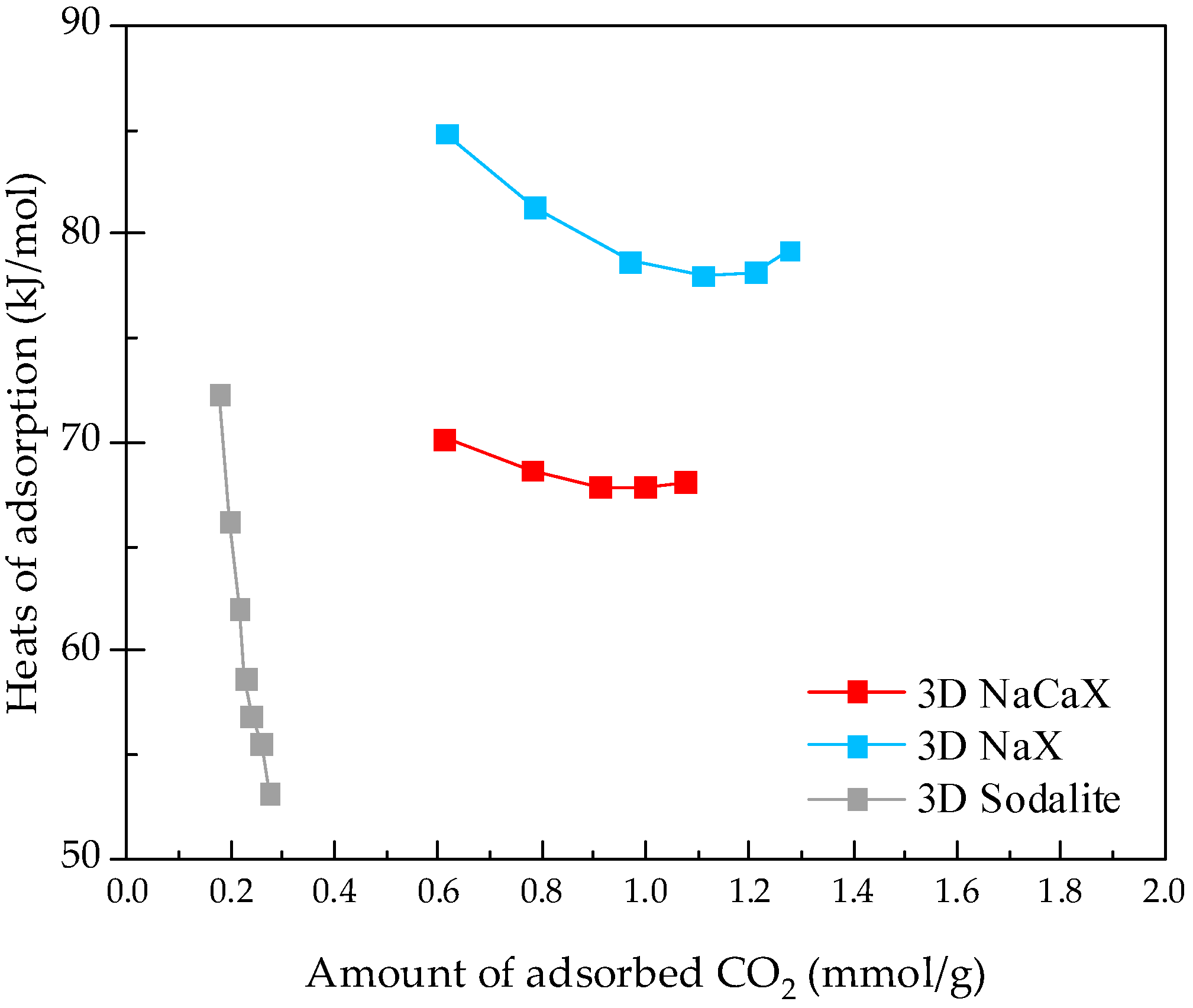
3.4. CO2 Adsorption Studies at Static Conditions
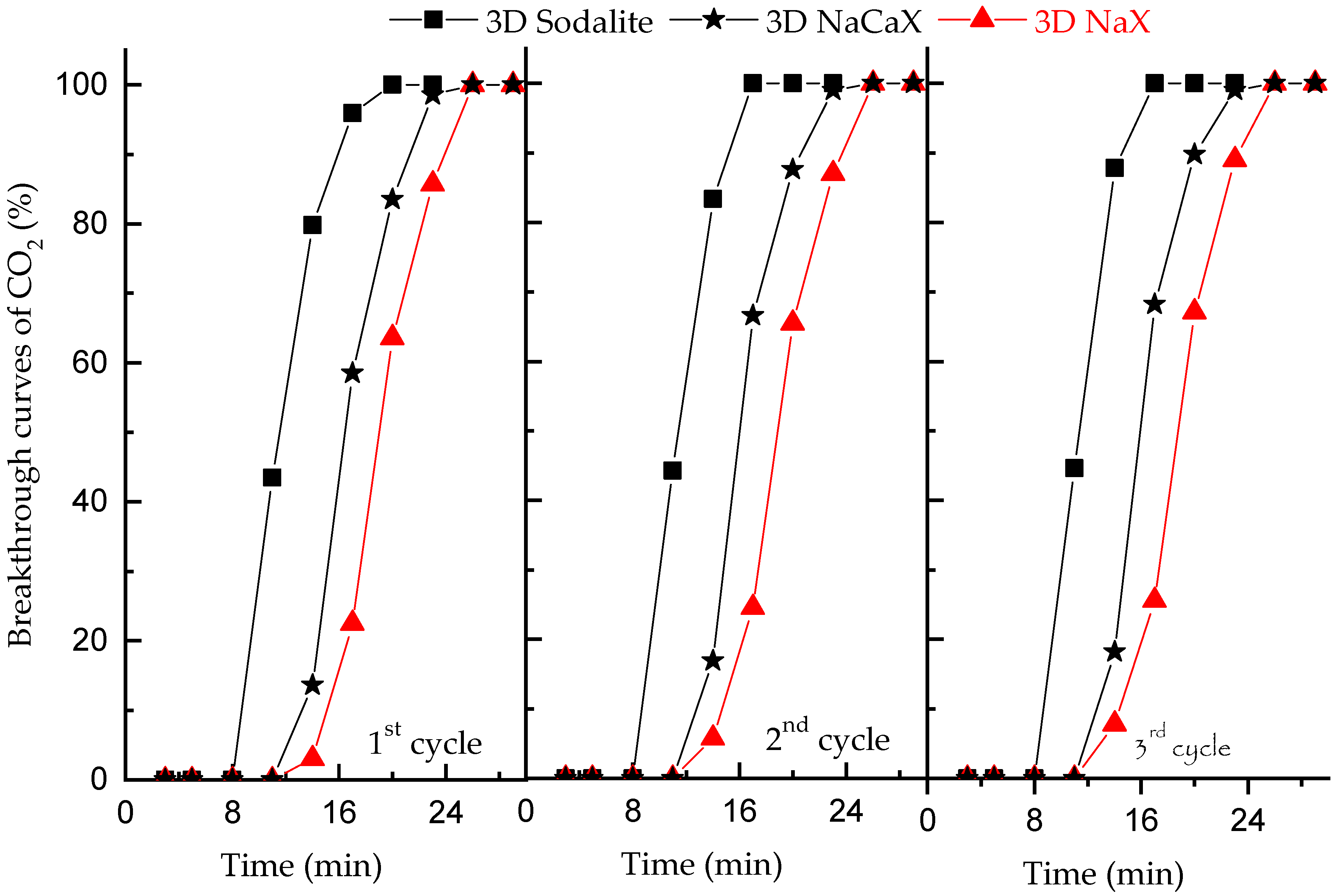
3.5. CO2 Adsorption Studies at Dynamic Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoro, K.O.; Michael, O.; Daramola, M.O. Chapter 1—CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture, 1st ed.; Rahimpour, M.R., Farsi, M., Makarem, M.A., Eds.; Woodhead Publishing: Sawston, UK; Elsevier Inc.: Duxford, UK, 2020; pp. 3–28. [Google Scholar] [CrossRef]
- Filonchyk, M.; Peterson, M.P.; Zhang, L.; Hurynovich, V.; He, Y. Greenhouse gases emissions and global climate change: Examining the influence of CO2, CH4, and N2O. Sci. Total Environ. 2024, 935, 173359. [Google Scholar] [CrossRef]
- Slameršak, A.; Kallis, G.; O’Neill, D.W. Energy requirements and carbon emissions for a low-carbon energy transition. Nat. Commun. 2022, 13, 6932. [Google Scholar] [CrossRef]
- Geweda, A.E.; Zayed, M.E.; Khan, M.Y.; Alquaity, A.B.S. Mitigating CO2 emissions: A review on emerging technologies/strategies for CO2 capture. J. Energy Inst. 2025, 118, 101911. [Google Scholar] [CrossRef]
- Baskaran, D.; Saravanan, P.; Nagarajan, L.; Byun, H.-S. An overview of technologies for capturing, storing, and utilizing carbon dioxide: Technology readiness, large-scale demonstration, and cost. Chem. Eng. J. 2024, 491, 151998. [Google Scholar] [CrossRef]
- Hanson, E.; Nwakile, C.; Hammed, V.O. Carbon capture, utilization, and storage (CCUS) technologies: Evaluating the effectiveness of advanced CCUS solutions for reducing CO2 emissions. Results Surf. Interfaces 2025, 18, 100381. [Google Scholar] [CrossRef]
- Muthuraj, R.; Mekonnen, T. Recent progress in carbon dioxide (CO2) as feedstock for sustainable materials development: Co-polymers and polymer blends. Polymer 2018, 145, 348–373. [Google Scholar] [CrossRef]
- Valluri, S.; Claremboux, V.; Kawatra, S. Opportunities and challenges in CO2 utilization. J. Environ. Sci. 2022, 113, 322–344. [Google Scholar] [CrossRef]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. Methods and Techniques for CO2 Capture: Review of Potential Solutions and Applications in Modern Energy Technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Meng, F.; Meng, Y.; Ju, T.; Han, S.; Lin, L.; Jiang, J. Research progress of aqueous amine solution for CO2 capture: A review. Renew. Sustain. Energy Rev. 2022, 168, 112902. [Google Scholar] [CrossRef]
- Loachamin, D.; Casierra, J.; Calva, V.; Palma-Cando, A.; Ávila, E.E.; Ricaurte, M. Amine-Based Solvents and Additives to Improve the CO2 Capture Processes: A Review. Chem. Eng. 2024, 8, 129. [Google Scholar] [CrossRef]
- Dziejarski, B.; Serafin, J.; Andersson, K.; Krzyżyńska, R. CO2 capture materials: A review of current trends and future challenges. Mater. Today Sustain. 2023, 24, 100483. [Google Scholar] [CrossRef]
- Soo, X.Y.D.; Lee, J.J.C.; Wu, W.-Y.; Tao, L.; Wang, C.; Zhu, Q.; Bu, J. Advancements in CO2 capture by absorption and adsorption: A comprehensive review. J. CO2 Util. 2024, 81, 102727. [Google Scholar] [CrossRef]
- Das, A.; Peu, S.D.; Hossain, M.S.; Nahid, M.M.A.; Karim, F.R.B.; Chowdhury, H.; Porag, M.H.; Argha, D.B.P.; Saha, S.; Islam, A.R.M.T.; et al. Advancements in adsorption based carbon dioxide capture technologies—A comprehensive review. Heliyon 2023, 9, e22341. [Google Scholar] [CrossRef]
- Khosrowshahi, M.S.; Aghajari, A.A.; Rahimi, M.; Maleki, F.; Ghiyabi, E.; Rezanezhad, A.; Bakhshi, A.; Salari, E.; Shayesteh, H.; Mohammadi, H. Recent progress on advanced solid adsorbents for CO2 capture: From mechanism to machine learning. Mater. Today Sustain. 2024, 27, 100900. [Google Scholar] [CrossRef]
- Ye, W.; Liang, W.; Luo, Q.; Liang, X.; Chen, C. Recent progress in CO2 capture by porous solid materials. Ind. Eng. Chem. Res. 2025, 64, 4148–4178. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, R. A critical review on new and efficient adsorbents for CO2 capture. Chem. Eng. J. 2024, 485, 149495. [Google Scholar] [CrossRef]
- Rezaei, F.; Sakwa-Novak, M.A.; Bali, S.; Duncanson, D.M.; Jones, C.W. Shaping amine-based solid CO2 adsorbents: Effects of pelletization pressure on the physical and chemical properties. Micropor. Mesopor. Mat. 2015, 204, 34–42. [Google Scholar] [CrossRef]
- Akhtar, F.; Kaiser, A. Design and structuring of porous sorbents for CO2 capture and separation. Curr. Opin. Green Sustain. Chem. 2024, 50, 100966. [Google Scholar] [CrossRef]
- Nedoma, M.; Azzan, H.; Yio, M.; Danaci, D.; Itskou, I.; Kia, A.; Pini, R.; Petit, C. The effect of adsorbent shaping on the equilibrium and kinetic CO2 adsorption properties of ZIF-8. Micropor. Mesopor. Mat. 2024, 380, 113303. [Google Scholar] [CrossRef]
- Popova, M.; Boycheva, S.; Lazarova, H.; Zgureva, D.; Lázár, K.; Szegedi, Á. VOC oxidation and CO2 adsorption on dual adsorption/catalytic system based on fly ash zeolites. Catal. Today 2020, 357, 518–525. [Google Scholar] [CrossRef]
- Boycheva, S.; Zgureva, D.; Lazarova, H.; Popova, M. Comparative studies of carbon capture onto coal fly ash zeolites Na-X and Na–Ca-X. Chemosphere 2021, 271, 129505. [Google Scholar] [CrossRef]
- Zgureva, D.; Boycheva, S. Experimental and model investigations of CO2 adsorption onto fly ash zeolite surface in dynamic conditions. Sustain. Chem. Pharm. 2020, 15, 100222. [Google Scholar] [CrossRef]
- Boycheva, S.; Marinov, I.; Zgureva-Filipova, D. Studies on the CO2 capture by coal fly ash zeolites: Process design and simulation. Energies 2021, 14, 8279. [Google Scholar] [CrossRef]
- Boycheva, S.; Zgureva, D.; Lazarova, K.; Babeva, T.; Popov, C.; Lazarova, H.; Popova, M. Progress in the Utilization of Coal Fly Ash by Conversion to Zeolites with Green Energy Applications. Materials 2020, 13, 2014. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Jiang, X.; Zhao, Y.; Yan, J. Zeolite greenly synthesized from fly ash and its resource utilization: A review. Sci. Total Environ. 2022, 851, 158182. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Liu, Z.; Yu, L.; Wang, W. Fly ash-based zeolites: From waste to value—A comprehensive overview of synthesis, properties, and applications. Chem. Eng. Res. Des. 2024, 212, 240–260. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Shu, C.M.; Gautam, S. Turning coal fly ash into zeolite for effective waste management. In Pollutants from Energy Sources. Energy, Environment, and Sustainability; Agarwal, R., Agarwal, A., Gupta, T., Sharma, N., Eds.; Springer: Singapore, 2019; pp. 269–290. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. A new approach for the classification of coal fly ashes based on their origin, composition, properties, and behaviour. Fuel 2007, 86, 1490–1512. [Google Scholar] [CrossRef]
- Bhatt, A.; Priyadarshini, S.; Mohanakrishnan, A.A.; Abri, A.; Sattler, M.; Techapaphawit, S. Physical, chemical, and geotechnical properties of coal fly ash: A global review. Case Stud. Constr. Mater. 2019, 11, e00263. [Google Scholar] [CrossRef]
- Alterary, S.S.; Marei, N.H. Fly ash properties, characterization, and applications: A review. J. King Saud Univ. Sci. 2021, 33, 101536. [Google Scholar] [CrossRef]
- Yue, B.; Liu, S.; Chai, Y.; Wu, G.; Guan, N.; Li, L. Zeolites for separation: Fundamental and application. J. Energy Chem. 2022, 71, 288–303. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J. Emerging applications of zeolites in catalysis, separation and host–guest assembly. Nat. Rev. Mater. 2021, 6, 1156–1174. [Google Scholar] [CrossRef]
- Rahman, R.O.A.; El-Kamash, A.M.; Hung, Y.-T. Applications of nano-zeolite in wastewater treatment: An overview. Water 2022, 14, 137. [Google Scholar] [CrossRef]
- Kordala, N.; Wyszkowski, M. Zeolite Properties, Methods of synthesis, and selected applications. Molecules 2024, 29, 1069. [Google Scholar] [CrossRef]
- Dzwigaj, S.; Massiani, P.; Davidson, A.; Che, M. Role of silanol groups in the incorporation of V in β zeolite. J. Mol. Catal. A Chem. 2000, 155, 169–182. [Google Scholar] [CrossRef]
- Steenbruggen, G.; Hollman, G.G. The synthesis of zeolites from fly ash the properties of the zeolite products. J. Geochem. Explor. 1998, 62, 305–309. [Google Scholar] [CrossRef]
- Querol, X.; Moreno, N.; Umaña, J.C.; Alastuey, A.; Hernández, E.; López-Soler, A.; Plana, F. Synthesis of zeolites from coal fly ash: An overview. Int. J. Coal Geol. 2002, 50, 413–423. [Google Scholar] [CrossRef]
- Koshlak, H. Synthesis of zeolites from coal fly ash using alkaline fusion and its applications in removing heavy metals. Materials 2023, 16, 4837. [Google Scholar] [CrossRef] [PubMed]
- Boycheva, S.; Marinov, I.; Miteva, S.; Zgureva, D. Conversion of coal fly ash into nanozeolite Na-X by applying ultrasound assisted hydrothermal and fusion-hydrothermal alkaline activation. Sustain. Chem. Pharm. 2020, 15, 100217. [Google Scholar] [CrossRef]
- Zgureva, D.; Boycheva, S.; Behunová, D.; Václavíková, M. Smart- and zero-energy utilization of coal ash from Thermal Power Plants in the context of circular economy and related to soil recovery. J. Environ. Eng. 2020, 146, 04020081. [Google Scholar] [CrossRef]
- Zgureva, D.; Stoyanova, V.; Shoumkova, A.; Boycheva, S.; Avdeev, G. Quasi natural approach for crystallization of zeolites from different fly ashes and their application as adsorbent media for malachite green removal from polluted waters. Crystals 2020, 10, 1064. [Google Scholar] [CrossRef]
- Jedli, H.; Bouzgarrou, S.M.; Hassani, R.; Sabi, E.; Slimi, K. Adsorption of CO2, CH4 and H2 onto zeolite 13 X: Kinetic and equilibrium studies. Heliyon 2024, 10, e40672. [Google Scholar] [CrossRef]
- Bahmanzadegan, F.; Ghaemi, A. Modification and functionalization of zeolites to improve the efficiency of CO2 adsorption: A review. Case Stud. Chem. Environ. Eng. 2024, 9, 100564. [Google Scholar] [CrossRef]
- Boer, D.G.; Langerak, J.; Pescarmona, P.P. Zeolites as selective adsorbents for CO2 separation. ACS Appl. Energy Mater. 2023, 6, 2634–2656. [Google Scholar] [CrossRef]
- de Aquino, T.F.; Estevam, S.T.; Viola, V.O.; Marques, C.R.M.; Zancan, F.L.; Vasconcelos, L.B.; Riella, H.G.; Pires, M.J.R.; Morales-Ospino, R.; Torres, A.E.B.; et al. CO2 adsorption capacity of zeolites synthesized from coal fly ashes. Fuel 2020, 276, 118143. [Google Scholar] [CrossRef]
- Indira, V.; Abhitha, K. A review on recent developments in Zeolite A synthesis for improved carbon dioxide capture: Implications for the water-energy nexus. Energy Nexus 2022, 7, 100095. [Google Scholar] [CrossRef]
- Che, S.; Fang, X.; Li, S.; Chen, X.; Du, T. Modification of potassium chabazites derived from fly ash by dosing extra cations: Promoted CO2 adsorption capacities and fine-tuned frameworks. ZAAC 2019, 645, 1365–1371. [Google Scholar] [CrossRef]
- Sluijter, S.N.; Boon, J.; James, J.; Krishnamurthy, S.; Lind, A.; Blom, R.; Andreassen, K.A.; Cormos, A.M.; Sandu, V.C.; de Boer, R. 3D-printing of adsorbents for increased productivity in carbon capture applications (3D-CAPS). Int. J. Greenh. Gas Control 2021, 112, 103512. [Google Scholar] [CrossRef]
- Lawson, S.; Li, X.; Thakkar, H.; Rownaghi, A.A.; Rezaei, F. Recent advances in 3D printing of structured materials for adsorption and catalysis applications. Chem. Rev. 2021, 121, 6246–6291. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, H.; Tan, W.S.; Chuah, C.Y.; Tan, M.J.; Bae, T.-H.; Song, J. Enhancing CO2 capture efficiency using optimized 3D-Printed structured adsorbents. Sep. Purif. Technol. 2025, 361, 131495. [Google Scholar] [CrossRef]
- Chisăliță, D.-A.; Boon, J.; Lücking, L. Adsorbent shaping as enabler for intensified pressure swing adsorption (PSA): A critical review. Sep. Purif. Technol. 2025, 353, 128466. [Google Scholar] [CrossRef]
- Jandyal, A.; Chaturvedi, I.; Wazir, I.; Raina, A.; Haq, M.I.U. 3D printing—A review of processes, materials and applications in industry 4.0. Sustain. Oper. Comput. 2022, 3, 33–42. [Google Scholar] [CrossRef]
- Popova, M.; Mladenov, B.; Dimitrov, I.; Dimitrov, M.; Mitova, V.; Mitrev, Y.; Kovacheva, D.; Velinov, N.; Karashanova, D.; Boycheva, S. 3D Printed Ni–Cu Sodalite Catalysts for Sustainable γ-Valerolactone Production from Levulinic Acid—Effect of the Copper Content and the Method of Preparation. Processes 2025, 13, 72. [Google Scholar] [CrossRef]
- Popova, M.; Boycheva, S.; Dimitrov, I.; Dimitrov, M.; Kovacheva, D.; Karashanova, D.; Velinov, N.; Atanasova, G.; Szegedi, A. The Formation of γ-Valerolactone from Renewable Levulinic Acid over Ni-Cu Fly Ash Zeolite Catalysts. Molecules 2024, 29, 5753. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, I.; Boycheva, S.; Dimitrov, M.; Mladenov, B.; Kovacheva, D.; Karashanova, D.; Mitrev, Y.; Popova, M. 3D printed nickel-copper modified CaNaX and NaX zeolites obtained from coal fly ash for sustainable levulinic acid hydrogenation. Catal. Today 2025, 459, 115441. [Google Scholar] [CrossRef]
- Boycheva, S.; Chakarova, K.; Mihaylov, M.; Hadjiivanov, K.; Popova, M. Effect of calcium on enhanced carbon capture potential of coal fly ash zeolites. Part II: A study on the adsorption mechanisms. Environ. Sci. Process. Impacts 2022, 24, 1934–1944. [Google Scholar] [CrossRef]
- Tao, Z.; Tian, Y.; Wu, W.; Liu, Z.; Fu, W.; Kung, C.-W.; Shang, J. Development of zeolite adsorbents for CO2 separation in achieving carbon neutrality. npj Mater. Sustain. 2024, 2, 20. [Google Scholar] [CrossRef]
- Chena, C.; Parka, D.-W.; Ahna, W.-S. CO2 capture using zeolite 13X prepared from bentonite. Appl. Surf. Sci. 2014, 292, 63–67. [Google Scholar] [CrossRef]
- Khoramzadeh, E.; Mofarahi, M.; Lee, C.-H. Equilibrium Adsorption Study of CO2 and N2 on Synthesized Zeolites 13X, 4A, 5A, and Beta. J. Chem. Eng. Data 2019, 64, 5648–5664. [Google Scholar] [CrossRef]
- Liu, J.; Sun, X.; Li, N.; Tan, T.; Zhang, F.; Sun, M.; Liu, Q. Effect of synthesis conditions on the properties of 13X zeolites for CO2 adsorption. Environ. Pollut. Bioavailab. 2024, 36, 2387683. [Google Scholar] [CrossRef]
- Aghaei, M.; Anbia, M.; Salehi, S. Measurements and modeling of CO2 adsorption behaviors on granular zeolite 13X: Impact of temperature and time of calcination on granules properties in granulation process using organic binders. Environ. Prog. Sustain. Energy 2022, 41, e13866. [Google Scholar] [CrossRef]
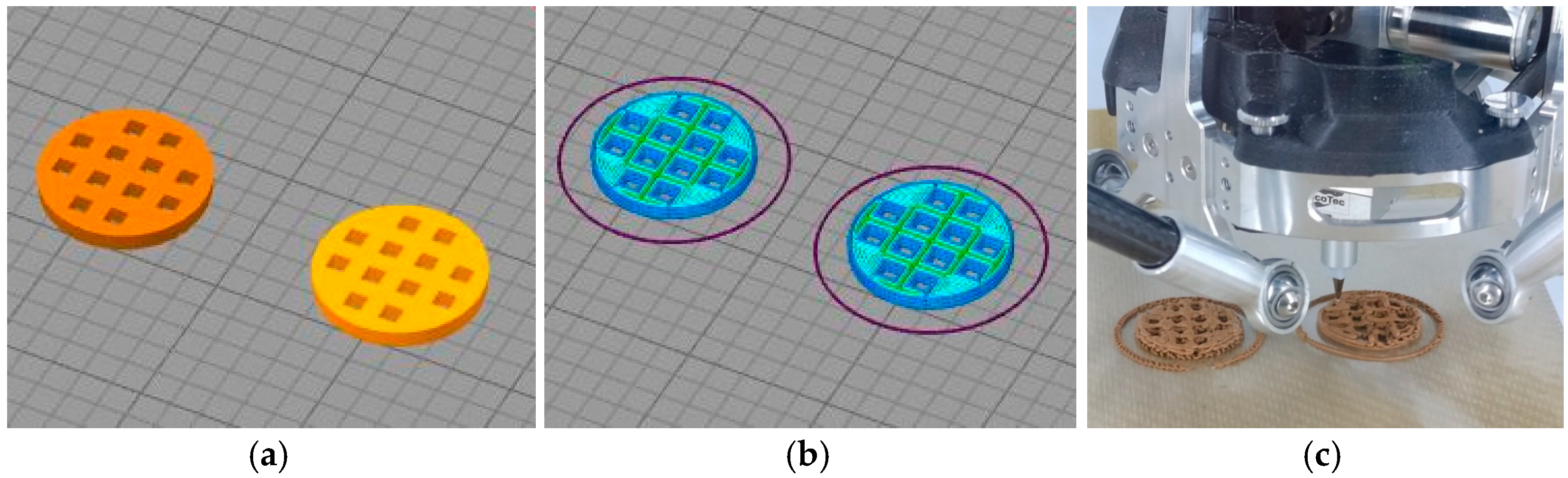
| Samples | Components, wt % | Zeolite Obtained | ||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Others * | LOI | ||
| CFAAES | 50.2 | 23.8 | 13.0 | 4.5 | 2.3 | 3.8 | 2.2 | NaX; Sodalite |
| CFADM3 | 50.8 | 21.3 | 4.7 | 9.4 | 0.8 | 4.8 | 7.8 | NaCaX |
| Structure Description | Technical Drawing | 3D Model | External Surface Area, mm2 | Structure Volume, mm2 |
|---|---|---|---|---|
| The model has a hexagonal shape, composed of concentric hexagons connected by vertical and horizontal support elements. It features an inscribed circle diameter of 30 mm, a circumscribed circle diameter of 25.98 mm, and a height of 5 mm. | 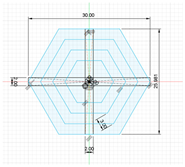 | 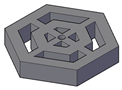 | 2133.47 | 2041.66 |
| Cylindrical in shape, with symmetrical rectangular holes. The model has a diameter of 27 mm and a height of 3 mm. | 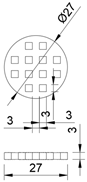 |  | 1615.58 | 1393.66 |
| Cylindrical shape, with symmetrical rectangular holes. The model has a diameter of 30 mm, and a height of 3 mm. | 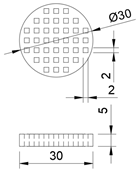 |  | 1795.10 | 1548.51 |
| The model has a cylindrical shape characterized by concentric circles, reinforced with vertical and horizontal support elements. Its outer diameter is 42 mm, featuring a central hole of 12 mm diameter and 5 mm height. Additional holes in the model are defined by arcs with a width of 3 mm and a thickness of 5 mm. | 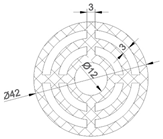 |  | 3707.85 | 3782.33 |
| 3D Structure | Advantages | Disadvantages |
|---|---|---|
| Hexagonal (concentric hexagons) |
|
|
| Cylindrical Ø 27 mm with rectangular holes |
|
|
| Cylindrical Ø 30 mm with rectangular holes |
|
|
| Concentric circular structure (Ø 42 mm, inner Ø 12 mm) |
|
|
| Samples | SBET (m2/g) | Smicro (m2/g) | Total Pore Volume (cm3/g) | Vmicro (cm3/g) | Average Pore Diameter (nm) | DFT Pore Diameter (nm) |
|---|---|---|---|---|---|---|
| NaX | 552 | 433 | 0.408 | 0.171 | 2.95 | 1.0; 5.1 |
| NaCaX | 320 | 224 | 0.256 | 0.090 | 3.20 | 1.5; 5.1 |
| Sodalite | 60 | 6 | 0.145 | 0.003 | 9.653 | 1.3; 5.3 |
| 3D NaX | 336 | 233 | 0.311 | 0.092 | 3.705 | 0.6; 5.3 |
| 3D NaCaX | 300 | 189 | 0.495 | 0.076 | 6.600 | 0.6; 1.3 |
| 3D Sodalite | 60 | 6 | 0.145 | 0.003 | 9.653 | 1.3; 5.3 |
| Sample | Cads, mmol/g 0 °C, 104 kPa | Cads,eq mmol/g | b | n | R2 |
|---|---|---|---|---|---|
| NaX | 3.79 | 4.09 | 0.11 * | - | >0.999 |
| NaCaX | 3.12 | 3.40 | 0.12 * | - | >0.990 |
| Sodalite | 0.49 | 0.47 | 8.03 | - | >0.980 |
| 3D NaX | 2.37 | 4.77 | 0.97 | 0.34 | >0.999 |
| 3D NaCaX | 2.10 | 3.11 | 2.04 | 0.45 | >0.999 |
| 3D Sodalite | 0.49 | 0.47 | 8.03 | - | >0.980 |
| Sample | Cads,flow mmol/g | Time for Passing of 5 vol% CO2, min | Time for Passing of 100 vol% CO2, min |
|---|---|---|---|
| NaX * | 2.9 | 3.5 | 14 |
| NaCaX * | 4.2 | 1.2 | 14 |
| Sodalite | - | - | - |
| 3D NaX | 4.3 | 8.4 | 20 |
| 3D NaCaX | 3.5 | 12.0 | 26 |
| 3D Sodalite | 2.1 | 14.5 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boycheva, S.; Mladenov, B.; Dimitrov, I.; Popova, M. Development of 3D-Printed Carbon Capture Adsorbents by Zeolites Derived from Coal Fly Ash. J. Compos. Sci. 2025, 9, 524. https://doi.org/10.3390/jcs9100524
Boycheva S, Mladenov B, Dimitrov I, Popova M. Development of 3D-Printed Carbon Capture Adsorbents by Zeolites Derived from Coal Fly Ash. Journal of Composites Science. 2025; 9(10):524. https://doi.org/10.3390/jcs9100524
Chicago/Turabian StyleBoycheva, Silviya, Boian Mladenov, Ivan Dimitrov, and Margarita Popova. 2025. "Development of 3D-Printed Carbon Capture Adsorbents by Zeolites Derived from Coal Fly Ash" Journal of Composites Science 9, no. 10: 524. https://doi.org/10.3390/jcs9100524
APA StyleBoycheva, S., Mladenov, B., Dimitrov, I., & Popova, M. (2025). Development of 3D-Printed Carbon Capture Adsorbents by Zeolites Derived from Coal Fly Ash. Journal of Composites Science, 9(10), 524. https://doi.org/10.3390/jcs9100524








