Fabrication and Characterization of Granulated β-Tricalcium Phosphate and Bioactive Glass Powders by Spray Drying
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of β-TCP and BG Starting Powders
2.2. Characterization of β-TCP and BG Starting Powders
2.3. Preparation of Granulated β-TCP/BG Powders
2.4. Characterization of Granulated β-TCP/BG Powders
2.5. Statistical Analysis
3. Results
3.1. SP-Derived β-TCP and BG Starting Powders
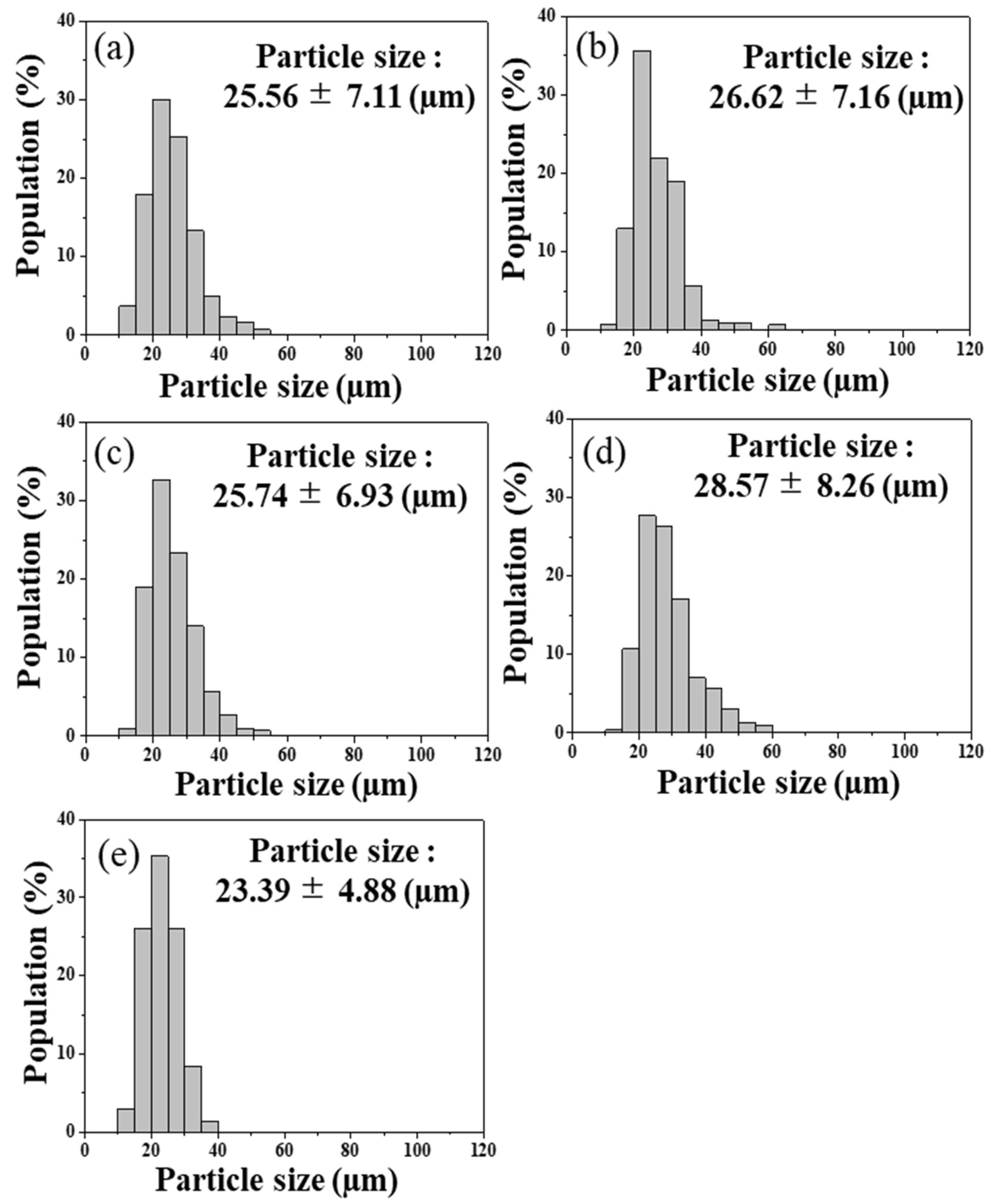
3.2. Granulated β-TCP/BG Powders
3.3. Cytotoxicity Test
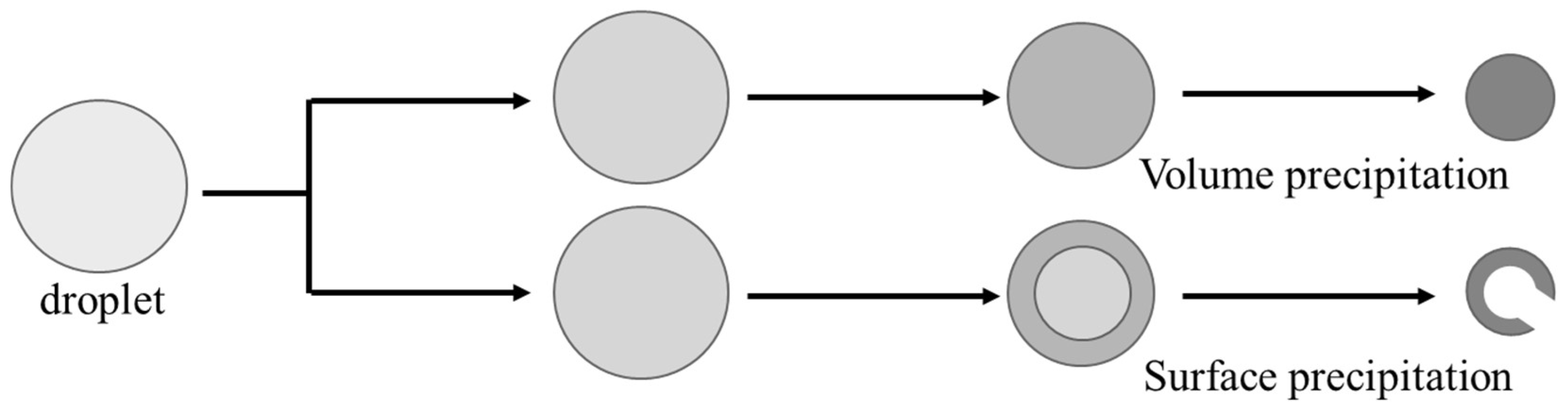
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brunello, G.; Panda, S.; Schiavon, L.; Sivolella, S.; Biasetto, L.; Del Fabbro, M. The impact of bioceramic scaffolds on bone regeneration in preclinical in vivo studies: A systematic review. Materials 2020, 13, 1500. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Ji, L.; Jell, G.; Dong, Y.; Jones, J.R.; Stevens, M.M. Template synthesis of ordered macroporous hydroxyapatite bioceramics. Chem. Commun. 2011, 47, 9048–9050. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.; Santos, A.; Neves, G.; Menezes, R. A brief review on hydroxyapatite production and use in biomedicine. Cerâmica 2019, 65, 282–302. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone grafts and substitutes in dentistry: A review of current trends and developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef] [PubMed]
- Eggli, P.; Moller, W.; Schenk, R. Porous hydroxyapatite and tricalcium phosphate cylinders with two different pore size ranges implanted in the cancellous bone of rabbits: A comparative histomorphometric and histologic study of bony ingrowth and implant substitution. Clin. Orthop. Relat. Res. 1988, 232, 127–138. [Google Scholar] [CrossRef]
- Cai, S.; Xu, G.; Yu, X.; Zhang, W.; Xiao, Z.; Yao, K. Fabrication and biological characteristics of β-tricalcium phosphate porous ceramic scaffolds reinforced with calcium phosphate glass. J. Mater. Sci. Mater. Med. 2009, 20, 351–358. [Google Scholar] [CrossRef]
- Hsu, P.-Y.; Kuo, H.-C.; Syu, M.-L.; Tuan, W.-H.; Lai, P.-L. A head-to-head comparison of the degradation rate of resorbable bioceramics. Mater. Sci. Eng. C 2020, 106, 110175. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Huang, Y.-K.; Hsu, P.-Y.; Tuan, W.-H.; Naito, M. Sintering of degradable bone substitutes at room temperature. Ceram. Int. 2021, 47, 21714–21720. [Google Scholar] [CrossRef]
- Uzun Kart, E. A Novel Method to Synthesis of Calcium Sulphate Anhydrite Self-Doped with SiO2 from Red Mud as a Bioceramic. Ceram. Silik. 2021, 65, 344–353. [Google Scholar] [CrossRef]
- Chou, Y.-J.; Hsiao, C.-W.; Tsou, N.-T.; Wu, M.-H.; Shih, S.-J. Preparation and in vitro bioactivity of micron-sized bioactive glass particles using spray drying method. Appl. Sci. 2018, 9, 19. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.; Greenlee, T. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Xin, F.; Chen, J.; Ruan, J.; Zhou, Z.; Zou, J. Synthesis and degradation properties of TCP/BG porous composite materials. Bull. Mater. Sci. 2011, 34, 357–364. [Google Scholar] [CrossRef]
- Spirandeli, B.; Ribas, R.; Amaral, S.; Martins, E.; Esposito, E.; Vasconcellos, L.; Campos, T.; Thim, G.; Trichês, E. Incorporation of 45S5 bioglass via sol-gel in β-TCP scaffolds: Bioactivity and antimicrobial activity evaluation. Mater. Sci. Eng. C 2021, 131, 112453. [Google Scholar] [CrossRef] [PubMed]
- Bakare, F.F.; Chou, Y.-J.; Huang, Y.-H.; Tesfay, A.H.; Moriga, T.; Shih, S.-J. Correlation of morphology and in-vitro degradation behavior of spray pyrolyzed bioactive glasses. Materials 2019, 12, 3703. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-J.; Ningsih, H.S.; Shih, S.-J. Preparation, characterization and investigation of antibacterial silver-zinc co-doped β-tricalcium phosphate by spray pyrolysis. Ceram. Int. 2020, 46, 16708–16715. [Google Scholar] [CrossRef]
- Janackovic, D.; Jokanovic, V.; Kostic-Gvozdenovic, L.; Zec, S.; Uskokovic, D. Synthesis and formation mechanism of submicrometre spherical cordierite powders by ultrasonic spray pyrolysis. J. Mater. Sci. 1997, 32, 163–168. [Google Scholar] [CrossRef]
- Messing, G.L.; Zhang, S.C.; Jayanthi, G.V. Ceramic powder synthesis by spray pyrolysis. J. Am. Ceram. Soc. 1993, 76, 2707–2726. [Google Scholar] [CrossRef]
- Chen, J.; Yang, H.; Xu, C.-M.; Cheng, J.-G.; Lu, Y.-W. Preparation of ZrO2 microspheres by spray granulation. Powder Technol. 2021, 385, 234–241. [Google Scholar] [CrossRef]
- Chen, L.G.; Huang, Y.H.; Chou, Y.J. Preparation and characterization of spray-dried granulated bioactive glass micron spheres. Int. J. Appl. Ceram. Technol. 2021, 18, 1743–1750. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Ogi, T.; Wang, W.-N.; Gradon, L.; Okuyama, K. Template-assisted spray-drying method for the fabrication of porous particles with tunable structures. Adv. Powder Technol. 2019, 30, 2908–2924. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Soe, T.N.; Malakhinsky, A.; Makhadilov, I.; Romanov, V.; Kuznetsova, E.; Smirnov, A.; Podrabinnik, P.; Khmyrov, R.; Solís Pinargote, N.W. Granulation of Silicon Nitride Powders by Spray Drying: A Review. Materials 2022, 15, 4999. [Google Scholar] [CrossRef]
- Nampi, P.P.; Kume, S.; Hotta, Y.; Watari, K.; Itoh, M.; Toda, H.; Matsutani, A. The effect of polyvinyl alcohol as a binder and stearic acid as an internal lubricant in the formation, and subsequent sintering of spray-dried alumina. Ceram. Int. 2011, 37, 3445–3450. [Google Scholar] [CrossRef]
- Tosaka, M.; Murakami, S.; Poompradub, S.; Kohjiya, S.; Ikeda, Y.; Toki, S.; Sics, I.; Hsiao, B.S. Orientation and crystallization of natural rubber network as revealed by WAXD using synchrotron radiation. Macromolecules 2004, 37, 3299–3309. [Google Scholar] [CrossRef]
- King, P.; McMillan, P.; Moore, G.; Ramsey, M.; Swayze, G. Infrared spectroscopy of silicate glasses with application to natural systems. Infrared Spectrosc. Geochem. Explor. Geochem. Remote Sens. 2004, 33, 93–133. [Google Scholar]
- Siddharthan, A.; Seshadri, S.; Kumar, T.S. Microwave accelerated synthesis of nanosized calcium deficient hydroxyapatite. J. Mater. Sci. Mater. Med. 2004, 15, 1279–1284. [Google Scholar] [CrossRef]
- Nahar, U.; Shovon, B.; Chandra, R.; Shukanta, B.; Chandra, S. Characterization of beta-tricalcium phosphate (β-TCP) produced at different process conditions. J. Bioeng. Biomed. Sci. 2017, 7, 2. [Google Scholar] [CrossRef]
- Wallin, R.F.; Arscott, E. A practical guide to ISO 10993-5: Cytotoxicity. Med. Device Diagn. Ind. 1998, 20, 96–98. Available online: https://www.namsa.com/wp-content/uploads/2015/10/A-Practical-Guide-to-ISO-10993-5_Cytotoxicity.pdf (accessed on 26 February 2021).
- Bellucci, D.; Cannillo, V.; Sola, A. Coefficient of thermal expansion of bioactive glasses: Available literature data and analytical equation estimates. Ceram. Int. 2011, 37, 2963–2972. [Google Scholar] [CrossRef]
- Tsuru, Y.; Shinzato, Y.; Saito, Y.; Shimazu, M.; Shiono, M.; Morinaga, M. Estimation of linear thermal expansion coefficient from cohesive energy obtained by ab-initio calculation of metals and ceramics. J. Ceram. Soc. Jpn. 2010, 118, 241–245. [Google Scholar] [CrossRef]
- Sivamma, M.; Snehitha, R. Atomization techniques in spray drying: A Review. Pharma Innov. J. 2021, 10, 454–461. [Google Scholar]
- Ningsih, H.S.; Tannesia, L.; Chen, H.-H.; Shih, S.-J. Fabrication, characterization and in vitro cytotoxicity of mesoporous β-tricalcium phosphate using the spray drying method. Crystals 2021, 11, 252. [Google Scholar] [CrossRef]
- Naz, S.; Gul, A.; Zia, M. Toxicity of copper oxide nanoparticles: A review study. IET Nanobiotechnol. 2020, 14, 1–13. [Google Scholar] [CrossRef]
- Addison, W.N.; Azari, F.; Sørensen, E.S.; Kaartinen, M.T.; McKee, M.D. Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J. Biol. Chem. 2007, 282, 15872–15883. [Google Scholar] [CrossRef]








| Pre-Heating (°C) | Calcination (°C) | Cooling (°C) | |
|---|---|---|---|
| β-TCP | 300 | 1050 | 350 |
| BG | 400 | 700 | 500 |
| Sample (Weight Ratio: β-TCP/BG) | β-TCP (g) | BG (g) | PVA (g) | PNVA (g) | Water (g) | Total (g) |
|---|---|---|---|---|---|---|
| 100/0 | 26 | 0 | 1.3 | 1.3 | 101.4 | 130 |
| 75/25 | 19.5 | 6.5 | 1.3 | 1.3 | 101.4 | 130 |
| 50/50 | 13 | 13 | 1.3 | 1.3 | 101.4 | 130 |
| 25/75 | 6.5 | 19.5 | 1.3 | 1.3 | 101.4 | 130 |
| 0/100 | 0 | 26 | 1.3 | 1.3 | 101.4 | 130 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakanishi, A.; Ningsih, H.S.; Putra, D.F.A.; Moriga, T.; Shih, S.-J. Fabrication and Characterization of Granulated β-Tricalcium Phosphate and Bioactive Glass Powders by Spray Drying. J. Compos. Sci. 2024, 8, 111. https://doi.org/10.3390/jcs8030111
Nakanishi A, Ningsih HS, Putra DFA, Moriga T, Shih S-J. Fabrication and Characterization of Granulated β-Tricalcium Phosphate and Bioactive Glass Powders by Spray Drying. Journal of Composites Science. 2024; 8(3):111. https://doi.org/10.3390/jcs8030111
Chicago/Turabian StyleNakanishi, Akihiro, Henni Setia Ningsih, Dwi Fortuna Anjusa Putra, Toshihiro Moriga, and Shao-Ju Shih. 2024. "Fabrication and Characterization of Granulated β-Tricalcium Phosphate and Bioactive Glass Powders by Spray Drying" Journal of Composites Science 8, no. 3: 111. https://doi.org/10.3390/jcs8030111
APA StyleNakanishi, A., Ningsih, H. S., Putra, D. F. A., Moriga, T., & Shih, S.-J. (2024). Fabrication and Characterization of Granulated β-Tricalcium Phosphate and Bioactive Glass Powders by Spray Drying. Journal of Composites Science, 8(3), 111. https://doi.org/10.3390/jcs8030111






