CeF3-YF3-TbF3 Nanoparticle-Polymer–“Radachlorin” Conjugates for Combined Photodynamic Therapy: Synthesis, Characterization, and Biological Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Classification
2.3. Characterization of the Samples
2.4. Cells Preparation and Cytotoxicity Assessment of CeF3-YF3-TbF3 Nanoparticles
2.5. Flow Cytometry
2.6. Transmission Electron Microscopy
3. Results
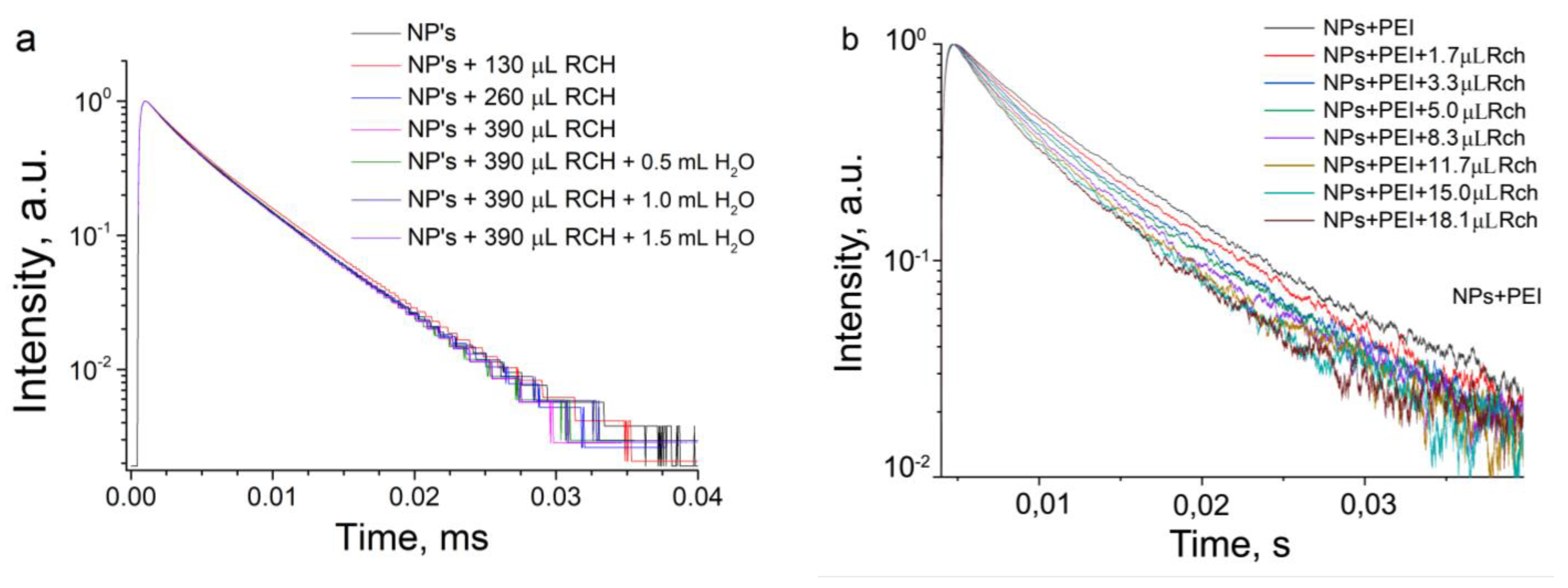
3.1. Optical Spectroscopy of the Samples
3.2. Cytotoxicity of PEI-NP and PEI-NP-RCH Samples
3.3. Visualization of Nanoparticles Internalization by A549 Cells Using TEM
3.4. Flow Cytometry Study of Cellular Uptake of PEI-NP and PEI-NP-RCH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shanazarov, N.; Zinchenko, S.; Zhapparov, E.; Muratov, N.; Turzhanova, D.; Bilyalov, A.; Tashpulatov, T. The Clinical Case of Successful Application of Photodynamic Therapy in the Skin Metastases Treatment of Breast Cancer. BioNanoScience 2021, 11, 957–961. [Google Scholar] [CrossRef]
- Clement, S.; Campbell, J.M.; Deng, W.; Guller, A.; Nisar, S.; Liu, G.; Goldys, E.M. Mechanisms for tuning engineered nanomaterials to enhance radiation therapy of cancer. Adv. Sci. 2020, 7, 2003584. [Google Scholar] [CrossRef] [PubMed]
- Gadzhimagomedova, Z.; Zolotukhin, P.; Kit, O.; Kirsanova, D.; Soldatov, A. Nanocomposites for X-ray photodynamic therapy. Int. J. Mol. Sci. 2020, 21, 4004. [Google Scholar] [CrossRef] [PubMed]
- Chong, L.M.; Tng, D.J.H.; Tan, L.L.Y.; Chua, M.L.K.; Zhang, Y. Recent advances in radiation therapy and photodynamic therapy. Appl. Phys. Rev. 2021, 8, 041322. [Google Scholar] [CrossRef]
- Cooper, D.R.; Capobianco, J.A.; Seuntjens, J. Radioluminescence studies of colloidal oleate-capped β-Na (Gd, Lu) F4:Ln3+ nanoparticles (Ln = Ce, Eu, Tb). Nanoscale 2018, 10, 7821–7832. [Google Scholar] [CrossRef] [PubMed]
- Clement, S.; Deng, W.; Camilleri, E.; Wilson, B.C.; Goldys, E.M. X-ray induced singlet oxygen generation by nanoparticle-photosensitizer conjugates for photodynamic therapy: Determination of singlet oxygen quantum yield. Sci. Rep. 2016, 6, 19954. [Google Scholar] [CrossRef] [PubMed]
- Rzigalinski, B.A.; Carfagna, C.S.; Ehrich, M. Cerium oxide nanoparticles in neuroprotection and considerations for efficacy and safety. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1444. [Google Scholar] [CrossRef]
- Nizamutdinov, A.S.; Madirov, E.I.; Lukinova, E.V.; Kiyamov, A.G.; Andreeva, D.D.; Pudovkin, M.S.; Korableva, S.L.; Semashko, V.V. Spectral-Kinetic Properties and Energy Transfer in Nanoparticles of Y0.5–x Ce0.5 Tbx F3 Solid Solution. J. Appl. Spectrosc. 2020, 87, 481–487. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Dong, L.; Liu, D.; Qi, Z. Low temperature molten salt synthesis of CeF3 and CeF3: Tb3+ phosphors with efficient luminescence properties. J. Lumin. 2019, 205, 122–128. [Google Scholar] [CrossRef]
- Kochneva, E.V.; Filonenko, E.V.; Vakulovskaya, E.G.; Scherbakova, E.G.; Seliverstov, O.V.; Markichev, N.A.; Reshetnickov, A.V. Photosensitizer Radachlorin®: Skin cancer PDT phase II clinical trials. Photodiagn. Photodyn. Ther. 2010, 7, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Efendiev, K.T.; Alekseeva, P.M.; Shiryaev, A.A.; Skobeltsin, A.S.; Solonina, I.L.; Fatyanova, A.S.; Loschenov, V.B. Preliminary low-dose photodynamic exposure to skin cancer with chlorin e6 photosensitizer. Photodiagn. Photodyn. Ther. 2022, 38, 102894. [Google Scholar] [CrossRef]
- Krasnyuk, I.I.; Babenyshev, A.V.; Maslov, I.V.; Loshkarev, A.A.; Krasnyuk, I.I. Radachlorin-Containing Microparticles for Photodynamic Therapy. Adv. Pharm. Bull. 2021, 11, 458–468. [Google Scholar]
- Zhikhoreva, A.A.; Belashov, A.V.; Belyaeva, T.N.; Salova, A.V.; Litvinov, I.K.; Kornilova, E.S.; Vasyutinskii, O.S. Comparative analysis of Radachlorin accumulation, localization, and photobleaching in three cell lines by means of holographic and fluorescence microscopy. Photodiagn. Photodyn. Ther. 2022, 39, 102973. [Google Scholar] [CrossRef] [PubMed]
- Alakshin, E.M.; Klochkov, A.V.; Kondratyeva, E.I.; Korableva, S.L.; Kiiamov, A.G.; Nuzhina, D.S.; Kodjikian, S. Microwave-assisted hydrothermal synthesis and annealing of DyF3 nanoparticles. J. Nanomater. 2016, 2016, 7148307. [Google Scholar] [CrossRef]
- Wang, X.; Sheng, T.; Fu, Z.; Li, W.; Jeong, J.H. Highly uniform YF3: Ln3+ (Ln = Ce3+, Tb3+) walnut-like microcrystals: Hydrothermal synthesis and luminescent properties. Mater. Res. Bull. 2013, 48, 2143–2148. [Google Scholar] [CrossRef]
- Bekah, D.; Cooper, D.; Kudinov, K.; Hill, C.; Seuntjens, J.; Bradforth, S.; Nadeau, J. Synthesis and characterization of biologically stable, doped LaF3 nanoparticles co-conjugated to PEG and photosensitizers. J. Photochem. Photobiol. A Chem. 2016, 329, 26–33. [Google Scholar] [CrossRef]
- Freshney, R.R.I.; Capes-Davis, A.; Gregory, C.; Przyborski, S. Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications; John Wiley: Hoboken, NJ, USA, 2021; p. 758. [Google Scholar]
- Garg, S.; Chandra, A. Green Photocatalytic Semiconductors; Springer International Publishing: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Rojas-Hernandez, R.E.; Santos, L.F.; Almeida, R.M. Tb3+/Yb3+ doped aluminosilicate phosphors for near infrared emission and efficient down-conversion. J. Lumin. 2018, 197, 180–186. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, Z.W.; Jiang, D.G.; Li, Y.N.; Sun, X.Y. Generation of bright white-light by energy-transfer strategy in Ca19Zn2(PO4)14: Ce3+, Tb3+, Mn2+ phosphors. J. Lumin. 2019, 206, 244–249. [Google Scholar] [CrossRef]
- Sahoo, H. Förster resonance energy transfer–A spectroscopic nanoruler: Principle and applications. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 20–30. [Google Scholar] [CrossRef]
- Clegg, R.M. Förster resonance energy transfer—FRET what is it, why do it, and how it’s done. Lab. Tech. Biochem. Mol. Biol. 2009, 33, 1–57. [Google Scholar]
- Sun, X.; Sun, J.; Dong, B.; Huang, G.; Zhang, L.; Zhou, W.; Song, H. Noninvasive temperature monitoring for dual-modal tumor therapy based on lanthanide-doped up-conversion nanocomposites. Biomaterials 2019, 201, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; He, F.; Liu, B.; Yang, G.; Gai, S.; Yang, P.; Lin, J. g-C3N4 coated upconversion nanoparticles for 808 nm near-infrared light triggered phototherapy and multiple imaging. Chem. Mater. 2016, 28, 7935–7946. [Google Scholar] [CrossRef]
- Stearns, R.C.; Paulauskis, J.D.; Godleski, J.J. Endocytosis of ultrafine particles by A549 cells. Am. J. Respir. Cell Mol. Biol. 2001, 24, 108–115. [Google Scholar] [CrossRef]
- Pudovkin, M.S.; Zelenikhin, P.V.; Shtyreva, V.V.; Evtugyn, V.G.; Salnikov, V.V.; Nizamutdinov, A.S.; Semashko, V.V. Cellular uptake and cytotoxicity of unmodified Pr3+:LaF3 nanoparticles. J. Nanopart. Res. 2019, 21, 184. [Google Scholar] [CrossRef]
- Pudovkin, M.S.; Shamsutdinov, N.I.; Zelenikhin, P.V.; Nizamutdinov, A.S. Transmission electron microscopy and flow cytometry study of cellular uptake of unmodified Pr3+:LaF3 nanoparticles in dynamic. J. Nanopart. Res. 2021, 23, 124. [Google Scholar] [CrossRef]
- Canton, I.; Battaglia, G. Endocytosis at the nanoscale. Chem. Soc. Rev. 2012, 41, 2718–2739. [Google Scholar] [CrossRef]
- Zarei, H.; Kazemi Oskuee, R.; Hanafi-Bojd, M.Y.; Gholami, L.; Ansari, L.; Malaekeh-Nikouei, B. Enhanced gene delivery by polyethyleneimine coated mesoporous silica nanoparticles. Pharm. Dev. Technol. 2019, 24, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Kerr, M.C.; Teasdale, R.D. Defining macropinocytosis. Traffic 2009, 10, 364–371. [Google Scholar] [CrossRef]
- Gorry, M.; Yoneyama, T.; Vujanovic, L.; Moss, M.L.; Garlin, M.A.; Miller, M.A.; Vujanovic, N.L. Development of flow cytometry assays for measuring cell-membrane enzyme activity on individual cells. J. Cancer 2020, 11, 702–715. [Google Scholar] [CrossRef]
- Zucker, R.M.; Massaro, E.J.; Sanders, K.M.; Degn, L.L.; Boyes, W.K. Detection of TiO2 nanoparticles in cells by flow cytometry. Cytom. Part A 2010, 77, 677–685. [Google Scholar] [CrossRef]
- Jin, J.; Gu, Y.J.; Man, C.W.Y.; Cheng, J.; Xu, Z.; Zhang, Y.; Wong, W.T. Polymer-coated NaYF4: Yb3+, Er3+ upconversion nanoparticles for charge-dependent cellular imaging. ACS Nano 2011, 5, 7838–7847. [Google Scholar] [CrossRef] [PubMed]
- Douillard, S.; Lhommeau, I.; Olivier, D.; Patrice, T. In vitro evaluation of Radachlorin® sensitizer for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2010, 98, 128–137. [Google Scholar] [CrossRef] [PubMed]








| Sample | NPs+PEI 1.67 μL Rch | NPs+PEI 3.34 μL Rch | NPs+PEI 5.01 μL Rch | NPs+PEI 8.35 μL Rch | NPs+PEI 11.69 μL Rch | NPs+PEI 15.03 μL Rch | NPs+PEI 18.1 μL Rch |
|---|---|---|---|---|---|---|---|
| , nm | 7.1 | 5.9 | 5.8 | 5.4 | 5.3 | 5.2 | 5.1 |
| Concentration, g/L | Survival, % | Error Bar. % |
|---|---|---|
| 0 | 100 | 1.3 |
| 0.1 | 96.9 | 0.5 |
| 0.5 | 96.5 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nizamutdinov, A.; Lukinova, E.; Shamsutdinov, N.; Zelenikhin, P.; Khusainova, A.; Gafurov, M.; Zinchenko, S.; Safin, D.; Pudovkin, M. CeF3-YF3-TbF3 Nanoparticle-Polymer–“Radachlorin” Conjugates for Combined Photodynamic Therapy: Synthesis, Characterization, and Biological Activity. J. Compos. Sci. 2023, 7, 255. https://doi.org/10.3390/jcs7060255
Nizamutdinov A, Lukinova E, Shamsutdinov N, Zelenikhin P, Khusainova A, Gafurov M, Zinchenko S, Safin D, Pudovkin M. CeF3-YF3-TbF3 Nanoparticle-Polymer–“Radachlorin” Conjugates for Combined Photodynamic Therapy: Synthesis, Characterization, and Biological Activity. Journal of Composites Science. 2023; 7(6):255. https://doi.org/10.3390/jcs7060255
Chicago/Turabian StyleNizamutdinov, Alexey, Elena Lukinova, Nail Shamsutdinov, Pavel Zelenikhin, Alina Khusainova, Marat Gafurov, Sergey Zinchenko, Damir Safin, and Maksim Pudovkin. 2023. "CeF3-YF3-TbF3 Nanoparticle-Polymer–“Radachlorin” Conjugates for Combined Photodynamic Therapy: Synthesis, Characterization, and Biological Activity" Journal of Composites Science 7, no. 6: 255. https://doi.org/10.3390/jcs7060255
APA StyleNizamutdinov, A., Lukinova, E., Shamsutdinov, N., Zelenikhin, P., Khusainova, A., Gafurov, M., Zinchenko, S., Safin, D., & Pudovkin, M. (2023). CeF3-YF3-TbF3 Nanoparticle-Polymer–“Radachlorin” Conjugates for Combined Photodynamic Therapy: Synthesis, Characterization, and Biological Activity. Journal of Composites Science, 7(6), 255. https://doi.org/10.3390/jcs7060255









