To Shed Light on the UV Curable Coating Technology: Current State of the Art and Perspectives
Abstract
1. Introduction
2. Photo-Curing Polymerization Mechanism
2.1. Free Radical Polymerization
2.1.1. Unimolecular Photoinitiators (Type I)
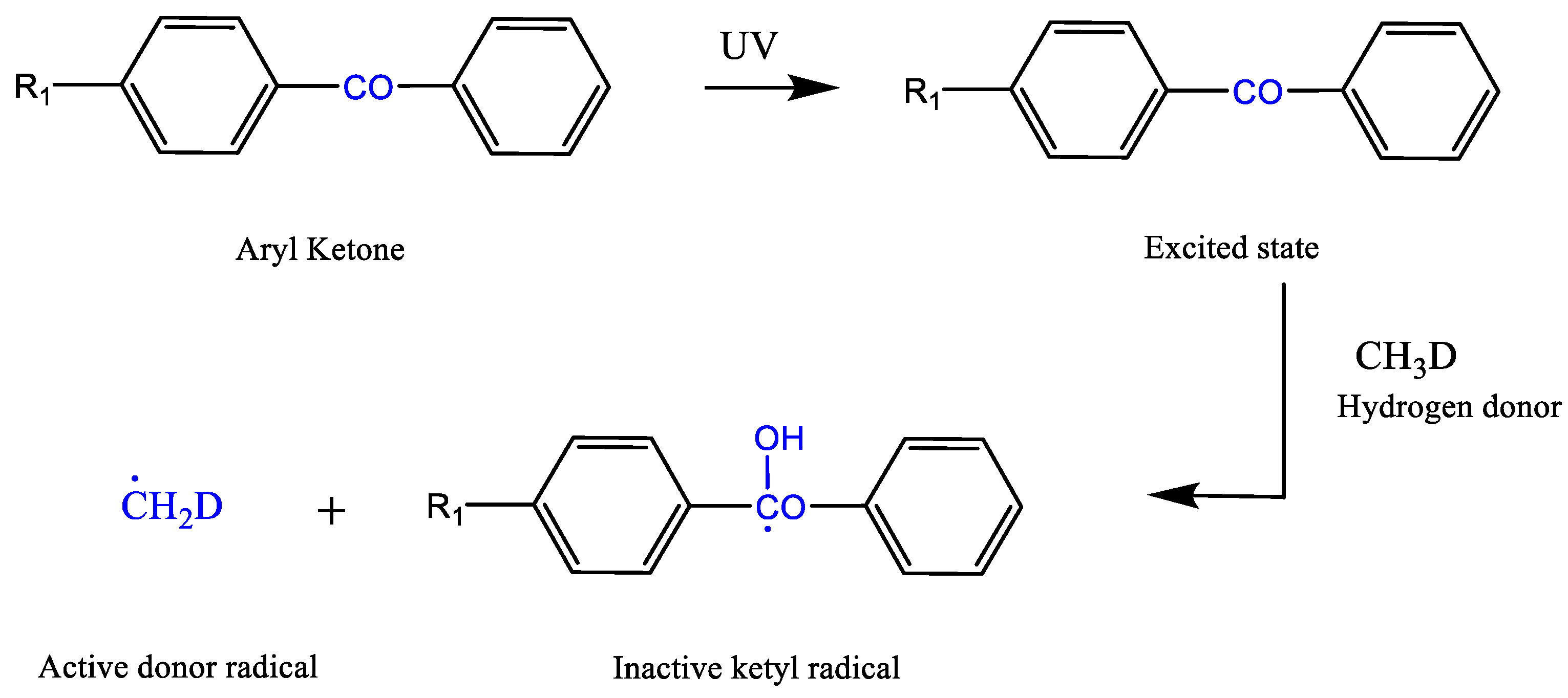
2.1.2. Bimolecular Photoinitiator (Type II)
2.1.3. Oxygen Inhibition
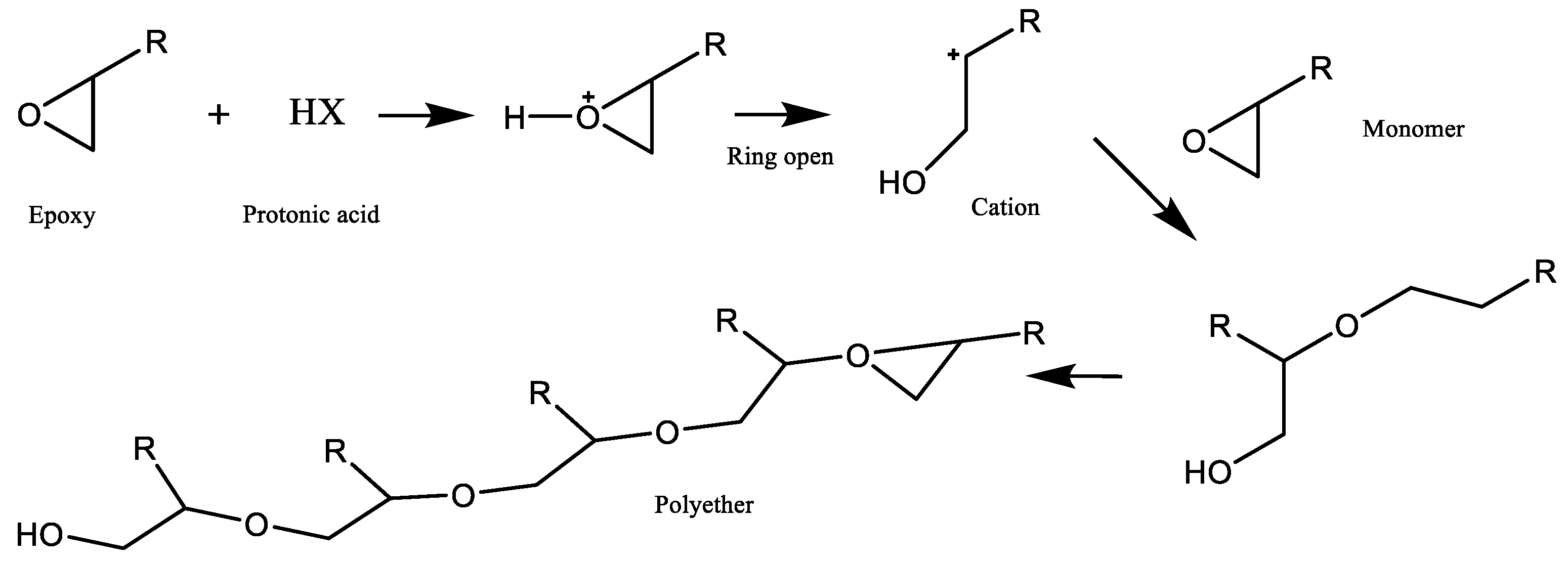
2.2. Cationic Polymerization
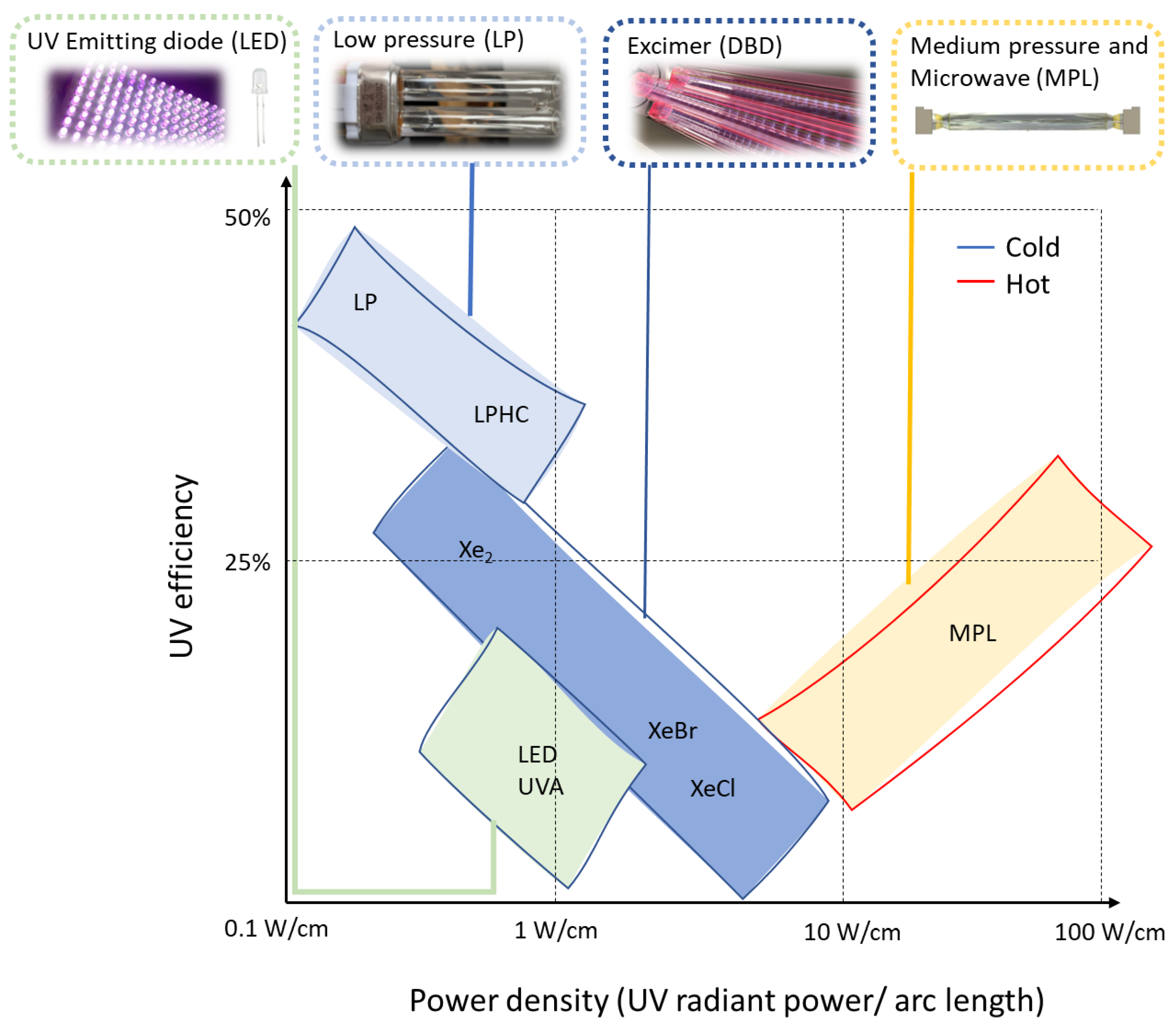
3. Lamps for UV-Curable Systems
4. UV Curing Chemistries
5. UV Coating Technology Types
6. Evolving Opportunities
7. The Aspect of Academia and Industry Research
8. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Javadi, A.; Mehr, H.S.; Sobani, M.; Soucek, M.D. Cure-on-command technology: A review of the current state of the art. Prog. Org. Coat. 2016, 100, 2–31. [Google Scholar] [CrossRef]
- Baburaj, S.; Valloli, L.K.; Parthiban, J.; Garg, D.; Sivaguru, J. Manipulating excited state reactivity and selectivity through hydrogen bonding-from solid state reactivity to Brønsted acid photocatalysis. Chem. Commun. 2022, 58, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Decker, C. Kinetic study and new applications of UV radiation curing. Macromol. Rapid Commun. 2002, 23, 1067–1093. [Google Scholar] [CrossRef]
- Fouassier, J.P. Radiation Curing in Polymer Science and Technology; Elsevier Ltd.: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Drobny, J.G. Radiation Technology for Polymers, 3rd ed.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: New York, NY, USA, 2021. [Google Scholar]
- UV Coatings Value Market Analysis, PCI-Paint Coatings Ind. 2023, pp. 1–6. Available online: https://www.researchandmarkets.com/reports/5892815/uv-curable-coatings-global-market (accessed on 19 November 2023).
- Global UV-Cured Coatings Market Size by Product (Wood, Plastics), by Application (Industrial Coatings, Electronic Coatings), by Geographic Scope and Forecast. Available online: https://www.globenewswire.com/news-release/2023/11/14/2779791/0/en/Global-UV-Curable-Coatings-Market-Size-to-Worth-USD-19-63-Billion-By-2032 (accessed on 19 November 2023).
- Thomas, J.; Patil, R. The Road to Sustainable Tire Materials: Current State-of-the-Art and Future Prospectives. Environ. Sci. Technol. 2023, 57, 2209–2216. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Patil, R. Enabling Green Manufacture of Polymer Products via Vegetable Oil Epoxides. Ind. Eng. Chem. Res. 2023, 62, 1725–1735. [Google Scholar] [CrossRef]
- Thomas, J.; Soucek, M.D. Cationic Copolymers of Norbornylized Seed Oils for Fiber-Reinforced Composite Applications. ACS Omega 2022, 7, 33949–33962. [Google Scholar] [CrossRef]
- Thomas, J.; Nwosu, J.; Soucek, M.D. Sustainable biobased composites from norbornylized linseed oil and biomass sorghum fillers. Compos. Commun. 2023, 42, 101695. [Google Scholar] [CrossRef]
- Ren, X.; Xu, T.; Thomas, J.; Soucek, M.D. Isoprene Soya Diels–Alder Adduct and Epoxidation for Photopolymerization. Macromol. Chem. Phys. 2021, 222, 2100054. [Google Scholar] [CrossRef]
- Thomas, J.; Patil, R.S.; John, J.; Patil, M. A Comprehensive Outlook of Scope within Exterior Automotive Plastic Substrates and Its Coatings. Coatings 2023, 13, 1569. [Google Scholar] [CrossRef]
- Hoge, S. Why LED is the Only Sustainable Choice for UV Curing. Phoseon Technol. 2022. Available online: https://phoseon.com/in-the-news/why-led-is-the-only-sustainable-choice-for-uv-curing (accessed on 19 November 2023).
- Su, Y.; Zhang, S.; Zhou, X.; Yang, Z.; Yuan, T. A novel multi-functional bio-based reactive diluent derived from cardanol for high bio-content UV-curable coatings application. Prog. Org. Coat. 2020, 148, 105880. [Google Scholar] [CrossRef]
- Scharnweber, D.; Makhlouf, A. Handbook of Nanoceramic and Nanocomposite Coatings and Materials; Elsevier B.H.: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Hoyle, C.E. Photocurable coatings. In Radiation Curing of Polymeric Materials; American Chemical Society: Washington, DC, USA, 1990; p. 16. [Google Scholar]
- Shukla, V.; Bajpai, M.; Singh, D.K.; Singh, M.; Shukla, R. Review of basic chemistry of UV-curing technology. Pigment Resin Technol. 2004, 33, 272–279. [Google Scholar] [CrossRef]
- Khudyakov, I.V. Fast photopolymerization of acrylate coatings: Achievements and problems. Prog. Org. Coat. 2018, 121, 151–159. [Google Scholar] [CrossRef]
- Kumar, P.; Hegde, K.; Brar, S.K.; Cledon, M.; Pour, A.K. Physico-chemical treatment for the degradation of cyanotoxins with emphasis on drinking water treatment—How far have we come? J. Environ. Chem. Eng. 2018, 6, 5369–5388. [Google Scholar] [CrossRef]
- Sangermano, M.; Razza, N.; Crivello, J.V. Cationic UV-curing: Technology and applications. Macromol. Mater. Eng. 2014, 299, 775–793. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Chen, Y.; Pi, J.; Liu, R.; Zhu, Y. Recent Advances and Challenges in Long Wavelength Sensitive Cationic Photoinitiating Systems. Polymers 2023, 15, 2524. [Google Scholar] [CrossRef]
- Mendes-Felipe, C.; Oliveira, J.; Etxebarria, I.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. State-of-the-Art and Future Challenges of UV Curable Polymer-Based Smart Materials for Printing Technologies. Adv. Mater. Technol. 2019, 4, 1–16. [Google Scholar] [CrossRef]
- Decker, C. The use of UV irradiation in polymerization. Polym. Int. 1998, 45, 133–141. [Google Scholar] [CrossRef]
- Lazare, S.; Granier, V. Ultraviolet Laser Photoablation of Polymers: A Review and Recent Results. Laser Chem. 1989, 10, 25–40. [Google Scholar] [CrossRef]
- Jones, F.N.; Nichols, M.E.; Pappas, S.P. Organic Coatings: Science and Technology, 4th ed.; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Davidson, R.S. Radiation Curing; Rapra Technology Limited: Shropshire, UK, 2001. [Google Scholar]
- Schwalm, R. UV Coatings: Basics, Recent Developments and New Applications; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Green, W.A. Industrial Photoinitiators—A Technical Guide; Taylor & Francis: Oxfordshire, UK, 2010. [Google Scholar]
- Lee, Z.H.; Yen, S.C.; Hammoud, F.; Hijazi, A.; Graff, B.; Lalevée, J.; Chen, Y.C. Naphthalene-Based Oxime Esters as Type I Photoinitiators for Free Radical Photopolymerization. Polymers 2022, 14, 5261. [Google Scholar] [CrossRef]
- Hageman, H.J. Photoinitiators for free radical polymerization. Prog. Org. Coat. 1985, 13, 123–150. [Google Scholar] [CrossRef]
- Kowalska, A.; Sokolowski, J.; Bociong, K. The photoinitiators used in resin based dental composite—A review and future perspectives. Polymers 2021, 13, 470. [Google Scholar] [CrossRef]
- Dietlin, C.; Trinh, T.T.; Schweizer, S.; Graff, B.; Morlet-Savary, F.; Noirot, P.A.; Lalevée, J. New phosphine oxides as high performance near-UV type I photoinitiators of radical polymerization. Molecules 2020, 25, 1671. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.Y.; Hu, Y.; Song, L.; Chen, X.L.; Zhang, P.; Ni, J.X. Thermal degradation and combustion of a novel UV curable coating containing phosphorus. Polym. Degrad. Stab. 2009, 94, 1176–1182. [Google Scholar] [CrossRef]
- Green, W.A. Boosting the cure of phosphine oxide photoinitiators. Radtech Conf. 2016. Available online: https://www.radtech.org/proceedings/2016/papers/technical-conference/Photoinitiator/ (accessed on 19 November 2023).
- Maurel, A.; Martinez, A.C.; Grugeon, S.; Panier, S.; Dupont, L.; Cortes, P.; Sherrard, C.G.; Small, I.; Sreenivasan, S.T.; MacDonald, E. Toward High Resolution 3D Printing of Shape-Conformable Batteries via Vat Photopolymerization: Review and Perspective. IEEE Access 2021, 9, 140654–140666. [Google Scholar] [CrossRef]
- Ciba IRGACURE 2959, Coating. 2001, pp. 2–4. Available online: http://www.xtgchem.cn/upload/20110629045632.PDF (accessed on 19 November 2023).
- Molina-gutiérrez, S.; Vacche, S.D.; Vitale, A.; Caillol, S.; Bongiovanni, R.; Lacroix-desmazes, P.; Molina-gutiérrez, S.; Vacche, S.D.; Vitale, A.; Ladmiral, V.; et al. Photoinduced Polymerization of Eugenol-Derived Methacrylates. Molecules 2020, 25, 3444. [Google Scholar] [CrossRef] [PubMed]
- Ciba DAROCUR 1173, Ciba. 2001, pp. 3–5. Available online: http://xtgchem.cn/upload/20110629045246.PDF (accessed on 19 November 2023).
- IGM Resins, Low Migration Product Guide, 2020. (n.d.). Available online: https://www.igmresins.com/Product_documentation/IGM_Low_Migration_Product_Guide_V2.pdf (accessed on 19 November 2023).
- Wu, Y.; Dai, C.; Ke, J.; Tang, R.; Huang, C.; Wang, J.; Situ, Y.; Huang, H. Synthesis and characterization of low-migration bisacylphenylphosphine oxide photoinitiators. Prog. Org. Coat. 2023, 176, 107396. [Google Scholar] [CrossRef]
- Lalevée, J.; El-Roz, M.; Allonas, X.; Fouassier, J.P. Surface modification of a UV curable acrylate coating: In situ introduction of hydrophobic properties. Prog. Org. Coat. 2009, 65, 457–461. [Google Scholar] [CrossRef]
- Huang, T.L.; Chen, Y.C. Synthesis and free radical photopolymerization of one-component type II photoinitiator based on benzophenone segment. J. Photochem. Photobiol. A Chem. 2022, 429, 113900. [Google Scholar] [CrossRef]
- Sanai, Y.; Ninomiya, T.; Arimitsu, K. Improvements in the physical properties of UV-curable coating by utilizing type II photoinitiator. Prog. Org. Coat. 2021, 151, 106038. [Google Scholar] [CrossRef]
- Balta, D.K.; Keskin, S.; Karasu, F.; Arsu, N. Quinoxaline derivatives as photoinitiators in UV-cured coatings. Prog. Org. Coat. 2007, 60, 207–210. [Google Scholar] [CrossRef]
- Metin, E.; Arsu, N.; Catak, S.; Aviyente, V. Photophysical, kinetic and thermodynamic study of one-component Type II thioxanthone acetic acid photoinitiators. Eur. Polym. J. 2020, 136, 109909. [Google Scholar] [CrossRef]
- Yen, S.C.; Ni, J.S.; Chen, Y.C. Triphenylamine-functionalized chalcones as one-component type II visible-light-absorbing photoinitiators for free radical photopolymerization. Eur. Polym. J. 2023, 187, 111885. [Google Scholar] [CrossRef]
- Balcerak, A.; Kabatc-Borcz, J.; Czech, Z.; Bartkowiak, M. Latest Advances in Highly Efficient Dye-Based Photoinitiating Systems for Radical Polymerization. Polymers 2023, 15, 1148. [Google Scholar] [CrossRef]
- Segurola, J.; Allen, N.S.; Edge, M.; McMahon, A.; Wilson, S. Photoyellowing and discolouration of UV cured acrylated clear coatings systems: Influence of photoinitiator type. Polym. Degrad. Stab. 1999, 64, 39–48. [Google Scholar] [CrossRef]
- Ligon, S.C.; Husár, B.; Wutzel, H.; Holman, R.; Liska, R. Strategies to reduce oxygen inhibition in photoinduced polymerization. Chem. Rev. 2014, 114, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Studer, K.; Decker, C.; Beck, E.; Schwalm, R. Overcoming oxygen inhibition in UV-curing of acrylate coatings by carbon dioxide inerting, Part I. Prog. Org. Coat. 2003, 48, 92–100. [Google Scholar] [CrossRef]
- Husár, B.; Ligon, S.C.; Wutzel, H.; Hoffmann, H.; Liska, R. The formulator’s guide to anti-oxygen inhibition additives. Prog. Org. Coat. 2014, 77, 1789–1798. [Google Scholar] [CrossRef]
- Hoyle, C.E. An Overview of Oxygen Inhibition in Photocuring. Radtech Conf. 2004. Available online: https://www.radtech.org/proceedings/2004/papers/104.pdf (accessed on 19 November 2023).
- Goodrich, J.E.; Nunez, J. Acrylated amine oligomers to enhance cure with uv led sources. PCI-Paint Coat. Ind. 2020, 36, 53–58. [Google Scholar]
- Thomas, J.; Bouscher, R.F.; Nwosu, J.; Soucek, M.D. Sustainable Thermosets and Composites Based on the Epoxides of Norbornylized Seed Oils and Biomass Fillers. ACS Sustain. Chem. Eng. 2022, 10, 12342–12354. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lam, J.H.W. Diaryliodonium Salts. A New Class of Photoinitiators for Cationic Polymerization. J. Polym. Sci. Polym. Symp. 1977, 10, 383–395. [Google Scholar] [CrossRef]
- Pierau, L.; Elian, C.; Akimoto, J.; Ito, Y.; Caillol, S.; Versace, D.L. Bio-sourced monomers and cationic photopolymerization–The green combination towards eco-friendly and non-toxic materials. Prog. Polym. Sci. 2022, 127, 101517. [Google Scholar] [CrossRef]
- Thomas, J.; Nwosu, J.; Soucek, M.D. Acid-Cured Norbornylized Seed Oil Epoxides for Sustainable, Recyclable, and Reprocessable Thermosets and Composite Application. ACS Appl. Polym. Mater. 2023, 5, 2230–2242. [Google Scholar] [CrossRef]
- Sari, E.; Mitterbauer, M.; Liska, R.; Yagci, Y. Visible light induced free radical promoted cationic polymerization using acylsilanes. Prog. Org. Coat. 2019, 132, 139–143. [Google Scholar] [CrossRef]
- Sangermano, M.; Roppolo, I.; Chiappone, A. New horizons in cationic photopolymerization. Polymers 2018, 10, 136. [Google Scholar] [CrossRef]
- Shi, S.; Croutxé-Barghorn, C.; Allonas, X. Photoinitiating systems for cationic photopolymerization: Ongoing push toward long wavelengths and low light intensities. Prog. Polym. Sci. 2017, 65, 1–41. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lam, J.H.W. Photoinitiated Cationic Polymerization of Epoxy Resins. Am. Chem. Soc. Div. Org. Coat. Plast. Chem. Prepr. 1978, 39, 31–35. [Google Scholar] [CrossRef]
- Martysz, D.; Antoszczyszyn, M.; Urbala, M.; Krompiec, S.; Fabrycy, E. Synthesis of 1-propenyl ethers and their using as modifiers of UV-cured coatings in radical and cationic polymerization. Prog. Org. Coat. 2003, 46, 302–311. [Google Scholar] [CrossRef]
- Işin, D.; Kayaman-Apohan, N.; Güngör, A. Preparation and characterization of UV-curable epoxy/silica nanocomposite coatings. Prog. Org. Coat. 2009, 65, 477–483. [Google Scholar] [CrossRef]
- Li, F.; Guo, X.; Wang, Y.; Jin, M. From soft hyperbranched polymers to hard crosslinkers: UV curable macromers that contained oxetane on and around hyperbranched frameworks. Eur. Polym. J. 2023, 192, 112074. [Google Scholar] [CrossRef]
- Thanamongkollit, N.; Miller, K.R.; Soucek, M.D. Synthesis of UV-curable tung oil and UV-curable tung oil based alkyd. Prog. Org. Coat. 2012, 73, 425–434. [Google Scholar] [CrossRef]
- Chittavanich, P.; Miller, K.; Soucek, M.D. A photo-curing study of a pigmented UV-curable alkyd. Prog. Org. Coat. 2012, 73, 392–400. [Google Scholar] [CrossRef]
- IGM Resins, Omnicat. 2021, pp. 1–3. Available online: https://www.igmresins.com/Product_documentation/IGM_Cationic_product_guide_V1.pdf (accessed on 19 November 2023).
- Elliott, D.J. Ultraviolet Laser Technology and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 1995. [Google Scholar] [CrossRef]
- Allen, N.S. Photoinitiators for UV and visible curing of coatings: Mechanisms and properties. J. Photochem. Photobiol. A Chem. 1996, 100, 101–107. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, J.; Long, L.; Gong, J.; Chen, M.; Liu, R. A novel self-wrinkled polyurethane-acrylate wood coating with self-matting, anti-fingerprint performance and skin-tactile feeling via excimer lamp/UV curing. RSC Adv. 2023, 13, 7300–7311. [Google Scholar] [CrossRef] [PubMed]
- Arceneaux, J.A.; Buono, C.; Poelmans, K. Cure Speed Improvements and Yellowing Effects in UV LED-Curable OPVs and Clear Coats Using Acrylated Amines. Uvebtechnology Com + Radtech.Org UV+EB Technol. 2018, 3, 69. [Google Scholar]
- Chen, Y.; Liu, R.; Luo, J. Enhancing weathering resistance of UV-curable coatings by using TiO2 particles as filler. Prog. Org. Coat. 2022, 169, 106936. [Google Scholar] [CrossRef]
- Patil, R.S.; Sancaktar, E. Fabrication of pH-Responsive Polyimide Polyacrylic Acid Smart Gating Membranes: Ultrafast Method Using 248 nm Krypton Fluoride Excimer Laser. ACS Appl. Mater. Interfaces 2021, 13, 24431–24441. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.S.; Sancaktar, E. Effect of solution parameters on pH-response of polyacrylic acid grafted polyimide smart membrane fabricated using 248 nm krypton fluoride excimer laser. Polymer 2021, 233, 124181. [Google Scholar] [CrossRef]
- Patil, R.S.; Narayanan, A.; Tantisuwanno, C.; Sancaktar, E. Immobilization of Glucose Oxidase on pH-Responsive Polyimide-Polyacrylic Acid Smart Membranes Fabricated Using 248 nm KrF Excimer Laser for Drug Delivery. Biointerface Res. Appl. Chem. 2023, 13, 1–10. [Google Scholar] [CrossRef]
- Patil, R.S. Fabrication of pH Responsive Membranes Using 248 Nanometer Krypton Fluoride Excimer Laser, University of Akron. 2021. Available online: http://rave.ohiolink.edu/etdc/view?acc_num=akron1626866059729935 (accessed on 19 November 2023).
- Scherzer, T.; Knolle, W.; Naumov, S.; Mehnert, R. Direct initiation of the photopolymerization of acrylates by short-wavelength excimer UV radiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2003, 208, 271–276. [Google Scholar] [CrossRef]
- Marson, J.; Mills, P. A Novel Approach to UV Curing for PVC and Wood Applications. Radtech Conf. 2008. Available online: https://www.radtech.org/proceedings/2008/papers/088.pdf (accessed on 19 November 2023).
- Kiyoi, E. The State of UV-LED Curing: An Investigation of Chemistry and Applications. Radtech Rep. 2013, 2, 32–36. [Google Scholar]
- Eta Plus, Medium Pressure UV Lamp. 2021. Available online: https://www.eta-uv.de/en/products/uv-lamps/overview-uv-lamps (accessed on 19 November 2023).
- Wu, J.; Ren, X.; Soucek, M.D. Synthesis and characterization of UV-curable maleimide-terminated imide oligomers. Prog. Org. Coat. 2016, 100, 129–140. [Google Scholar] [CrossRef]
- Wang, X.; Soucek, M.D. Investigation of non-isocyanate urethane dimethacrylate reactive diluents for UV-curable polyurethane coatings. Prog. Org. Coat. 2013, 76, 1057–1067. [Google Scholar] [CrossRef]
- Krongauz, V.V.; Tortorello, A.J. Reactive diluents and properties of ultraviolet-cured polycarbonate urethane acrylates. J. Appl. Polym. Sci. 1995, 57, 1627–1636. [Google Scholar] [CrossRef]
- Palanisamy, A.; Rao, B.S. Photo-DSC and dynamic mechanical studies on UV curable compositions containing diacrylate of ricinoleic acid amide derived from castor oil. Prog. Org. Coat. 2007, 60, 161–169. [Google Scholar] [CrossRef]
- Black, M.; Rawlins, J.W. Thiol-ene UV-curable coatings using vegetable oil macromonomers. Eur. Polym. J. 2009, 45, 1433–1441. [Google Scholar] [CrossRef]
- Resetco, C.; Hendriks, B.; Badi, N.; Prez, F.D. Thiol-ene chemistry for polymer coatings and surface modification-building in sustainability and performance. Mater. Horiz. 2017, 4, 1041–1053. [Google Scholar] [CrossRef]
- Thomas, J. A Methodological Outlook on Bioplastics from Renewable Resources. Open J. Polym. Chem. 2020, 10, 21–47. [Google Scholar] [CrossRef]
- Pezzana, L.; Mousa, M.; Malmström, E.; Johansson, M.; Sangermano, M. Bio-based monomers for UV-curable coatings: Allylation of ferulic acid and investigation of photocured thiol-ene network. Prog. Org. Coat. 2021, 150, 105986. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, Y.; Liu, X.; Fan, L.; Zhu, J. Bio-based tetrafunctional crosslink agent from gallic acid and its enhanced soybean oil-based UV-cured coatings with high performance. RSC Adv. 2014, 4, 23036–23042. [Google Scholar] [CrossRef]
- Li, P.; Chu, Z.; Chen, Y.; Yuan, T.; Yang, Z. One-pot and solvent-free synthesis of castor oil-based polyurethane acrylate oligomers for UV-curable coatings applications. Prog. Org. Coat. 2021, 159, 106398. [Google Scholar] [CrossRef]
- Pezzana, L.; Sangermano, M. Fully biobased UV-cured thiol-ene coatings. Prog. Org. Coat. 2021, 157, 106295. [Google Scholar] [CrossRef]
- Wang, Q.; Thomas, J.; Soucek, M.D. Investigation of UV-curable alkyd coating properties. J. Coat. Technol. Res. 2023, 20, 545–557. [Google Scholar] [CrossRef]
- Babahan-bircan, I.; Thomas, J.; Soucek, M.D. Environment-friendly UV-curable alkyd-based non-isocyanate urethanes. J. Coat. Technol. Res. 2022, 19, 1507–1522. [Google Scholar] [CrossRef]
- Thomas, J.; Singh, V.; Jain, R. Synthesis and characterization of solvent free acrylic copolymer for polyurethane coatings. Prog. Org. Coat. 2020, 145, 105677. [Google Scholar] [CrossRef]
- Thomas, J.; Patil, R.S.; Patil, M.; John, J. Addressing the Sustainability Conundrums and Challenges within the Polymer Value Chain. Sustainability 2023, 15, 15758. [Google Scholar] [CrossRef]
- Decker, C.; Masson, F.; Schwalm, R. Dual-curing of waterborne urethane-acrylate coatings by UV and thermal processing. Macromol. Mater. Eng. 2003, 288, 17–28. [Google Scholar] [CrossRef]
- Hwang, H.D.; Park, C.H.; Moon, J.I.; Kim, H.J.; Masubuchi, T. UV-curing behavior and physical properties of waterborne UV-curable polycarbonate-based polyurethane dispersion. Prog. Org. Coat. 2011, 72, 663–675. [Google Scholar] [CrossRef]
- Dsouza, R.F.; Parthiban, A. UV-curable polyurethane-acrylate hybrids made by a prepolymer-free process and free-standing polymer-metal oxide films made in a wholly water-based UV curing process. Polym. Chem. 2023, 14, 2670–2674. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, Q.; Li, L.; Wang, Q.; Guo, C. Construction of waterborne flame-retardant itaconate-based unsaturated polyesters and application for UV-curable hybrid coatings on wood. Prog. Org. Coat. 2023, 183, 107826. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, W.; Li, Z.; Feng, Y.; Qi, W.; Li, S.; Wang, X.; Chen, M. Enhancement of Wood Coating Properties by Adding Silica Sol to UV-Curable Waterborne Acrylics. Forests 2023, 14, 335. [Google Scholar] [CrossRef]
- Luo, H.; Wei, H.; Wang, Z.; Li, H.; Chen, Y.; Xiang, J.; Fan, H. Fabrication of UV-curable waterborne polyurethane coatings with self-cleaning, anti-graffiti performance, and corrosion resistance. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132177. [Google Scholar] [CrossRef]
- Iso, T.; Ninomiya, T.; Kagami, S.; Kubota, K.; Sanai, Y. Environmentally-friendly UV-curable coatings utilizing bio-based polyester acrylates. Prog. Org. Coat. 2023, 175, 107356. [Google Scholar] [CrossRef]
- Chen, J.; Ke, Q.; Li, S.; Wang, X.; Zeng, Z.; Liu, C.; Mo, R.; Karmaker, P.G.; Xie, Z.; Yong, Q. Robust UV-curable anti-smudge electrodeposition coating for self-cleaning, anti-graffiti and corrosion protection. Prog. Org. Coat. 2023, 179, 107526. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, L.; Xie, X.; Zhou, M.; Fu, C.; Chen, S. High-Hardness, Water-Stable, and UV-Resistant Conductive Coatings Based on Waterborne PEDOT:PSS/Epoxy/(KH560/SiO2) Composite. J. Compos. Sci. 2023, 7, 51. [Google Scholar] [CrossRef]
- Cui, Y.T.; Wei, B.X.; Wang, Y.J.; Guo, X.; Xiao, J.; Li, W.; Pang, A.; Bai, Y.P. Fabrication of UV/moisture dual curing coatings based on fluorinated polyoxetanes for anti-fouling applications. Prog. Org. Coat. 2022, 163, 106656. [Google Scholar] [CrossRef]
- Shen, Z.; Wu, Y.; Qiu, S.; Deng, H.; Hou, R.; Zhu, Y. UV-thermal dual-cured polymers with degradable and anti-bacterial function. Prog. Org. Coat. 2020, 148, 105783. [Google Scholar] [CrossRef]
- Yao, B.; Zhao, H.; Wang, L.; Liu, Y.; Zheng, C.; Li, H.; Sun, C. Synthesis of acrylate-based UV/thermal dual-cure coatings for antifogging. J. Coat. Technol. Res. 2018, 15, 149–158. [Google Scholar] [CrossRef]
- Okamura, A.M.H.; Ueda, Y.; Shirai, M. Dual UV curing system using a dimethacrylate containing a chalcone moiety. Radtech Conf. 2014, 4, 29–34. [Google Scholar]
- Bednarczyk, P.; Wróblewska, A.; Markowska-Szczupak, A.; Ossowicz-Rupniewska, P.; Nowak, M.; Kujbida, M.; Kamińska, A.; Czech, Z. UV curable coatings based on urethane acrylates containing eugenol and evaluation of their antimicrobial activity. Coat 2021, 11, 1556. [Google Scholar] [CrossRef]
- Lee, S.; Gavande, V.; Chun, J.H.; Cheon, J.M.; Jin, Y.; Lee, W.K. Synthesis and properties of UV-curable polyurethane acrylates with reactive silicones. Mol. Cryst. Liq. Cryst. 2020, 706, 86–93. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Preparation and Properties of Poly(urethane acrylate) Films for Ultraviolet-Curable Coatings. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Cheon, J.; Park, S.Y.; Jeong, B.Y.; Chun, J.H. Preparation and properties of UV-curable polyurethane-acrylate coatings of pre-coated metal (PCM): Effect of polyol type/contents on adhesive property. Mol. Cryst. Liq. Cryst. 2020, 706, 62–71. [Google Scholar] [CrossRef]
- Czachor-Jadacka, D.; Pilch-Pitera, B. Progress in development of UV curable powder coatings. Prog. Org. Coat. 2021, 158, 106355. [Google Scholar] [CrossRef]
- McGinniss, V.D. Ultraviolet Curable Powder Paints. United. States Patent 4163810, 7 August 1979. [Google Scholar]
- Czachor-Jadacka, D.; Pilch-Pitera, B.; Kisiel, M.; Thomas, J. Polyurethane powder coatings with low curing temperature: Research on the effect of chemical structure of crosslinking agent on the properties of coatings. Prog. Org. Coat. 2023, 182, 107662. [Google Scholar] [CrossRef]
- Zeren, S.; Huguenard, S. UV-curable powder coatings: Formulation of thick white finish for MDF. Surf. Coat. Int. Part B Coat. Trans. 2004, 87, 97–101. [Google Scholar] [CrossRef]
- Misev, L.; Schmid, O.; Udding-Louwrier, S.; De Jong, E.S.; Bayards, R. Weather stabilization and pigmentation of UV-curable powder coatings. J. Coat. Technol. 1999, 71, 37–44. [Google Scholar] [CrossRef]
- Chemtob, A.; Versace, D.L.; Belon, C.; Croutxé-Barghorn, C.; Rigolet, S. Concomitant organic-inorganic UV-curing catalyzed by photoacids. Macromolecules 2008, 41, 7390–7398. [Google Scholar] [CrossRef]
- Asemani, H.R.; Luo, L.; Mannari, V. Corrosion-resistant organic-inorganic hybrid pretreatments obtained by UV-initiated process suitable for primer-less coating systems. Prog. Org. Coat. 2020, 147, 105878. [Google Scholar] [CrossRef]
- İnan, T.; Seden, M.G.; Baştürk, E.; Al-Shafei, M. UV-curable sol–gel based protective hybrid coatings. Polym. Eng. Sci. 2019, 59, E146–E154. [Google Scholar] [CrossRef]
- Senani, S.; Campazzi, E.; Villatte, M.; Druez, C. Potentiality of UV-cured hybrid sol-gel coatings for aeronautical metallic substrate protection. Surf. Coat. Technol. 2013, 227, 32–37. [Google Scholar] [CrossRef]
- Balbay, S.; Acıkgoz, C. Anti-Yellowing UV-Curable hybrid coatings prepared by the Sol–Gel method on polystyrene. Prog. Org. Coat. 2020, 140, 105499. [Google Scholar] [CrossRef]
- Manchanda, H.; Mannari, V. Super photo-base initiated organic-inorganic hybrid coatings by plural-cure mechanisms. Prog. Org. Coat. 2019, 127, 222–230. [Google Scholar] [CrossRef]
- Thomas, J.; Moosavian, S.K.; Cutright, T.; Pugh, C.; Soucek, M.D. Method Development for Separation and Analysis of Tire and Road Wear Particles from Roadside Soil Samples. Environ. Sci. Technol. 2022, 56, 11910–11921. [Google Scholar] [CrossRef] [PubMed]
- Yaneff, P.V.; Adamsons, K.; Cliff, N.; Kanouni, M. Migration of reactable UVAs and HALS in automotive plastic coatings. J. Coat. Technol. Res. 2004, 1, 201–212. [Google Scholar] [CrossRef]
- Thomas, J.; Moosavian, S.K.; Cutright, T.; Pugh, C.; Soucek, M.D. Investigation of abiotic degradation of tire cryogrinds. Polym. Degrad. Stab. 2022, 195, 109814. [Google Scholar] [CrossRef]
- Thomas, J.; Cutright, T.; Pugh, C.; Soucek, M.D. Quantitative assessment of additive leachates in abiotic weathered tire cryogrinds and its application to tire wear particles in roadside soil samples. Chemosphere 2023, 311, 137132. [Google Scholar] [CrossRef]
- Dreyer, C.; Motoc, D.L.; Koehler, M.; Goldenberg, L. UV-LED curable perfluoropolyether (PFPE)-urethane methacrylate transparent coatings for photonic applications: Synthesis and characterization. Polymers 2023, 15, 2983. [Google Scholar] [CrossRef]
- Ghazali, S.K.; Adrus, N.; Majid, R.A.; Ali, F.; Jamaluddin, J. UV-LED as a new emerging tool for curable polyurethane acrylate hydrophobic coating. Polymers 2021, 13, 487. [Google Scholar] [CrossRef]
- Karasu, F.; Rocco, C.; Zhang, Y.; Croutxé-Barghorn, C.; Allonas, X.; Van Der Ven, L.G.J.; Van Benthem, R.A.T.M.; Esteves, A.C.C.; De With, G. LED-cured self-replenishing hydrophobic coatings based on interpenetrating polymer networks (IPNs). RSC Adv. 2016, 6, 33971–33982. [Google Scholar] [CrossRef]
- Condini, A.; Morozov, V.; Trentalange, C.; Rossi, S. Modeling LEDs radiation patterns for curing UV coatings inside of pipes. Opt. Mater. 2023, 144, 114275. [Google Scholar] [CrossRef]
- Schmitt, M. Method to analyse energy and intensity dependent photo-curing of acrylic esters in bulk. RSC Adv. 2015, 5, 67284–67298. [Google Scholar] [CrossRef][Green Version]
- Liu, S.; Borjigin, T.; Schmitt, M.; Morlet-Savary, F.; Xiao, P.; Lalevée, J. High-Performance Photoinitiating Systems for LED-Induced Photopolymerization. Polymers 2023, 15, 342. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hao, Y.; Zhong, M.; Tang, L.; Nie, J.; Zhu, X. Synthesis of furan derivative as LED light photoinitiator: One-pot, low usage, photobleaching for light color 3D printing. Dye. Pigment. 2019, 165, 467–473. [Google Scholar] [CrossRef]
- Calvez, I.; Szczepanski, C.R.; Landry, V. Hybrid Free-Radical/Cationic Phase-Separated UV-Curable System: Impact of Photoinitiator Content and Monomer Fraction on Surface Morphologies and Gloss Appearance. Macromolecules 2022, 55, 3129–3139. [Google Scholar] [CrossRef] [PubMed]
- Tataru, G.; Coqueret, X. Hybrid free-radical and cationic photo-polymerization of bio-based monomers derived from seed oils-control of competitive processes by experimental design. Polym. Chem. 2020, 11, 5067–5077. [Google Scholar] [CrossRef]
- Wei, H.; Li, Q.; Ojelade, M.; Madbouly, S.; Otaigbe, J.U.; Hoyle, C.E. Thiol-ene free-radical and vinyl ether cationic hybrid photopolymerization. Macromolecules 2007, 40, 8788–8793. [Google Scholar] [CrossRef]
- Oxman, J.D.; Jacobs, D.W.; Trom, M.C.; Sipani, V.; Ficek, B.; Scranton, A.B. Evaluation of initiator systems for controlled and sequentially curable free-radical/cationic hybrid photopolymerizations. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 1747–1756. [Google Scholar] [CrossRef]
- Hasa, E.; Scholte, J.P.; Jessop, J.L.P.; Stansbury, J.W.; Guymon, C.A. Kinetically Controlled Photoinduced Phase Separation for Hybrid Radical/Cationic Systems. Macromolecules 2019, 52, 2975–2986. [Google Scholar] [CrossRef]
- Asemani, H.R.; Mannari, V. Ambient temperature and UV-cured hybrid coatings from acetoacetylated non-isocyanate polyurethanes. J. Coat. Technol. Res. 2021, 18, 469–488. [Google Scholar] [CrossRef]
- Hermann, A.; Giljean, S.; Pac, M.J.; Marsiquet, C.; Burr, D.; Landry, V. Physico-mechanical characterisation of basecoats for tailored UV-cured multilayered wood coating systems. Prog. Org. Coat. 2023, 182, 107673. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, H.; Tang, L. Facile fabrication of high performance hydrophilic anti-icing polyurethane methacrylate coatings cured via UV irradiation. Prog. Org. Coat. 2023, 182, 107657. [Google Scholar] [CrossRef]
- Li, X.; Bian, F.; Li, S.; Gui, X.; Yao, M.; Hu, J.; Lin, S. Preparation of siloxymethyl-modified silicone acrylate prepolymers with UV/moisture dual curability for applications in anti-smudge and anti-fingerprint coatings. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130669. [Google Scholar] [CrossRef]
- Shu, P.; Ai, L.; Kong, Y.; Ji, H.; Lu, Y.; Zhang, J.; Song, W. UV-cured organic–inorganic composites for highly durable and flexible antireflection coatings. Appl. Surf. Sci. 2022, 584, 152600. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, H.; Im, H.G.; Jo, W.; Choi, G.M.; Kim, T.S.; Jang, J.; Bae, B.S. Transparent and flexible hybrid cover window film: Hard coating/substrate all-in-one composite film for reliable foldable display. Compos. Part B Eng. 2022, 247, 110336. [Google Scholar] [CrossRef]
- Wu, L.; Kang, Y.; Deng, Y.; Yang, F.; He, R.; Yu, X.F. Long-Term Antifogging Coating Based on Black Phosphorus Hybrid Super-Hydrophilic Polymer Hetero-Network. Nanomaterials 2023, 13, 86. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, C.; Peng, J.; Chen, B.; Pan, X.; Xiong, W.; Zhang, X.; Xu, Z.; Luo, X.; Liu, Y. A substrate-independent transparent UV-curable coating with excellent anti-smudge performance. Prog. Org. Coat. 2022, 173, 107185. [Google Scholar] [CrossRef]
- Kraśkiewicz, A.; Kowalczyk, A.; Kowalczyk, K.; Schmidt, B. Novel solvent-free UV-photocurable varnish coatings based on acrylic telomers—Synthesis and properties. Prog. Org. Coat. 2023, 175, 107365. [Google Scholar] [CrossRef]
- Cui, Y.T.; Liu, H.H.; Wei, B.X.; Deng, C.; Bai, Y.P. Fabrication of UV-curable Anti-fouling coating based on fluorinated polyoxetane and long Side-Chain Polysilcone. Eur. Polym. J. 2022, 172, 111227. [Google Scholar] [CrossRef]
- Cataldi, A.; Corcione, C.E.; Frigione, M.; Pegoretti, A. Photocurable resin/nanocellulose composite coatings for wood protection. Prog. Org. Coat. 2017, 106, 128–136. [Google Scholar] [CrossRef]
- Barletta, M.; Vesco, S.; Puopolo, M.; Tagliaferri, V. High performance composite coatings on plastics: UV-curable cycloaliphatic epoxy resins reinforced by graphene or graphene derivatives. Surf. Coat. Technol. 2015, 272, 322–336. [Google Scholar] [CrossRef]
- Long, T.R.; Ilenda, C.S. UV Cure Top Coatings for Medical Devices. United. States Patent US 2021/0115350A1, 22 April 2021. [Google Scholar]
- Huang, C.Y.; Yu, Z.; Zhao, Y.; Pokorny, R.J.; Chen, X.-H. UV-Cure Coating Composition and Flexible Hard Coating Formed by the Composition. WO 2022/208399A1, 6 October 2022. [Google Scholar]
- Owenj, S.; Owen, T. UV Curable Coating and Application Method International. AU 2021102994A4, 22 July 2021. [Google Scholar]
- Habibpour, M.; Shouldice, G. Ultra-Fast Curing Scratch-Resistant Headlight Restoration Coating. US 2021/0129801A1, 6 May 2021. [Google Scholar]
- Sandqvist, L.; Wede, C.; Drougge, R.; Sandstrom, P. Method of Curing a Waterborne Coating. WO2022/235604A1, 10 November 2022. [Google Scholar]
- Becker, A.; Starzmann, O.; Stengle, M.; Feil, F. Method for the UV Curing of Lacquers Contaning Non-Aqueous poly(meth)acrylates without a Photoinitiator. WO 2022043181A1, 3 March 2022. [Google Scholar]
- Becker, A.; Starzmann, O.; Stengle, M.; Feil, F. Method for UV Curing of Water Based Polyurethane Paint Dispersions without UV-C Activatible Surface Initiators. WO 2022043180A1, 3 March 2022. [Google Scholar]







| Ingredient | Amount (%) |
|---|---|
| Oligomer | 0–90 |
| Monomer/diluent | 0–80 |
| Photoinitiators | 0.25–5 |
| Additives like Stabilizers, surfactant, adhesion promoter etc. | 0–5 |
| Fillers and pigment | 0–45 |
| Chemical Name | Structure | Activation Wavelength (nm) | Physical Form | Ref. |
|---|---|---|---|---|
| 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (Omnirad 2959) | 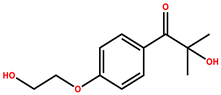 | 276 | White powder | [36,37] |
| 2-Hydroxy-2-methylpropiophenone (Omnirad 1173) | 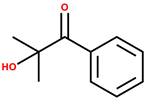 | 245 | Colorless to slightly yellow | [36,38,39] |
| 2-methyl-1-[4-(methylthio)phenyl]-2-morpholinopropan-1-one (Omnirad 907) | 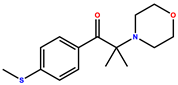 | 230, 303 | Off-white powder | [40] |
| diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide (TPO) | 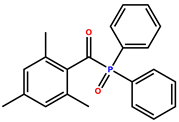 | 275, 379 | Pale yellow crystal powder | [32,36,40] |
| Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate (TPO-L) | 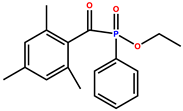 | 299, 366, 380, 393 | Yellow liquid | [36,40,41] |
| Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide (Omnirad 819) | 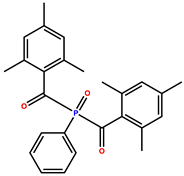 | 295, 370 | Light yellow powder | [38,40] |
| Chemical Name | Structure | Physical Form | Activation Wavelength (nm) | Ref. |
|---|---|---|---|---|
| Benzopehenone (BP) | 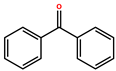 | White flakes | 250 | [40,48,49] |
| 4-Phenylbenzophenone (PBZ) |  | White powder | 248 | |
| Methyl 2-Benzoylbenzoate (OMBB) | 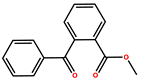 | liquid | 260 | |
| Isopropylthioxanthone (ITX) | 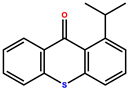 | Pale yellow powder | 244, 330 | |
| 1-[4-(4-Benzoylphenylsulfanyl)phenyl]-2-methyl-2-[(4-methylphenyl)sulfonyl]propan-1-one (Esacure 1001 M) |  | Off-white to pale pink | 315 |
| Chemical Name | Structure | Activation Wavelength (nm) | Ref. |
|---|---|---|---|
| triarylsulfonium hexafluorophosphate | 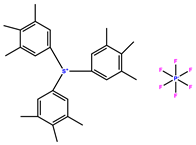 | 350 | [68] |
| Iodonium, (4-methylphenyl)[4-(2-methylpropyl) phenyl-, hexafluorophosphate |  | 240 | [68] |
| diphenyliodonium borontetrafluoride |  | 227 | [22] |
| 6-bromobenzo[de]isochromene-1,3-dione Diphenyliodonium Hexafluorophosphate |  | 340, 360 | |
| pyrenylmetyl triphenylphosphonium hexafluoroantimonate | 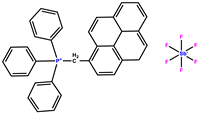 | 280, 350 | |
| benzoyl triphenylphosphonium Hexafluoroantimonate | 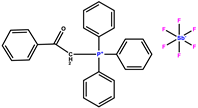 | 257 | |
| methoxy pyridinium hexafluorophosphate |  | 266 | |
| N,N′-diethoxy-4,4′-azobis(pyridinium) hexafluorophosphate |  | 289, 459 | |
| diphenyl [4-(phenylthio)phenyl]sulfonium Hexafluoroantimonate salts |  | 245, 312 | [68] |
| Oligomers/Chemistry | Advantages |
|---|---|
| Polyacrylates and acrylates | Good exterior durability, good chemical resistance |
| Epoxy acrylate | Good adhesion, good chemical resistance, good flexibility, high gloss, and good chemical resistance |
| Urethane acrylates | Good for flexibility, abrasion resistance, good weatherability |
| Polyether acrylates | Excellent film |
| Silicone acrylates | Excellent resistance to heat, moisture, shear forces, and radiation degradation |
| Amine modified acrylate | Increased reactivity |
| Polyester acrylates | Hard, tough, and solvent resistant film |
| Thiol-ene systems | No oxygen inhibition, delayed gel points, uniform networks, low polymerization shrinkage, and reduced stress |
| UV System and Lamp | Substrate and Application | Ref. |
|---|---|---|
| Urethane and epoxy acrylate coating cured by a mercury lamp | Multilayer protective wood coating | [142] |
| Polyurethane acrylate cured using an excimer lamp and a UV mercury lamp | Anti-fingerprint coating for wood application | [71] |
| Polyurethane methacrylate coating cured using LED | Anti-icing coating for tin | [143] |
| Water-based polyurethane acrylate coating cured using LED | Corrosion resistance for metal; can also be applied on glass | [99] |
| Siloxymethyl-modified silicone acrylate cured using a mercury lamp and a moisture cure | Anti-smudge coating and ant fingerprint coating for PET film | [144] |
| Silsesquioxanes and an acidic silica sol composite coating film cured by a mercury lamp | Antireflection coating for a PET substrate | [145] |
| Epoxy-functionalized siloxane hybrid coating matrix cured using LED | Glass fabric-reinforced siloxane hybrid composite | [146] |
| Polyurethane acrylate coating | Antifogging coating for plastic substrates like PET, PC, PMMA, PBS | [147] |
| Polyurethan coating cured using a mercury lamp | Anti-smudge coating for glass, wood, tin thermoplastic urethanes | [148] |
| Acrylic–styrene coating cured using a mercury lamp | Varnish coating for glass | [149] |
| Fluorinated polyoxetane and polysiloxane coating cured using LED | Antifouling coating for glass and PET | [150] |
| UV System and Lamp | Substrate and Application | Ref. |
|---|---|---|
| Polyacrylate cured using an LED lamp | Medical implants | [153] |
| Carbamate acrylate cured with a mercury lamp | Coating for computer and mobile devices | [154] |
| Epoxy acrylate coating cured with an LED lamp | Concrete floor | [155] |
| Polyester and polyurethane acrylate cured with an LED lamp | Scratch-resistant coating for automotive headlight | [156] |
| Waterborne coating cured with a mercury lamp | Wood, metal, paper, ceramic, leather, fabric substrate | [157] |
| Polymethacrylate coating cured using a mercury lamp | Paint application on paper | [158] |
| Waterborne polyurethane paint cured using a mercury lamp | Paint application for wood, paper, metal, and plastic | [159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, R.S.; Thomas, J.; Patil, M.; John, J. To Shed Light on the UV Curable Coating Technology: Current State of the Art and Perspectives. J. Compos. Sci. 2023, 7, 513. https://doi.org/10.3390/jcs7120513
Patil RS, Thomas J, Patil M, John J. To Shed Light on the UV Curable Coating Technology: Current State of the Art and Perspectives. Journal of Composites Science. 2023; 7(12):513. https://doi.org/10.3390/jcs7120513
Chicago/Turabian StylePatil, Renuka Subhash, Jomin Thomas, Mahesh Patil, and Jacob John. 2023. "To Shed Light on the UV Curable Coating Technology: Current State of the Art and Perspectives" Journal of Composites Science 7, no. 12: 513. https://doi.org/10.3390/jcs7120513
APA StylePatil, R. S., Thomas, J., Patil, M., & John, J. (2023). To Shed Light on the UV Curable Coating Technology: Current State of the Art and Perspectives. Journal of Composites Science, 7(12), 513. https://doi.org/10.3390/jcs7120513










