A Review on the Electrospinning of Polymer Nanofibers and Its Biomedical Applications
Abstract
1. Introduction
2. Methods for the Fabrication of Polymer Nanofiber Synthesis and Constructing Materials
2.1. Template Synthesis
2.2. Phase Separation
- At a normal temperature or at a higher temperature, polymer dissolution in a solvent occurs.
- The most challenging phase in controlling nanofiber shape is gelation (porosity). The gelation time varies depending on the polymer content and the gelation temperature.
- Using water to extract the solvent from the gel.
- The last step is vacuum freezing and freeze-drying.
2.3. Drawing
2.4. Self-Assembly
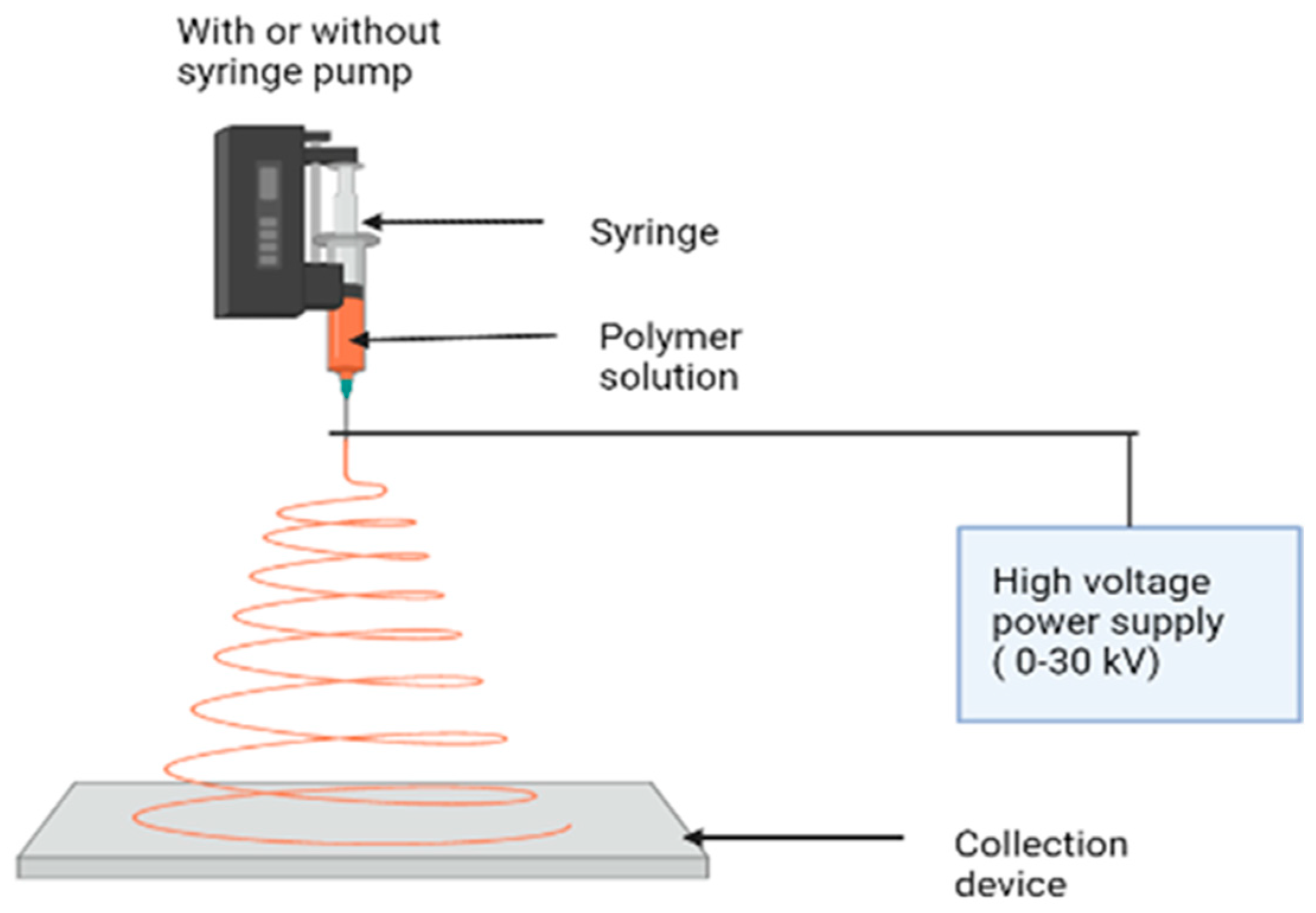
3. Electrospinning
- A proper solvent for dissolving the polymer should be provided.
- The vapor pressure of the solvent needs to be high enough for the fiber to maintain its integrity when it reaches the target but not too high that it hardens before it reaches the nanoscale range.
- The solvent surface tension and viscosity must be neither too high, to prevent the formation of a jet, nor too low, to enable the solution to drain easily from the pipette.
- The power source should be sufficient to overcome the viscosity and surface tension of the polymer solution and sustain the jet from the pipette.
- Maintaining the distance between the pipette and the surface to avoid sparks between the electrodes allows for the evaporation of the solvent to produce fibers.
3.1. Melt Electrospinning
3.2. Near-Field Electrospinning
3.3. Coaxial Electrospinning
3.4. Solution Blow Spinning
3.5. Force Spinning
3.6. Microfluidic Spinning
4. Modification of Polymer Nanofibers
5. Biomedical Applications
5.1. Scaffolds
5.1.1. Regeneration of Bone
5.1.2. Cartilage Regeneration
5.1.3. Use in Peripheral Nerve Regeneration
5.1.4. Regeneration of Ligaments and Tendons
5.1.5. Regeneration of Cardiovascular
5.2. Dressings for Wound Healing
5.3. Dental Restoration Nanocomposites
5.4. Controlled Drug Release
5.5. Medical Implants
5.6. Cancer Therapy
5.7. Biosensors
6. Advantages and Disadvantages of Polymer Nanofibers
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roco, M.C.; Mirkin, C.A.; Hersam, M.C. Nanotechnology Research Directions for Societal Needs in 2020: Retrospective and Outlook; Springer Science & Business Media: Berlin, Germany, 2011; Volume 1. [Google Scholar]
- Camposeo, A.; Moffa, M.; Persano, L. Electrospun fluorescent nanofibers and their application in optical sensing. In Electrospinning for High Performance Sensors; Springer: Cham, Switzerland, 2015; pp. 129–155. [Google Scholar]
- Zhu, M.; Han, J.; Wang, F.; Shao, W.; Xiong, R.; Zhang, Q.; Pan, H.; Yang, Y.; Samal, S.K.; Zhang, F.; et al. Electrospun nanofibers membranes for effective air filtration. Macromol. Mater. Eng. 2017, 302, 1600353. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Goyal, R.; Vega, M.E.; Pastino, A.K.; Singh, S.; Guvendiren, M.; Kohn, J.; Murthy, N.S.; Schwarzbauer, J.E. Development of hybrid scaffolds with natural extracellular matrix deposited within synthetic polymeric fibers. J. Biomed. Mater. Res. Part A 2017, 105, 2162–2170. [Google Scholar] [CrossRef]
- Chen, C.; Hu, L. Nanoscale Ion Regulation in Wood-Based Structures and Their Device Applications. Adv. Mater. 2021, 33, 2002890. [Google Scholar] [CrossRef]
- Ferrari, M.; Cirisano, F.; Morán, M.C. Mammalian cell behaviour on hydrophobic substrates: Influence of surface properties. Colloids Interfaces 2019, 3, 48. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Luo, D.; Xue, Z.; Yang, X.; Gu, L.; Zhou, Y.; Wang, T. Hierarchically staggered nanostructure of mineralized collagen as a bone-grafting scaffold. Adv. Mater. 2016, 28, 8740–8748. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Huang, C.-C.; Chin, W.-T. Carbon dioxide adsorption on Carbon nanofibers with different porous structures. Appl. Sci. 2021, 11, 7724. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Y. Centrifugal spinning: An alternative approach to fabricate nanofibers at high speed and low cost. Polym. Rev. 2014, 54, 677–701. [Google Scholar] [CrossRef]
- Luo, C.J.; Stoyanov, S.D.; Stride, E.; Pelan, E.; Edirisinghe, M. Electrospinning versus fiber production methods: From specifics to technological convergence. Chem. Soc. Rev. 2012, 41, 4708–4735. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Zagho, M.M.; Elzatahry, A.A. Polymer-based electrospun nanofibers for biomedical applications. Nanomaterials 2018, 8, 259. [Google Scholar] [CrossRef]
- Wu, H.; Pan, W.; Lin, D.; Li, H. Electrospinning of ceramic nanofibers: Fabrication, assembly and applications. J. Adv. Ceram. 2012, 1, 2–23. [Google Scholar] [CrossRef]
- Dos Santos, D.M.; Correa, D.S.; Medeiros, E.S.; Oliveira, J.E.; Mattoso, L.H. Advances in functional polymer nanofibers: From spinning fabrication techniques to recent biomedical applications. ACS Appl. Mater. Interfaces 2020, 12, 45673–45701. [Google Scholar] [CrossRef]
- Chen, S.; Li, R.; Li, X.; Xie, J. Electrospinning: An enabling nanotechnology platform for drug delivery and regenerative medicine. Adv. Drug Deliv. Rev. 2022, 132, 188–213. [Google Scholar] [CrossRef]
- Nayak, R.; Padhye, R.; Kyratzis, I.L.; Truong, Y.B.; Arnold, L. Recent advances in nanofiber fabrication techniques. Text. Res. J. 2012, 82, 129–147. [Google Scholar] [CrossRef]
- Beachley, V.; Wen, X. Polymer nanofibrous structures: Fabrication, biofunctionalization, and cell interactions. Prog. Polym. Sci. 2010, 35, 868–892. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.; Trabzon, L.; Kilic, A.; Zaidi, S.J. Recent advances in nanofibrous membranes: Production and applications in water treatment and desalination. Desalination 2020, 478, 114178. [Google Scholar] [CrossRef]
- Shaulsky, E.; Nejati, S.; Boo, C.; Perreault, F.; Osuji, C.O.; Elimelech, M. Post-fabrication modification of electrospun nanofiber mats with polymer coating for membrane distillation applications. J. Membr. Sci. 2017, 530, 158–165. [Google Scholar] [CrossRef]
- Li, M.; Ishihara, S.; Ji, Q.; Akada, M.; Hill, J.P.; Ariga, K. Paradigm shift from self-assembly to commanded assembly of functional materials: Recent examples in porphyrin/fullerene supramolecular systems. Sci. Technol. Adv. Mater. 2012, 13, 053001. [Google Scholar] [CrossRef]
- Ekrami, E.; Khodabandeh Shahraky, M.; Mahmoudifard, M.; Mirtaleb, M.S.; Shariati, P. Biomedical applications of electrospun nanofibers in industrial world: A review. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 561–575. [Google Scholar] [CrossRef]
- Tarus, B.; Fadel, N.; Al-Oufy, A.; El-Messiry, M. Effect of polymer concentration on the morphology and mechanical characteristics of electrospun cellulose acetate and poly (vinyl chloride) nanofiber mats. Alex. Eng. J. 2016, 55, 2975–2984. [Google Scholar] [CrossRef]
- Kang, J.; Gi, H.; Choe, R.; Yun, S.I. Fabrication and characterization of poly (3-hydroxybutyrate) gels using non-solvent-induced phase separation. Polymer 2016, 104, 61–71. [Google Scholar] [CrossRef]
- da Silva Parize, D.D.; Foschini, M.M.; de Oliveira, J.E.; Klamczynski, A.P.; Glenn, G.M.; Marconcini, J.M.; Mattoso, L.H.C. Solution blow spinning: Parameters optimization and effects on the properties of nanofibers from poly (lactic acid)/dimethyl carbonate solutions. J. Mater. Sci. 2016, 51, 4627–4638. [Google Scholar] [CrossRef]
- Mitsi, M.; Handschin, S.; Gerber, I.; Schwartländer, R.; Klotzsch, E.; Wepf, R.; Vogel, V. The ultrastructure of fibronectin fibers pulled from a protein monolayer at the air-liquid interface and the mechanism of the sheet-to-fiber transition. Biomaterials 2015, 36, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Alghoraibi, I.; Alomari, S. Different methods for nanofiber design and fabrication. In Handbook of Nanofibers; Springer: Cham, Switzerland, 2018; pp. 1–46. [Google Scholar]
- SalehHudin, H.S.; Mohamad, E.N.; Mahadi, W.N.L.; Muhammad Afifi, A. Multiple-jet electrospinning methods for nanofiber processing: A review. Mater. Manuf. 2018, 33, 479–498. [Google Scholar] [CrossRef]
- Mishra, R.; Militky, J.; Venkataraman, M. Electrospun nanofibers. In Nanotechnology in Textiles; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 35–161. [Google Scholar]
- Rest, C.; Kandanelli, R.; Fernández, G. Strategies to create hierarchical self-assembled structures via cooperative non-covalent interactions. Chem. Soc. Rev. 2015, 44, 2543–2572. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Calandra, P.; Pasqua, L.; Magazù, S. Self-assembly of organic nanomaterials and biomaterials: The bottom-up approach for functional nanostructures formation and advanced applications. Materials 2020, 13, 1048. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, W.; Jiang, X. Biomimetic collagen nanofibrous materials for bone tissue engineering. Adv. Eng. Mater. 2010, 12, B451–B466. [Google Scholar] [CrossRef]
- Silva, M.L.; Martins, A.; Pinto, A.R.; Monteiro, N.; Reis, R.L.; Neves, N.M. Indirect co-cultures of stem cells with chondrocytes for cartilage tissue engineering using PCL electrospun nanofiber meshes. J. Tissue Eng. Regen. Med. 2013, 7 Suppl. S1, 6–52. [Google Scholar] [CrossRef]
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Melt electrospinning today: An opportune time for an emerging polymer process. Prog. Polym. Sci. 2016, 56, 116–166. [Google Scholar] [CrossRef]
- Abadi, B.; Goshtasbi, N.; Bolourian, S.; Tahsili, J.; Adeli-Sardou, M.; Forootanfar, H. Electrospun hybrid nanofibers: Fabrication, characterization, and biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 986975. [Google Scholar] [CrossRef]
- Sencadas, V.; Correia, D.M.; Areias, A.; Botelho, G.; Fonseca, A.M.; Neves, I.C.; Ribelles, J.G.; Mendez, S.L. Determination of the parameters affecting electrospun chitosan fiber size distribution and morphology. Carbohydr. Polym. 2012, 87, 1295–1301. [Google Scholar] [CrossRef]
- Kong, L.; Ziegler, G.R. Quantitative relationship between electrospinning parameters and starch fiber diameter. Carbohydr. Polym. 2013, 92, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Ghosh, B.; Biswas, S. Nanocarriers for cancer-targeted drug delivery. J. Drug Target. 2016, 24, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Guo, Q.; Zhang, Z.; Xu, Q. High-Speed Centrifugal Spinning Polymer Slip Mechanism and PEO/PVA Composite Fiber Preparation. Nanomaterials 2023, 13, 1277. [Google Scholar] [CrossRef]
- Agarwal, S.; Greiner, A.; Wendorff, J.H. Functional materials by electrospinning of polymers. Prog. Polym. Sci. 2013, 38, 963–991. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Z.; Gu, J.; Zhou, W.; Liang, X.; Zhou, G.; Han, C.C.; Xu, S.; Liu, Y. Mechanism of a long-term controlled drug release system based on simple blended electrospun fibers. J. Control. Release 2020, 320, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Englert, C.; Brendel, J.C.; Majdanski, T.C.; Yildirim, T.; Schubert, S.; Gottschaldt, M.; Windhab, N.; Schubert, U.S. Pharmapolymers in the 21st century: Synthetic polymers in drug delivery applications. Prog. Polym. Sci. 2018, 87, 107–164. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Yadav, D.; Amini, F.; Ehrmann, A. Recent advances in carbon nanofibers and their applications—A review. Eur. Polym. J. 2020, 138, 109963. [Google Scholar] [CrossRef]
- Wang, P.; Lv, H.; Cao, X.; Liu, Y.; Yu, D.G. Recent progress of the preparation and application of electrospun porous nanofibers. Polymers 2023, 15, 921. [Google Scholar] [CrossRef]
- Li, K.; Clarkson, C.M.; Wang, L.; Liu, Y.; Lamm, M.; Pang, Z.; Zhou, Y.; Qian, J.; Tajvidi, M.; Gardner, D.J.; et al. Alignment of cellulose nanofibers: Harnessing nanoscale properties to macroscale benefits. ACS Nano 2021, 15, 3646–3673. [Google Scholar] [CrossRef] [PubMed]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial upscaling of electrospinning and applications of polymer nanofibers: A review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Sadeghian, Z.; Hadidi, M.R.; Salehzadeh, D.; Nemati, A. Hydrophobic octadecylamine-functionalized graphene/TiO2 hybrid coating for corrosion protection of copper bipolar plates in simulated proton exchange membrane fuel cell environment. Int. J. Hydrogen Energy 2020, 45, 15380–15389. [Google Scholar] [CrossRef]
- Xu, H.; Yagi, S.; Ashour, S.; Du, L.; Hoque, M.E.; Tan, L. A Review on Current Nanofiber Technologies: Electrospinning, Centrifugal Spinning, and Electro-Centrifugal Spinning. Macromol. Mater. Eng. 2023, 308, 2200502. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun poly (ε-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: Characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 2006, 7, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, F.; Cheng, Z.; Raad, R.; Xi, J.; Foroughi, J. Piezofibers to smart textiles: A review on recent advances and future outlook for wearable technology. J. Mater. Chem. A 2020, 8, 9496–9522. [Google Scholar] [CrossRef]
- Bhattarai, D.P.; Aguilar, L.E.; Park, C.H.; Kim, C.S. A review on properties of natural and synthetic based electrospun fibrous materials for bone tissue engineering. Membranes 2018, 8, 62. [Google Scholar] [CrossRef]
- Han, W.; Wang, L.; Li, Q.; Ma, B.; He, C.; Guo, X.; Nie, J.; Ma, G. A Review: Current Status and Emerging Developments on Natural Polymer-Based Electrospun Fibers. Macromol. Rapid Commun. 2022, 43, 2200456. [Google Scholar] [CrossRef]
- Dos Santos, A.E.A.; Dos Santos, F.V.; Freitas, K.M.; Pimenta, L.P.S.; de Oliveira Andrade, L.; Marinho, T.A.; de Avelar, G.F.; da Silva, A.B.; Ferreira, R.V. Cellulose acetate nanofibers loaded with crude annatto extract: Preparation, characterization, and in vivo evaluation for potential wound healing applications. Mater. Sci. Eng. C 2021, 118, 111322. [Google Scholar] [CrossRef]
- Lu, T.; Cui, J.; Qu, Q.; Wang, Y.; Zhang, J.; Xiong, R.; Huang, C. Multistructured electrospun nanofibers for air filtration: A review. ACS Appl. Mater. Interfaces 2021, 13, 23293–23313. [Google Scholar] [CrossRef]
- Nemati, S.; Kim, S.J.; Shin, Y.M.; Shin, H. Current progress in application of polymeric nanofibers to tissue engineering. Nano Converg. 2019, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.M.; Klingner, A. A review on electrospun polymeric nanofibers: Production parameters and potential applications. Polym. Test. 2020, 90, 106647. [Google Scholar] [CrossRef]
- Barhoum, A.; Pal, K.; Rahier, H.; Uludag, H.; Kim, I.S.; Bechelany, M. Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications. Appl. Mater. Today 2019, 17, 1–35. [Google Scholar] [CrossRef]
- Huang, Y.; Song, J.; Yang, C.; Long, Y.; Wu, H. Scalable manufacturing and applications of nanofibers. Mater. Today 2019, 28, 98–113. [Google Scholar] [CrossRef]
- Guarino, V.; Altobelli, R.; Cirillo, V.; Cummaro, A.; Ambrosio, L. Additive electrospraying: A route to process electrospun scaffolds for controlled molecular release. Polym. Adv. Technol. 2015, 26, 1359–1369. [Google Scholar] [CrossRef]
- Hashimdeen, S.H. A Prototype for 3D Electrohydrodynamic Printing. Doctoral Dissertation, UCL (University College London), London, UK, 2016. [Google Scholar]
- Di Camillo, D.; Fasano, V.; Ruggieri, F.; Santucci, S.; Lozzi, L.; Camposeo, A.; Pisignano, D. Near-field electrospinning of conjugated polymer light-emitting nanofibers. arXiv 2013, arXiv:1310.5101. [Google Scholar] [CrossRef] [PubMed]
- Torres-Giner, S. Electrospun nanofibers for food packaging applications. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Woodhead Publishing: New Delhi, India, 2011; pp. 108–125. [Google Scholar]
- Pant, B.; Park, M.; Park, S.J. Drug delivery applications of core-sheath nanofibers prepared by coaxial electrospinning: A review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef]
- Elahi, M.F.; Lu, W.; Guoping, G.; Khan, F. Core-shell fibers for biomedical applications—A review. J. Bioeng. Biomed. Sci. 2013, 3, 121. [Google Scholar] [CrossRef]
- Fadil, F.; Affandi, N.D.N.; Misnon, M.I.; Bonnia, N.N.; Harun, A.M.; Alam, M.K. Review on electrospun nanofiber-applied products. Polymers 2021, 13, 2087. [Google Scholar] [CrossRef]
- George, D.; Garcia, A.; Pham, Q.; Perez, M.R.; Deng, J.; Nguyen, M.T.; Madou, M. Fabrication of patterned graphitized carbon wires using low voltage near-field electrospinning, pyrolysis, electrodeposition, and chemical vapor deposition. Microsyst. Nanoeng. 2020, 6, 7. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, H.; Yue, L.; Fan, G.; Li, T.; Lu, S.; Chen, G.; Gao, S.; Asiri, A.M.; Sun, X. Recent advances in electrospun nanofibers for supercapacitors. J. Mater. Chem. A 2020, 8, 16747–16789. [Google Scholar] [CrossRef]
- Zhao, Z.; Fang, R.; Rong, Q.; Liu, M. Bioinspired nanocomposite hydrogels with highly ordered structures. Adv. Mater. 2017, 29, 1703045. [Google Scholar] [CrossRef] [PubMed]
- Chakrapani, G.; Ramakrishna, S.; Zare, M. Functionalization of electrospun nanofiber for biomedical application. J. Appl. Polym. Sci. 2023, 140, e53906. [Google Scholar] [CrossRef]
- Dias, F.T.G.; Rempel, S.P.; Agnol, L.D.; Bianchi, O. The main blow spun polymer systems: Processing conditions and applications. J. Polym. Res. 2020, 27, 205. [Google Scholar] [CrossRef]
- Dadol, G.C.; Kilic, A.; Tijing, L.D.; Lim, K.J.A.; Cabatingan, L.K.; Tan, N.P.B.; Stojanovska, E.; Polat, Y. Solution blow spinning (SBS) and SBS-spun nanofibers: Materials, methods, and applications. Mater. Today Commun. 2020, 25, 101656. [Google Scholar] [CrossRef]
- Abdal-Hay, A.; Oh, Y.S.; Yousef, A.; Pant, H.R.; Vanegas, P.; Lim, J.K. In vitro deposition of Ca-P nanoparticles on air jet spinning Nylon 6 nanofibers scaffold for bone tissue engineering. Appl. Surf. Sci. 2014, 307, 69–76. [Google Scholar] [CrossRef]
- Zou, W.; Chen, R.Y.; Zhang, G.Z.; Zhang, H.C.; Qu, J.P. Recent advances in centrifugal spinning preparation of nanofibers. Adv. Mater. Res. 2014, 1015, 170–176. [Google Scholar] [CrossRef]
- Marjuban, S.M.H.; Rahman, M.; Duza, S.S.; Ahmed, M.B.; Patel, D.K.; Rahman, M.S.; Lozano, K. Recent Advances in Centrifugal Spinning and Their Applications in Tissue Engineering. Polymers 2023, 15, 1253. [Google Scholar] [CrossRef]
- Badrossamay, M.R.; McIlwee, H.A.; Goss, J.A.; Parker, K.K. Nanofiber assembly by rotary jet-spinning. Nano Lett. 2010, 10, 2257–2261. [Google Scholar] [CrossRef]
- Venugopal, J.; Ramakrishna, S. Applications of polymer nanofibers in biomedicine and biotechnology. Appl. Biochem. Biotechnol. 2005, 125, 147–157. [Google Scholar] [CrossRef]
- Mokhtari, F.; Salehi, M.; Zamani, F.; Hajiani, F.; Zeighami, F.; Latifi, M. Advances in electrospinning: The production and application of nanofibers and nanofibrous structures. Text. Prog. 2016, 48, 119–219. [Google Scholar] [CrossRef]
- Jun, Y.; Kang, E.; Chae, S.; Lee, S.H. Microfluidic spinning of micro-and nano-scale fibers for tissue engineering. Lab Chip 2014, 14, 2145–2160. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Mun, C.H.; Lee, S.H. Microfluidic spinning of fibrous alginate carrier having highly enhanced drug loading capability and delayed release profile. RSC Adv. 2015, 5, 15172–15181. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.S.; Li, C.W.; Nguyen, N.C.; Nguyen, H.T. A comprehensive review: Electrospinning technique for fabrication and surface modification of membranes for water treatment application. RSC Adv. 2016, 6, 85495–85514. [Google Scholar] [CrossRef]
- Amani, H.; Arzaghi, H.; Bayandori, M.; Dezfuli, A.S.; Pazoki-Toroudi, H.; Shafiee, A.; Moradi, L. Controlling cell behavior through the design of biomaterial surfaces: A focus on surface modification techniques. Adv. Mater. Interfaces 2019, 6, 1900572. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, L.; Wang, J.; Jin, M.; Tang, Q.; Chen, Z.; Zhao, G. Electrospun nanofibers promote wound healing: Theories, techniques, and perspectives. J. Mater. Chem. B 2021, 9, 3106–3130. [Google Scholar] [CrossRef]
- Tebyetekerwa, M.; Xu, Z.; Yang, S.; Ramakrishna, S. Electrospun nanofibers-based face masks. Adv. Fiber Mater. 2020, 2, 161–166. [Google Scholar] [CrossRef]
- Kajdič, S.; Planinšek, O.; Gašperlin, M.; Kocbek, P. Electrospun nanofibers for customized drug-delivery systems. J. Drug Deliv. Sci. Technol. 2019, 51, 672–681. [Google Scholar] [CrossRef]
- Abdul Hameed, M.M.; Mohamed Khan, S.A.P.; Thamer, B.M.; Rajkumar, N.; El-Hamshary, H.; El-Newehy, M. Electrospun nanofibers for drug delivery applications: Methods and mechanism. Polym. Adv. Technol. 2023, 34, 6–23. [Google Scholar] [CrossRef]
- Lee, S.J.; Yoo, J.J.; Atala, A. Biomaterials and tissue engineering. In Clinical Regenerative Medicine in Urology; Springer: Singapore, 2018; pp. 17–51. [Google Scholar]
- Balusamy, B.; Celebioglu, A.; Senthamizhan, A.; Uyar, T. Progress in the design and development of “fast-dissolving” electrospun nanofibers-based drug delivery systems—A systematic review. J. Control Release 2020, 326, 482–509. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of collagen scaffold in tissue engineering: Recent advances and new perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Cleeton, C.; Keirouz, A.; Chen, X.; Radacsi, N. Electrospun nanofibers for drug delivery and biosensing. ACS Biomater. Sci. Eng. 2019, 5, 4183–4205. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.A.M.; Guler, E.; Rayaman, E.; Cam, M.E.; Sahin, A.; Grinholc, M.; Mansuroglu, D.S.; Sahin, Y.M.; Gunduz, O.; Muhammed, M.; et al. Dual-drug delivery of Ag-chitosan nanoparticles and phenytoin via core-shell PVA/PCL electrospun nanofibers. Carbohydr. Polym. 2021, 270, 118373. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.Y.; Jia, X.W.; Liu, Q.; Kong, B.H.; Wang, H. Fast dissolving oral films for drug delivery prepared from chitosan/pullulan electrospinning nanofibers. Int. J. Biol. Macromol. 2019, 137, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, D.; Zhao, X.; Pakvasa, M.; Tucker, A.B.; Luo, H.; Qin, K.H.; Hu, D.A.; Wang, E.J.; Li, A.J.; et al. Stem Cell-Friendly Scaffold Biomaterials: Applications for Bone Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 598607. [Google Scholar] [CrossRef]
- Balagangadharan, K.; Dhivya, S.; Selvamurugan, N. Chitosan based nanofibers in bone tissue engineering. Int. J. Biol. Macromol. 2017, 104, 1372–1382. [Google Scholar] [CrossRef]
- Zhao, Q.; Du, X.; Wang, M. Electrospinning and Cell Fibers in Biomedical Applications. Adv. Biol. 2023, 7, 2300092. [Google Scholar] [CrossRef]
- Santo, V.E.; Gomes, M.E.; Mano, J.F.; Reis, R.L. From nano-to macro-scale: Nanotechnology approaches for spatially controlled delivery of bioactive factors for bone and cartilage engineering. Nanomedicine 2012, 7, 1045–1066. [Google Scholar] [CrossRef]
- Gadalla, D.M.A. Osteogenic Scaffolds for Enhanced Graft-Bone Integration in Ligament Tissue Engineering. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 2020. [Google Scholar]
- Yu, Y.; Hua, S.; Yang, M.; Fu, Z.; Teng, S.; Niu, K.; Zhao, Q.; Yi, C. Fabrication and characterization of electrospinning/3D printing bone tissue engineering scaffold. RSC Adv. 2016, 6, 110557–110565. [Google Scholar] [CrossRef]
- Frost, H.K.; Andersson, T.; Johansson, S.; Englund-Johansson, U.; Ekström, P.; Dahlin, L.B.; Johansson, F. Electrospun nerve guide conduits have the potential to bridge peripheral nerve injuries in vivo. Sci. Rep. 2018, 8, 16716. [Google Scholar] [CrossRef]
- Habre, S.B.; Bond, G.; Jing, X.L.; Kostopoulos, E.; Wallace, R.D.; Konofaos, P. The surgical management of nerve gaps: Present and future. Ann. Plast. Surg. 2018, 80, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.W.H.; Jiang, T.; Gagnon, K.A.; Nelson, C.; Laurencin, C.T. Small-molecule based musculoskeletal regenerative engineering. Trends Biotechnol. 2014, 32, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Carbone, E.J.; Lo, K.W.H.; Laurencin, C.T. Electrospinning of polymer nanofibers for tissue regeneration. Prog. Polym. Sci. 2015, 46, 1–24. [Google Scholar] [CrossRef]
- Brimo, N.; Serdaroğlu, D.Ç.; Uysal, B. Comparing antibiotic pastes with electrospun nanofibers as modern drug delivery systems for regenerative endodontics. Curr. Drug Deliv. 2022, 19, 904–917. [Google Scholar] [PubMed]
- Ponrasu, T.; Chen, B.H.; Chou, T.H.; Wu, J.J.; Cheng, Y.S. Fast dissolving electrospun nanofibers fabricated from jelly fig polysaccharide/pullulan for drug delivery applications. Polymers 2021, 13, 241. [Google Scholar] [CrossRef]
- Heusch, G.; Libby, P.; Gersh, B.; Yellon, D.; Böhm, M.; Lopaschuk, G.; Opie, L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 2014, 383, 1933–1943. [Google Scholar] [CrossRef]
- Kristen, M.; Ainsworth, M.J.; Chirico, N.; van der Ven, C.F.T.; Doevendans, P.A.; Sluijter, J.P.G.; Malda, J.; van Mil, A.; Castilho, M. Fiber scaffold patterning for mending hearts: 3D organization bringing the next step. Adv. Health. Mater. 2020, 9, 1900775. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: Regulation of local and global homeostasis by the skin neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 2012, 212, 7–26. [Google Scholar]
- Sarabahi, S. Recent advances in topical wound care. Indian J. Plast. Surg. 2012, 45, 379–387. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.S.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Khodadadi, M.; Alijani, S.; Montazeri, M.; Esmaeilizadeh, N.; Sadeghi-Soureh, S.; Pilehvar-Soltanahmadi, Y. Recent advances in electrospun nanofiber-mediated drug delivery strategies for localized cancer chemotherapy. J. Biomed. Mater. Res. A 2020, 108, 1444–1458. [Google Scholar] [CrossRef] [PubMed]
- Al-Tannak, N.F.; Alzoubi, F.; Kareem, F.M.; Novotny, L. Determination of Endocrine Disruptor Bisphenol—A Leakage from Different Matrices of Dental Resin-Based Composite Materials. Curr. Pharm. Anal. 2022, 18, 305–315. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, S.; Misra, M. Electrospun polymeric nanofibers: New horizons in drug delivery. Eur. J. Pharm. Sci. 2017, 107, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, P.; Lim, D.H.; Ahn, J.H.; Nah, C.; Sherrington, D.C.; Ryu, H.S.; Ahn, H.J. Electrospun polymer nanofibers: The booming cutting edge technology. React. Funct. Polym. 2012, 72, 915–930. [Google Scholar] [CrossRef]
- Ignatious, F.; Sun, L.; Lee, C.P.; Baldoni, J. Electrospun nanofibers in oral drug delivery. Pharm. Res. 2010, 27, 576–588. [Google Scholar] [CrossRef]
- Anand, U.; Bandyopadhyay, A.; Jha, N.K.; Pérez de la Lastra, J.M.; Dey, A. Translational aspect in peptide drug discovery and development: An emerging therapeutic candidate. Biofactors 2023, 49, 251–269. [Google Scholar] [CrossRef]
- Arinstein, A.; Zussman, E. Electrospun polymer nanofibers: Mechanical and thermodynamic perspectives. J. Polym. Sci. B Polym. Phys. 2011, 49, 691–707. [Google Scholar] [CrossRef]
- Valipour, P.; Ghasemi, S.E. Effect of non-Newtonian rheology on electrified jets of polymer nanofibers in electrospinning process based on bead–spring model. Int. J. Adv. Manuf. Technol. 2017, 91, 3535–3550. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, R.; das Neves, J.; Tang, J.; Xiao, J.; Ni, Q.; Liu, X.; Pan, G.; Li, D.; Cui, W.; et al. Advances in biomaterials for preventing tissue adhesion. J. Control Release 2017, 261, 318–336. [Google Scholar] [CrossRef]
- Cannata, A.; Petrella, D.; Russo, C.F.; Bruschi, G.; Fratto, P.; Gambacorta, M.; Martinelli, L. Postsurgical intrapericardial adhesions: Mechanisms of formation and prevention. Ann. Thorac. Surg. 2013, 95, 1818–1826. [Google Scholar] [CrossRef]
- Onoue, S.; Yamada, S.; Chan, H.K. Nanodrugs: Pharmacokinetics and safety. Int. J. Nanomed. 2014, 9, 1025. [Google Scholar] [CrossRef] [PubMed]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef]
- Chaudhary, S.; Umar, A.; Bhasin, K.K.; Baskoutas, S. Chemical sensing applications of ZnO nanomaterials. Materials 2018, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, M.; Shalaby, E.; Youssef, A. Nanomaterials and nanofibers as wound dressing mats: An overview of the fundamentals, properties and applications. Egypt. J. Chem. 2021, 64, 7447–7473. [Google Scholar] [CrossRef]
- Nabgan, W.; Jalil, A.A.; Nabgan, B.; Ikram, M.; Ali, M.W.; Lakshminarayana, P. A state-of-the-art overview of carbon-based composites applications for detecting and eliminating pharmaceuticals containing wastewater. Chemosphere 2022, 288, 132535. [Google Scholar] [CrossRef]
- Cho, C.J.; Chang, Y.S.; Lin, Y.Z.; Jiang, D.H.; Chen, W.H.; Lin, W.Y.; Kuo, C.C. Green electro spun nanofiber membranes filter prepared from novel biomass thermoplastic copolyester: Morphologies and filtration properties. J. Taiwan Inst. Chem. Eng. 2020, 106, 206–214. [Google Scholar] [CrossRef]
- Muthuraj, R.; Mekonnen, T. Recent progress in carbon dioxide (CO2) as feedstock for sustainable materials development: Co-polymers and polymer blends. Polymer 2018, 145, 348–373. [Google Scholar] [CrossRef]
- Venmathi Maran, B.A.; Iqbal, M.; Gangadaran, P.; Ahn, B.-C.; Rao, P.V.; Shah, M.D. Hepatoprotective potential of Malaysian medicinal plants: A review on phytochemicals, oxidative stress, and antioxidant mechanisms. Molecules 2022, 27, 1533. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.D.; Venmathi Maran, B.A.; Shaleh, S.R.M.; Zuldin, W.H.; Gnanaraj, C.; Yong, Y.S. Therapeutic potential and nutraceuticals profiling of North Bornean seaweeds: A review. Mar. Drugs 2022, 20, 101. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, R.K.; Nunzi, J.M.; Mahajan, A.; Sharma, D.P.; Pathak, D. Electrospun Polymer Nanofibers for Technology Applications: A Short Review. Curr. Mater. Sci. Former. Recent Pat. Mater. Sci. 2023, 16, 376–399. [Google Scholar]
- Savva, I.; Evaggelou, E.; Papaparaskeva, G.; Leontiou, T.; Stylianopoulos, T.; Mpekris, F.; Stylianou, K.; Krasia-Christoforou, T. Alignment of electrospun polymer fibers using a concave collector. RSC Adv. 2015, 5, 104400–104407. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef] [PubMed]



| Techniques Used to Prepare Polymer Nanofibers | Biomedical Applications | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Template synthesis | Not applicable; more analysis needed | A template can be used as a nanoporous membrane | Unable to create continuous nanofibers; fibers a few micrometers long are obtained | [37,38] |
| Self assembly | Designing peptide sequences | A good way to make nanofibers that are less than 100 nm of diameter | Biomaterials’ uses are limited by their high cost of manufacture; engineered peptide nanofibers could be broken and are sensitive to endocytosis | [37,39] |
| Drawing method | Not applicable; more analysis needed | These processes can be carried out several times on each droplet | Limited to viscoelastic materials; depends on extrusion molds pore size; challenging to generate fiber diameters smaller than 100 nm | [16,28] |
| Phase separation | Used in drug delivery systems | A requirement for the minimal essentials in terms of equipment | Long continuous fibers cannot be made; just a few polymers are available | [40,41] |
| Electrospinning | Tissue engineering, medication release, wound healing, enzyme immobilization, etc. | Fibers ranging from nm in size to a few microns, low-cost approach, high aspect ratio, improved mechanical qualities | Difficult to construct a huge volume scaffold | [26] |
| Solution blow spinning | Used in medicine formulation | Large-scale fabrication, random deposition, good industrialization prospect | Random deposition; poor fiber morphology; solvent treatment is necessary; the fiber diameter has low reproducibility | [42,43,44] |
| Centrifugal spinning (force spinning) | Used in tissue engineering, etc. | The most promising for a high production rate, low cost, and high production safety | The main issue with centrifugal spinning is material qualities | [45,46,47,48] |
| Drum and mandrel spinning | Extracellular matrix formation of the wound in animal models | Well-aligned polymer | Poor mechanical motion | [49,50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venmathi Maran, B.A.; Jeyachandran, S.; Kimura, M. A Review on the Electrospinning of Polymer Nanofibers and Its Biomedical Applications. J. Compos. Sci. 2024, 8, 32. https://doi.org/10.3390/jcs8010032
Venmathi Maran BA, Jeyachandran S, Kimura M. A Review on the Electrospinning of Polymer Nanofibers and Its Biomedical Applications. Journal of Composites Science. 2024; 8(1):32. https://doi.org/10.3390/jcs8010032
Chicago/Turabian StyleVenmathi Maran, Balu Alagar, Sivakamavalli Jeyachandran, and Masanari Kimura. 2024. "A Review on the Electrospinning of Polymer Nanofibers and Its Biomedical Applications" Journal of Composites Science 8, no. 1: 32. https://doi.org/10.3390/jcs8010032
APA StyleVenmathi Maran, B. A., Jeyachandran, S., & Kimura, M. (2024). A Review on the Electrospinning of Polymer Nanofibers and Its Biomedical Applications. Journal of Composites Science, 8(1), 32. https://doi.org/10.3390/jcs8010032








