Trimethoxy Silyl End-Capped Hyperbranched Polyglycidol/Polycaprolactone Particle Gels for Cell Delivery and Tissue Repair: Mechanical Properties, Biocompatibility, and Biodegradability Studies
Abstract
:1. Introduction
2. Results
2.1. Mechanical Properties of Gels
2.1.1. Frequency-Sweep Data
2.1.2. Strain-Sweep Data
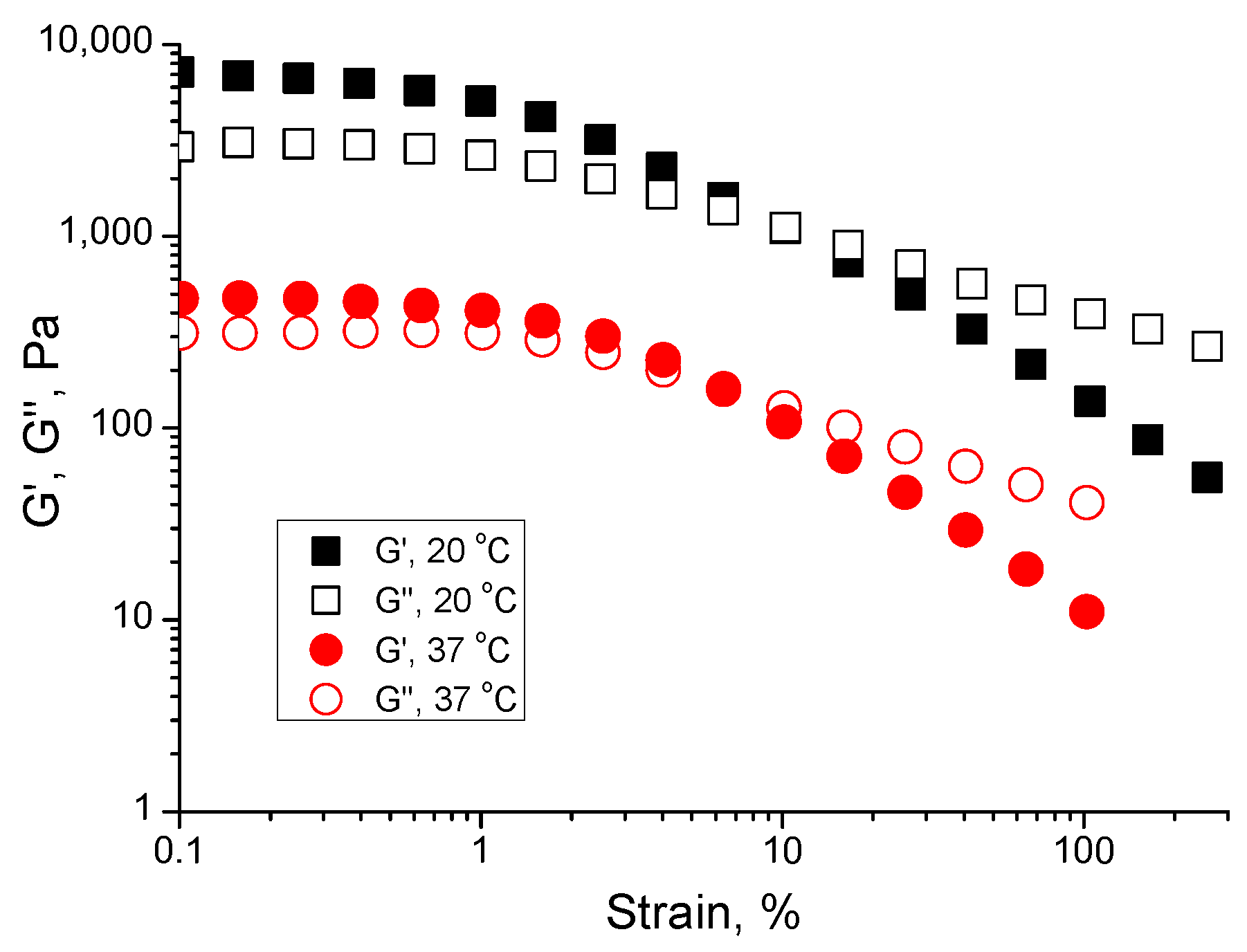
2.1.3. Temperature-Sweep Data
2.2. Covalent Crosslinking of the PCL-HBPG/1SiHBPG Particle Dispersions
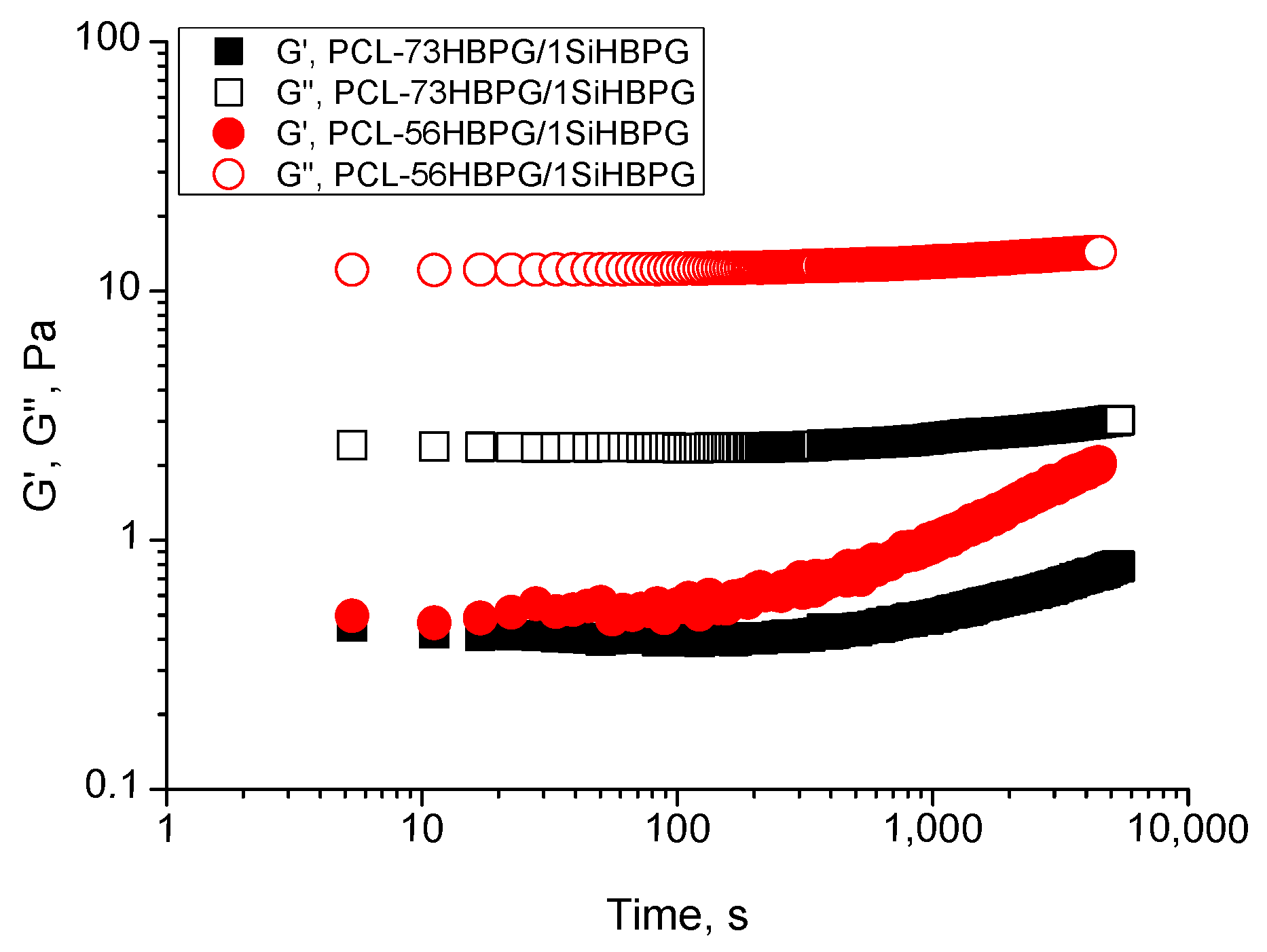
Time-Sweep Rheological Data
2.3. SAXS Analysis
2.4. Hydrolytic Disassembly of the PCL-HBPG/1SiHBPG Gels
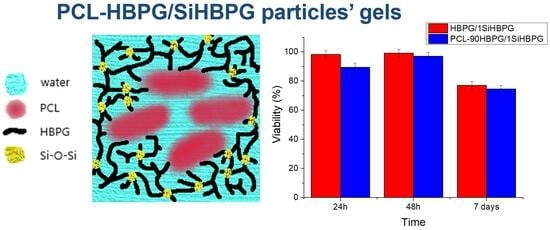
2.5. Biocompatibility Studies with PCL-HBPG/1SiHBPG
3. Discussion
Structure–Properties Relationship in PCL-HBPG/1SiHBPG Concentrated Polymer Dispersions
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation
4.3. Methods and Analysis
4.4. Gel Cytotoxicity Studies
4.5. Scaffold Degradation Studies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mellati, A.; Hasanzadeh, E.; Gholipourmalekabadi, M.; Enderami, S.E. Injectable Nanocomposite Hydrogels as an Emerging Platform for Biomedical Applications: A Review. Mater. Sci. Eng. C 2021, 131, 112489–112514. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Spicer, C.D. Hydrogel Scaffolds for Tissue Engineering: The Importance of Polymer Choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Hou, Q.; De Bank, P.A.; Shakesheff, K.M. Injectable Scaffolds for Tissue Regeneration. J. Mater. Chem. 2004, 14, 1915–1923. [Google Scholar] [CrossRef]
- Fu, S.Z.; Ni, P.Y.; Wang, B.Y.; Chu, B.Y.; Zheng, L.; Luo, F.; Luo, J.C.; Qian, Z.Y. Injectable and Thermo-Sensitive PEG-PCL-PEG Copolymer/Collagen/n-HA Hydrogel Composite for Guided Bone Regeneration. Biomaterials 2012, 33, 4801–4809. [Google Scholar] [CrossRef]
- Diniz, I.M.A.; Chen, C.; Xu, X.; Ansari, S.; Zadeh, H.H.; Marques, M.M.; Shi, S.; Moshaverinia, A. Pluronic F-127 Hydrogel as a Promising Scaffold for Encapsulation of Dental-Derived Mesenchymal Stem Cells. J. Mater. Sci. Mater. Med. 2015, 26, 153–163. [Google Scholar] [CrossRef]
- Li, G.; Wu, J.; Wang, B.; Yan, S.; Zhang, K.; Ding, J.; Yin, J. Self-Healing Supramolecular Self-Assembled Hydrogels Based on Poly(l-Glutamic Acid). Biomacromolecules 2015, 16, 3508–3518. [Google Scholar] [CrossRef]
- Payyappilly, S.; Dhara, S.; Chattopadhyay, S. Thermoresponsive Biodegradable PEG-PCL-PEG Based Injectable Hydrogel for Pulsatile Insulin Delivery. J. Biomed. Mater. Res. Part A 2014, 102, 1500–1509. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of Physical and Chemical Crosslinked Hydrogels for Bone Tissue Engineering. Bioact. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef]
- Calderón, M.; Quadir, M.A.; Sharma, S.K.; Haag, R. Dendritic Polyglycerols for Biomedical Applications. Adv. Mater. 2010, 22, 190–218. [Google Scholar] [CrossRef]
- Fedorovich, N.E.; Oudshoorn, M.H.; van Geemen, D.; Hennink, W.E.; Alblas, J.; Dhert, W.J.A. The Effect of Photopolymerization on Stem Cells Embedded in Hydrogels. Biomaterials 2009, 30, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, H.; Yang, X.; Luo, P.; Wu, Z.; Zhang, X. The Supramolecular Hydrogel Based on Hyperbranched Polyglycerol and Dextran as a Scaffold for Living Cells and Drug Delivery. RSC Adv. 2015, 5, 86730–86739. [Google Scholar] [CrossRef]
- de Queiroz, A.A.A.; Bressiani, J.C.; Bressiani, A.H.A.; Higa, O.Z.; Abraham, G.A. A Novel Bone Scaffolds Based on Hyperbranched Polyglycerol Fibers Filled with Hydroxyapatite Nanoparticles: In Vitro Cell Response. Key Eng. Mater. 2008, 396–398, 633–636. [Google Scholar] [CrossRef]
- Wu, C.; Strehmel, C.; Achazi, K.; Chiappisi, L.; Dernedde, J.; Lensen, M.C.; Gradzielski, M.; Ansorge-Schumacher, M.B.; Haag, R. Enzymatically Cross-Linked Hyperbranched Polyglycerol Hydrogels as Scaffolds for Living Cells. Biomacromolecules 2014, 15, 3881–3890. [Google Scholar] [CrossRef]
- Haryanto; Singh, D.; Huh, P.H.; Kim, S.C. Hyperbranched Poly(Glycidol)/Poly(Ethylene Oxide) Crosslinked Hydrogel for Tissue Engineering Scaffold Using e-Beams. J. Biomed. Mater. Res. Part A 2016, 104, 48–56. [Google Scholar] [CrossRef]
- John, Ł.; Janeta, M.; Rajczakowska, M.; Ejfler, J.; Łydżba, D.; Szafert, S. Synthesis and Microstructural Properties of the Scaffold Based on a 3-(Trimethoxysilyl)Propyl Methacrylate–POSS Hybrid towards Potential Tissue Engineering Applications. RSC Adv. 2016, 6, 66037–66047. [Google Scholar] [CrossRef]
- Li, P.; Luo, Z.; Li, X.; Wang, R.; Chen, H.; Zhao, Y.; Wang, J.; Huang, N. Preparation, Evaluation and Functionalization of Biomimetic Block Copolymer Coatings for Potential Applications in Cardiovascular Implants. Appl. Surf. Sci. 2020, 502, 144085. [Google Scholar] [CrossRef]
- Linn, J.D.; Liberman, L.; Neal, C.A.P.; Calabrese, M.A. Role of Chain Architecture in the Solution Phase Assembly and Thermoreversibility of Aqueous PNIPAM/Silyl Methacrylate Copolymers. Polym. Chem. 2022, 13, 3840–3855. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, J.; Gao, X.; Luo, Y. Semi-Spontaneous Post-Crosslinking Triblock Copolymer Electrolyte for Solid-State Lithium Battery. Batteries 2023, 9, 465. [Google Scholar] [CrossRef]
- Osváth, Z.; Tóth, T.; Iván, B. Sustained Drug Release by Thermoresponsive Sol–Gel Hybrid Hydrogels of Poly(N-Isopropylacrylamide-Co-3-(Trimethoxysilyl)Propyl Methacrylate) Copolymers. Macromol. Rapid Commun. 2017, 38, 1600724. [Google Scholar] [CrossRef]
- Osváth, Z.; Tóth, T.; Iván, B. Synthesis, Characterization, LCST-Type Behavior and Unprecedented Heating-Cooling Hysteresis of Poly(N-Isopropylacrylamide-Co-3-(Trimethoxysilyl)Propyl Methacrylate) Copolymers. Polymer 2017, 108, 395–399. [Google Scholar] [CrossRef]
- Fan, X.; Gu, S.; Wu, L.; Yang, L. Preparation and Characterization of Thermoresponsive Poly(N-Isopropylacrylamide) Copolymers with Enhanced Hydrophilicity. e-Polymers 2020, 20, 561–570. [Google Scholar] [CrossRef]
- Du, J.; Armes, S.P. PH-Responsive Vesicles Based on a Hydrolytically Self-Cross-Linkable Copolymer. J. Am. Chem. Soc. 2005, 127, 12800–12801. [Google Scholar] [CrossRef] [PubMed]
- González-Chomón, C.; Garamus, V.M.; Rangelov, S.; Ebdon, J.R.; Novakov, C.; Halacheva, S.S. Trimethoxysilyl End-Capped Hyperbranched Polyglycidol/Polycaprolactone Copolymers for Cell Delivery and Tissue Repair: Synthesis, Characterisation and Aqueous Solution Properties. Eur. Polym. J. 2019, 112, 648–659. [Google Scholar] [CrossRef]
- Indumathy, B.; Sathiyanathan, P.; Prasad, G.; Reza, M.S.; Prabu, A.A.; Kim, H. A Comprehensive Review on Processing, Development and Applications of Organofunctional Silanes and Silane-Based Hyperbranched Polymers. Polymers 2023, 15, 2517. [Google Scholar] [CrossRef] [PubMed]
- Sterman, S.; Marsden, J.G. Silane Coupling Agents. Ind. Eng. Chem. 1966, 58, 33–37. [Google Scholar] [CrossRef]
- Halacheva, S.; Rangelov, S.; Tsvetanov, C. Rheology of Aqueous Solutions of Polyglycidol-Based Analogues to Pluronic Block Copolymers. J. Phys. Chem. B 2008, 112, 1899–1905. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Béland, F.; Ilharco, L.M.; Pagliaro, M. The Sol–Gel Route to Advanced Silica-Based Materials and Recent Applications. Chem. Rev. 2013, 113, 6592–6620. [Google Scholar] [CrossRef]
- Avnir, D.; Coradin, T.; Lev, O.; Livage, J. Recent Bio-Applications of Sol–Gel Materials. J. Mater. Chem. 2006, 16, 1013–1030. [Google Scholar] [CrossRef]
- Arkles, B.; Steinmetz, J.; Zazyczny, J.; Mehta, P. Factors Contributing to the Stability of Alkoxysilanes in Aqueous Solution. J. Adhes. Sci. Technol. 1992, 6, 193–206. [Google Scholar] [CrossRef]
- Bianco, S.; Panja, S.; Adams, D.J. Using Rheology to Understand Transient and Dynamic Gels. Gels 2022, 8, 132. [Google Scholar] [CrossRef]
- Avnir, D.; Gutfraind, R.; Farin, D. Fractal Analysis in Heterogeneous Chemistry. In Fractals in Science; Bunde, A., Havlin, S., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 229–256. ISBN 978-3-642-77953-4. [Google Scholar]
- Ko, J.; Cho, J.; Petrov, M.S. Low Serum Amylase, Lipase, and Trypsin as Biomarkers of Metabolic Disorders: A Systematic Review and Meta-Analysis. Diabetes Res. Clin. Pract. 2020, 159, 107974. [Google Scholar] [CrossRef] [PubMed]
- Brockman, H.L. Lipases. In Encyclopedia of Biological Chemistry, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 729–732. [Google Scholar]
- Singh, R.S.; Singh, T.; Pandey, A. Microbial Enzymes—An Overview. In Advances in Enzyme Technology, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–40. [Google Scholar]
- Blackwell, C.J.; Haernvall, K.; Guebitz, G.M.; Groombridge, M.; Gonzales, D.; Khosravi, E. Enzymatic Degradation of Star Poly(ε-Caprolactone) with Different Central Units. Polymers 2018, 10, 1266. [Google Scholar] [CrossRef]
- Glosz, K.; Stolarczyk, A.; Jarosz, T. Siloxanes—Versatile Materials for Surface Functionalisation and Graft Copolymers. Int. J. Mol. Sci. 2020, 21, 6387. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, R. Constructing a Molecular Theory of Self-Assembly: Interplay of Ideas from Surfactants and Block Copolymers. Adv. Colloid Interface Sci. 2017, 244, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Panja, S.; Dietrich, B.; Trabold, A.; Zydel, A.; Qadir, A.; Adams, D.J. Varying the Hydrophobic Spacer to Influence Multicomponent Gelation. Chem. Commun. 2021, 57, 7898–7901. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Marchant, R.E. Design Properties of Hydrogel Tissue-Engineering Scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic Polymer Scaffolds for Tissue Engineering. Chem. Soc. Rev. 2009, 38, 1139–1151. [Google Scholar] [CrossRef]
- Nguyen, K.T.; West, J.L. Photopolymerizable Hydrogels for Tissue Engineering Applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Chen, D.R.; Bei, J.Z.; Wang, S.G. Polycaprolactone Microparticles and Their Biodegradation. Polym. Degrad. Stab. 2000, 67, 455–459. [Google Scholar] [CrossRef]
- Cushing, M.C.; Anseth, K.S. Hydrogel Cell Cultures. Science 2007, 316, 1133–1134. [Google Scholar] [CrossRef] [PubMed]
- Manoukian, O.S.; Arul, M.R.; Sardashti, N.; Stedman, T.; James, R.; Rudraiah, S.; Kumbar, S.G. Biodegradable Polymeric Injectable Implants for Long-Term Delivery of Contraceptive Drugs. J. Appl. Polym. Sci. 2018, 135, 46068. [Google Scholar] [CrossRef]
- Aurand, E.R.; Wagner, J.; Lanning, C.; Bjugstad, K.B. Building Biocompatible Hydrogels for Tissue Engineering of the Brain and Spinal Cord. J. Funct. Biomater. 2012, 3, 839–863. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Aigner, A.; Czubayko, F.; Kissel, T.; Wendorff, J.H.; Greiner, A. Poly(Vinyl Alcohol) Nanofibers by Electrospinning as a Protein Delivery System and the Retardation of Enzyme Release by Additional Polymer Coatings. Biomacromolecules 2005, 6, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- Gonen-Wadmany, M.; Oss-Ronen, L.; Seliktar, D. Protein–Polymer Conjugates for Forming Photopolymerizable Biomimetic Hydrogels for Tissue Engineering. Biomaterials 2007, 28, 3876–3886. [Google Scholar] [CrossRef]
- Picout, D.R.; Ross-Murphy, S.B. Rheology of Biopolymer Solutions and Gels. Sci. World J. 2003, 3, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Murata, H. Rheology—Theory and Application to Biomaterials; Gomes, A.D.S., Ed.; IntechOpen: Rijeka, Yugoslavia, 2012; Chapter 17; pp. 403–426. [Google Scholar]










| Sample | T (°C) | Slope |
|---|---|---|
| PCL-45HBPG/1SiHBPG | 25 | 3.10 ± 0.01 |
| PCL-45HBPG/1SiHBPG | 60 | 2.70 ± 0.01 |
| PCL-56HBPG/1SiHBPG | 30 | 2.90 ± 0.01 |
| PCL-73HBPG/1SiHBPG | 30 | 2.71 ± 0.01 |
| PCL-90HBPG/1SiHBPG | 30 | 2.67 ± 0.03 |
| Sample | T, °C | a, Å | b, Å | Rg, Å | V, Å3 | NaggPCL |
|---|---|---|---|---|---|---|
| PCL-45HBPG/1SiHBPG | 25 | 57 ± 14 | 18 ± 1 | 28 | 77,320 | 25 |
| PCL-45HBPG/1SiHBPG | 60 | 47 ± 5 | 4.3 ± 1.9 | 21 | 3640 | 2 |
| PCL-56HBPG/1SiHBPG | 30 | 29 ± 1 | 8.3 ± 0.7 | 14 | 8368 | 3 |
| PCL-70HBPG/1SiHBPG | 30 | 56 ± 19 | 4.7 ± 2.2 | 25 | 5182 | 2 |
| PCL-90HBPG/1SiHBPG | 30 | 59 ± 32 | 6.1 ± 3.9 | 27 | 9196 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Chomón, C.; Garamus, V.M.; Hoyland, J.; Halacheva, S.S. Trimethoxy Silyl End-Capped Hyperbranched Polyglycidol/Polycaprolactone Particle Gels for Cell Delivery and Tissue Repair: Mechanical Properties, Biocompatibility, and Biodegradability Studies. J. Compos. Sci. 2023, 7, 451. https://doi.org/10.3390/jcs7110451
González-Chomón C, Garamus VM, Hoyland J, Halacheva SS. Trimethoxy Silyl End-Capped Hyperbranched Polyglycidol/Polycaprolactone Particle Gels for Cell Delivery and Tissue Repair: Mechanical Properties, Biocompatibility, and Biodegradability Studies. Journal of Composites Science. 2023; 7(11):451. https://doi.org/10.3390/jcs7110451
Chicago/Turabian StyleGonzález-Chomón, Clara, Vasil M. Garamus, Judith Hoyland, and Silvia S. Halacheva. 2023. "Trimethoxy Silyl End-Capped Hyperbranched Polyglycidol/Polycaprolactone Particle Gels for Cell Delivery and Tissue Repair: Mechanical Properties, Biocompatibility, and Biodegradability Studies" Journal of Composites Science 7, no. 11: 451. https://doi.org/10.3390/jcs7110451
APA StyleGonzález-Chomón, C., Garamus, V. M., Hoyland, J., & Halacheva, S. S. (2023). Trimethoxy Silyl End-Capped Hyperbranched Polyglycidol/Polycaprolactone Particle Gels for Cell Delivery and Tissue Repair: Mechanical Properties, Biocompatibility, and Biodegradability Studies. Journal of Composites Science, 7(11), 451. https://doi.org/10.3390/jcs7110451