Electrochemical Oxidation of Phenol Released from Spent Coordination Polymer Impregnated with Ionic Liquid
Abstract
:1. Introduction
2. Experimental
2.1. Reagents
2.2. Preparation of Adsorbent—Spent Adsorbent
2.3. Desorption of Phenol from Spent Adsorbent
2.4. Electrochemical Characterization by Cyclic Voltammetry
2.5. Electrochemical Impedance Spectroscopy
2.6. Degradation of Phenol
2.7. FTIR Spectroscopy of Spent Adsorbent
2.8. Efficiency of Electrooxidation
2.9. Reuse of Treated Adsorbents
3. Results and Discussion
3.1. Desorption Kinetics
3.2. Electrochemical Oxidation of Phenol
3.3. UV-Vis Spectra
3.4. Electrochemical Impedance Spectroscopy (EIS)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, C.; Qian, Y.; Zhang, L.; Feng, J. Solvent extraction process development and on-site trial-plant for phenol removal from industrial coal-gasification wastewater. Chem. Eng. J. 2006, 117, 179–185. [Google Scholar] [CrossRef]
- Asghar, A.; Raman, A.A.A.; Daud, W.M. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]
- Dong, H.; Su, H.; Chen, Z.; Yu, H.; Yu, H. Fabrication of electrochemically reduced graphene oxide modified gas diffusion electrode for in-situ electrochemical advanced oxidation process under mild conditions. Electrochim. Acta 2016, 222, 1501–1509. [Google Scholar] [CrossRef]
- Mengoli, G.; Daolio, S.; Musiani, M.M. The influence of amines on the anodic coupling of phenols to polyoxyphenylene films. J. Appl. Electrochem. 1980, 10, 459–471. [Google Scholar] [CrossRef]
- Quiroz, M.A.; Sánchez-Salas, J.L.; Reyna, S.; Bandala, E.R.; Peralta-Hernández, J.M.; Martínez-Huitle, C.A. Degradation of 1-hydroxy-2,4-dinitrobenzene from aqueous solutions by electrochemical oxidation: Role of anodic material. J. Hazard. Mater. 2014, 268, 6–13. [Google Scholar] [CrossRef]
- Barisci, S.; Turkay, O.; Ozturk, H.; Sekar, M.G. Anodic oxidation of phenol by mixed-metal oxide electrodes: Identification of transformation by-products and toxicity assessment. J. Electrochem. Soc. 2017, 164, E129. [Google Scholar] [CrossRef]
- Xing, X.; Ni, J.; Zhu, X.; Jiang, Y.; Xia, J. Maximization of current efficiency for organic pollutants oxidation at BDD, Ti/SnO2-Sb/PbO2, and Ti/SnO2-Sb anodes. Chemosphere 2018, 205, 361–368. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, L.; Liu, J.; Logan, B.E. Electrochemical technologies for wastewater treatment and resource reclamation. Environ. Sci. Water Res. Technol. 2016, 2, 800–831. [Google Scholar] [CrossRef]
- Tasic, Z.; Gupta, V.K.; Antonijevic, M.M. The mechanism and kinetics of degradation of phenolics in wastewaters using electrochemical oxidation. Int. J. Electrochem. Sci. 2014, 9, 3473–3490. [Google Scholar] [CrossRef]
- Liu, X.; You, S.; Ma, F.; Zhou, H. Characterization of electrode fouling during electrochemical oxidation of phenolic pollutant. Front. Environ. Sci. Eng. 2021, 15, 53. [Google Scholar] [CrossRef]
- Weiss, E.; Groenen-Serrano, K.; Savall, A. A comparison of electrochemical degradation of phenol on boron doped diamond and lead dioxide anodes. J. Appl. Electrochem. 2008, 38, 329–337. [Google Scholar] [CrossRef]
- Đuričić, T.; Prosen, H.; Kravos, A.; Mićin, S.; Kalčíková, G.; Malinović, B.N. Electrooxidation of phenol on boron-doped diamond and mixed-metal oxide anodes: Process evaluation, transformation by-products, and ecotoxicity. J. Electrochem. Soc. 2023, 170, 023503. [Google Scholar] [CrossRef]
- Feng, Y.J.; Li, X.Y. Electro-catalytic oxidation of phenol on several metal-oxide electrodes in aqueous solution. Water Res. 2003, 37, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Iniesta, J.; González-García, J.; Expósito, E.; Montiel, V.; Aldaz, A. Influence of chloride ion on electrochemical degradation of phenol in alkaline medium using bismuth doped and pure PbO2 anodes. Water Res. 2001, 35, 3291–3300. [Google Scholar] [CrossRef] [PubMed]
- Zhi, J.-F.; Wang, H.-B.; Nakashima, T.; Rao, T.N.; Fujishima, A. Electrochemical incineration of organic pollutants on boron-doped diamond electrode. Evidence for direct electrochemical oxidation pathway. J. Phys. Chem. B 2003, 107, 13389–13395. [Google Scholar] [CrossRef]
- Chen, H.; Chen, S.; Quan, X.; Yu, H.; Zhao, H.; Zhang, Y. Fabrication of TiO2−Pt coaxial nanotube array schottky structures for enhanced photocatalytic degradation of phenol in aqueous solution. J. Phys. Chem. C 2008, 112, 9285–9290. [Google Scholar] [CrossRef]
- Kawde, A.N.; Morsy, M.A.; Odewunmi, N.; Mahfou, W. From electrode surface fouling to sensitive electroanalytical determination of phenols. Electroanalysis 2013, 25, 1547–1555. [Google Scholar] [CrossRef]
- Parisheva, Z.; Demirev, A. Ozonation of aqueous solutions of resorcinol and catechol. Environ. Prot. Eng. 2001, 2, 17–25. [Google Scholar]
- Martínez-Huitle, C.A.; Rodrigo, M.A.; Sirés, I.; Scialdone, O. A critical review on latest innovations and future challenges of electrochemical technology for the abatement of organics in water. Appl. Catal. B Environ. 2023, 328, 122430. [Google Scholar] [CrossRef]
- Urbansky, E.T.; Schock, M. Issues in managing the risks associated with perchlorate in drinking water. J. Environ. Manag. 1999, 56, 79–95. [Google Scholar] [CrossRef]
- Zhao, J.; Dang, Z.; Muddassir, M.; Raza, S.; Zhong, A.; Wang, X.; Jin, J. A New Cd(II)-Based Coordination Polymer for Efficient Photocatalytic Removal of Organic Dyes. Molecules 2023, 28, 6848. [Google Scholar] [CrossRef]
- Li, L.; Zou, J.; Han, Y.; Liao, Z.; Lu, P.; Nezamzadeh-Ejhieh, A.; Peng, Y. Recent advances in Al(iii)/In(iii)-based MOFs for the detection of pollutants. New J. Chem. 2022, 46, 19577–19592. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal organic frameworks as efficient adsorbents for drugs from wastewater. Mater. Today Commun. 2022, 31, 103514. [Google Scholar] [CrossRef]
- Balasubramanian, A.; Venkatesan, S. Removal of phenolic compounds from aqueous solutions using Aliquat 336 as a carrier in emulsion liquid membrane. Korean J. Chem. Eng. 2012, 29, 1622–1627. [Google Scholar] [CrossRef]
- Bergmann, M.; Koparal, A.; Iourtchouk, T. Electrochemical advanced oxidation processes, formation of halogenate and perhalogenate species: A critical review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 348–390. [Google Scholar] [CrossRef]
- Maranescu, B.; Lupa, L.; Mihali, M.T.L.; Plesu, N.; Maranescu, V.; Visa, A. The corrosion inhibitor behavior of iron in saline solution by the action of magnesium carboxyphosphonate. Pure Appl. Chem. 2018, 90, 1713–1722. [Google Scholar] [CrossRef]
- Simulescu, V.; Crasmareanu, E.; Ilia, G. Synthesis, Properties and Structures of Phosphorus-Nitrogen Heterocycles. Heterocycles 2011, 83, 275–291. [Google Scholar] [CrossRef]
- Maranescu, B.; Visa, A.; Ilia, G.; Simon, Z.; Demadis, K.; Colodrero, R.M.P.; Cabeza, A.; Vallcorba, O.; Rius, J.; Choquesillo-Lazarte, D. Synthesis and characterization of styryl phosphonic acid and its use as new ligand for phosphonate metal organic framework. J. Coord. Chem. 2014, 67, 1562–1572. [Google Scholar] [CrossRef]
- Vélez, G.Q.; Carmona-Sarabia, L.; Rodríguez-Silva, W.A.; Rivera Raíces, A.A.; Feliciano Cruz, L.; Hu, T.; Peterson, E.; López-Mejías, V. Potentiating bisphosphonate-based coordination complexes to treat osteolytic metastases. J. Mater. Chem. B 2020, 8, 2155–2168. [Google Scholar] [CrossRef]
- Visa, A.; Mracec, M.; Maranescu, B.; Ilia, G.; Popa, A.; Mracec, M. Structure simulation into a lamellar supramolecular network and calculation of the metal ions/ligands ratio. Chem. Cent. J. 2012, 6, 91. [Google Scholar] [CrossRef]
- Slepokura, K.; Piatkowska, A.; Lis, T. Isomorphous phosphonoacetic acid salts with magnesium (II), manganese(II), cobalt(II), zinc(II) and copper(II). Z. Krist.-Cryst. Mater. 2002, 217, 614–621. [Google Scholar] [CrossRef]
- Iliescu, S.; Ilia, G.; Pascariu, A.; Popa, A.; Plesu, N. Novel synthesis of phosphorus containing polymers under inverse phase transfer catalysis. Polymer 2006, 47, 6509–6512. [Google Scholar] [CrossRef]
- Iliescu, S.; Pascariu, A.; Plesu, N.; Popa, A.; Macarie, L.; Ilia, G. Unconventional method used in synthesis of polyphosphoesters. Polym. Bull. 2009, 63, 485–495. [Google Scholar] [CrossRef]
- Rocha, J.H.B.; Gomes, M.M.S.; Fernandes, N.S.; Da Silva, D.R.; Martínez-Huitle, C.A. Application of electrochemical oxidation as alternative treatment of produced water generated by Brazilian petrochemical industry. Fuel Process. Technol. 2012, 96, 80–87. [Google Scholar] [CrossRef]
- Krawczyk, P.; Skowroński, J.M. Multiple anodic regeneration of exfoliated graphite electrodes spent in the process of phenol electrooxidation. J. Solid State Electrochem. 2014, 18, 917–928. [Google Scholar] [CrossRef]
- Singh, R.N.; Anindita, D.M. Pd-1%Ni Composite Electrodes for Electrooxidation of Phenol in Acid Solution. Int. J. Electrochem. Sci. 2009, 4, 1638–1649. [Google Scholar] [CrossRef]
- Grabowska, E.; Reszczyńska, J.; Zaleska, A. Mechanism of phenol photodegradation in the presence of pure and modified-TiO2: A review. Water Res. 2012, 46, 5453–5471. [Google Scholar] [CrossRef] [PubMed]
- Stets, S.; Amaral, B.; Bach, L.; Nagata, N.; Peralta-Zamora, P.G. New insight into monitoring degradation products during the TiO2-photocatalysis process by multivariate molecular spectroscopy. Environ. Sci. Pollut. Res. 2017, 24, 6040–6046. [Google Scholar] [CrossRef]
- Leuaa, P.; Priyadarshani, D.; Tripathi, A.K.; Neergat, M. Internal and External Transport of Redox Species across the Porous Thin-Film Electrode/Electrolyte Interface. J. Phys. Chem. C 2019, 123, 21440–21447. [Google Scholar] [CrossRef]
- Leuaa, P.; Priyadarshani, D.; Choudhury, D.; Maurya, R.; Neergat, M. Resolving charge-transfer and mass-transfer processes of VO2+/VO2+ redox species across the electrode/electrolyte interface using electrochemical impedance spectroscopy for vanadium redox flow battery. RSC Adv. 2020, 10, 30887–30895. [Google Scholar] [CrossRef]
- Radjenovic, J.; Sedlak, D.L. Challenges and Opportunities for Electrochemical Processes as Next-Generation Technologies for the Treatment of Contaminated Water. Environ. Sci. Technol. 2015, 49, 11292–11302. [Google Scholar] [CrossRef]
- Zambrano, J.; Min, B. Comparison on efficiency of electrochemical phenol oxidation in two different supporting electrolytes (NaCl and Na2SO4) using Pt/Ti electrode. Environ. Technol. Innov. 2019, 15, 100382. [Google Scholar] [CrossRef]
- Iniesta, J.; Expósito, E.; González-Garcıá, J.; Montiel, V.; Aldaz, A. Electrochemical treatment of industrial wastewater containing phenols. J. Electrochem. Soc. 2002, 149, D57–D62. [Google Scholar] [CrossRef]
- Britto-Costa, P.H.; Ruotolo, L.A.M. Phenol removal from wastewaters by electrochemical oxidation using boron doped diamond (BDD) and Ti/Ti0.7Ru0.3O2 dsa® electrodes. Braz. J. Chem. Eng. 2012, 29, 763–773. [Google Scholar] [CrossRef]
- Crisan, L.; Bora, A. Small Molecules of Natural Origin as Potential Anti-HIV Agents: A Computational Approach. Life 2021, 11, 722. [Google Scholar] [CrossRef]
- Menachekanian, S.; Perez, C.M.; Pennathur, A.K.; Voegtle, M.J.; Blauth, D.; Prezhdo, O.V.; Dawlaty, J.M. Phenol as a tethering groupto gold surface: Stark response and comparison to benzenethiol. J. Phys. Chem. Lett. 2023, 14, 8353–8359. [Google Scholar] [CrossRef]
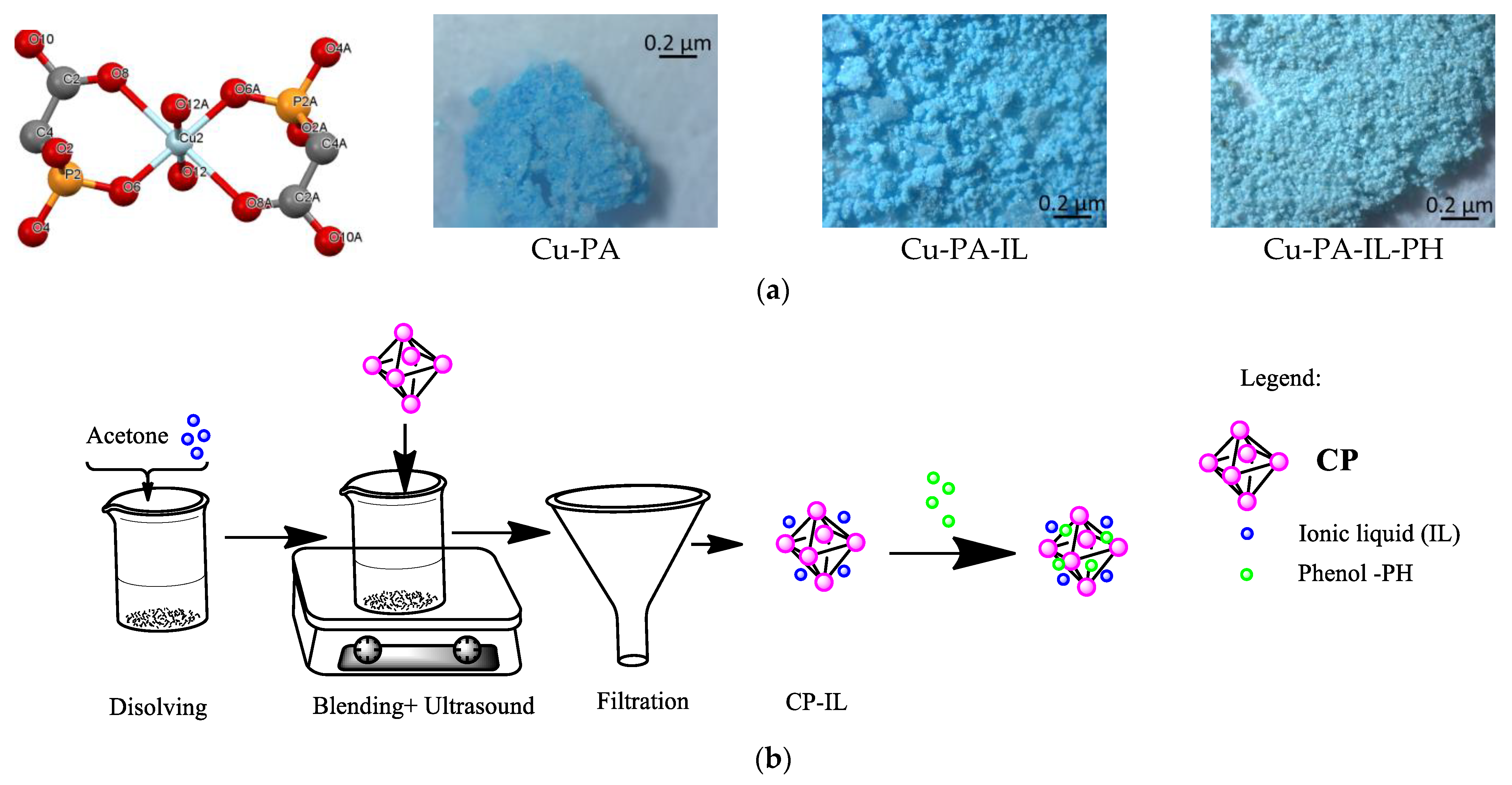

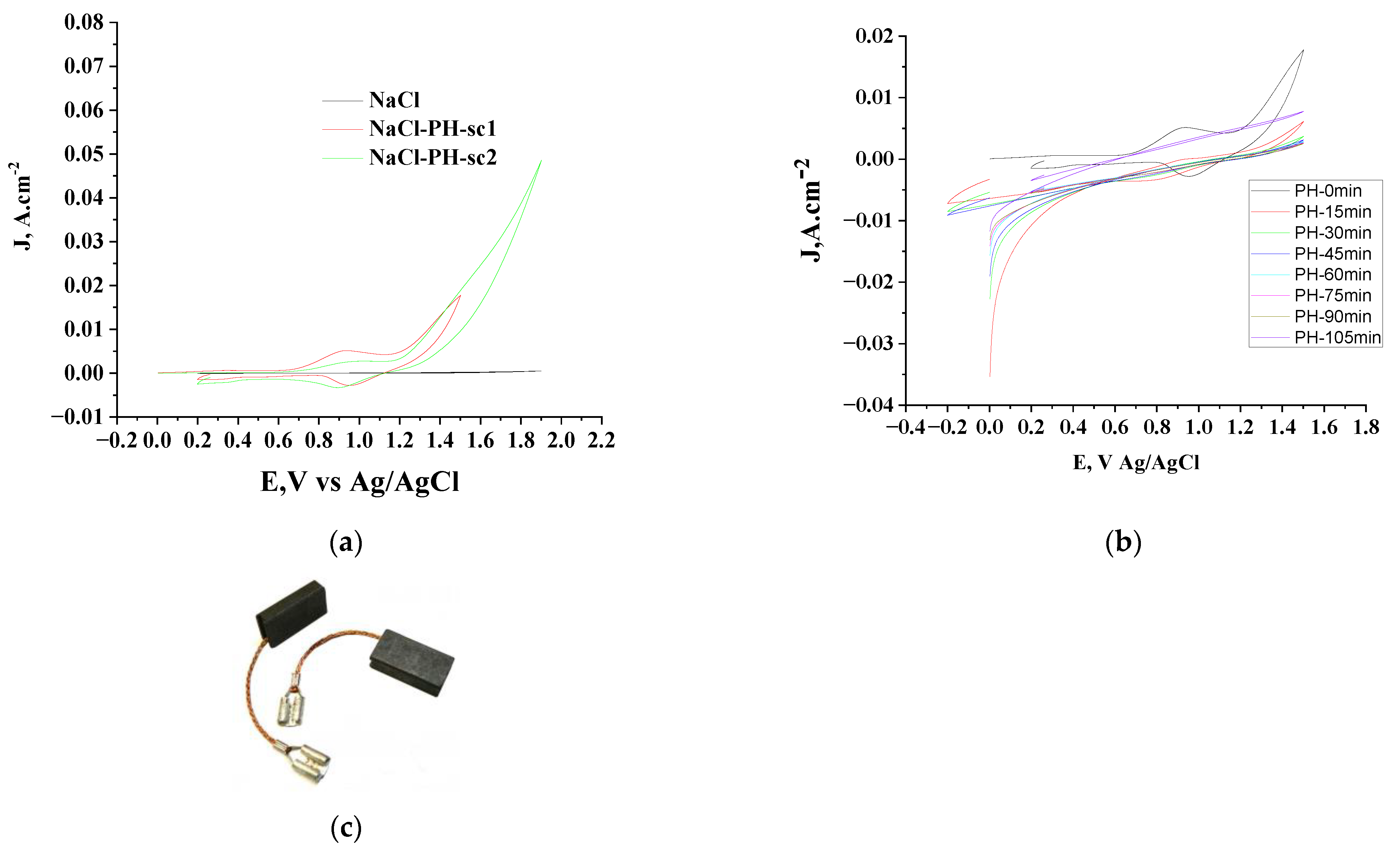

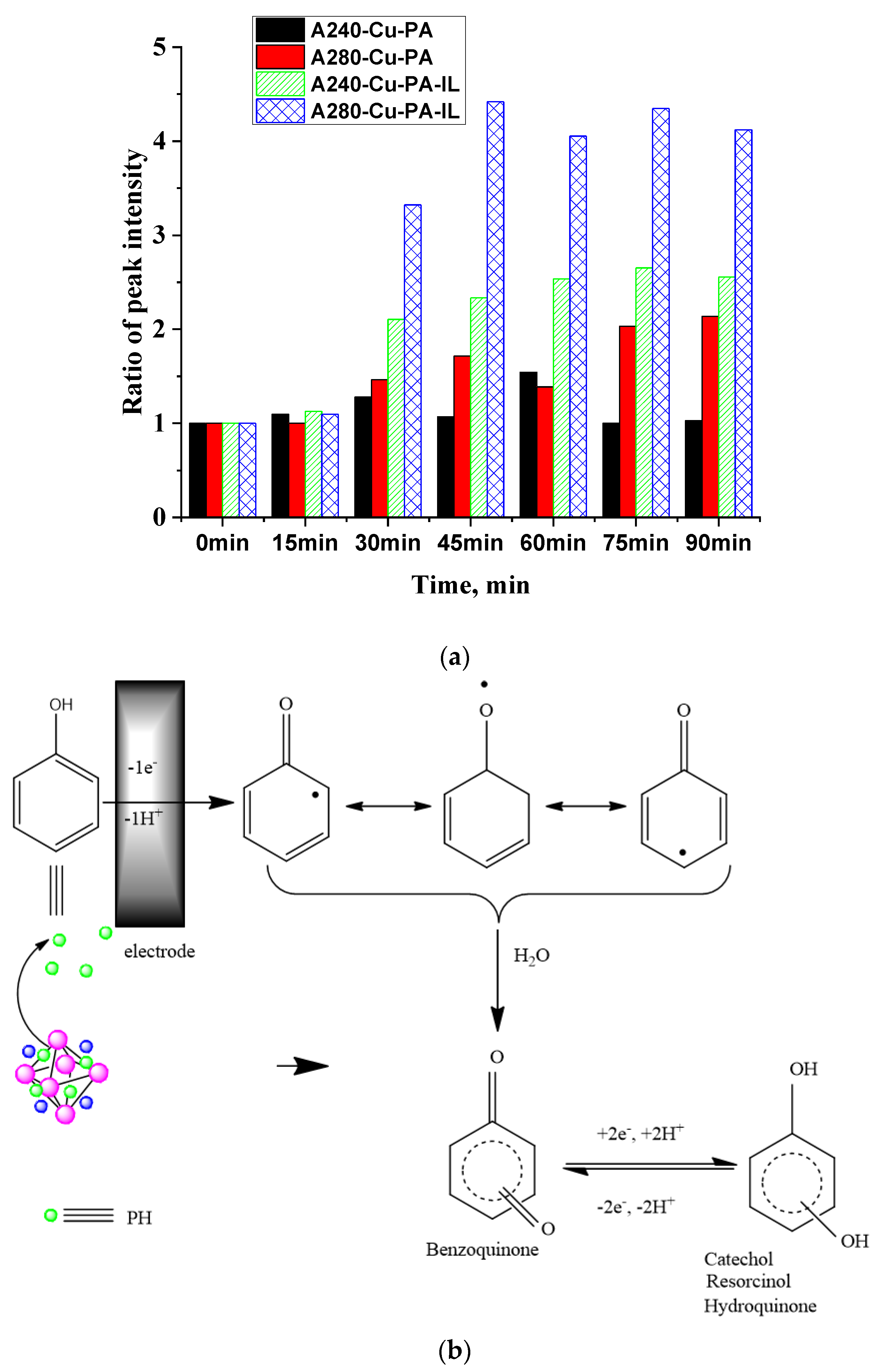


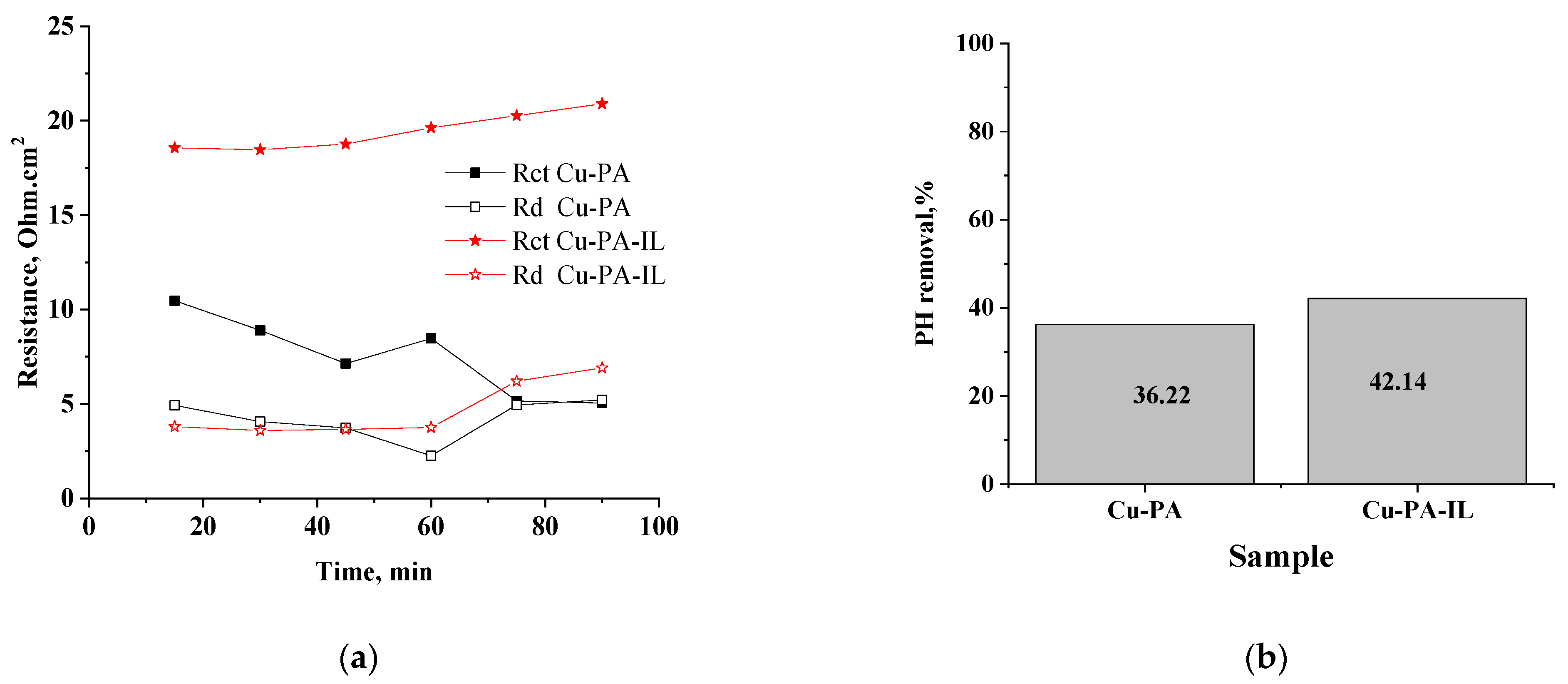
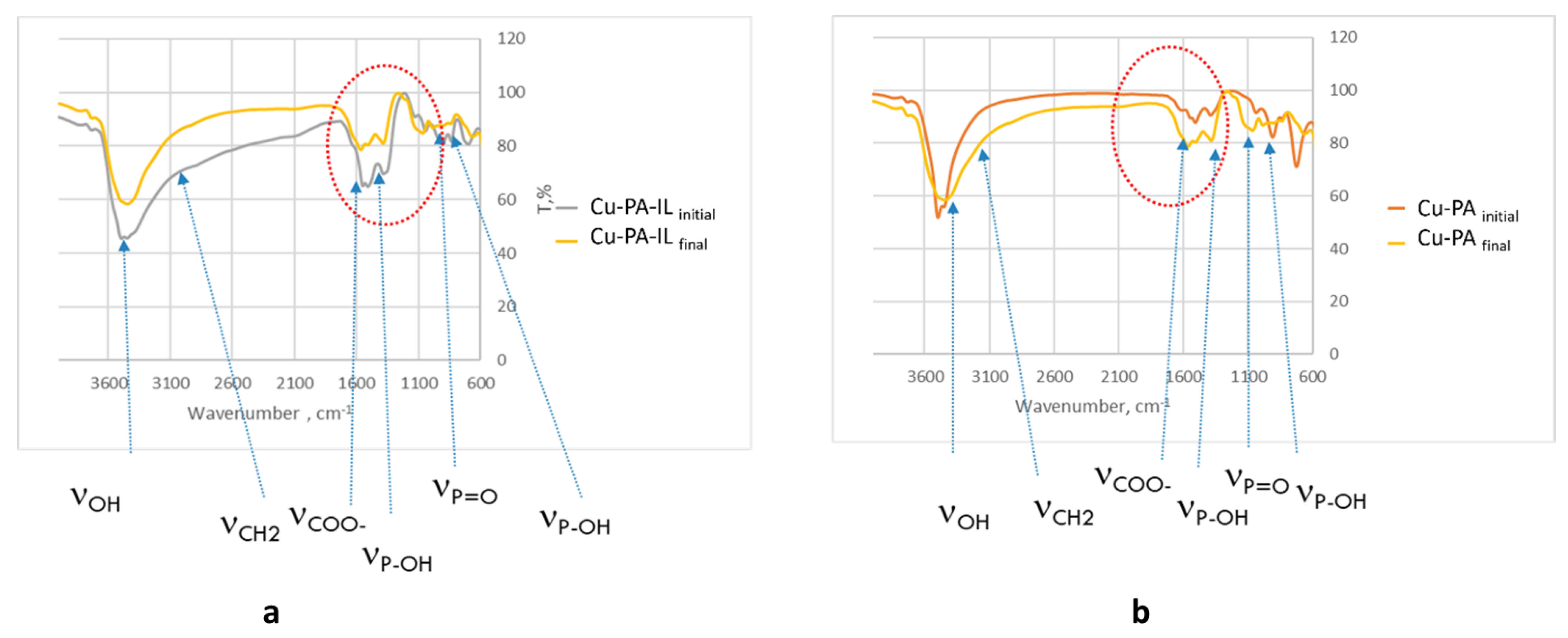
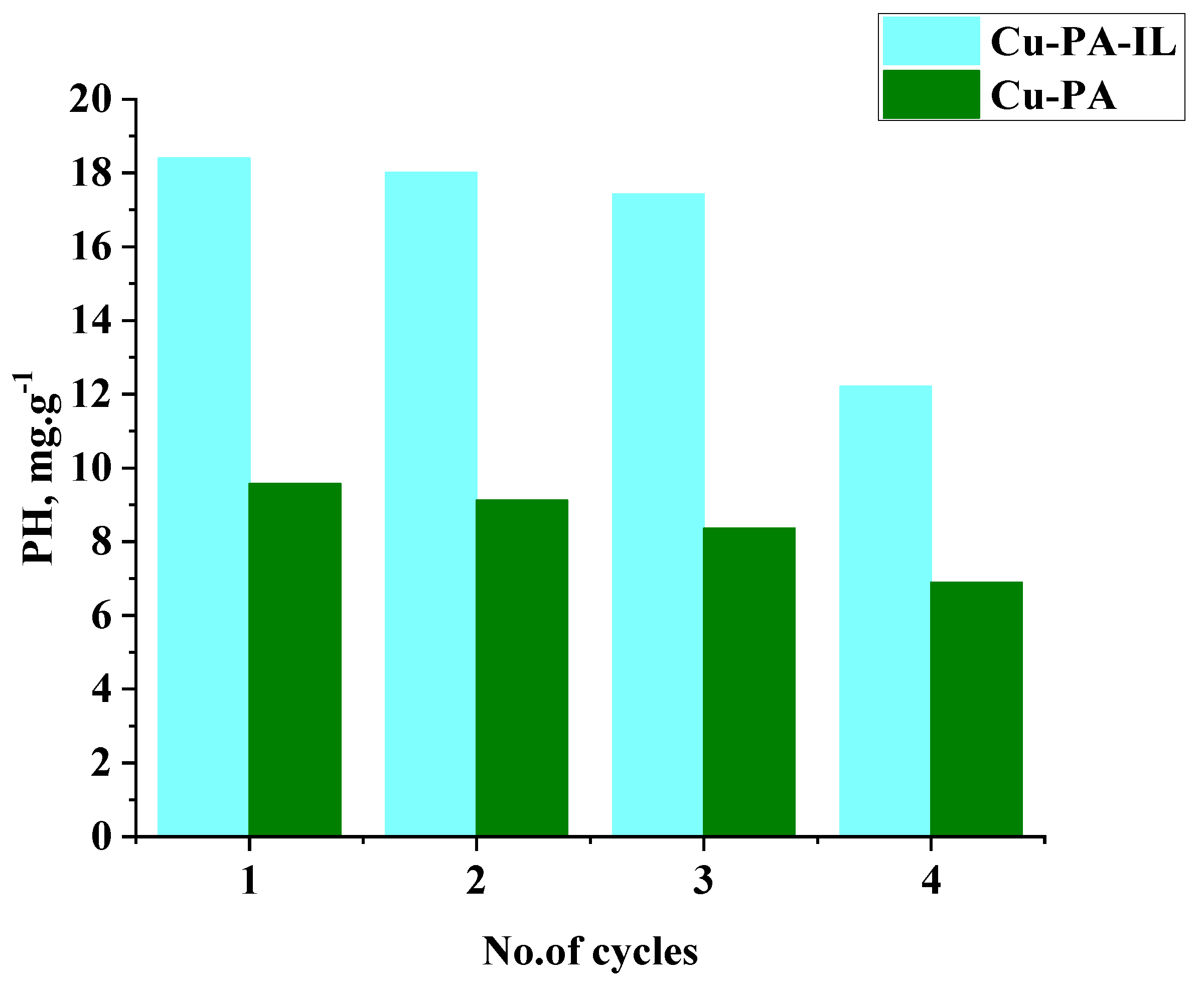
| Adsorbent | qe exp. mg/g | Pseudo-First Order | Second Order | Intraparticle Diffusion Model | |||
|---|---|---|---|---|---|---|---|
| K1, min−1 | R2 | K2, g mg−1 min−1 | R2 | KI, mg g−1 min−1/2 | R2 | ||
| Cu-PA | 9.57 | 0.0147 | 0.9818 | 1.202 × 10−4 | 0.9571 | 8.523 × 10−5 | 0.9597 |
| Cu-PA-IL | 18.4 | 0.086 | 0.9628 | 0.93 × 10−4 | 0.9659 | 2.04 × 10−4 | 0.9797 |
| Electrode | Electrolyte | Phenol mg L−2 | pH | Time, h | Cell Potential, V | Current Density, mA cm2 | Energy Consumption, kW/L | Ref |
|---|---|---|---|---|---|---|---|---|
| BOD | NaCl | 100 | 7 | 6.7 | 4.5 | 5 | 0.002 | [41] |
| Ti-Pt | NaCl/ Na2SO4 | 100 | 6 | 6 | 6 | 9.6 | 0.025 | [42] |
| Ti-IrO2 | NaCl | 100 | 5.5 | 2 | 3.5 | 10 | 28 | [43] |
| Ti/Pt/SnO5/ SbO5 | NaCl | 100 | 6.2 | 2 | 3.5 | 20 | 40 | |
| DSA® (Ti/Ti0.7Ru0.3O2) | NaCl | 3035 | 0.76–0.87 | 8 | 150 | 123 | [44] | |
| BBD (boron-doped diamond) | NaCl | 607 | 0.76–0.87 | 8 | 150 | 248 | [44] | |
| Graphite | NaCl | 2.35 | 6.8 | 5 | 1.5 | 10 | 0.29–0.44 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plesu, N.; Maranescu, B.; Tara-Lunga Mihali, M.; Visa, A. Electrochemical Oxidation of Phenol Released from Spent Coordination Polymer Impregnated with Ionic Liquid. J. Compos. Sci. 2023, 7, 510. https://doi.org/10.3390/jcs7120510
Plesu N, Maranescu B, Tara-Lunga Mihali M, Visa A. Electrochemical Oxidation of Phenol Released from Spent Coordination Polymer Impregnated with Ionic Liquid. Journal of Composites Science. 2023; 7(12):510. https://doi.org/10.3390/jcs7120510
Chicago/Turabian StylePlesu, Nicoleta, Bianca Maranescu, Milica Tara-Lunga Mihali, and Aurelia Visa. 2023. "Electrochemical Oxidation of Phenol Released from Spent Coordination Polymer Impregnated with Ionic Liquid" Journal of Composites Science 7, no. 12: 510. https://doi.org/10.3390/jcs7120510
APA StylePlesu, N., Maranescu, B., Tara-Lunga Mihali, M., & Visa, A. (2023). Electrochemical Oxidation of Phenol Released from Spent Coordination Polymer Impregnated with Ionic Liquid. Journal of Composites Science, 7(12), 510. https://doi.org/10.3390/jcs7120510







