PEBAX® 5533D Formulation for Enhancement of Mechanical and Thermal Properties of Material Used in Medical Device Manufacturing
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanchez, D. Advanced Extrusion Techniques: How Engineered Extrusion Helps Medical Device Makers Improve Device Designs and Patient Experiences. Med. Des. Briefs 2021, 1, 6–9. [Google Scholar]
- Yin, J.; Luan, S. Opportunities and challenges for the development of polymer-based biomaterials and medical devices. Regen. Biomater. 2016, 3, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Toh, H.W.; Toong, D.W.Y.; Ng, J.C.K.; Ow, V.; Lu, S.; Tan, L.P.; Wong, P.E.H.; Venkatraman, S.; Huang, Y.; Ang, H.Y. Polymer blends and polymer composites for cardiovascular implants. Eur. Polym. J. 2021, 146, 110249. [Google Scholar] [CrossRef]
- Touris, A.; Turcios, A.; Mintz, E.; Pulugurtha, S.R.; Thor, P.; Jolly, M.; Jalgaonkar, U. Effect of molecular weight and hydration on the tensile properties of polyamide 12. Results Mater. 2020, 8, 100149. [Google Scholar] [CrossRef]
- Lewis, P.R. Manufacturing defects in polymeric medical devices. In Durability and Reliability of Medical Polymers; Woodhead Publishing: Cambridge, UK, 2012; pp. 225–268. [Google Scholar] [CrossRef]
- Megaly, M.; Morcos, R.; Kucharik, M.; Tawadros, M.; Basir, M.B.; Pershad, A.; Maini, B.; Khalili, H.; Alaswad, K.; Brilakis, E.S. Complications and Failure Modes of Polymer-Jacketed Guidewires; Insights from the MAUDE Database. Cardiovasc. Revascularization Med. 2022, 36, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Ram, M. Hoja Técnica Estearato de Calcio. 2022. Available online: https://mineralesram.com/wp-content/uploads/2020/11/HOJA-TE%cc%81CNICA-ESTEARATO-DE-CALCIO-.pdf (accessed on 6 December 2023).
- Silva, L.; Ricardo, J.; Macedo, N. Evaluation of the Enzymatic Degradation of PLA and Their Compounds with Thermoplastic Starch. 2014. Available online: https://www.researchgate.net/publication/312503004 (accessed on 28 December 2023).
- Salahshoori, I.; Jorabchi, M.N.; Valizadeh, K.; Yazdanbakhsh, A.; Bateni, A.; Wohlrab, S. A deep insight of solubility behavior, mechanical quantum, thermodynamic, and mechanical properties of Pebax-1657 polymer blends with various types of vinyl polymers: A mechanical quantum and molecular dynamics simulation study. J. Mol. Liq. 2022, 363, 119793. [Google Scholar] [CrossRef]
- Jamarun, N.; Amelia, D.; Rahmayeni; Septiani, U.; Sisca, V. The effect of temperature on the synthesis and characterization of hydroxyapatite-polyethylene glycol composites by in-situ process. Hybrid Adv. 2023, 2, 100031. [Google Scholar] [CrossRef]
- Mikhailova, O.; Rovnaník, P. Effect of Polyethylene Glycol Addition on Metakaolin-based Geopolymer. In Procedia Engineering; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 222–228. [Google Scholar] [CrossRef]
- Li, F.J.; Liang, J.Z.; Zhang, S.D.; Zhu, B. Tensile Properties of Polylactide/Poly(ethylene glycol) Blends. J. Polym. Environ. 2015, 23, 407–415. [Google Scholar] [CrossRef]
- Athanasoulia, I.-G.; Tarantili, P.A. Preparation and characterization of polyethylene glycol/poly(L-lactic acid) blends. Pure Appl. Chem. 2017, 89, 141–152. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Balaji, K.V.; Yadav, R.; Zabihi, O.; Ahmadi, M.; Adetunji, P.; Naebe, M. Balancing the toughness and strength in polypropylene composites. Compos. B Eng. 2021, 223, 109121. [Google Scholar] [CrossRef]
- Tso, C.Y.; Chao, C.Y.H. Activated carbon, silica-gel and calcium chloride composite adsorbents for energy efficient solar adsorption cooling and dehumidification systems. Int. J. Refrig. 2012, 35, 1626–1638. [Google Scholar] [CrossRef]
- Garofalo, E.; Scarfato, P.; Di Maio, L.; Protopapa, A.; Incarnato, L. Zeolites as effective desiccants to solve hygroscopicity issue of post-consumer mixed recycled polyolefins. J. Clean. Prod. 2021, 295, 126379. [Google Scholar] [CrossRef]
- Yang, I.-K.; Tsai, P.-H. Rheology and structure change of poly(ether-block-amide) segmented block copolymer. J. Cent. South Univ. Technol. 2007, 14, 146–150. [Google Scholar] [CrossRef]
- ASTM D3850; Standard Test Method for Rapid Thermal Degradation of Solid Electrical Insulating Materials by Thermogravimetric Method (TGA); Volume 10.02, Book of Standards. ASTM International: West Conshohocken, PA, USA, 2019. Available online: https://www.astm.org/d3850-19.html (accessed on 19 January 2024).
- ASTM D792; Standard Test Methods for Density and Specific Gravity (Relative Density) of Plastics by Displacement; Volume 08.01, Book of Standards. ASTM International: West Conshohocken, PA, USA, 2020. Available online: https://www.astm.org/d0792-20.html (accessed on 13 December 2023).
- ASTM D3418; Standard Test Method for Transition Temperatures and Enthalpies of Fusion and Crystallization of Polymers by Differential Scanning Calorimetry; Volume 08.02, Book of Standards. ASTM International: West Conshohocken, PA, USA, 2021. Available online: https://www.astm.org/d3418-21.html (accessed on 19 January 2024).
- ASTM D638; Standard Test Method for Tensile Properties of Plastics; Volume 08.01, Book of Standards. ASTM International: West Conshohocken, PA, USA, 2022. Available online: https://www.astm.org/d0638-22.html (accessed on 19 January 2024).
- ASTM D790; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials; Volume 08.01, Book of Standards. ASTM International: West Conshohocken, PA, USA, 2017. Available online: https://www.astm.org/d0790-17.html (accessed on 19 January 2024).
- Haudin, J.-M.; Chenot, J.-L. Numerical and Physical Modeling of Polymer Crystallization. Int. Polym. Process. 2004, 19, 267–274. [Google Scholar] [CrossRef]
- Palacios, J.K.; Michell, R.M.; Müller, A.J. Crystallization, morphology and self-assembly of double, triple and tetra crystalline block polymers. Polym. Testing 2023, 121, 107995. [Google Scholar] [CrossRef]
- Lemanowicz, M.; Mielańczyk, A.; Walica, T.; Kotek, M.; Gierczycki, A. Application of Polymers as a Tool in Crystallization—A Review. Polymers 2021, 13, 2695. [Google Scholar] [CrossRef]
- Sadeghi, F.; Le, D. Characterization of polymeric biomedical balloon: Physical and mechanical properties. J. Polym. Eng. 2021, 41, 799–807. [Google Scholar] [CrossRef]
- Tien, N.-D.; Prud’homme, R.E. Crystallization Behavior of Semicrystalline Immiscible Polymer Blends. In Crystallization in Multiphase Polymer Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 181–212. [Google Scholar] [CrossRef]
- Bertolla, M.; Cecchetto, M.; Comotto, M.; Moro, A.D. Comparison of the Properties of a Random Copolymer and a Molten Blend PA6/PA6.9. Polymers 2022, 14, 4115. [Google Scholar] [CrossRef] [PubMed]
- Thanakkasaranee, S.; Kim, D.; Seo, J. Preparation and Characterization of Poly(ether-block-amide)/Polyethylene Glycol Composite Films with Temperature-Dependent Permeation. Polymers 2018, 10, 225. [Google Scholar] [CrossRef]
- Amstutz, C.; Weisse, B.; Valet, S.; Haeberlin, A.; Burger, J.; Zurbuchen, A. Temperature-dependent tensile properties of polyamide 12 for the use in percutaneous transluminal coronary angioplasty balloon catheters. BioMed. Eng. Online 2021, 20, 110. [Google Scholar] [CrossRef]
- Peng, J.; Snyder, G.J. A figure of merit for flexibility. Science 2019, 366, 690–691. [Google Scholar] [CrossRef] [PubMed]
- Arkema, G.M. Improvements in local mechanical property measurements of polymers. Plast. Res. Online 2010. [Google Scholar] [CrossRef]
- Wang, X.-Z.; Wang, J.-W.; Wang, H.-Q.; Zhuang, G.-C.; Yang, J.-B.; Ma, Y.-J.; Zhang, Y.; Ren, H. Effects of a new compatibilizer on the mechanical properties of TPU/PEBA blends. Eur. Polym. J. 2022, 175, 111358. [Google Scholar] [CrossRef]
- Yu, T.; Luo, F.; Zhao, Y.; Wang, D.; Wang, F. Improving the processability of biodegradable polymer by stearate additive. J. Appl. Polym. Sci. 2011, 120, 692–700. [Google Scholar] [CrossRef]
- Laboulfie, F.; Hémati, M.; Lamure, A.; Diguet, S. Effect of the plasticizer on permeability, mechanical resistance and thermal behaviour of composite coating films. Powder Technol. 2013, 238, 14–19. [Google Scholar] [CrossRef]
- Jeong, S. Investigation of intrinsic characteristics of polymer blends via molecular simulation: A review. Korea Aust. Rheol. J. 2023, 35, 249–266. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Walkowiak, K.; Irska, I.; Zubkiewicz, A.; Figiel, P.; Gorący, K.; El Fray, M. Furan-based copoly(ester-ethers) and copoly(ester-amide-ethers). Comparison study on the phase structure, mechanical and thermal properties. Polymer 2023, 269, 125740. [Google Scholar] [CrossRef]
- Roland, C.M.; Casalini, R. The role of density and temperature in the dynamics of polymer blends. Macromolecules 2005, 38, 8729–8733. [Google Scholar] [CrossRef][Green Version]
- Kim, M.; Kang, S.W. PEBAX-1657/Ag nanoparticles/7,7,8,8-tetracyanoquinodimethane complex for highly permeable composite membranes with long-term stability. Sci. Rep. 2019, 9, 4266. [Google Scholar] [CrossRef]
- Umar, M.; Ofem, M.I.; Anwar, A.S.; Salisu, A.G. Thermo gravimetric analysis (TGA) of PA6/G and PA6/GNP composites using two processing streams. J. King Saud Univ.—Eng. Sci. 2022, 34, 77–87. [Google Scholar] [CrossRef]
- Zhao, D.; Ren, J.; Wang, Y.; Qiu, Y.; Li, H.; Hua, K.; Li, X.; Ji, J.; Deng, M. High CO2 separation performance of Pebax®/CNTs/GTA mixed matrix membranes. J. Memb. Sci. 2017, 521, 104–113. [Google Scholar] [CrossRef]
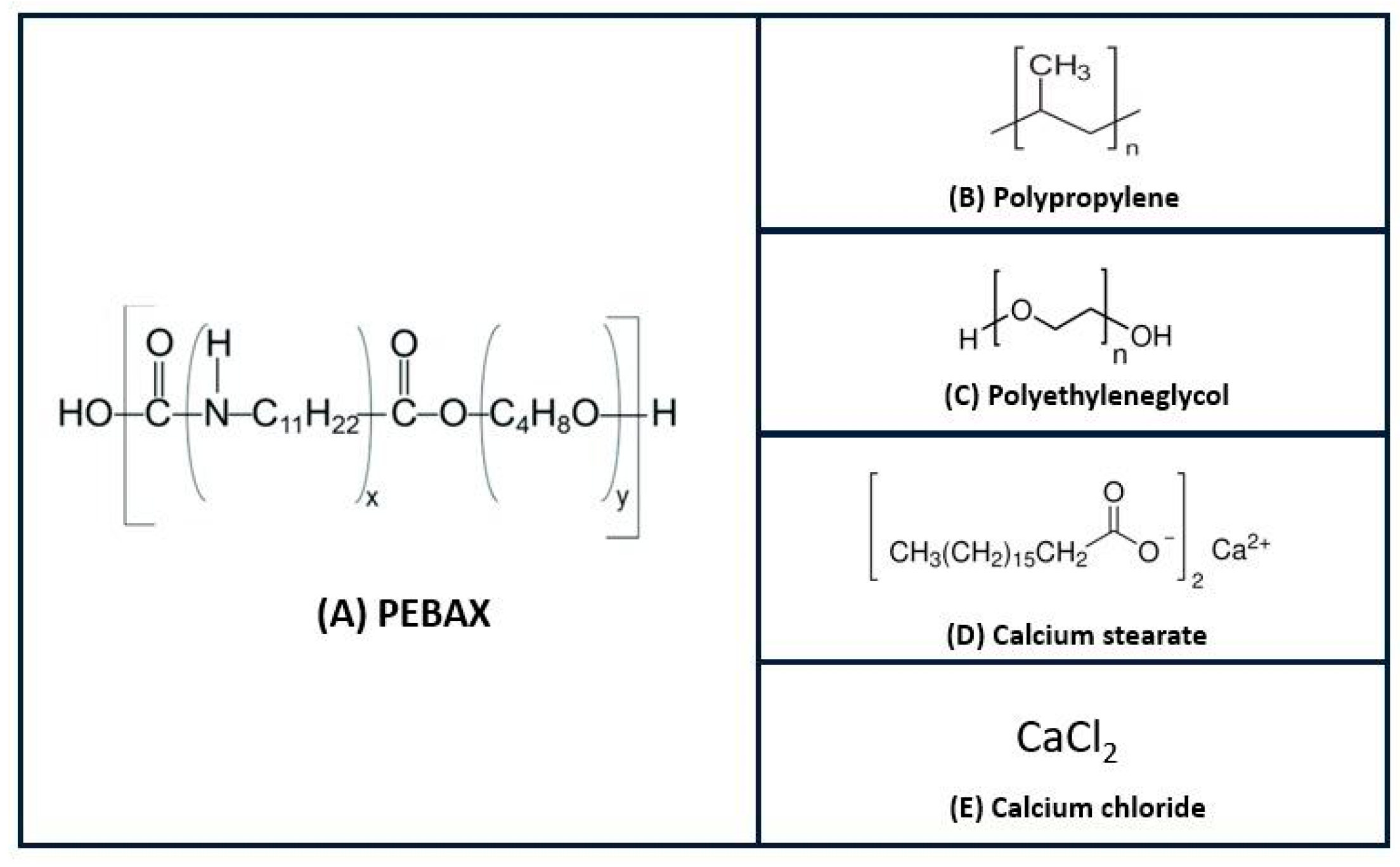


| Ref. Run | Formula | A (%) | B (%) | C (%) | D (%) | E (%) |
|---|---|---|---|---|---|---|
| P1 | 4 | 89.17 | 7.50 | 2.50 | 0.63 | 0.21 |
| P2 | 5 | 89.29 | 10.00 | 0.00 | 0.50 | 0.21 |
| P3 | 11 | 88.79 | 0.00 | 10.00 | 1.00 | 0.21 |
| P4 | 16 | 79.79 | 10.00 | 10.00 | 0.00 | 0.21 |
| P5 | 15 | 99.29 | 0.00 | 0.00 | 0.50 | 0.21 |
| P6 | 3 | 98.79 | 0.00 | 0.00 | 1.00 | 0.21 |
| P7 | 7 | 93.92 | 2.50 | 2.50 | 0.88 | 0.21 |
| Std | 18 | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 |
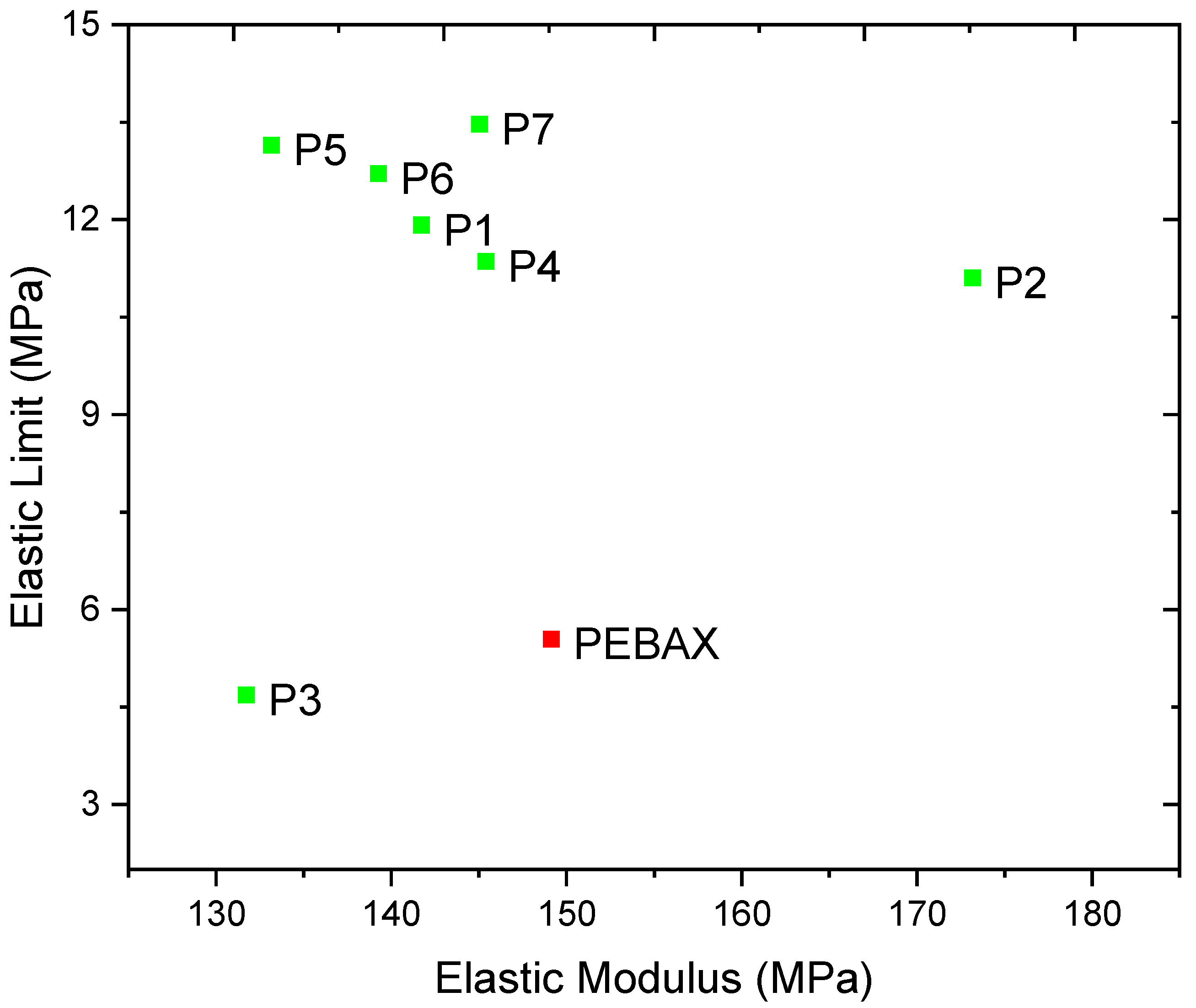
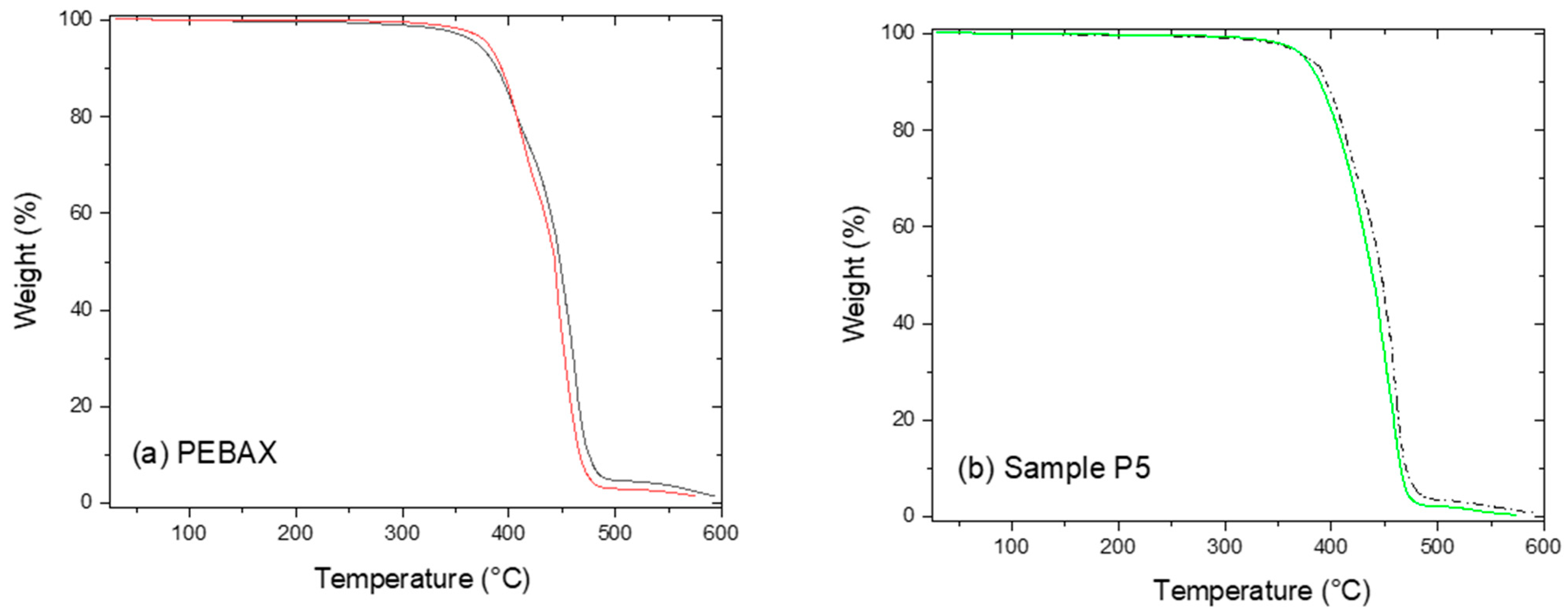
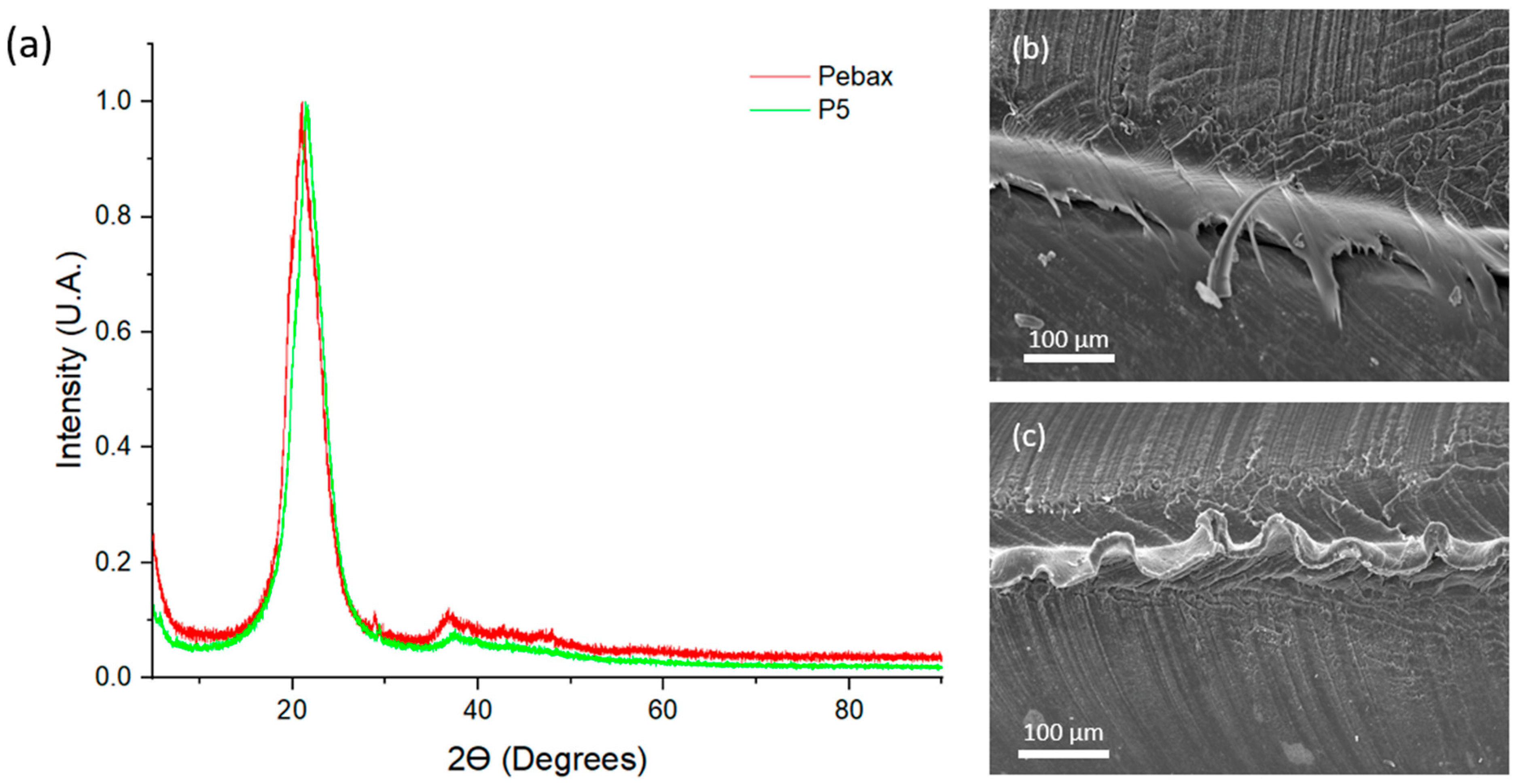
| Ref. Run | Crystallinity Temperature (°C) ± 0.025 Accuracy | Tm (°C) ± 0.025 Accuracy | Ultimate Tensile Strength (MPa) | Elastic Modulus (MPa) | Elastic Limit (MPa) | Flexural Strength (MPa) | Density @ 23 °C (g/cm3) | Moisture Content (%) |
|---|---|---|---|---|---|---|---|---|
| P1 | 91.010 | 155.150 | 23.20 | 141.71 | 11.92 | 8.14 ± 0.40 | 1.0153 | 0.186 |
| P2 | 92.670 | 155.520 | 26.10 | 173.16 | 11.11 | 10.67 ± 0.07 | 1.0057 | 0.339 |
| P3 | 76.670 | 154.160 | 12.90 | 131.72 | 4.69 | 10.74 ± 0.22 | 1.0017 | 0.279 |
| P4 | 96.060 | 157.280 | 19.80 | 145.38 | 11.36 | 9.69 ± 0.24 | 1.0223 | 0.262 |
| P5 | 94.740 | 158.010 | 24.20 | 133.16 | 13.15 | 7.56 ± 0.18 | 1.0147 | 0.276 |
| P6 | 90.510 | 154.790 | 23.60 | 139.26 | 12.71 | 7.32 ± 0.06 | 1.0157 | 0.272 |
| P7 | 93.720 | 154.730 | 21.70 | 145.02 | 13.47 | 7.83 ± 0.16 | 1.0120 | 0.147 |
| Std (Pebax) | 100.82 | 157.13 | 22.00 | 149.12 | 5.55 | 9.14 ± 0.29 | 1.0057 | 0.351 |
| Response Variable | R-Squared | Pebax Coefficient | PP Coefficient | PEG Coefficient | EC Coefficient | ANOVA p-Value |
|---|---|---|---|---|---|---|
| UTS | 93.19% | 23.2748 | 63.5181 | −62.8362 | −40.119 | 0.009 |
| Elastic modulus | 64.44% | 143.407 | 343.892 | −2.399 | −79.109 | 0.207 |
| Elastic limit | 39.00% | 8.474 | 48.27 | −28.652 | 311.809 | 0.534 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillén-Espinoza, M.; Sancho, F.V.; Starbird-Perez, R.; Zamora-Sequeira, R. PEBAX® 5533D Formulation for Enhancement of Mechanical and Thermal Properties of Material Used in Medical Device Manufacturing. J. Compos. Sci. 2024, 8, 314. https://doi.org/10.3390/jcs8080314
Guillén-Espinoza M, Sancho FV, Starbird-Perez R, Zamora-Sequeira R. PEBAX® 5533D Formulation for Enhancement of Mechanical and Thermal Properties of Material Used in Medical Device Manufacturing. Journal of Composites Science. 2024; 8(8):314. https://doi.org/10.3390/jcs8080314
Chicago/Turabian StyleGuillén-Espinoza, Mildred, Fabián Vásquez Sancho, Ricardo Starbird-Perez, and Roy Zamora-Sequeira. 2024. "PEBAX® 5533D Formulation for Enhancement of Mechanical and Thermal Properties of Material Used in Medical Device Manufacturing" Journal of Composites Science 8, no. 8: 314. https://doi.org/10.3390/jcs8080314
APA StyleGuillén-Espinoza, M., Sancho, F. V., Starbird-Perez, R., & Zamora-Sequeira, R. (2024). PEBAX® 5533D Formulation for Enhancement of Mechanical and Thermal Properties of Material Used in Medical Device Manufacturing. Journal of Composites Science, 8(8), 314. https://doi.org/10.3390/jcs8080314








