Current Trends in the Use of Biomass in the Manufacture of Rigid Polyurethane Foams: A Review
Abstract
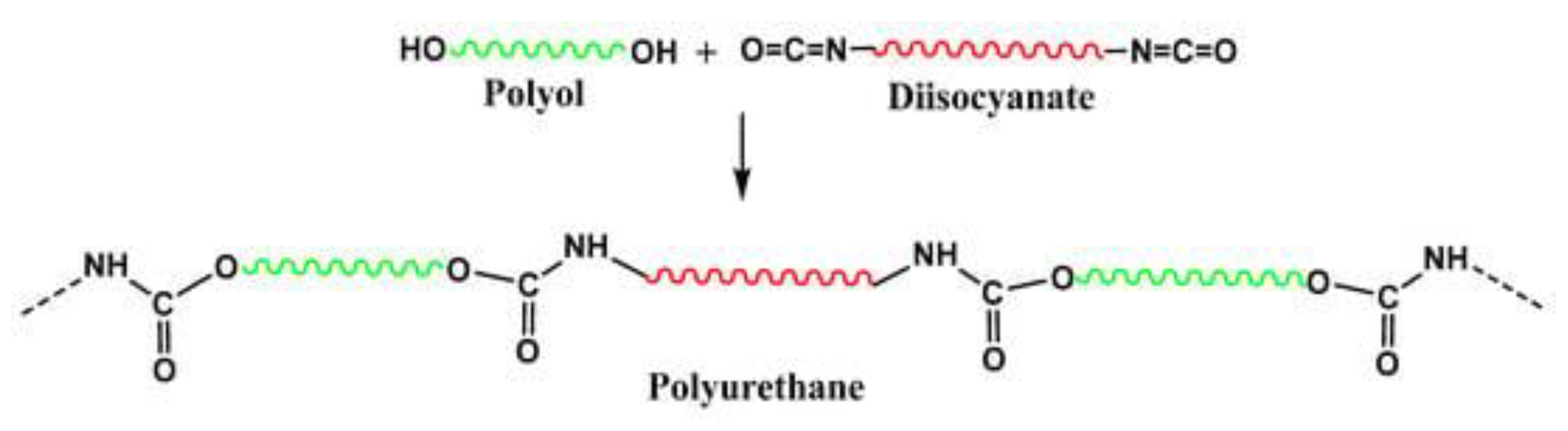
1. Introduction
2. Diversity of Biomass Used in the RPUR Foam Manufacturing Process
3. Biomass as a Raw Material for the Production of Bio-Polyols
3.1. Oil-Based Bio-Polyols

3.2. Bio-Polyol Synthesis in the Biomass Liquefaction Process
3.3. Lignin-Based Bio-Polyols
4. Effectiveness of Using Biomass as a Filler for RPUR Foams
4.1. Application Perspectives for Agricultural Biomass as RPUR Foam Fillers
4.2. Food Industry Waste as RPUR Foam Fillers
4.3. Forest Biomass as PUR Foam Fillers
5. Environmental Benefits Arising from the Use of Biomass and Challenges Associated with Biomass Utilization in the RPUR Foam Industry
5.1. Positive Environmental Aspects of Biomass Application
5.2. Barriers and Difficulties in the Application of Biomass in the Production of RPUR Foams
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- White, W.R. New Concepts for Polyurethane Foams in Automotive Body Design; SAE Technical Paper: Warrendale, PA, USA, 1969. [Google Scholar]
- Morris, A.M.; Black, R.G.; Runk, R.H.; Minter, H.F. Dependence of Physical Properties on Composition in a Series of High Load-Bearing Polyurethane Foams. J. Appl. Polym. Sci. 1965, 9, 2715–2728. [Google Scholar] [CrossRef]
- John, J.; Bhattacharya, M.K.; Turner, R.B. Characterization of Polyurethane Foams from Soybean Oil. J. Appl. Polym. Sci. 2002, 86, 3097–3107. [Google Scholar] [CrossRef]
- Ahmed, N.; Kausar, A.; Muhammad, B. Advances in Shape Memory Polyurethanes and Composites: A Review. Polym.-Plast. Technol. Eng. 2015, 54, 1410–1423. [Google Scholar] [CrossRef]
- Kausar, A. Polyurethane Composite Foams in High-Performance Applications: A Review. Polym.-Plast. Technol. Eng. 2018, 57, 346–369. [Google Scholar] [CrossRef]
- Jin, F.-L.; Zhao, M.; Park, M.; Park, S.-J. Recent Trends of Foaming in Polymer Processing: A Review. Polymers 2019, 11, 953. [Google Scholar] [CrossRef]
- Dawson, J.R.; Shortall, J.B. The Microstructure of Rigid Polyurethane Foams. J. Mater. Sci. 1982, 17, 220–224. [Google Scholar] [CrossRef]
- Valenzuela, J.A.; Glicksman, L.R. Thermal Resistance and Aging of Rigid Urethane Foam Insulation. In Thermal Insulation, Materials, and Systems for Energy Conservation in the ‘80s; Govan, F., Greason, D., McAllister, J., Eds.; ASTM International: West Conshohocken, PA, USA, 1983. [Google Scholar] [CrossRef]
- Norton, F.J. Diffusion of Chlorofluorocarbon Gases in Polymer Films And Foams. J. Cell. Plast. 1982, 18, 300–315. [Google Scholar] [CrossRef]
- Reitz, D.W.; Schuetz, M.; Glicksman, L.R. A Basic Study of Aging of Foam Insulation. J. Cell. Plast. 1984, 20, 104–113. [Google Scholar] [CrossRef]
- Ostrogorsky, A.G.; Glicksman, L.R.; Reitz, D.W. Aging of Polyurethane Foams. Int. J. Heat Mass Transf. 1986, 29, 1169–1176. [Google Scholar] [CrossRef]
- Cunningham, A.; Jeffs, G.M.F.; Rosbotham, I.D.; Sparrow, D.J. Recent Advances in the Development of Rigid Polyurethane Foams of Improved Thermal Insulation Efficiency. Cell. Polym. 1988, 7, 1–15. [Google Scholar] [CrossRef]
- Lifshitz, J.M. Some Mechanical Properties of Rigid Polyurethane Structural Foam. Polym. Eng. Sci. 1983, 23, 144–154. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Deng, Y.; Zhang, P. A Review of Research on the Effect of Temperature on the Properties of Polyurethane Foams. Polymers 2022, 14, 4586. [Google Scholar] [CrossRef] [PubMed]
- Jackovich, D.; O’Toole, B.; Hawkins, M.C.; Sapochak, L.S. Temperature and Mold Size Effects on Physical and Mechanical Properties of a Polyurethane Foam. J. Cell. Plast. 2005, 41, 153–168. [Google Scholar] [CrossRef]
- Park, S.-B.; Lee, C.-S.; Choi, S.-W.; Kim, J.-H.; Bang, C.-S.; Lee, J.-M. Polymeric Foams for Cryogenic Temperature Application: Temperature Range for Non-Recovery and Brittle-Fracture of Microstructure. Compos. Struct. 2016, 136, 258–269. [Google Scholar] [CrossRef]
- Menges, G.P.D.-I.; Knipschild, F.W. Estimation of Mechanical Properties for Rigid Polyurethane Foams. Polym. Eng. Sci. 1975, 15, 623–627. [Google Scholar] [CrossRef]
- Siegmann, A.; Kenig, S.; Alperstein, D.; Narkis, M. Mechanical Behavior of Reinforced Polyurethane Foams. Polym. Compos. 1983, 4, 113–119. [Google Scholar] [CrossRef]
- Goods, S.H.; Neuschwanger, C.L.; Whinnery, L.L. Mechanical Properties of a Structural Polyurethane Foam and the Effect of Particulate Loading. MRS Proc. 1998, 521, 15. [Google Scholar] [CrossRef]
- Saint-Michel, F.; Chazeau, L.; Cavaillé, J.-Y.; Chabert, E. Mechanical Properties of High Density Polyurethane Foams: I. Effect of the Density. Compos. Sci. Technol. 2006, 66, 2700–2708. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.S.; Naik, Y. Effect of Foam Density on the Properties of Water Blown Rigid Polyurethane Foam. J. Appl. Polym. Sci. 2008, 108, 1810–1817. [Google Scholar] [CrossRef]
- Yakushin, V.A.; Zhmud’, N.P.; Stirna, U.K. Physicomechanical Characteristics of Spray-On Rigid Polyurethane Foams at Normal and Low Temperatures. Mech. Compos. Mater. 2002, 38, 273–280. [Google Scholar] [CrossRef]
- Stirna, U.; Beverte, I.; Yakushin, V.; Cabulis, U. Mechanical Properties of Rigid Polyurethane Foams at Room and Cryogenic Temperatures. J. Cell. Plast. 2011, 47, 337–355. [Google Scholar] [CrossRef]
- Linul, E.; Marşavina, L.; Vălean, C.; Bănică, R. Static and Dynamic Mode I Fracture Toughness of Rigid PUR Foams under Room and Cryogenic Temperatures. Eng. Fract. Mech. 2020, 225, 106274. [Google Scholar] [CrossRef]
- Bureau, M.N.; Gendron, R. Mechanical-Morphology Relationship of PS Foams. J. Cell. Plast. 2003, 39, 353–367. [Google Scholar] [CrossRef]
- Hawkins, M.C.; O’Toole, B.; Jackovich, D. Cell Morphology and Mechanical Properties of Rigid Polyurethane Foam. J. Cell. Plast. 2005, 41, 267–285. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Wang, C.; Zhang, C.; Fang, H.; Deng, Y. Quantitative Analysis of the Representative Volume Element of Polymer Grouting Materials Based on Geometric Homogenization. Constr. Build. Mater. 2021, 300, 124223. [Google Scholar] [CrossRef]
- Li, M.; Du, M.; Wang, F.; Xue, B.; Zhang, C.; Fang, H. Study on the Mechanical Properties of Polyurethane (PU) Grouting Material of Different Geometric Sizes under Uniaxial Compression. Constr. Build. Mater. 2020, 259, 119797. [Google Scholar] [CrossRef]
- Andersons, J.; Modniks, J.; Kirpluks, M.; Cabulis, U. The Effect of Cell Shape Anisotropy on Fracture Toughness of Low-Density Brittle Foams. Eng. Fract. Mech. 2022, 269, 108565. [Google Scholar] [CrossRef]
- Maji, A.; Schreyer, H.L.; Donald, S.; Zuo, Q.; Satpathi, D. Mechanical Properties of Polyurethane-Foam Impact Limiters. J. Eng. Mech. 1995, 121, 528–540. [Google Scholar] [CrossRef]
- Ridha, M.; Shim, V.P.W. Microstructure and Tensile Mechanical Properties of Anisotropic Rigid Polyurethane Foam. Exp. Mech. 2008, 48, 763–776. [Google Scholar] [CrossRef]
- Şerban, D.A.; Linul, E.; Voiconi, T.; Marşavina, L.; Modler, N. Numerical Evaluation of Two-Dimensional Micromechanical Structures of Anisotropic Cellular Materials: Case Study for Polyurethane Rigid Foams. Iran. Polym. J. 2015, 24, 515–529. [Google Scholar] [CrossRef]
- Şerban, D.A.; Linul, E.; Sărăndan, S.; Marșavina, L. Development of Parametric Kelvin Structures Will Closed Cells. Solid State Phenom. 2016, 254, 49–54. [Google Scholar] [CrossRef]
- Emanoil, L.; Liviu, M. Prediction Of Fracture Toughness For Open Cell Polyurethane Foams By Finite-Element Micromechanical Analysis. Iran. Polym. J. 2011, 20, 735–746. [Google Scholar]
- Ridha, M. Mechanical and Failure Properties of Rigid Polyurethane Foam under Tension. Ph.D. Thesis, National University of Singapore, Singapore, 2007. [Google Scholar]
- Burbank, S.; Smith, L.V. Dynamic Characterization of Rigid Foam Used in Finite Element Sports Ball Simulations. Proc. IMechE Part P J. Sports Eng. Technol. 2012, 226, 77–85. [Google Scholar] [CrossRef]
- Pozorski, Z. Numerical Modelling of the Rigid Polyurethane Foam Microstructure. MATEC Web Conf. 2018, 157, 06008. [Google Scholar] [CrossRef]
- He, Y.; Wu, J.; Qiu, D.; Yu, Z. Experimental and Numerical Analyses of Thermal Failure of Rigid Polyurethane Foam. Mater. Chem. Phys. 2019, 233, 378–389. [Google Scholar] [CrossRef]
- Anderson, J.J. Retention of Flame Properties of Rigid Polyurethane Foams. Ind. Eng. Chem. Prod. Res. Dev. 1963, 2, 260–263. [Google Scholar] [CrossRef]
- Zhu, M.; Ma, Z.; Liu, L.; Zhang, J.; Huo, S.; Song, P. Recent Advances in Fire-Retardant Rigid Polyurethane Foam. J. Mater. Sci. Technol. 2022, 112, 315–328. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Y.H. Preparation and Characterization of Excellent Flame Retarded Rigid Polyurethane Foams. Adv. Mater. Res. 2011, 374–377, 1563–1566. [Google Scholar] [CrossRef]
- Wang, J.P. The Research on Structure Fire Resistance Test of Fire Retardant Rigid Polyurethane. App. Mech. Mater. 2014, 580–583, 2646–2648. [Google Scholar] [CrossRef]
- Nametz, R.C.; Deanin, R.D.; Lambert, P.M. Flame-resistant Rigid Polyurethance Foams from Monobrominated Toluene Diisocyanate. Polym. Eng. Sci. 1964, 4, 251–255. [Google Scholar] [CrossRef]
- Borowicz, M.; Paciorek-Sadowska, J.; Lubczak, J.; Czupryński, B. Biodegradable, Flame-Retardant, and Bio-Based Rigid Polyurethane/Polyisocyanurate Foams for Thermal Insulation Application. Polymers 2019, 11, 1816. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Oyedeji, O.; Leal, J.H.; Donohoe, B.S.; Semelsberger, T.A.; Li, C.; Hoover, A.N.; Webb, E.; Bose, E.A.; Zeng, Y.; et al. Characterizing Variability in Lignocellulosic Biomass: A Review. ACS Sustain. Chem. Eng. 2020, 8, 8059–8085. [Google Scholar] [CrossRef]
- Terzopoulou, P.; Kamperidou, V. Chemical Characterization of Wood and Bark Biomass of the Invasive Species of Tree-of-Heaven (Ailanthus Altissima (Mill.) Swingle), Focusing on Its Chemical Composition Horizontal Variability Assessment. Wood Mater. Sci. Eng. 2022, 17, 469–477. [Google Scholar] [CrossRef]
- Monono, E.M.; Nyren, P.E.; Berti, M.T.; Pryor, S.W. Variability in Biomass Yield, Chemical Composition, and Ethanol Potential of Individual and Mixed Herbaceous Biomass Species Grown in North Dakota. Ind. Crops Prod. 2013, 41, 331–339. [Google Scholar] [CrossRef]
- de Moraes Rocha, G.J.; Nascimento, V.M.; Goncalves, A.R.; Nunes Silva, V.F.; Martin, C. Influence of Mixed Sugarcane Bagasse Samples Evaluated by Elemental and Physical-Chemical Composition. Ind. Crops Prod. 2015, 64, 52–58. [Google Scholar] [CrossRef]
- de Abreu, I.B.S.; de Sousa, M.H.; da Silva, A.P.; Padilha, C.E.d.A.; Sales, A.T.; da Silva, A.S.A.; Dutra, E.D.; Menezes, R.S.C. Global Variability of Food Waste Chemical Composition and Its Consequences on the Production of Biofuels and Chemical Compounds. J. Mater. Cycles Waste Manag. 2023, 25, 1309–1324. [Google Scholar] [CrossRef]
- Ivdre, A.; Abolins, A.; Sevastyanova, I.; Kirpluks, M.; Cabulis, U.; Merijs-Meri, R. Rigid Polyurethane Foams with Various Isocyanate Indices Based on Polyols from Rapeseed Oil and Waste PET. Polymers 2020, 12, 738. [Google Scholar] [CrossRef]
- Bontaş, M.G.; Diacon, A.; Călinescu, I.; Rusen, E. Lignocellulose Biomass Liquefaction: Process and Applications Development as Polyurethane Foams. Polymers 2023, 15, 563. [Google Scholar] [CrossRef]
- Janik, H.; Sienkiewicz, M.; Kucinska-Lipka, J. 9—Polyurethanes. In Handbook of Thermoset Plastics, 3rd ed.; Dodiuk, H., Goodman, S.H., Eds.; William Andrew Publishing: Boston, MA, USA, 2014; pp. 253–295. ISBN 978-1-4557-3107-7. [Google Scholar]
- Wang, G.; Li, K.; Zou, W.; Hu, A.; Hu, C.; Zhu, Y.; Chen, C.; Guo, G.; Yang, A.; Drumright, R.; et al. Synthesis of HDI/IPDI Hybrid Isocyanurate and Its Application in Polyurethane Coating. Prog. Org. Coat. 2015, 78, 225–233. [Google Scholar] [CrossRef]
- Bresolin, D.; Valério, A.; de Oliveira, D.; Lenzi, M.K.; Sayer, C.; de Araújo, P.H.H. Polyurethane Foams Based on Biopolyols from Castor Oil and Glycerol. J. Polym. Environ. 2018, 26, 2467–2475. [Google Scholar] [CrossRef]
- de Oliveira, B.P.; Balieiro, L.C.S.; Maia, L.S.; Zanini, N.C.; Teixeira, E.J.O.; da Conceição, M.O.T.; Medeiros, S.F.; Mulinari, D.R. Eco-Friendly Polyurethane Foams Based on Castor Polyol Reinforced with Açaí Residues for Building Insulation. J. Mater. Cycles Waste Manag. 2022, 24, 553–568. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.H.; Oh, K.W. Bio-Based Polyurethane Foams with Castor Oil Based Multifunctional Polyols for Improved Compressive Properties. Polymers 2021, 13, 576. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A. The Influence of Rapeseed Oil-Based Polyols on the Foaming Process of Rigid Polyurethane Foams. Ind. Crops Prod. 2016, 89, 182–187. [Google Scholar] [CrossRef]
- Leszczyńska, M.; Malewska, E.; Ryszkowska, J.; Kurańska, M.; Gloc, M.; Leszczyński, M.K.; Prociak, A. Vegetable Fillers and Rapeseed Oil-Based Polyol as Natural Raw Materials for the Production of Rigid Polyurethane Foams. Materials 2021, 14, 1772. [Google Scholar] [CrossRef] [PubMed]
- Zieleniewska, M.; Leszczyński, M.K.; Kurańska, M.; Prociak, A.; Szczepkowski, L.; Krzyżowska, M.; Ryszkowska, J. Preparation and Characterisation of Rigid Polyurethane Foams Using a Rapeseed Oil-Based Polyol. Ind. Crops Prod. 2015, 74, 887–897. [Google Scholar] [CrossRef]
- Chian, K.S.; Gan, L.H. Development of a Rigid Polyurethane Foam from Palm Oil. J. Appl. Polym. Sci. 1998, 68, 509–5015. [Google Scholar] [CrossRef]
- Gomez, J.C.; Zakaria, R.; Aung, M.M.; Mokhtar, M.N.; Yunus, R.B. Characterization of Novel Rigid-Foam Polyurethanes from Residual Palm Oil and Algae Oil. J. Mater. Res. Technol. 2020, 9, 16303–16316. [Google Scholar] [CrossRef]
- Malewska, E.; Kurańska, M.; Tenczyńska, M.; Prociak, A. Application of Modified Seed Oils of Selected Fruits in the Synthesis of Polyurethane Thermal Insulating Materials. Materials 2024, 17, 158. [Google Scholar] [CrossRef]
- Okieimen, F.E.; Pavithran, C.; Bakare, I.O. Epoxidation and Hydroxlation of Rubber Seed Oil: One-Pot Multi-Step Reactions. Eur. J. Lipid Sci. Technol. 2005, 107, 330–336. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Zhang, W.; Javni, I. Structure and Properties of Polyurethanes Prepared from Triglyceride Polyols by Ozonolysis. Biomacromolecules 2005, 6, 713–719. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Adhikari, B. Polyurethanes from Seed Oil-Based Polyols: A Review of Synthesis, Mechanical and Thermal Properties. Ind. Crops Prod. 2019, 142, 111841. [Google Scholar] [CrossRef]
- Kaikade, D.S.; Sabnis, A.S. Polyurethane Foams from Vegetable Oil-Based Polyols: A Review. Polym. Bull. 2023, 80, 2239–2261. [Google Scholar] [CrossRef]
- Sharma, C.; Kumar, S.; Unni, A.R.; Aswal, V.K.; Rath, S.K.; Harikrishnan, G. Foam Stability and Polymer Phase Morphology of Flexible Polyurethane Foams Synthesized from Castor Oil. J. Appl. Polym. Sci. 2014, 131, 8420–8427. [Google Scholar] [CrossRef]
- Ji, D.; Fang, Z.; He, W.; Luo, Z.; Jiang, X.; Wang, T.; Guo, K. Polyurethane Rigid Foams Formed from Different Soy-Based Polyols by the Ring Opening of Epoxidised Soybean Oil with Methanol, Phenol, and Cyclohexanol. Ind. Crops Prod. 2015, 74, 76–82. [Google Scholar] [CrossRef]
- Zhang, C.; Kessler, M.R. Bio-Based Polyurethane Foam Made from Compatible Blends of Vegetable-Oil-Based Polyol and Petroleum-Based Polyol. ACS Sustain. Chem. Eng. 2015, 3, 743–749. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Isbrandt, M. Effect of Evening Primrose Oil-Based Polyol on the Properties of Rigid Polyurethane–Polyisocyanurate Foams for Thermal Insulation. Polymers 2018, 10, 1334. [Google Scholar] [CrossRef]
- Hejna, A. Application of Vegetable Oil-Based Biopolyols in Manufacturing of Rigid Polyurethane Foams—Short Review. J. Polym. Sci. Eng. 2018, 1, 1–9. [Google Scholar] [CrossRef]
- Prociak, A. Właściwości Termoizolacyjne Sztywnych Pianek Poliuretanowych Syntetyzowanych z Udziałem Polioli z Olejów Roślinnych. Polimery 2008, 53, 195–200. [Google Scholar] [CrossRef]
- Prociak, A.; Kurańska, M.; Cabulis, U.; Ryszkowska, J.; Leszczyńska, M.; Uram, K.; Kirpluks, M. Effect of Bio-Polyols with Different Chemical Structures on Foaming of Polyurethane Systems and Foam Properties. Ind. Crops Prod. 2018, 120, 262–270. [Google Scholar] [CrossRef]
- Hu, Y.H.; Gao, Y.; Wang, D.N.; HU, C.P.; Zu, S.; Vanoverloop, L.; Randall, D. Rigid Polyurethane Foam Prepared from a Rape Seed Oil Based Polyol. J. Appl. Polym. Sci. 2002, 84, 591–597. [Google Scholar] [CrossRef]
- Fan, H.; Tekeei, A.; Suppes, G.J.; Hsieh, F.-H. Rigid Polyurethane Foams Made from High Viscosity Soy-Polyols. J. Appl. Polym. Sci. 2013, 127, 1623–1629. [Google Scholar] [CrossRef]
- Volkman, J.K.; Jeffrey, S.W.; Nichols, P.D.; Rogers, G.I.; Garland, C.D. Fatty Acid and Lipid Composition of 10 Species of Microalgae Used in Mariculture. J. Exp. Mar. Biol. Ecol. 1989, 128, 219–240. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Tomaszewska, E.; Liszkowska, J. New Bio-Polyol Based on White Mustard Seed Oil for Rigid PUR-PIR Foams. Pol. J. Chem. Technol. 2018, 20, 24–31. [Google Scholar] [CrossRef]
- Liang, H.; Feng, Y.; Lu, J.; Liu, L.; Yang, Z.; Luo, Y.; Zhang, Y.; Zhang, C. Bio-Based Cationic Waterborne Polyurethanes Dispersions Prepared from Different Vegetable Oils. Ind. Crops Prod. 2018, 122, 448–455. [Google Scholar] [CrossRef]
- Hejna, A.; Kirpluks, M.; Kosmela, P.; Cabulis, U.; Haponiuk, J.; Piszczyk, Ł. The Influence of Crude Glycerol and Castor Oil-Based Polyol on the Structure and Performance of Rigid Polyurethane-Polyisocyanurate Foams. Ind. Crops Prod. 2017, 95, 113–125. [Google Scholar] [CrossRef]
- EBB STATISTICAL REPORT 2023; European Biodiesel Board EBB: Brussels, Belgium, 2023.
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of Biodiesel Composition, Properties, and Specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Knothe, G.; Van Gerpen, J.; Krahl, J. The Biodiesel Handbook, 2nd ed.; AOCS Press: Champaing, IL, USA, 2005. [Google Scholar]
- Puri, M.; Abraham, R.E.; Barrow, C.J. Biofuel Production: Prospects, Challenges and Feedstock in Australia. Renew. Sustain. Energy Rev. 2012, 16, 6022–6031. [Google Scholar] [CrossRef]
- Ma, Q.; Fu, K.; Zhang, J.; Li, M.; Han, X.; Chen, Z.; Ma, L.; Chang, C. New Bio-Based Polyurethane (PU) Foams Synthesized Using Crude Glycerol-Based Biopolyol and Humin-Based Byproducts from Biomass Hydrolysis. Ind. Crops Prod. 2023, 205, 117548. [Google Scholar] [CrossRef]
- Pawar, M.S.; Kadam, A.S.; Dawane, B.S.; Yemul, O.S. Synthesis and Characterization of Rigid Polyurethane Foams from Algae Oil Using Biobased Chain Extenders. Polym. Bull. 2016, 73, 727–741. [Google Scholar] [CrossRef]
- Kosmela, P.; Hejna, A.; Suchorzewski, J.; Piszczyk, Ł.; Haponiuk, J.T. Study on the Structure-Property Dependences of Rigid PUR-PIR Foams Obtained from Marine Biomass-Based Biopolyol. Materials 2020, 13, 1257. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Wan, X.; Bilić, O.; Zlatanic, A.; Hong, J.; Javni, I.; Ionescu, M.; Milić, J.; Degruson, D. Polyols and Polyurethanes from Crude Algal Oil. J. Am. Oil Chem. Soc. 2013, 90, 1073–1078. [Google Scholar] [CrossRef]
- Arbenz, A.; Perrin, R.; Avérous, L. Elaboration and Properties of Innovative Biobased PUIR Foams from Microalgae. J. Polym. Environ. 2017, 26, 254–262. [Google Scholar] [CrossRef]
- Olejnik, A.; Kosmela, P.; Piszczyk, Ł. Enhancement of PUR/PIR Foam Thermal Stability after Addition of Zostera Marina Biomass Component Investigated via Thermal Analysis and Isoconversional Kinetics. J. Polym. Sci. 2021, 59, 1095–1108. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Kairytė, A. Application of Walnut Shells-Derived Biopolyol in the Synthesis of Rigid Polyurethane Foams. Materials 2020, 13, 2687. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, J.; Zhao, J.; Tu, S. Liquefaction of Bamboo Shoot Shell for the Production of Polyols. Bioresour. Technol. 2014, 153, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.H.; Lee, E.Y. Production of Polyols and Polyurethane from Biomass: A Review. Environ. Chem. Lett. 2023, 21, 2199–2223. [Google Scholar] [CrossRef]
- Olszewski, A.; Kosmela, P.; Vēvere, L.; Kirpluks, M.; Cabulis, U.; Piszczyk, Ł. Effect of Bio-Polyol Molecular Weight on the Structure and Properties of Polyurethane-Polyisocyanurate (PUR-PIR) Foams. Sci. Rep. 2024, 14, 812. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Li, Y. Two-Step Sequential Liquefaction of Lignocellulosic Biomass by Crude Glycerol for the Production of Polyols and Polyurethane Foams. Bioresour. Technol. 2014, 161, 410–415. [Google Scholar] [CrossRef]
- Kosmela, P.; Hejna, A.; Formela, K.; Haponiuk, J.T.; Piszczyk, Ł. Biopolyols Obtained via Crude Glycerol-Based Liquefaction of Cellulose: Their Structural, Rheological and Thermal Characterization. Cellulose 2016, 23, 2929–2942. [Google Scholar] [CrossRef]
- Zhang, J.; Hori, N.; Takemura, A. Optimization of Agricultural Wastes Liquefaction Process and Preparing Bio-Based Polyurethane Foams by the Obtained Polyols. Ind. Crops Prod. 2019, 138, 111455. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, H.; Guo, H.; Huang, C.; Xiong, L.; Chen, X. Kinetic Study on the Liquefaction of Wood and Its Three Cell Wall Component in Polyhydric Alcohols. Appl. Energy 2014, 113, 1596–1600. [Google Scholar] [CrossRef]
- Lee, S.-H.; Yoshioka, M.; Shiraishi, N. Liquefaction of Corn Bran (CB) in the Presence of Alcohols and Preparation of Polyurethane Foam from Its Liquefied Polyol. J. Appl. Polym. Sci. 2000, 78, 319–325. [Google Scholar] [CrossRef]
- Zheng, Z.F.; Pan, H.; Huang, Y.B.; Chung, Y.H. Bio-Based Rigid Polyurethane Foam from Liquefied Products of Wood in the Presence of Polyhydric Alcohols. Adv. Mater. Res. 2010, 168–170, 1281–1284. [Google Scholar] [CrossRef]
- Ionescu, M.; Wan, X.; Bilić, N.; Petrović, Z.S. Polyols and Rigid Polyurethane Foams from Cashew Nut Shell Liquid. J. Polym. Environ. 2012, 20, 647–658. [Google Scholar] [CrossRef]
- Tran, H.T.T.; Deshan, A.D.K.; Doherty, W.; Rackemann, D.; Moghaddam, L. Production of Rigid Bio-Based Polyurethane Foams from Sugarcane Bagasse. Ind. Crops Prod. 2022, 188, 115578. [Google Scholar] [CrossRef]
- Amran, U.A.; Zakaria, S.; Chia, C.H.; Roslan, R.; Jaafar, S.N.S.; Salleh, K.M. Polyols and Rigid Polyurethane Foams Derived from Liquefied Lignocellulosic and Cellulosic Biomass. Cellulose 2019, 26, 3231–3246. [Google Scholar] [CrossRef]
- Gao, L.L.; Liu, Y.; Lei, H.; Hong, P.; Ruan, R.R. Preparation of Semirigid Polyurethane Foam with Liquefied Bamboo Residues. J. Appl. Polym. Sci. 2010, 116, 1694–1699. [Google Scholar] [CrossRef]
- Sendijarevic, I.; Pietrzyk, K.W.; Schiffman, C.M.; Sendijarevic, V.; Kiziltas, A.; Mielewski, D. Polyol from Spent Coffee Grounds: Performance in a Model Pour-in-Place Rigid Polyurethane Foam System. J. Cell. Plast. 2020, 56, 630–645. [Google Scholar] [CrossRef]
- Passoni, V.; Scarica, C.; Levi, M.; Turri, S.; Griffini, G. Fractionation of Industrial Softwood Kraft Lignin: Solvent Selection as a Tool for Tailored Material Properties. ACS Sustain. Chem. Eng. 2016, 4, 2232–2242. [Google Scholar] [CrossRef]
- Vishtal, A.; Kraslawski, A. Challenges in Industrial Applications of Technical Lignins. BioRes 2011, 6, 3547–3568. [Google Scholar] [CrossRef]
- Pan, X.; Saddler, J.N. Effect of Replacing Polyol by Organosolv and Kraft Lignin on the Property and Structure of Rigid Polyurethane Foam. Biotechnol. Biofuels 2013, 6, 12. [Google Scholar] [CrossRef]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C. (Charles) Depolymerization of Lignins and Their Applications for the Preparation of Polyols and Rigid Polyurethane Foams: A Review. Renew. Sustain. Energy Rev. 2016, 60, 317–329. [Google Scholar] [CrossRef]
- Quinsaat, J.E.Q.; Feghali, E.; van de Pas, D.J.; Vendamme, R.; Torr, K.M. Preparation of Mechanically Robust Bio-Based Polyurethane Foams Using Depolymerized Native Lignin. ACS Appl. Polym. Mater. 2021, 3, 5845–5856. [Google Scholar] [CrossRef]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C. (Charles) Production of Polyols via Direct Hydrolysis of Kraft Lignin: Effect of Process Parameters. Bioresour. Technol. 2013, 139, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Glasser, W.G.; Leitheiser, R.H. Engineering Plastics from Lignin. Polym. Bull. 1984, 12, 1–5. [Google Scholar] [CrossRef]
- Cateto, C.A.; Barreiro, M.F.; Rodrigues, A.E.; Belgacem, M.N. Optimization Study of Lignin Oxypropylation in View of the Preparation of Polyurethane Rigid Foams. Ind. Eng. Chem. Res. 2009, 48, 2583–2589. [Google Scholar] [CrossRef]
- Nadji, H.; Bruzzese, C.; Belgacem, M.N.; Benaboura, A.; Gandini, A. Oxypropylation of Lignins and Preparation of Rigid Polyurethane Foams from the Ensuing Polyols. Macromol. Mater. Eng. 2005, 290, 1009–1016. [Google Scholar] [CrossRef]
- Duval, A.; Vidal, D.; Sarbu, A.; René, W.; Avérous, L. Scalable Single-Step Synthesis of Lignin-Based Liquid Polyols with Ethylene Carbonate for Polyurethane Foams. Mater. Today Chem. 2022, 24, 100793. [Google Scholar] [CrossRef]
- Perez-Arce, J.; Centeno-Pedrazo, A.; Labidi, J.; Ochoa-Gómez, J.R.; García-Suárez, E.J. A Novel and Efficient Approach to Obtain Lignin-Based Polyols with Potential Industrial Applications. Polym. Chem. 2020, 11, 7362–7369. [Google Scholar] [CrossRef]
- Evtiouguina, M.; Barros-Timmons, A.; Cruz-Pinto, J.J.C.; Neto, C.P.; Belgacem, M.N.; Gandini, A. Oxypropylation of Cork and the Use of the Ensuing Polyols in Polyurethane Formulations. Biomacromolecules 2002, 3, 57–62. [Google Scholar] [CrossRef]
- Serrano, L.; Briones, R.; Melus, A.; Herseczk, Z.; Labidi, J. Polyols from the Lignocellulosic Waste of Biodiesel Production Process. Chem. Eng. Trans. 2010, 21, 1339–1344. [Google Scholar] [CrossRef]
- Yoshioka, M.; Nishio, Y.; Daisuke, S.; Ohashi, H.; Hashimoto, M.; Shiraishi, N. Synthesis of Biopolyols by Mild Oxypropylation of Liquefied Starch and Its Application to Polyurethane Rigid Foams. J. Appl. Polym. Sci. 2013, 130, 622–630. [Google Scholar] [CrossRef]
- Arshanitsa, A.; Paberza, A.; Vēvere, L.; Cabulis, U.; Telysheva, G. Two Approaches for Introduction of Wheat Straw Lignin into Rigid Polyurethane Foams. In AIP Conference Proceedings; American Institute of Physics: Nuremberg, Germany, 2014; Volume 1593, pp. 388–391. [Google Scholar] [CrossRef]
- Ryszkowska, J. Materiały Poliuretanowe Wytwarzane z Zastosowaniem Surowców Odnawialnych; Oficyna Wydawnicza Politechniki Warszawskiej: Warsaw, Poland, 2019. [Google Scholar]
- George, J.; Sabapathi, S.N. Cellulose Nanocrystals: Synthesis, Functional Properties, and Application. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, P.; Zygmunt-Kowalska, B.; Kuźnia, M.; Szajding, A.; Telejko, T.; Wilk, M. Biomass Origin Waste as Activators of the Polyurethane Foaming Process. Energies 2023, 16, 1354. [Google Scholar] [CrossRef]
- Li, Y.; Ragauskas, A.J. Kraft Lignin-Based Rigid Polyurethane Foam. J. Wood Chem. Technol. 2012, 32, 210–224. [Google Scholar] [CrossRef]
- Kumar, S.; Lohan, S.K.; Parihar, D.S. Biomass Energy from Agriculture. In Handbook of Energy Management in Agriculture; Rakshit, A., Biswas, A., Sarkar, D., Meena, V.S., Datta, R., Eds.; Springer Nature: Singapore, 2023; pp. 181–199. [Google Scholar]
- Tao, Y.; Li, P.; Cai, L. Effect of Fiber Content on Sound Absorption, Thermal Conductivity, and Compression Strength of Straw Fiber-Filled Rigid Polyurethane Foams. BioResources 2016, 11, 4159–4167. [Google Scholar] [CrossRef]
- Li, P.; Tao, Y.; Shi, S.Q. Effect of Fiber Content and Temperature on the Dielectric Properties of Kenaf Fiber-Filled Rigid Polyurethane Foam. BioResources 2014, 9, 2681–2688. [Google Scholar] [CrossRef]
- Głowacz-Czerwonka, D.; Zakrzewska, P.; Oleksy, M.; Pielichowska, K.; Kuźnia, M.; Telejko, T. The Influence of Biowaste-Based Fillers on the Mechanical and Fire Properties of Rigid Polyurethane Foams. Sustain. Mater. Technol. 2023, 36, e00610. [Google Scholar] [CrossRef]
- Paberza, A.; Cabulis, U.; Arshanitsa, A. Wheat Straw Lignin as Filler for Rigid Polyurethane Foams on the Basis of Tall Oil Amide. Polimery 2014, 59, 477–481. [Google Scholar] [CrossRef]
- Wang, F.L.; Meu, Q.L.; Huang, Z.X.; Qin, Y.; Du, M. Study of Properties of Rigid Polyurethane Foam Reinforced with Treated Jute Fiber. Polyurethane Ind. 2006, 21, 12–14. [Google Scholar]
- Miedzińska, K.; Członka, S.; Strąkowska, A.; Strzelec, K. Biobased Polyurethane Composite Foams Reinforced with Plum Stones and Silanized Plum Stones. Int. J. Mol. Sci. 2021, 22, 4757. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Lee, L.J.; Widya, T.; Macosko, C. Polyurethane/Clay Nanocomposites Foams: Processing, Structure and Properties. Polymer 2005, 46, 775–783. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Kairytė, A. Effect of Walnut Shells and Silanized Walnut Shells on the Mechanical and Thermal Properties of Rigid Polyurethane Foams. Polym. Test. 2020, 87, 106534. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Kairytė, A. The Impact of Hemp Shives Impregnated with Selected Plant Oils on Mechanical, Thermal, and Insulating Properties of Polyurethane Composite Foams. Materials 2020, 13, 4709. [Google Scholar] [CrossRef] [PubMed]
- Kairytė, A.; Członka, S.; Šeputytė-Jucikė, J.; Vėjelis, S. Impact of Sunflower Press Cake and Its Modification with Liquid Glass on Polyurethane Foam Composites: Thermal Stability, Ignitability, and Fire Resistance. Polymers 2022, 14, 4543. [Google Scholar] [CrossRef] [PubMed]
- Kairytė, A.; Członka, S.; Boris, R.; Vėjelis, S. Evaluation of the Performance of Bio-Based Rigid Polyurethane Foam with High Amounts of Sunflower Press Cake Particles. Materials 2021, 14, 5475. [Google Scholar] [CrossRef] [PubMed]
- Zieleniewska, M.; Leszczyński, M.K.; Szczepkowski, L.; Bryśkiewicz, A.; Krzyżowska, M.; Bień, K.; Ryszkowska, J. Development and Applicational Evaluation of the Rigid Polyurethane Foam Composites with Egg Shell Waste. Polym. Degrad. Stab. 2016, 132, 78–86. [Google Scholar] [CrossRef]
- Członka, S.; Bertino, M.F.; Strzelec, K. Rigid Polyurethane Foams Reinforced with Industrial Potato Protein. Polym. Test. 2018, 68, 135–145. [Google Scholar] [CrossRef]
- Członka, S.; Sienkiewicz, N.; Strąkowska, A.; Strzelec, K. Keratin Feathers as a Filler for Rigid Polyurethane Foams on the Basis of Soybean Oil Polyol. Polym. Test. 2018, 72, 32–45. [Google Scholar] [CrossRef]
- Hejna, A.; Haponiuk, J.; Piszczyk, Ł.; Klein, M.; Formela, K. Performance Properties of Rigid Polyurethane-Polyisocyanurate/Brewers’ Spent Grain Foamed Composites as Function of Isocyanate Index. e-Polymers 2017, 17, 427–437. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Czupryński, B.; Liszkowska, J. Application of Waste Products from Agricultural-Food Industry for Production of Rigid Polyurethane-Polyisocyanurate Foams. J. Porous Mater. 2011, 18, 631–638. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Isbrandt, M.; Czupryński, B.; Apiecionek, Ł. The Use of Waste from the Production of Rapeseed Oil for Obtaining of New Polyurethane Composites. Polymers 2019, 11, 1431. [Google Scholar] [CrossRef] [PubMed]
- Członka, S.; Kairytė, A.; Miedzińska, K.; Strąkowska, A. Casein/Apricot Filler in the Production of Flame-Retardant Polyurethane Composites. Materials 2021, 14, 3620. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Kairytė, A.; Kremensas, A. Nutmeg Filler as a Natural Compound for the Production of Polyurethane Composite Foams with Antibacterial and Anti-Aging Properties. Polym. Test. 2020, 86, 106479. [Google Scholar] [CrossRef]
- Strąkowska, A.; Członka, S.; Kairytė, A. Rigid Polyurethane Foams Reinforced with POSS-Impregnated Sugar Beet Pulp Filler. Materials 2020, 13, 5493. [Google Scholar] [CrossRef] [PubMed]
- Liszkowska, J.; Borowicz, M.; Paciorek-Sadowska, J.; Isbrandt, M.; Czupryński, B.; Moraczewski, K. Assessment of Photodegradation and Biodegradation of RPU/PIR Foams Modified by Natural Compounds of Plant Origin. Polymers 2020, 12, 33. [Google Scholar] [CrossRef]
- Delucis, R.d.A.; Kerche, E.F.; Gatto, D.A.; Esteves, W.L.M.; Petzhold, C.L.; Amico, S.C. Surface Response and Photodegradation Performance of Bio-Based Polyurethane-Forest Derivatives Foam Composites. Polym. Test. 2019, 80, 106102. [Google Scholar] [CrossRef]
- Delucis, R.d.A.; Magalhães, W.L.E.; Petzhold, C.L.; Amico, S.C. Forest-based Resources as Fillers in Biobased Polyurethane Foams. J. Appl. Polym. Sci. 2018, 135, 45684. [Google Scholar] [CrossRef]
- Delucis, R.d.A.; Magalhães, W.L.E.; Petzhold, C.L.; Amico, S.C. Thermal and Combustion Features of Rigid Polyurethane Biofoams Filled with Four Forest-based Wastes. Polym. Compos. 2018, 39, E1770–E1777. [Google Scholar] [CrossRef]
- Bradai, H.; Koubaa, A.; Bouafif, H.; Langlois, A.; Samet, B. Synthesis and Characterization of Wood Rigid Polyurethane Composites. Materials 2022, 15, 4316. [Google Scholar] [CrossRef]
- Dukarska, D.; Walkiewicz, J.; Derkowski, A.; Mirski, R. Properties of Rigid Polyurethane Foam Filled with Sawdust from Primary Wood Processing. Materials 2022, 15, 5361. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xiao, Y.; Wu, Q.; Zeng, J. Development of High-Performance Biodegradable Rigid Polyurethane Foams Using All Bioresource-Based Polyols: Lignin and Soy Oil-Derived Polyols. Int. J. Biol. Macromol. 2018, 115, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, H.; Kosugi, R.; Hatakeyama, T. Thermal Properties of Lignin-and Molasses-Based Polyurethane Foams. J. Therm. Anal. Calorim. 2008, 92, 419–424. [Google Scholar] [CrossRef]
- Luo, S.; Gao, L.; Guo, W. Influence of Adding Lignin and Wood as Reactive Fillers on the Properties of Lightweight Wood–Polyurethane Composite Foams. For. Prod. J. 2020, 70, 420–427. [Google Scholar] [CrossRef]
- Mosiewicki, M.A.; Dell’Arciprete, G.A.; Aranguren, M.I.; Marcovich, N.E. Polyurethane Foams Obtained from Castor Oil-Based Polyol and Filled with Wood Flour. J. Compos. Mater. 2009, 43, 3057–3072. [Google Scholar] [CrossRef]
- Augaitis, N.; Vaitkus, S.; Członka, S.; Kairytė, A. Research of Wood Waste as a Potential Filler for Loose-Fill Building Insulation: Appropriate Selection and Incorporation into Polyurethane Biocomposite Foams. Materials 2020, 13, 5336. [Google Scholar] [CrossRef]
- Aranguren, M.I.; González, J.F.; Mosiewicki, M.A. Biodegradation of a Vegetable Oil Based Polyurethane and Wood Flour Composites. Polym. Test. 2012, 31, 7–15. [Google Scholar] [CrossRef]
- Plastics—The Fast Facts 2023. Available online: http://www.plasticseurope.org (accessed on 20 May 2024).
- Chen, F.; Lu, Z. Liquefaction of Wheat Straw and Preparation of Rigid Polyurethane Foam from the Liquefaction Products. J. Appl. Polym. Sci. 2009, 111, 508–516. [Google Scholar] [CrossRef]
- Ge, J.J.; Xu, J.T.; Zhang, Z.N. Environmental-Friendly Materials Based on Natural Polysaccharides (II)—Biodegradable Polyurethane Foams from Biomass Polyols of Banknote Paper and Pulp Paper. Acta Chim. Sin. 2002, 60, 732–736. [Google Scholar]
- Mu, Y.; Wan, X.; Han, Z.; Peng, Y.; Zhong, S. Rigid Polyurethane Foams Based on Activated Soybean Meal. J. Appl. Polym. Sci. 2012, 124, 4331–4338. [Google Scholar] [CrossRef]
- Furtwengler, P.; Avérous, L. Renewable Polyols for Advanced Polyurethane Foams from Diverse Biomass Resources. Polym. Chem. 2018, 9, 4258–4287. [Google Scholar] [CrossRef]
- Lambeth, R.H. Progress in Hybrid Non-isocyanate Polyurethanes. Polym. Int. 2020, 70, 696–700. [Google Scholar] [CrossRef]
- Cornille, A.; Auvergne, R.; Figovsky, O.; Boutevin, B.; Caillol, S. A Perspective Approach to Sustainable Routes for Non-Isocyanate Polyurethanes. Eur. Polym. J. 2017, 87, 535–552. [Google Scholar] [CrossRef]
- Vlcek, T.; Cabulis, U.; Holynska, M. Eco-Friendlier and Non-Isocyanate-Based Polyurethane Materials for Space Applications. CEAS Space J. 2023, 15, 253–264. [Google Scholar] [CrossRef]
- Li, Y.; Luo, X.; Hu, S. Lignocellulosic Biomass-Based Polyols for Polyurethane Applications. In Bio-Based Polyols and Polyurethanes; Springer International Publishing: Cham, Switzerland, 2015; pp. 45–64. [Google Scholar]
- Challenges Related to Biomass. Available online: https://www.eubia.org/cms/wiki-biomass/biomass-resources/challenges-related-to-biomass/ (accessed on 2 July 2024).







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dukarska, D.; Mirski, R. Current Trends in the Use of Biomass in the Manufacture of Rigid Polyurethane Foams: A Review. J. Compos. Sci. 2024, 8, 286. https://doi.org/10.3390/jcs8080286
Dukarska D, Mirski R. Current Trends in the Use of Biomass in the Manufacture of Rigid Polyurethane Foams: A Review. Journal of Composites Science. 2024; 8(8):286. https://doi.org/10.3390/jcs8080286
Chicago/Turabian StyleDukarska, Dorota, and Radosław Mirski. 2024. "Current Trends in the Use of Biomass in the Manufacture of Rigid Polyurethane Foams: A Review" Journal of Composites Science 8, no. 8: 286. https://doi.org/10.3390/jcs8080286
APA StyleDukarska, D., & Mirski, R. (2024). Current Trends in the Use of Biomass in the Manufacture of Rigid Polyurethane Foams: A Review. Journal of Composites Science, 8(8), 286. https://doi.org/10.3390/jcs8080286





