Reinforcement of Polyethylene with Potassium Hexatitanate Whiskers: Importance of Polyvinylpyrrolidone Additive
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PHT Whiskers
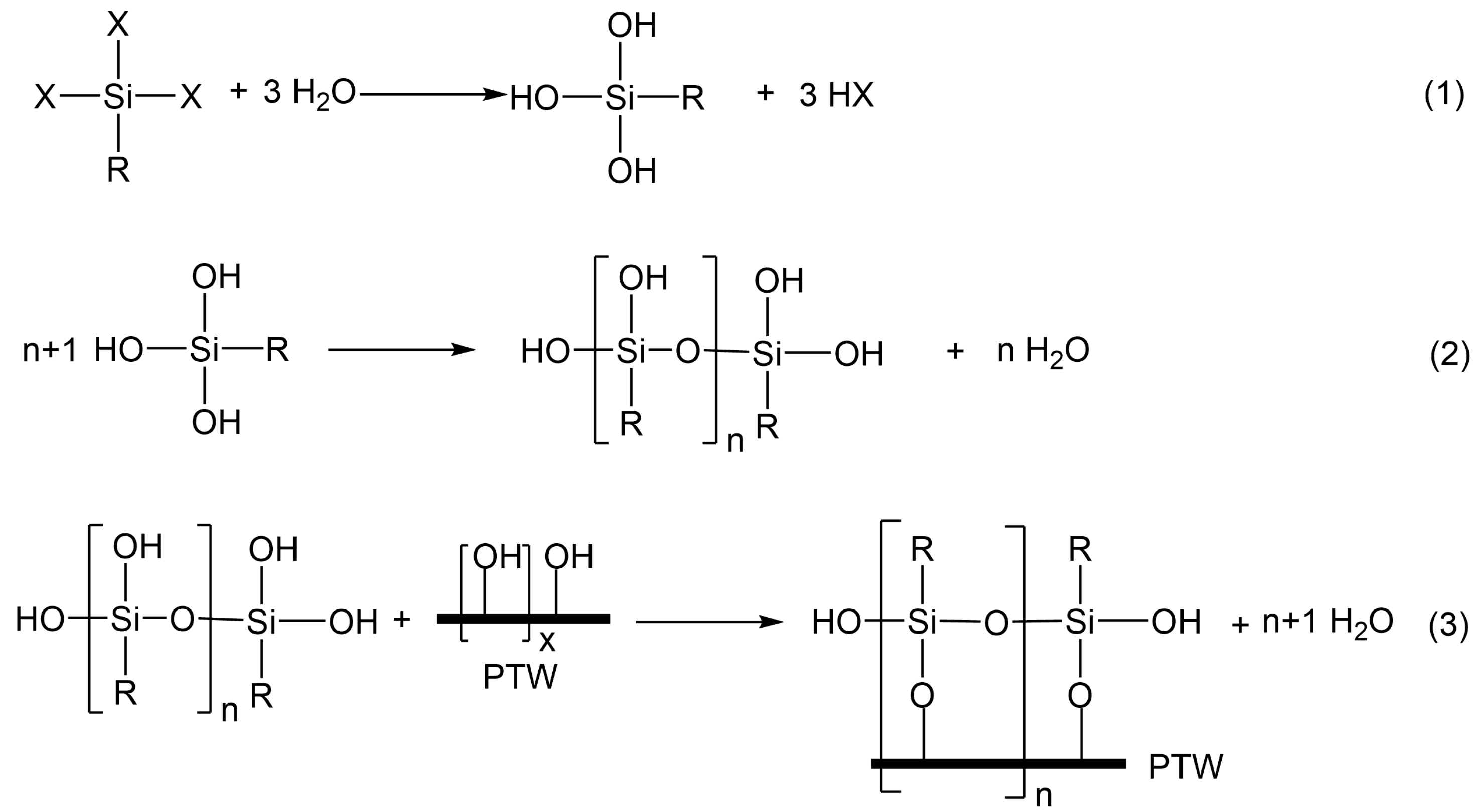
2.3. Surface Modification of PHT Whiskers
2.4. Reinforcement of Polyethylene (PE)
2.5. Characterization
3. Results
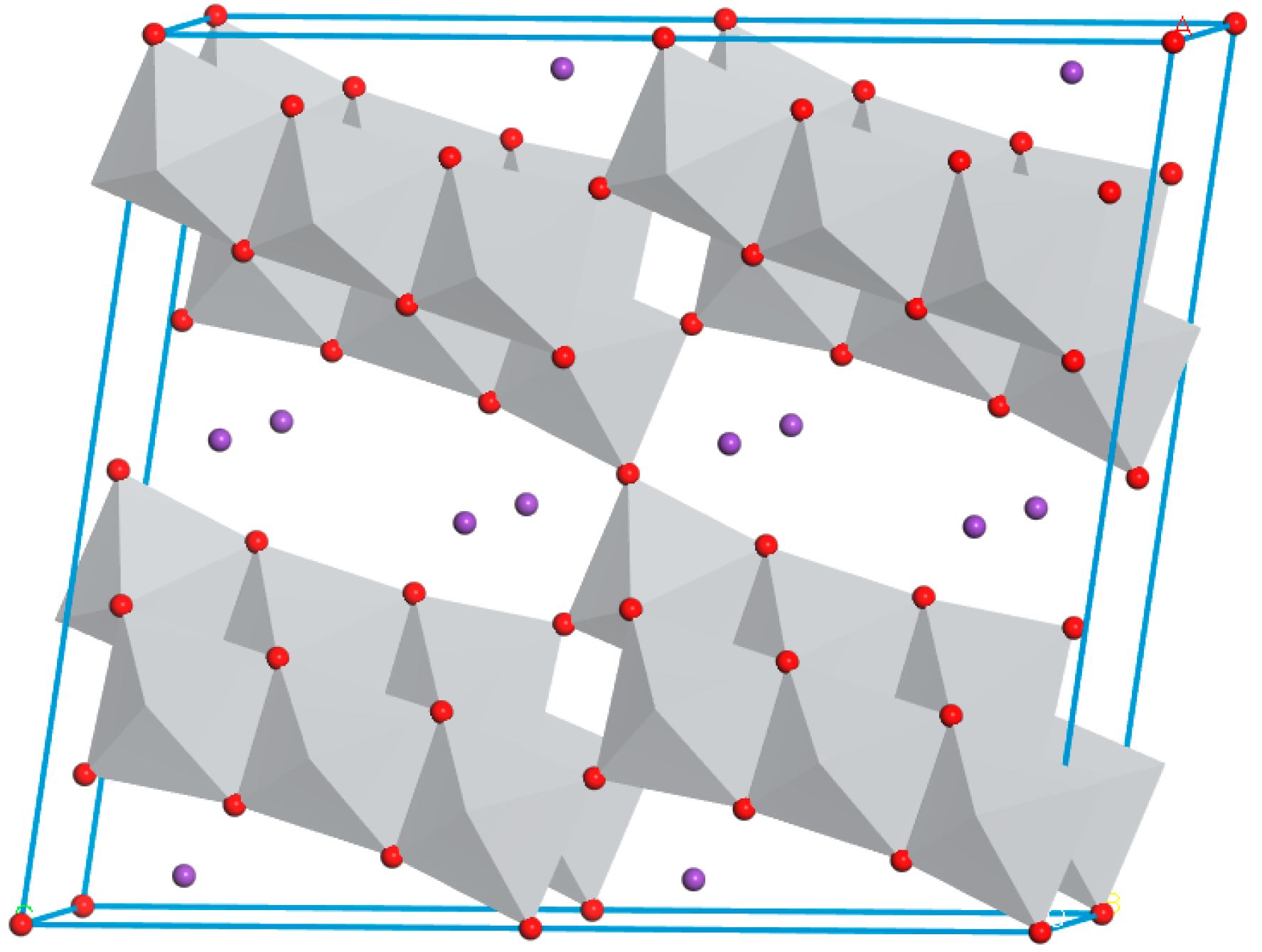
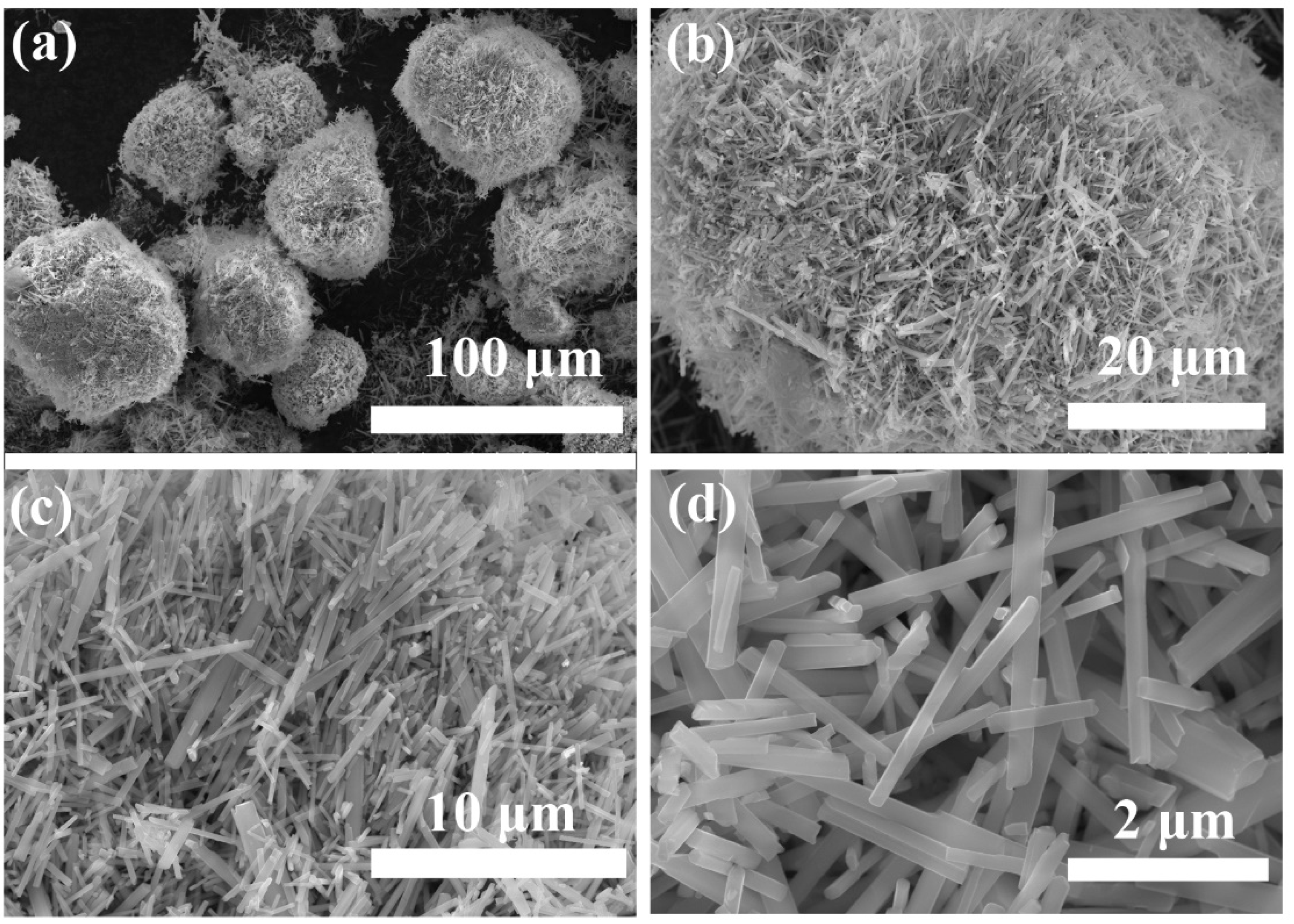
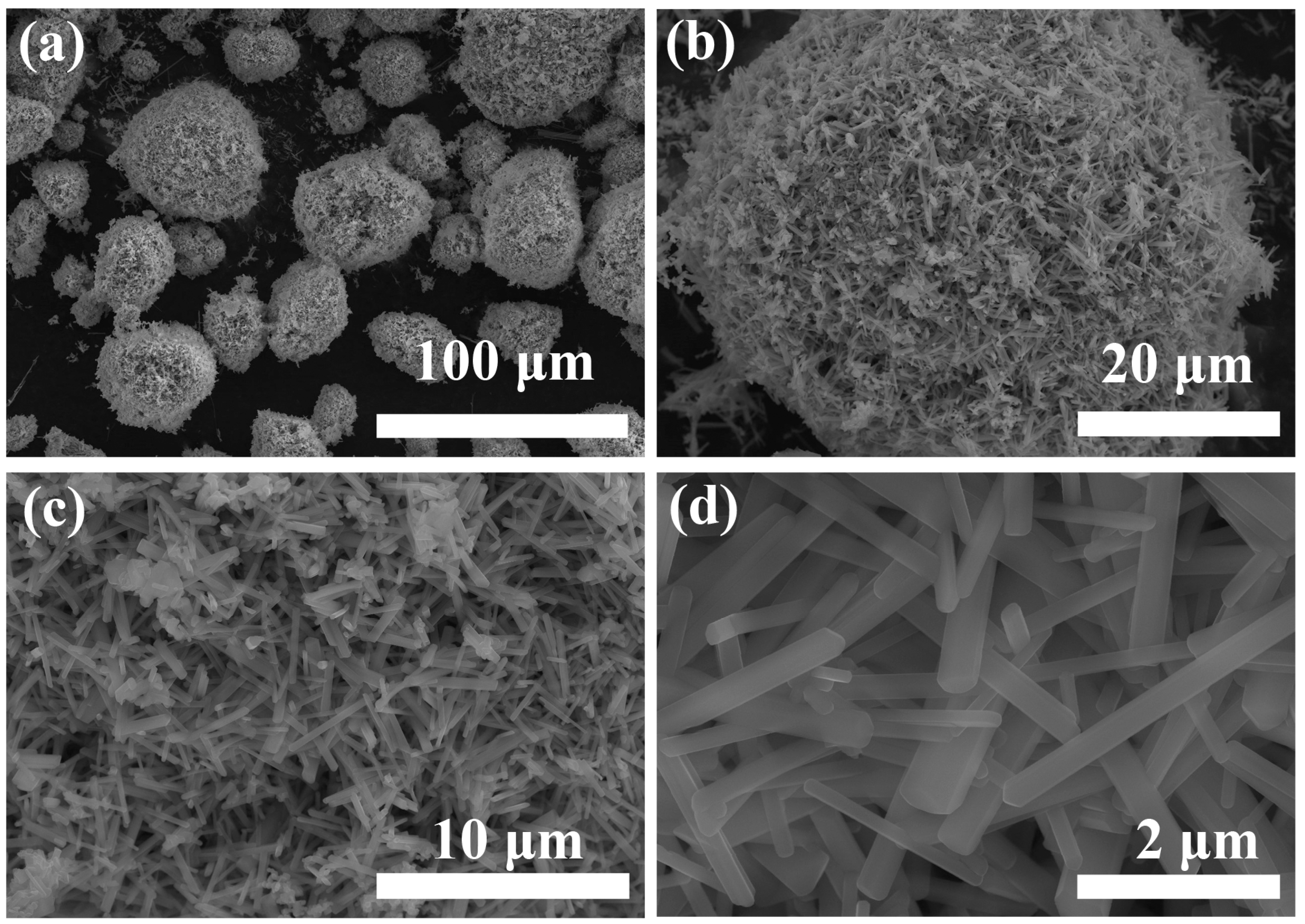
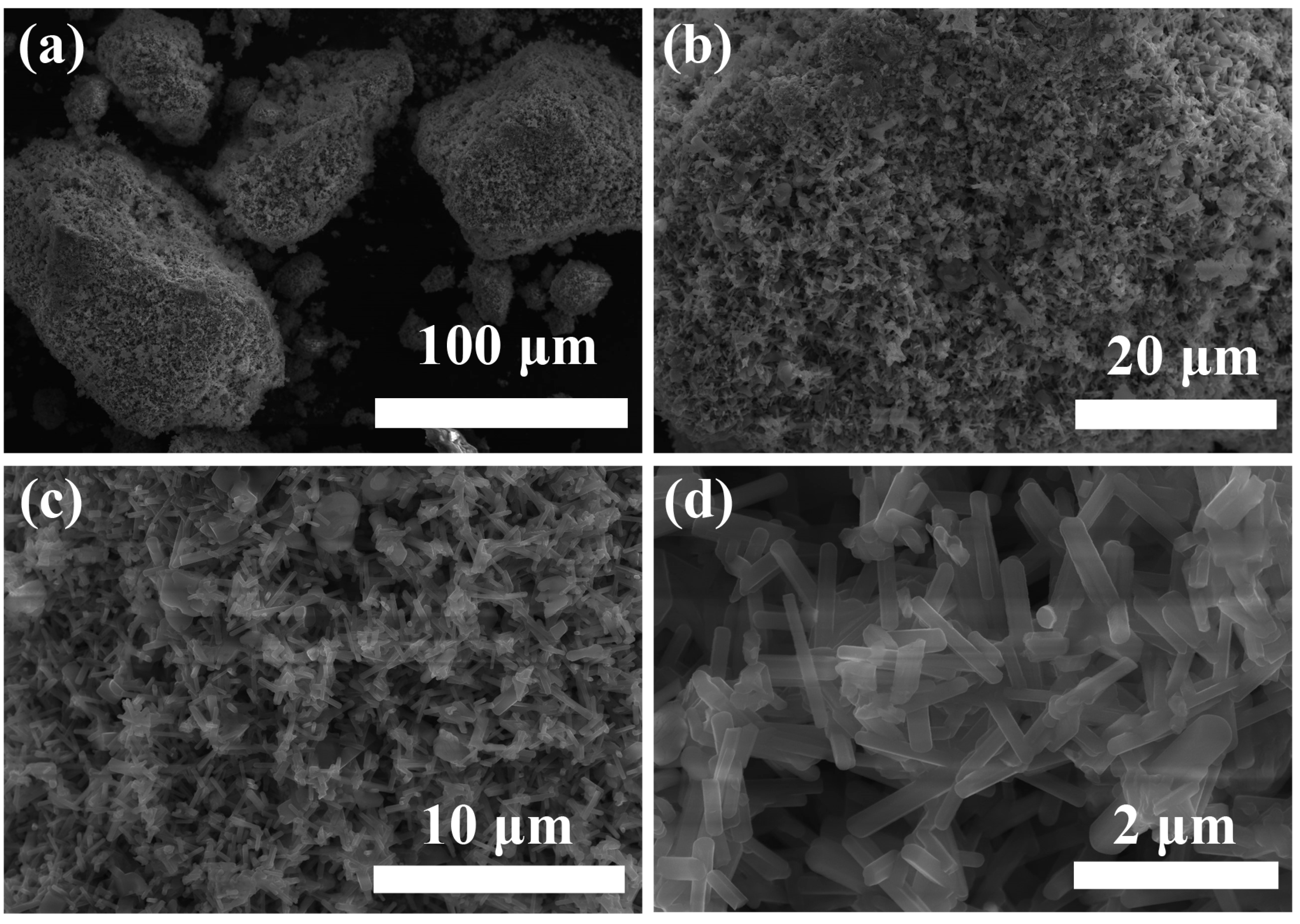
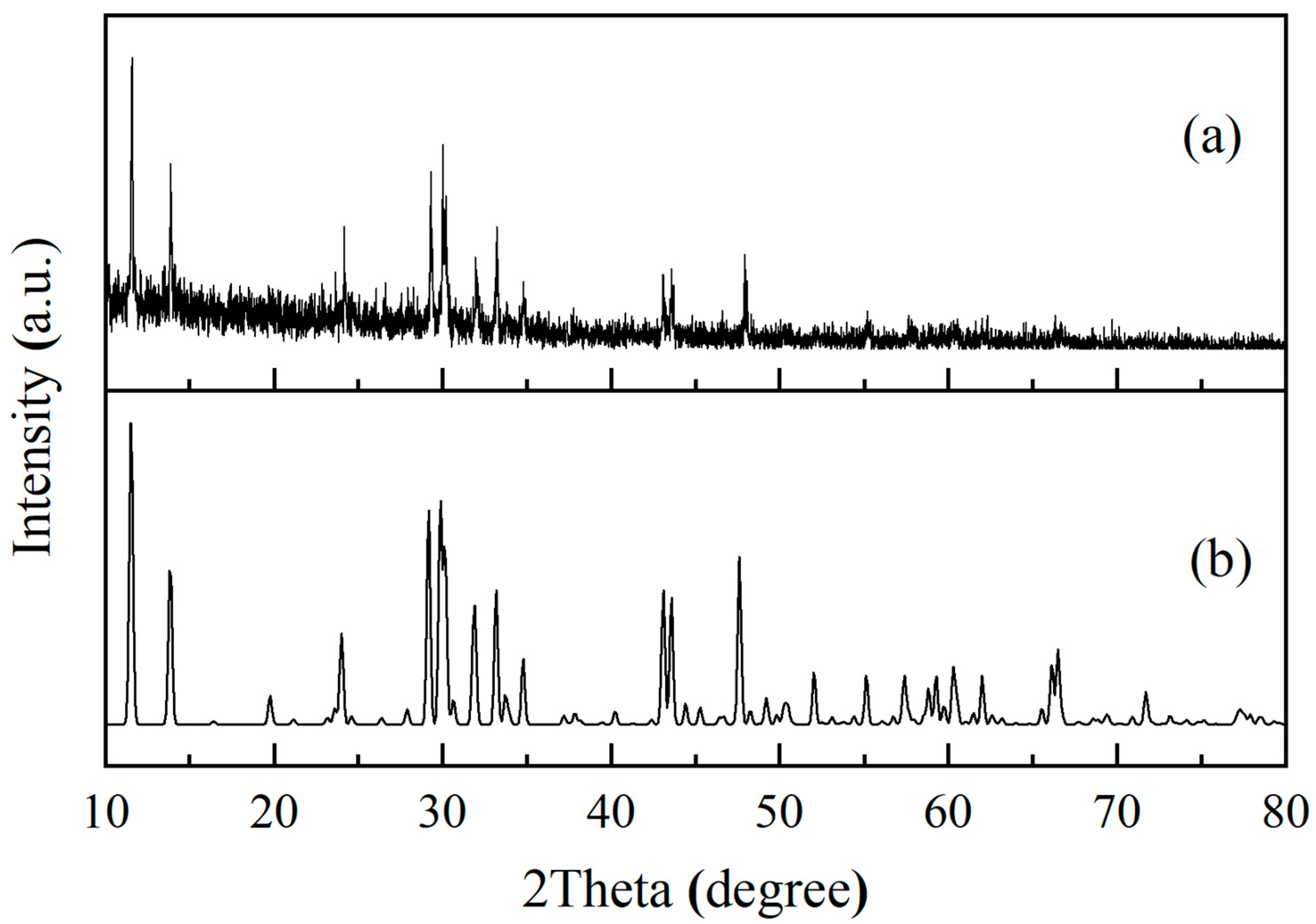
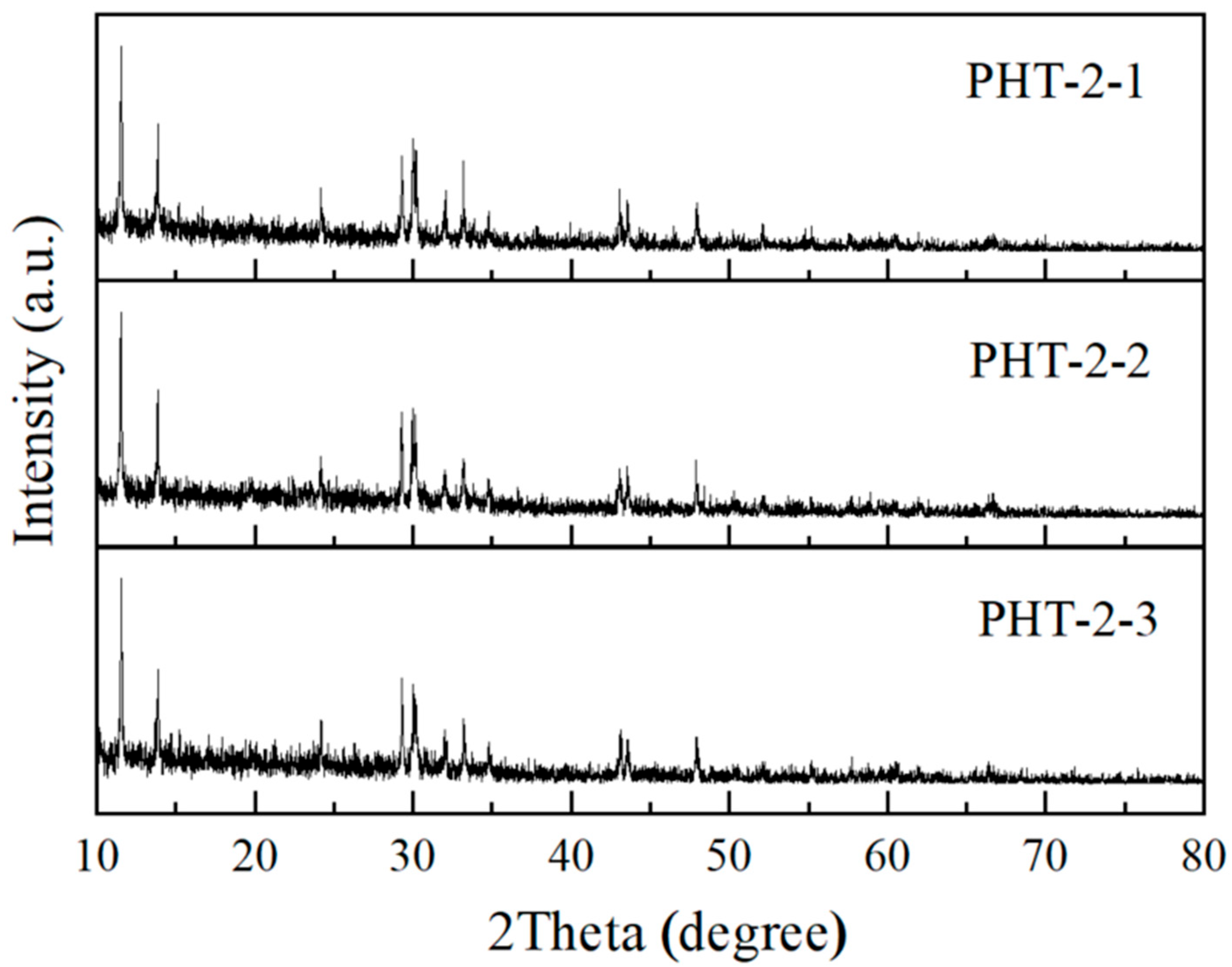
3.1. Characterization of PHT Whiskers
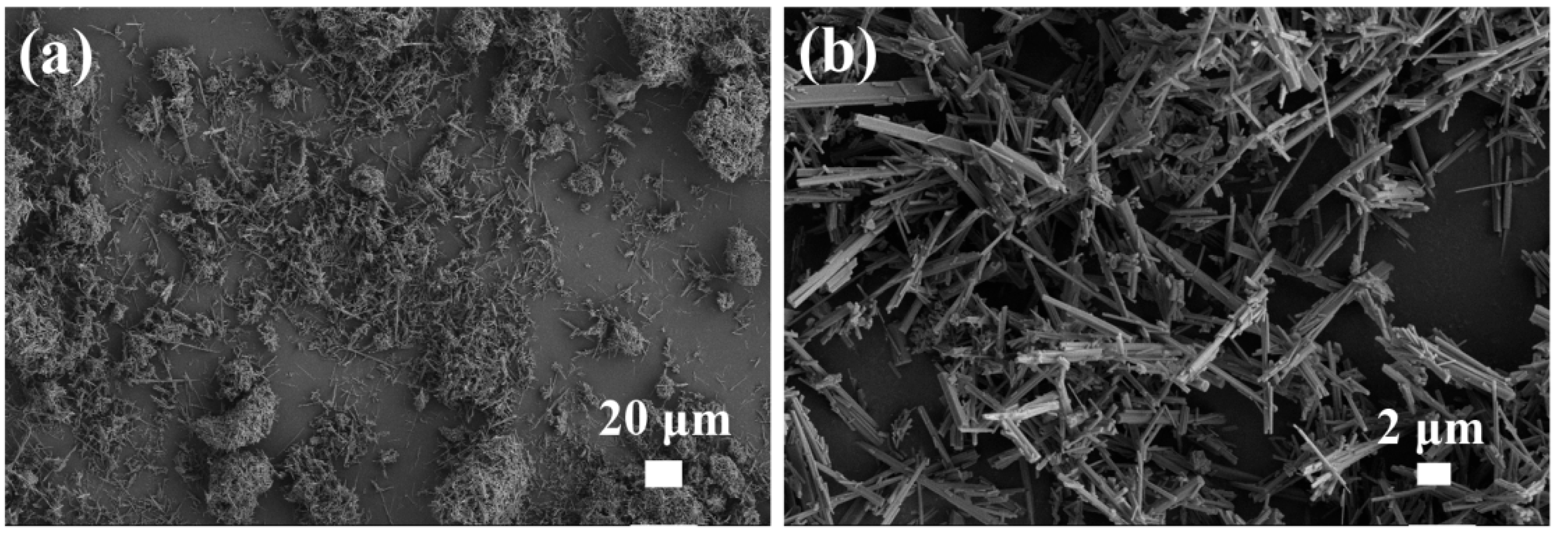
3.1.1. Morphology Studies
3.1.2. XPS Analysis
3.2. Characterization of Surface-Modified PHT Whiskers
3.3. Mechanical Test
4. Conclusions
- It was demonstrated that the PVP additive is highly effective in regulating the growth of crystals. The prepared potassium hexatitanate whiskers exhibit a slender, rod-like morphology with smooth and flat surfaces and a large length-to-diameter ratio.
- After the surface modification with silane coupling agents (KH-550, KH-560, and KH-570), the whiskers showed little change in shape. The results of the mechanical tests indicate that there is some improvement in the tensile strength, the modulus of elasticity, the shear strength, and the shear elasticity.
- Among the composites, the KH-550 modified composite (PE/PHT-2-1) exhibits the best performance. An increase of 18.6% in the tensile strength and 3.6% in the modulus of elasticity are observed. The modified PE composite shows enhanced softness and elasticity. The elongation at the break for PE/PHT-2-1 increases significantly to 38.1%.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, L.; Chen, X.; Song, H. Polymer Composites, 2nd ed.; China Light Industry Press: Beijing, China, 2017. [Google Scholar]
- Bao, N.; Feng, X.; Shen, L.; Lu, X. Calcination syntheses of a series of potassium titanates and their morphologic evolution. Cryst. Growth Des. 2022, 2, 437–442. [Google Scholar] [CrossRef]
- Ponce-Peña, P.; Poisot, M.; Rodríguez-Pulido, A.; González-Lozano, M.A. Crystalline structure, synthesis, properties and applications of potassium hexatitanate: A review. Materials 2019, 12, 4132. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, M. The formation of potassium titanate fibre with flux methods. J. Mater. Sci. 1987, 22, 1223–1227. [Google Scholar] [CrossRef]
- Yokoyama, M.; Ota, T.; Yamai, I. Preparation of potassium hexatitanate long fibre by the flux evaporation method. J. Mater. Sci. 1989, 24, 3787–3790. [Google Scholar] [CrossRef]
- Kajiwara, M. The synthesis of potassium titanate fibre by flux evaporation methods. J. Mater. Sci. 1988, 23, 3600–3602. [Google Scholar] [CrossRef]
- Liu, C.; He, M.; Lu, X.; Zhang, Q.; Xu, Z. Reaction and crystallization mechanism of potassium dititanate fibers synthesized by low-temperature calcination. Cryst. Growth Des. 2005, 5, 1399–1404. [Google Scholar] [CrossRef]
- Myeong-Heon, U. Thermal treatment of titanates derivatives synthesized by ion-exchange reaction. J. Am. Ceram. Soc. 2001, 84, 1181–1183. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Yang, J.; Chen, J.; Zhang, Z. Microwave-assisted synthesis of potassium titanate nanowires. Mater. Lett. 2006, 60, 3015–3017. [Google Scholar] [CrossRef]
- Bao, N.; Feng, X.; Lu, X.; Yang, Z. Study on the formation and growth of potassium titanate whiskers. J. Mater. Sci. 2002, 37, 3035–3043. [Google Scholar] [CrossRef]
- Choy, J.; Han, Y. A combinative flux evaporation-slow cooling route to potassium titanate fibers. Mater. Lett. 1998, 34, 111–118. [Google Scholar] [CrossRef]
- Ponce-Peña, P.; González-Lozano, M.A.; Escobedo-Bretado, M.A.; De Lira-Gómez, P.; García-Sánchez, E.; Rivera, E.; Alexandrova, L. Synthesis and characterization of potassium hexatitanate using boric acid as the flux. Ceram. Int. 2015, 41, 10051–10056. [Google Scholar] [CrossRef]
- Jung, K.T.; Shul, Y.G. Synthesis of high surface area potassium hexatitanate powders by sol-gel method. J. Sol-Gel Sci. Technol. 1996, 6, 227–233. [Google Scholar] [CrossRef]
- Kang, S.O.; Jang, H.S.; Kim, Y.I.; Kim, K.B.; Jung, M.J. Study on the growth of potassium titanate nanostructures prepared by sol-gel–calcination process. Mater. Lett. 2007, 61, 473–477. [Google Scholar] [CrossRef]
- Inoue, Y.; Kubokawa, T.; Sato, K. Photocatalytic activity of alkali-metal titanates combined with Ru in the decomposition of water. J. Phys. Chem. 1991, 95, 4059–4063. [Google Scholar] [CrossRef]
- Takaya, S.; Lu, Y.; Guan, S.; Miyazawa, K.; Yoshida, H.; Asanuma, H. Fabrication of the photocatalyst thin films of nano-structured potassium titanate by molten salt treatment and its photocatalytic activity. Surf. Coat. Technol. 2015, 275, 260–263. [Google Scholar] [CrossRef]
- Villalpando-Reyna, A.; Cortés-Hernádez, D.A.; Gorokhovsky, A.; Almanza-Robles, J.M.; Escobedo-Bocardo, J.C. In vitro bioactivity assessment and mechanical properties of novel calcium titanate/borosilicate glass composites. Ceram. Int. 2011, 37, 1625–1629. [Google Scholar] [CrossRef]
- Liu, Y.; Tsuru, K.; Hayakawa, S.; Osaka, A. Potassium titanate nanorods array grown on titanium substrates and their in vitro bioactivity. J. Ceram. Soc. Jpn. 2004, 112, 634–640. [Google Scholar] [CrossRef][Green Version]
- Song, H.; Jiang, H.; Liu, T.; Liu, X.; Meng, G. Preparation and photocatalytic activity of alkali titanate nano materials A2TinO2n+1 (A = Li, Na and K). Mater. Res. Bull. 2007, 42, 334–344. [Google Scholar] [CrossRef]
- Cid-Dersdner, H.; Buerger, M.J. The crystal structure of potassium hexatitanate K2Ti6O13. Z. Für Krist. 1962, 117, 411–430. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Chandela, V.S.; Azam, A. Comparative study of potassium hexatitanate (K2Ti6O13) whiskers prepared by sol–gel and solid state reaction routes. Appl. Surf. Sci. 2012, 258, 7354–7358. [Google Scholar] [CrossRef]
- Li, D.; Hagos, K.; Huang, L.; Lu, X.; Liu, C.; Quian, H. Self-propagating high-temperature synthesis of potassium hexatitanate whiskers. Ceram. Int. 2017, 43, 15505–15509. [Google Scholar] [CrossRef]
- Qian, Q.H.; Zhou, X.F.; Hu, Y.Y.; Liu, C.; Feng, X.; Lu, X.H. Preparation of smooth potassium hexatitanate nanofilms by sol-gel method. J. Matter Sci. 2007, 42, 8222–8229. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, C.; He, M.; Yang, Z.; Feng, X.; Lu, X. Preparation and characterization of alkaline resistant porous ceramics from potassium titanate whiskers. Chin. J. Chem. Eng. 2007, 15, 742–747. [Google Scholar] [CrossRef]
- Li, G.L.; Wang, G.H.; Hong, J.M. Synthesis and characterization of K2Ti6O13 whiskers with diameter on nanometer scale. J. Mater. Sci. Lett. 1999, 18, 1865–1867. [Google Scholar] [CrossRef]
- Meng, X.; Wang, D.; Liu, J.; Lin, B.; Fu, Z. Effects of titania different phases on the microstructure and properties of K2Ti6O13 nanowires. Solid State Commun. 2006, 137, 146–149. [Google Scholar] [CrossRef]
- Ishii, T.; Takioka, R.; Yasumura, H.; Yamamoto, A.; Yoshida, H. Fine crystals of potassium hexatitanate prepared by a sol-gel method for photocatalytic reduction of carbon dioxide with water. Catal. Today 2024, 429, 114476. [Google Scholar] [CrossRef]
- Yahya, R.B.; Hayashi, H.; Nagase, T.; Ebina, T.; Onodera, Y.; Saitoh, N. Hydrothermal synthesis of potassium hexatitanates under subcritical and supercritical water conditions and its application in photocatalysis. Chem. Mater. 2001, 13, 842–847. [Google Scholar] [CrossRef]
- Guan, G.; Kida, T.; Harada, T.; Isayama, M.; Yoshida, A. Photoreduction of carbon dioxide with water over K2Ti6O13 photocatalyst combined with Cu/ZnO catalyst under concentrated sunlight. Appl. Catal. A Gen. 2003, 249, 11–18. [Google Scholar] [CrossRef]
- Yoshida, H.; Takeuchi, M.; Sato, M.; Zhang, L.; Teshima, T.; Chaskar, M.G. Potassium hexatitanate photocatalysts prepared by a flux method for water splitting. Catal. Today 2014, 232, 158–164. [Google Scholar] [CrossRef]
- Bretado, E.M.A.; Lozano, G.M.A.; Martínez, C.V.; Ortiz, L.A.; Zaragoza, M.V.; Lara, R.H.; Medina, M.C.U. Synthesis, characterization and photocatalytic evaluation of potassium hexatitanate (K2Ti6O13) fibers. Int. J. Hydrogen Energy 2019, 44, 12470–12476. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, X.; Jing, Z.; Liu, H.; Tang, P.; Zhu, H.; Li, B. Recent advances in one-dimensional alkali-metal hexatitanate photocatalysts for environmental remediation and solar fuel production. J. Mater. Sci. Tchnol. 2024, 202, 201–239. [Google Scholar] [CrossRef]
- Hakuta, Y.; Hayashi, H.; Arai, K. Hydrothermal synthesis of photocatalyst potassium hexatitanate nanowires under supercritical conditions. J. Mater. Sci. 2004, 39, 4977–4980. [Google Scholar] [CrossRef]
- Yu, D.; Wu, J.; Zhou, L.; Xie, D.; Wu, S. The dielectric and mechanical properties of a potassium-titanate whisker-reinforced PP/PA blend. Compos. Sci. Technol. 2000, 60, 499–508. [Google Scholar] [CrossRef]
- Zhao, G.; Lu, X.; Cao, L.; Zhi, J.; Yang, Y. Synergistically enhancing ablation and thermal isolation properties of EPDM insulation composites by introducing potassium hexatitanate whisker. Compos. Sci. Technol. 2024, 249, 110478. [Google Scholar] [CrossRef]
- Du, G.H.; Chen, Q.; Han, P.D.; Yu, Y.; Peng, L.M. Potassium titanates nanowires. Structure, growth and optical properties. Phys. Rev. B 2003, 67, 035323. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.C.; Zhang, M. Low temperature preparation and optical properties of K2Ti6O13. Mater. Lett. 2012, 79, 136–138. [Google Scholar] [CrossRef]
- Tjong, S.C.; Meng, Y.Z. Microstructural and mechanical characteristics of compatibilized polypropylene hybrid composites containing potassium titanate whisker and liquid crystalline copolyester. Polymer 1999, 40, 7275–7283. [Google Scholar] [CrossRef]
- Sanchez-Valdes, E.; Gorokhovsii, A.V.; Scherbakova, N.N.; Rodríguez-Galicia, J.L. Formation of composite structure in alumosilicate systems with the introduction of potassium titanates. Glass Ceram. 2010, 67, 169–172. [Google Scholar] [CrossRef]
- Kyungho, C.; Youngkeun, H. Friction and wear properties of scrap tire/potassium hexatitanate whisker composites. J. Ind. Eng. Chem. 2013, 19, 1234–1240. [Google Scholar] [CrossRef]
- Zhuang, G.S.; Sui, G.X.; Meng, H.; Sun, Z.S.; Yang, R. Mechanical properties of potassium titanate whiskers reinforced poly(ether ether ketone) composites using different compounding processes. Compos. Sci. Technol. 2007, 67, 1172–1181. [Google Scholar] [CrossRef]
- Yun, S.; Song, Q.Q.; Zhao, D.M.; Qian, G.M.; Li, X.N.; Li, W. Study on the inorganic–organic surface modification of potassium titanate whisker. Appl. Surf. Sci. 2012, 258, 4444–4448. [Google Scholar] [CrossRef]
- Asano, K.; Yoneda, H.; Agari, Y.; Matsumuro, M.; Higashi, K. Thermal and mechanical properties of aluminum alloy composite reinforced with potassium hexatitanate short fiber. Mater. Trans. 2015, 56, 160–166. [Google Scholar] [CrossRef]
- Gorokhovsky, A.V.; Escalante-Garcia, J.I.; Sanchez-Valdes, E.; Burmistrov, I.N.; Kuznetsov, D.V. Synthesis and characterization of high-strength ceramic composites in the system of potassium titanate-metallurgical slag. Ceram. Int. 2015, 41, 13294–13303. [Google Scholar] [CrossRef]
- Long, C.G.; He, L.P.; Zhong, Z.H.; Chen, S.G. Studies on the polypropylene composites reinforced by ramier fiber and K2Ti6O13 Whiskers. Res. Lett. Mater. Sci. 2007, 2007, 087072. [Google Scholar] [CrossRef]
- Tjong, S.C.; Meng, Y.Z. Morphology and performance of potassium titanate whisker-reinforced polypropylene composites. J. Appl. Polym. Sci. 1998, 70, 431–439. [Google Scholar] [CrossRef]
- Tjong, S.C.; Meng, Y.Z. Performance of potassium titanate whisker reinforced polyamide-6 composites. Polymer 1998, 39, 5461–5466. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Y.; Feng, X.; Lu, X. The effect of self-assembly modified potassium titanate whiskers on the friction and wear behaviors of PEEK composites. Wear 2010, 269, 139–144. [Google Scholar] [CrossRef]
- Feng, X.; Diao, X.; Shi, Y.; Wang, H.; Sun, S.; Lu, X. A study on the friction and wear behavior of polytetrafluoroethylene filled with potassium titanate whiskers. Wear 2006, 261, 1208–1212. [Google Scholar] [CrossRef]
- Feng, X.; Wang, H.; Shi, Y.; Chen, D.; Lu, X. The effects of size and content of potassium titanate whiskers on the properties of PTW/PTFE composites. Mater. Sci. Eng. A 2007, 448, 253–258. [Google Scholar] [CrossRef]
- Mu, L.; Feng, X.; Zhu, J.; Wang, H.; Sun, Q.; Shi, Y.; Lu, X. Comparative study of tribological properties of different fibers reinforced PTFE/PEEK composites at elevated temperatures. Tribol. Trans. 2010, 53, 189–194. [Google Scholar] [CrossRef]
- Ou, Y.C.; Yang, F.; Chen, J. Interfacial interaction and mechanical properties of nylon 6-potassium titanate composites prepared by in-situ polymerization. J. Appl. Polym. Sci. 1997, 64, 2317–2322. [Google Scholar] [CrossRef]
- Hao, X.; Gai, G.; Lu, F.; Zhao, X.; Zhang, Y.; Liu, J.; Yang, Y.; Gui, D.; Nan, C. Dynamic mechanical properties of whisker/PA66 composites at high strain rates. Polymer 2005, 46, 3528–3534. [Google Scholar] [CrossRef]
- Qu, M.; Jian, X.; He, W.; Liao, G. Performance of potassium titanate whisker reinforced PPESK composites. J. Mater. Sci. Technol. 2004, 20, 445–447. [Google Scholar] [CrossRef]
- Shishkin, R.A. Influence of the physical and chemical properties of particles on the thermal conductivity of polymer composite materials. High Temp. 2023, 61, 163–172. [Google Scholar] [CrossRef]
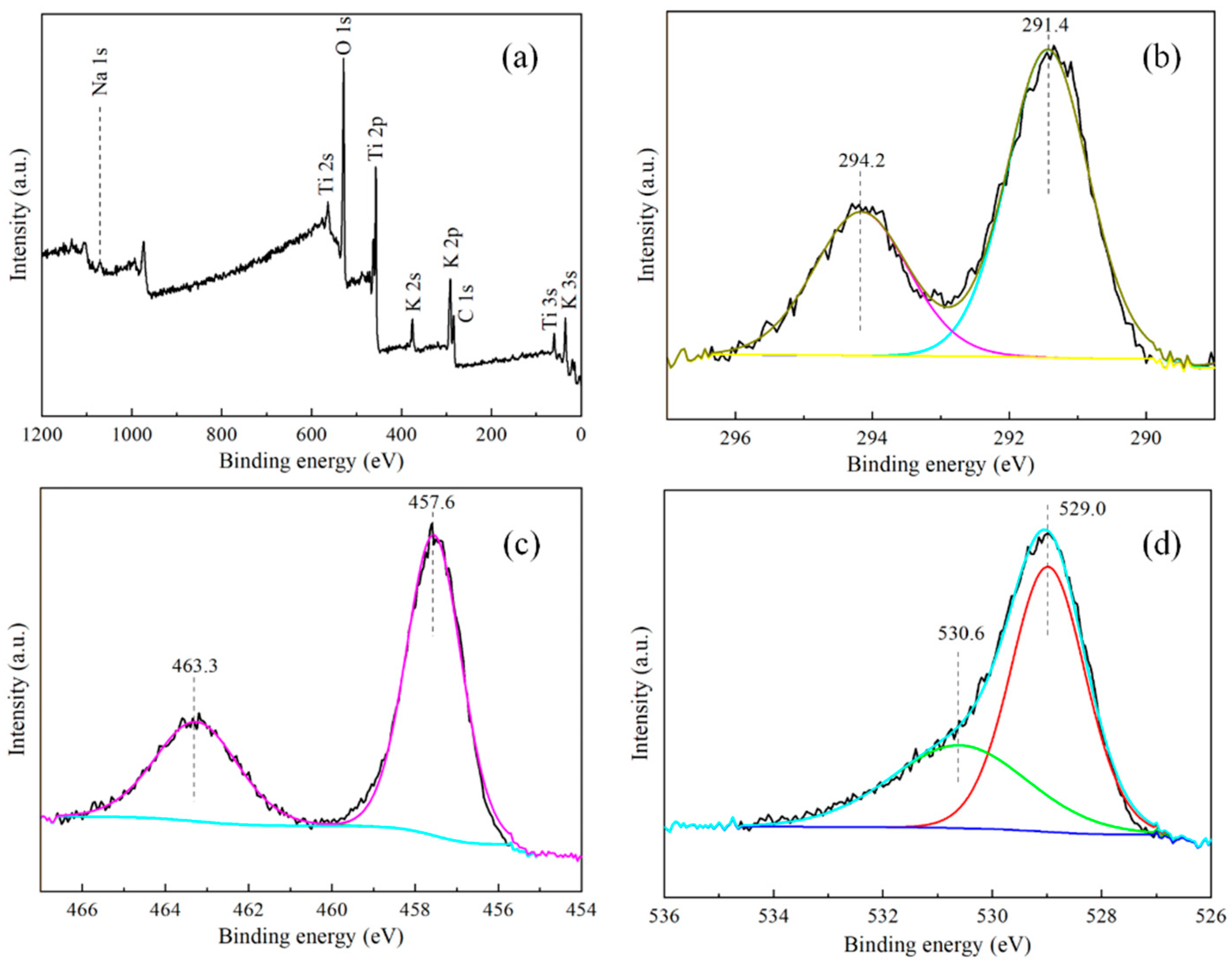
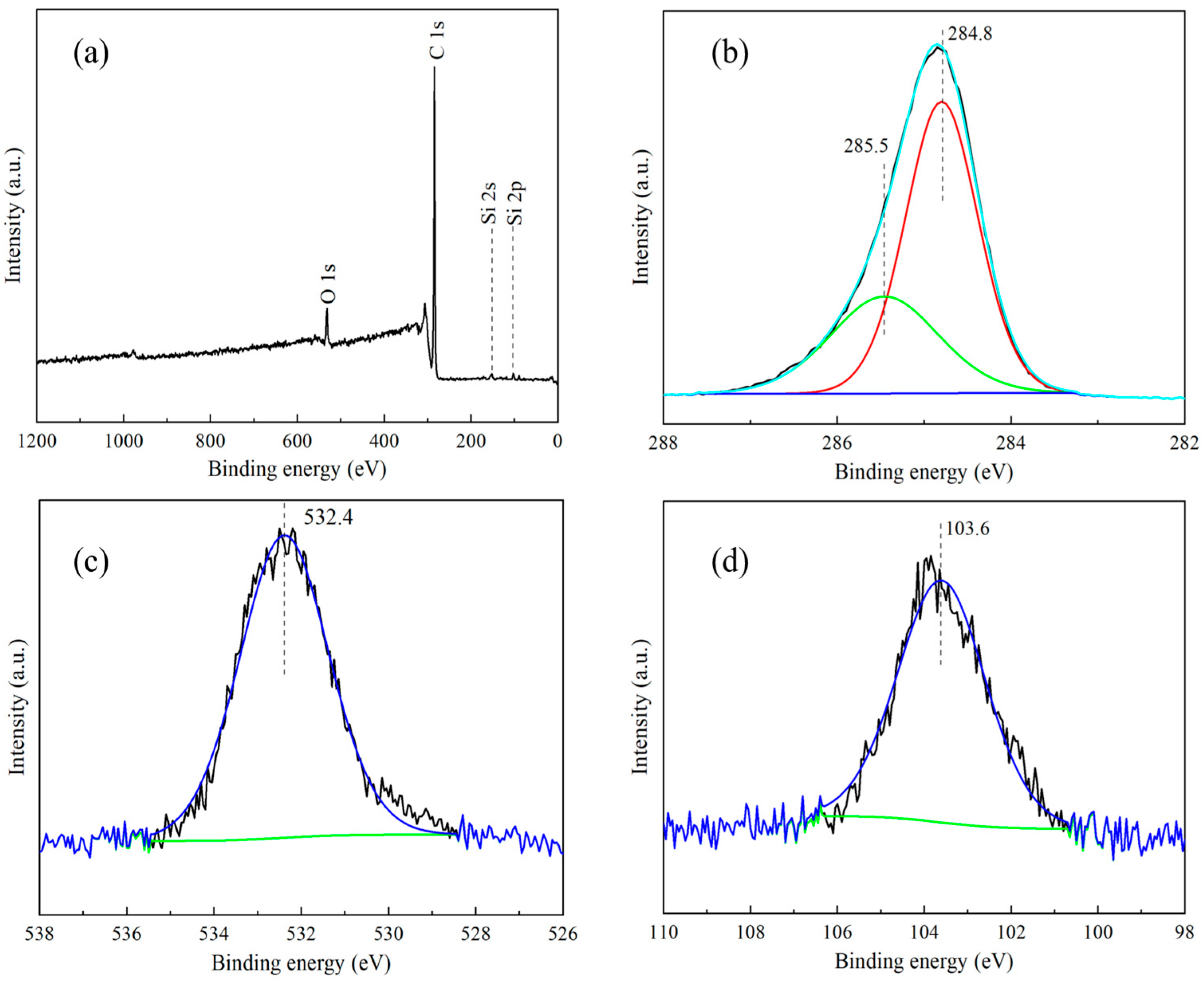
| O(1s) | C(1s) | Ti(2p) | Na(1s) | K(2p) | Si(2p) | |
|---|---|---|---|---|---|---|
| 528.8 | 290.8 | 456.8 | 1066.8 | 290.8 | 100.8 | |
| PHT-2 | 26.44 | 51.76 | 8.34 | 0.42 | 13.04 | - |
| PHT-2-1 | 27.49 | 49.20 | 6.81 | 0.60 | 12.39 | 3.51 |
| PHT-1-1 | 26.80 | 50.13 | 7.59 | 0.24 | 12.63 | 2.62 |
| Samples | Tensile Strength (MPa) | Elongation at Break (%) | Modulus of Elasticity (MPa) | Shear Strength (MPa) | Shear Elasticity (MPa) | Density (kg/m3) |
|---|---|---|---|---|---|---|
| PE | 18.3 | 6.84 | 1947 | 33.8 | 1900 | 1204 |
| PE/PHT-1-1 | 18.8 | 44.7 | 1810 | 30.7 | 1740 | 1242 |
| PE/PHT-1-2 | 17.1 | 9.02 | 1830 | 29.7 | 1800 | 1232 |
| PE/PHT-1-3 | 19.5 | 39.1 | 1740 | 31.2 | 1208 | 1229 |
| PE/PHT-2-1 | 21.7 | 38.1 | 2017 | 34.1 | 1910 | 1218 |
| PE/PHT-2-2 | 19.7 | 19.7 | 1970 | 32.8 | 1970 | 1232 |
| PE/PHT-2-2 | 21.2 | 36.4 | 1950 | 34.0 | 1530 | 1222 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Q.; Jia, Q.; Xie, J.; Li, X.; Wu, Y.; Sun, Y.; Chu, S.; Yang, B.; Chen, Z.; Peng, K. Reinforcement of Polyethylene with Potassium Hexatitanate Whiskers: Importance of Polyvinylpyrrolidone Additive. J. Compos. Sci. 2025, 9, 11. https://doi.org/10.3390/jcs9010011
Pan Q, Jia Q, Xie J, Li X, Wu Y, Sun Y, Chu S, Yang B, Chen Z, Peng K. Reinforcement of Polyethylene with Potassium Hexatitanate Whiskers: Importance of Polyvinylpyrrolidone Additive. Journal of Composites Science. 2025; 9(1):11. https://doi.org/10.3390/jcs9010011
Chicago/Turabian StylePan, Qin, Qinxiang Jia, Jin Xie, Xiaoyong Li, Yong Wu, Yang Sun, Suihong Chu, Bo Yang, Zhexi Chen, and Kewei Peng. 2025. "Reinforcement of Polyethylene with Potassium Hexatitanate Whiskers: Importance of Polyvinylpyrrolidone Additive" Journal of Composites Science 9, no. 1: 11. https://doi.org/10.3390/jcs9010011
APA StylePan, Q., Jia, Q., Xie, J., Li, X., Wu, Y., Sun, Y., Chu, S., Yang, B., Chen, Z., & Peng, K. (2025). Reinforcement of Polyethylene with Potassium Hexatitanate Whiskers: Importance of Polyvinylpyrrolidone Additive. Journal of Composites Science, 9(1), 11. https://doi.org/10.3390/jcs9010011








