1. Introduction
Technological advancements and innovations in dental materials have fundamentally transformed contemporary dental practices. Despite these advancements, dental cariers continue to present a significant public health challenge globally [
1]. The modern dental approach emphasizes the preservation of tooth structure and surrounding tissues, leading to the adoption of Minimally Invasive Dentistry (MID) as a standard practice [
2].
As dental caries progresses, notable loss of tooth structure occurs, beginning with the enamel and extending into the dentin, ultimately affecting the pulp. This progression can result in pulp inflammation, infection, and the development of periapical lesions, which pose increasingly complex treatment challenges. The MID concept seeks solutions that not only prevent the advancement of dental caries but also reverse the demineralization process and facilitate the remineralization of dentin [
2]. This approach alleviates the burden on patients and clinicians from the need to perform advanced, time-consuming, and costly procedures aimed at remedying lost tooth structure and affected tissues due to dental caries and its microbial consequences.
38% Silver Diamine Fluoride (SDF) is considered a favorable approach to caries management due to its minimally invasive application, antibacterial properties, patient ease, and affordability [
3]. Its rapid application makes it acceptable for a wide range of patients who struggle with traditional restorative procedures (i.e., pre-cooperative children with early childhood caries and patients with special health care needs). The efficacy of the application of SDF to halt the progression of cavitated caries before restorations has been demonstrated [
3]. This efficacy evidence, coupled with its recent regulatory approval in the United States, has attracted significant interest for the use of SDF in the management of dental caries. The main emphasis of policies, presentations, and publications has been on the ability to halt caries lesions due to the material’s distinctive capacity to non-invasively achieve this challenging and clinically significant objective [
4,
5]. Nevertheless, SDF has also demonstrated effectiveness in reducing the occurrence of new caries lesions through hardening of the tooth tissue and prevention of adhesion of cariogenic bacteria [
5]. A scoping review by Irmaleny et al. [
6] revealed that SDF improves the precipitation of minerals and increases mineral density, facilitates the remineralization of hydroxyapatite in enamel by elevating fluoride levels, and enhances the resistance of tooth structure against subsequent acid attacks.
Given that the application of SDF causes silver infiltration into tooth tissues while hardening them, several studies have investigated the impact of this treatment on the shear bond strength (SBS) of subsequent restorations [
7,
8,
9]. SDF application can greatly diminish the bond strength of resin composite to dentin, with the impact varying based on the adhesive system used and the condition of the dentin. For instance, a study showed higher SBS in sound dentin compared to demineralized dentin [
10]. Pretreatment of caries-affected dentin tissue with SDF in combination with potassium iodide has shown a significantly higher SBS of resin-modified glass ionomer (RMGIC) [
11]. However, a recent study demonstrated stronger SBS of resin-based composite to demineralized dentin surfaces of molar teeth pre-treated with SDF compared to resin-modified glass ionomer cement (RMGIC) [
12].
Nevertheless, the staining effect associated with SDF (Elevate Oral Care, LLC, West Palm Beach, FL, USA) has limited its application [
13]. Consequently, there has been a focused search for alternative materials that either do not stain or can mask the discoloration caused by SDF. Immediate application of potassium iodide (KI) solution following SDF application has been shown to mask the staining effect of SDF [
14,
15]. However, randomized controlled clinical studies demonstrated that the application of KI after SDF application does not reduce the black staining of arrested caries in the long term (30 months of the study) [
16]. For this reason, a non-staining chitosan-based nano-silver fluoride (CNSF) has recently become commercially available (Young
® ClearDefense™ Silver Fluoride; Young Dental Manufacturing, Earth City, MO, USA). But the effect of the non-staining silver fluoride on the SBS of subsequent restorations remains unexplored. Therefore, this study aims to investigate the influence of chitosan-based nano-silver fluoride (CNSF) treatment on tooth tissues concerning the SBS of resin composite (RC) and resin-modified glass ionomer (RMGI) restorations. The null hypothesis was that there would not be a difference among groups in the SBS of both restorations.
2. Materials and Methods
2.1. Sample Preparation
A total of 135 caries-free, extracted human permanent molars were collected for the study following the approval of the Institutional Review Board (IRB Approval: HSC20080233N) of our university. The teeth were thoroughly cleaned and examined with a handheld transilluminator to eliminate the cracked teeth. After this, 90 sound teeth were selected and stored in 0.1 thymol solution at room temperature till the study was conducted. The roots of each tooth were cut off, and the buccal surface of each tooth was flattened using a diamond bur till yellow dentin could be seen. The prepared flat dentinal surfaces were smoothed with 400 grit silicon carbide paper to achieve a standardized surface for all specimens. Then the specimens were embedded in auto-polymerizing polymethyl methacrylic (PMMA) resin in the aluminum molds, with the unprepared surface of the specimen embedded in resin and the prepared surface flush with the surface of the PMMA resin (
Figure 1a). The prepared samples are stored in humid containers to keep them moist, to prevent desiccation until the next step in the protocol.
2.2. Study Products
1. SDF: Advantage Arrest™ 38% Silver Diamine Fluoride (25% silver, 8% ammonium, 5% fluoride, 62% water), Elevate Oral Care, LLC, West Palm Beach, FL, USA. Lot#: 21396.
2. CNSF: Young® ClearDefense™ Silver Fluoride (3% sodium fluoride, 1.36% fluoride ion, 0.2% or 2000 ppm silver ion, chitosan—vegetally derived, acetic acid, water), Young Dental Manufacturing, Earth City, MO, USA. Lot#: 271581.
3. RMGI: GC Fuji II LC® resin-modified glass ionomer cement capsules (fluoro-aluminosilicate glass powder, polyacrylic acid, and methacrylate monomer (HEMA), water and a photo-initiator), GC Corporation, Tokyo, Japan. Lot#: 240822B.
4. RC: 3M Filtek Supreme Ultra Universal Restorative Capsules—Dentin B3 (bisphenol A diglycidyl ether dimethacrylate (Bis-GMA), bisphenol A polyethylene glycol diether dimethacrylate (BIS-EMA-6), diurethane dimethacrylate (UDMA), polyethylene glycol dimethacrylate (PEGDMA), triethylene glycol dimethacrylate (TEGDMA), silane-treated ceramic and silica nanoparticles, phenyl bis(2,4,6-trimethylbenzoyl)-phosphine oxide (a photoinitiator), 3M St. Paul, MN, USA. Lot#: 10752254.
2.3. Study Groups
The 90 specimens were randomly assigned to three treatment groups (30/group), each with two subgroups to give the following six experimental pretreatment subgroups (15/subgroup). (1) Non-pretreatment (NPT) group: prepared surfaces not pretreated but restored with either (a) resin composite with adhesive bond (NPT-RC) or (b) resin-modified glass ionomer (NPT-RMGI); (2) CNSF group: prepared surfaces pretreated with CNSF and restored with either (a) resin composite with adhesive bond (CNSF-RC) or (b) RMGI (CNSF-RMGI); and (3) SDF group: prepared surfaces pretreated with SDF and restored with either (a) resin composite with adhesive bond (SDF-RC) or (b) RMGI (SDF-RMGI).
2.4. Specimen Pretreatment and Restoration Bonding
NPT group (NPT-RC and NPT-RMGI): In NPT-RC subgroups, 32% phosphoric acid etchant gel (UNI-ETCH™ semi-gel; BISCO Dental Products, BISCO, Inc., Schaumburg, IL, USA; Lot# 2400013593) was applied on the prepared dentin surface of each sample for 15 s with agitation. Then the surface was rinsed with distilled water for 10 s with a dental air-water syringe, and excess moisture was removed by an air syringe. Then the surface was rubbed with 2% chlorhexidine gluconate solution (Concepsis©; Ultradent Products, Inc., South Jordan, UT, USA; Lot# 20251472) and left for 60 s and then dried to the same level of moistness that was performed after rinsing. Following this, the etched surfaces had adhesive (All-Bond Universal adhesive; BISCO Dental Products, BISCO, Inc., Schaumburg, IL, USA; Lot # 2400012165) applied on them as follows. The first coat of adhesive was applied to the tooth surfaces and scrubbed for 10 s (the force being enough to bend the Kerr applicator). This is followed by the application of the second coat of the adhesive and scrubbing for 10 s. Then the tooth surfaces were air-dried for at least 10 s to remove puddles and excess solvents. If the surface was not glossy, the adhesive application process was repeated as directed by the manufacturer. Then the adhesive was light-cured for 10 s using VALO™ X LED Curing Light (1000 mW/cm2; Ultradent Products, Inc., South Jordan, UT, USA). In NPT-RMGI subgroups, Cavity Conditioner (20% polyacrylic acid and 3% aluminum chloride hexahydrate; GC Corporation, Tokyo, Japan) was applied for 10 s, rinsed with distilled water, and gently dried (avoiding desiccation).
Following the above treatment, a cylindrical restoration build-up was constructed on all specimens in both subgroups (NPT-RC, NPT-RMGI) as follows. The PMMA base bearing the tooth specimen was positioned underneath the bonding clamp (
Figure 1b) with the restoration mold centered directly onto the dentin of the tooth sample, sitting on a bonding surface area of 2.38 mm. This bonding surface area was uniform in all samples since we used the same standardized mold, with a fixed bonding surface area, for all samples in all groups. The mold was tightened on the dentin surface by lowering the thumb knots until they pressed on the springs. Then the restorative material (resin composite or RMGI) was condensed into the mold to construct an RC or RMGI build-up of 2.38 mm diameter and 2 mm height on the treated surfaces of the specimens in all groups. The restoration build-up was then cured with dental curing light (1000 mW/cm
2, VALO™ X LED Curing Light; Ultradent Products, Inc., South Jordan, UT, USA) for 20 s. After light-curing, the thumb knots were loosened up and the mold lifted from the restoration build-up. The restoration build-up is ready for shear bond strength (SBS) testing (
Figure 1c).
CNSF group (CNSF-RC and CNSF-RMGI): In these subgroups, the prepared tooth surface of each specimen had CNSF (Young
® ClearDefense™ Silver Fluoride; Young Dental Manufacturing, Earth City, MO, USA; Lot#: 271581) applied according to the manufacturer’s instructions as follows. The dentin surface was dried as much as possible, then one drop (0.05 mL) of CNSF solution was applied and spread with a micro brush applicator and allowed to air dry. Following this, the surfaces of the specimens in CNSF-RC subgroups were etched and treated with adhesive as described in the NPT group above, while CNSF-RMGI subgroups were treated with Cavity Conditioner as described in the NPT group above. Then restoration build-up was constructed on all specimens in all subgroups as described above and ready for SBS testing (
Figure 1c).
SDF group (SDF-RC and SDF-RMGI): In these groups, the prepared tooth surface of each specimen was treated with SDF (Advantage Arrest™ 38% SDF; Elevate Oral Care, LLC, West Palm Beach, FL, USA; Lot#: 21396) according to the manufacturer’s instructions as follows. The dentin surface was dried as much as possible, then SDF solution was applied using a micro brush applicator and left for 60 s, after which it was dabbed with a cotton roll to remove all remaining SDF from the surface. Following this, the surfaces of the specimens in the SDF-RC and SDF-RMGI subgroups were treated as described in the CNSF group above. Then restoration build-up was constructed on all specimens in all groups as described above in the NPT group and ready for SBS testing (
Figure 1c).
2.5. Thermocycling
All PMMA bases bearing the tooth specimens were thermocycled 5000 times using a thermocycler (Applied Biosystem, automated thermocycler, Waltham, MA, USA) maintained at 5 °C and 55 °C for dwell times of 30 s intervals between cycles. These cycles simulate about 5 years of the fluctuating temperature of the oral environment that subjects the restorations to thermal stresses [
17,
18,
19]. After thermocycling, the PMMA base bearing the tooth specimens was stored in normal saline at room temperature for 24 h to simulate saliva, after which the shear bond strength was measured.
2.6. Shear Bond Strength Testing
After thermocycling, the shear bond strength of all specimens in all groups was evaluated using the Ultradent UltraTester (Ultradent Products, Inc., South Jordan, UT, USA). The PMMA base bearing the restoration build-up was positioned horizontally in a test-base clamp with the tooth root pointing upwards (
Figure 1d). The platform of the UltraTester (
Figure 1e) was raised so that the notch of the UltraTester crosshead engaged the cylindrical restoration build-up at the bonding position (
Figure 1f). To de-bond the restoration, the tester was switched on, and the platform traveled at a speed of 1 mm/min until the restoration de-bonded and the peak shear bond strength in Mega Pascal Units (MPa) was recorded.
2.7. Sample Size and Power Calculation
A power analysis was conducted to determine the sample size. GPower statistical software 3.1.9.7 for Windows was used for this determination. Based on data from previously available literature [
20,
21,
22,
23] with a 95% confidence interval and 80% statistical power and an effect size of 0.40, it was estimated that 12 samples were appropriate for each group to detect a significant difference among the groups. However, 15 samples were used for each subgroup to account for damage during processing.
2.8. Statistical Analysis
The data was analyzed using Statistical Package of Social Sciences (SPSS version 23, Chicago Inc., Chicago, IL, USA). A one-way analysis of variance (ANOVA) test was conducted to determine whether the SBS of restorations differed statistically significantly among the treatment conditions of the tooth surface (untreated, CNSF, SDF) with the use of different restorative materials (RC or RMGI). This was followed by a Tukey post hoc multiple comparison of the SBS of the treatment conditions. A value of p < 0.05 was considered significant.
3. Results
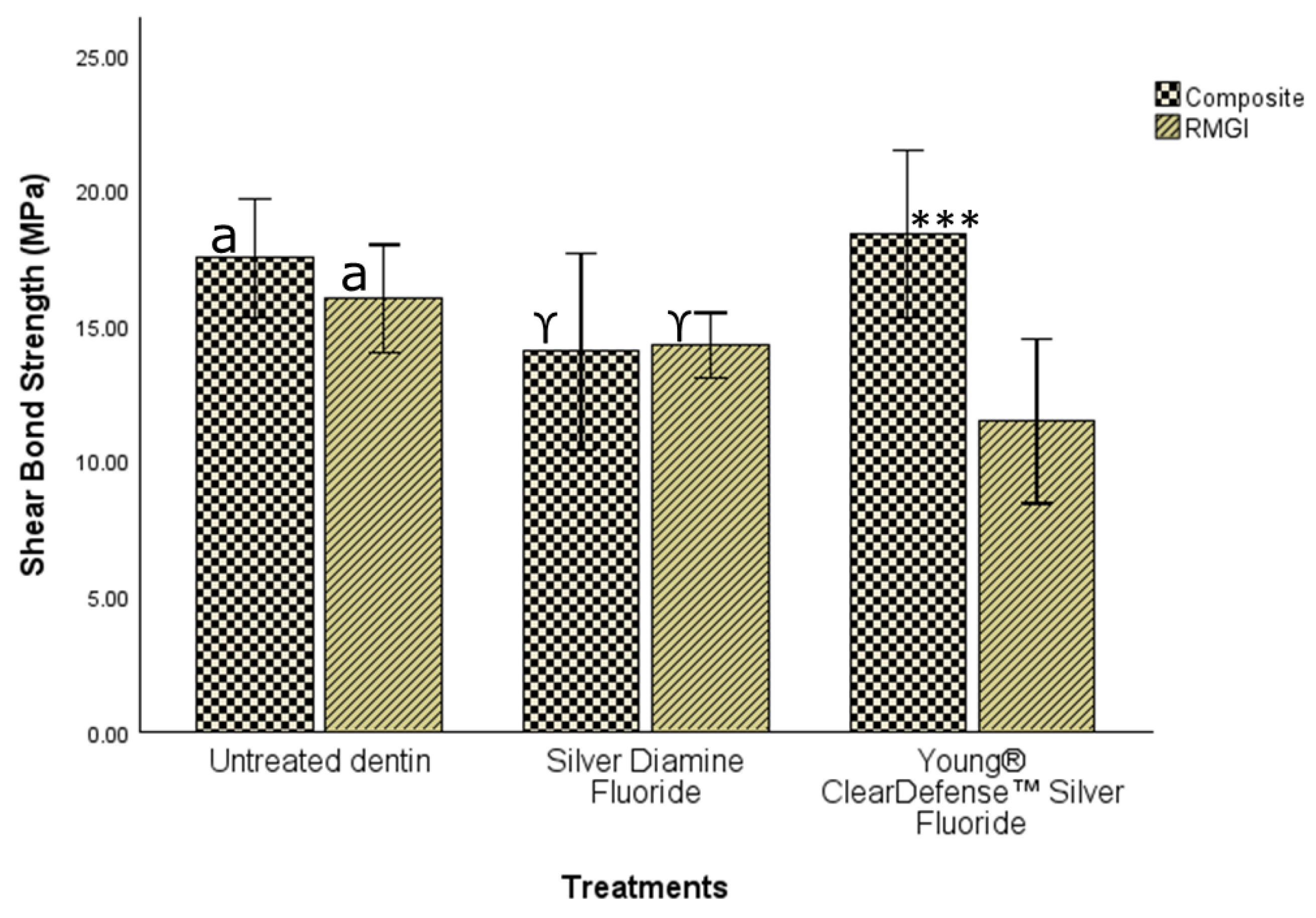
Figure 2 and
Table 1 showed that with the use of resin composite restoration, there was no statistically significant difference (ANOVA; F (2, 42) = 2.64,
p = 0.08) in SBS when the tooth tissue was not pretreated (17.48 ± 3.96; 95% CI 15.29–19.67), treated with either SDF (14.03 ± 6.56; 95% CI 10.39–17.66), or CNSF (18.38 ± 5.59; 95% CI 1 15.29–21.47). As a result, with RC, the research hypothesis that the shear bond strength of restorations would not be significantly different among groups was supported at the alpha level of 0.05.
However, with the use of RMGI restoration (without adhesive as recommended by the manufacturer), there was a statistically significant difference (ANOVA; F (2, 42) = 4.98,
p = 0.011) in SBS among the three treatment conditions (
Figure 2;
Table 2 and
Table 3). A Tukey post hoc comparison (
Table 2) showed that there was no significant difference in SBS when the tooth tissue received no pretreatment (15.99 ± 3.59; 95% CI 14.01–17.98) compared to when pretreated with SDF (14.27 ± 2.17; 95% CI 13.06–15.47). But the SBS was statistically significantly higher when the tooth tissue received no pretreatment compared to when pretreated with CNSF (11.45 ± 5.48; 95% CI 8.41–14.48). However, there was no significant difference in SBS when the tooth tissue was pretreated with either SDF or CNSF. These results suggest that with RMGI the research hypothesis was not supported.
When the dentin tissue was not pretreated with either SDF or CNSF, there was no statistically significant difference (ANOVA; F (2, 42) = 1.36,
p = 0.27) in SBS of the two restoration types (
Figure 3;
Table 4). Similarly, when the dentin tissue was pretreated with SDF, there was no statistically significant difference (ANOVA; F (2, 42) = 0.04,
p = 0.96) in SBS of the two restoration types (
Figure 3;
Table 4). As a result, the research hypothesis was supported at the alpha level of 0.05. However, when the dentin tissue was pretreated with CNSF, there was a statistically significant difference (ANOVA; F (2, 42) = 10.49,
p < 0.001) in SBS of the two restorations, with composite resin restoration having a statistically significant (
Figure 3,
Table 4; Tukey HSD;
p < 0.001; mean diff = 6.93333; 95% CI 2.5055–11.3611) higher SBS (17.48 ± 3.96) than RMGI (15.99 ± 3.59). The result failed to support the research hypothesis that there would not be a difference among groups in the SBS of both restorations at the alpha level of 0.05.
4. Discussion
The pursuit of a material that replicates the benefits of silver diamine fluoride (SDF) in halting caries and preventing the advancement of dental lesions, while eliminating the associated discoloration, has been a continuous endeavor among researchers, dental materials developers, and manufacturers. SDF has undergone extensive examination in various studies, demonstrating efficacy in arresting caries progression and facilitating the remineralization of demineralized dentin.
Additionally, numerous studies have focused on the impact of SDF on the Shear bonding strength (SBS) of composite restorations and resin-modified glass ionomer (RMGI) restorations to both enamel and dentin. In the current study, no significant difference in SBS was observed when tooth tissue was not subjected to pretreatment compared to instances where it was pretreated with SDF, particularly in relation to RMGI restorations.
In a study conducted by Abuljadayel et al. [
23], it was reported that silver ions precipitating from SDF enhance the ionic bond to glass ionomer cement (GIC) and improve bond strength to dentin. The observed increase in bond strength in relation to SDF treatment has been attributed to the chemical interaction between the carboxylic acid of RMGIC and the silver phosphate formed as a result of the reaction between the tooth surface and SDF. This may elucidate the findings of the current study, which indicate that the application of SDF did not significantly influence the SBS when RMGI was employed.
In a study conducted by Mondal et al. [
7], the assessment of SBS of glass ionomer cement (GIC) to demineralized dentin in primary teeth treated with SDF and potassium iodide (PI) revealed that higher SBS was achieved with the combination of SDF and PI followed by GIC restoration. This finding is consistent with the results of the current study. Rinsanthol et al. [
24] investigated the bond strength of GIC to artificial carious dentin, noting that SDF and various surface treatment procedures influenced the outcomes. They observed that the SDF application without a rinsing step resulted in reduced bond strength compared to that observed in untreated carious dentin surfaces. This contradicts the findings of the current study.
In the current study, when resin composite restorations were performed, no statistically significant difference in SBS was noted among the tooth tissues categorized as untreated, those treated with SDF, or those treated with CNSF.
Concerning bonding with resin composites, Shaheen et al. [
25] demonstrated that delaying the application of composite restorations for 14 days following SDF and potassium iodide treatments can enhance bond strength. This improvement was attributed to SDF’s capability of remineralizing demineralized carious dentin, which positively affects bonding efficacy. Furthermore, Shaheen et al.’s research indicated that resin composites applied to permanent teeth exhibited greater microtensile bond strength than those applied to deciduous teeth, likely due to the mineral composition of the dentin, as primary teeth contain lower concentrations of calcium and phosphorus. This finding may elucidate the results obtained in the current study regarding SDF and resin composite use.
Danaeifar et al. [
26] reported that SDF did not adversely affect the bonding effectiveness of tested bulk-fill materials to dentin, nor did prior conditioning impact the bond strength of high-viscosity glass ionomer to dentin. These results align with those observed in the present study.
However, manufacturers and users of SDF continue to face challenges associated with the staining effect of SDF, which arises from the penetration of silver particles into the dentinal tubules, leading to black staining immediately following light curing.
CNSF has been introduced by manufacturers as a non-staining fluoride treatment capable of halting the progression of dental caries and facilitating the remineralization of demineralized dentin. Chitosan is characterized as a weak base, insoluble in water and organic solvents, yet soluble in dilute aqueous acidic solutions. It is biocompatible and mucoadhesive, exhibiting a broad spectrum of antibacterial and antibiofilm properties against both Gram-positive and Gram-negative bacteria. A study by Debnath et al. evaluated the antibacterial and adhesive properties of chitosan-modified GIC versus conventional GIC. The study indicated that modifying the liquid component of conventional GIC with 10% chitosan significantly enhanced its antibacterial properties as well as its adhesion to enamel [
27].
This present study represents the first investigation into the effects of CNSF on SBS concerning various restorations. Zioti et al. [
28] reported that chitosan has been known to be an important biomaterial that can stabilize the adhesive interface and hence prevent the degradation of the organic dentin matrix by metalloproteinases by forming crosslinks with collagen fibrils. Their study concluded that a 2.5% chitosan solution improved the bond strength of the composite resin to dentin. This was attributed to the fact that chitosan cross-links with dental collagen, producing a mechanically strong fibril chain. This may explain the higher SBS when CNSF is used with composite resin in this study.
In a different study by Sodagar et al. [
29], the effect of nanoparticle incorporation on the antibacterial properties and SBS of composite resin was evaluated. They reported that the shear bond strength of the composite reduced as the percentage of nanoparticles, such as chitosan, increased. It may be important to look at how the concentration of chitosan in CNSF would affect the SBS when composite resin is used in future studies.
A previous study [
30] assessed the impact of chitosan incorporation into adhesives on the antibacterial activity and bond strength of single-bond adhesives modified with varying concentrations of chitosan. The findings revealed that adhesives with lower concentrations of chitosan exhibited heightened antibacterial effects, while higher concentrations negatively influenced microtensile bond strength.
In the present study, when dentin tissue was not pre-treated with either SDF or CNSF, there was no statistically significant difference in the SBS among the two types of restorations. Notably, when tooth tissue underwent no pretreatment, the SBS was statistically significantly higher when RMGI was utilized compared to cases pretreated with CNSF.
A study by Khudair et al. [
31] evaluated the effect of incorporating chitosan into RMGI and its effect on the SBS. They concluded that the addition of chitosan to the glass ionomer cement had a negative effect on the SBS when RMGI was used. They attributed this to the chitosan interacting with the polyacrylic acid in the RMGI and forming a polymer complex that could affect the chemical properties and setting reaction of the cement. The findings of the current study align with this research, suggesting that the reduced SBS observed when using CNSF in conjunction with RMGI may be attributable to this relationship.
Although the objective of the present in vitro study was achieved with its current design, there are still some limitations associated with the extent of the study. One limitation is that failure mode analysis (adhesive vs. cohesive) was not conducted, which may limit the understanding of the nature of bond failure. Also, the surface morphology post-treatment was not evaluated by scanning electron microscope to assess the physical interaction of CNSF/SDF with dentin. However, the findings garnered from the present in vitro study can be used as a foundation for developing further studies aimed at exploring the influence of differing chitosan concentrations in CNSF on the SBS of restorations, as well as the impact of timing between CNSF applications and subsequent restoration on SBS outcomes.