A Highly Promising Flower-Shaped WO2I2/Poly(1H-Pyrrole) Nanocomposite Thin Film as a Potentiometric Sensor for the Detection of Cd2+ Ions in Water
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. WO2I2/P1HP Thin Film Sensor Preparation
2.3. The Potentiometric Sensing
3. Results and Discussion
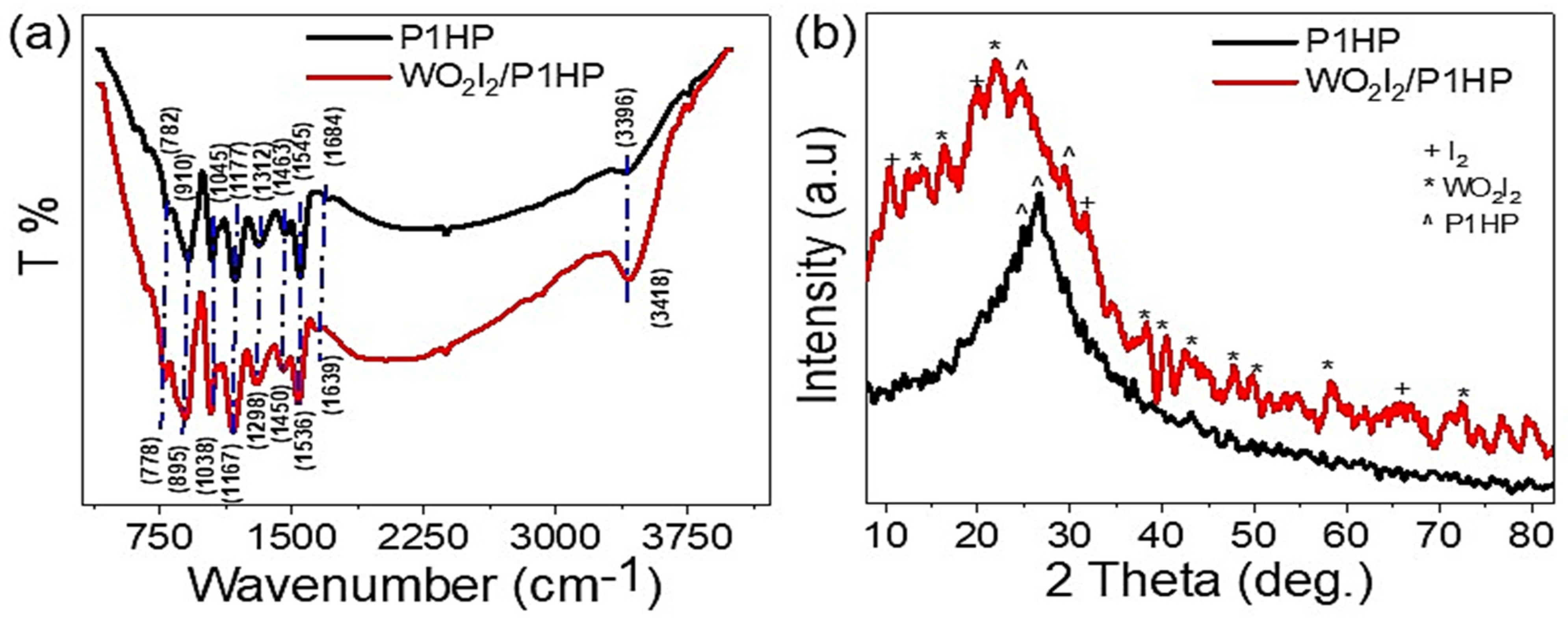
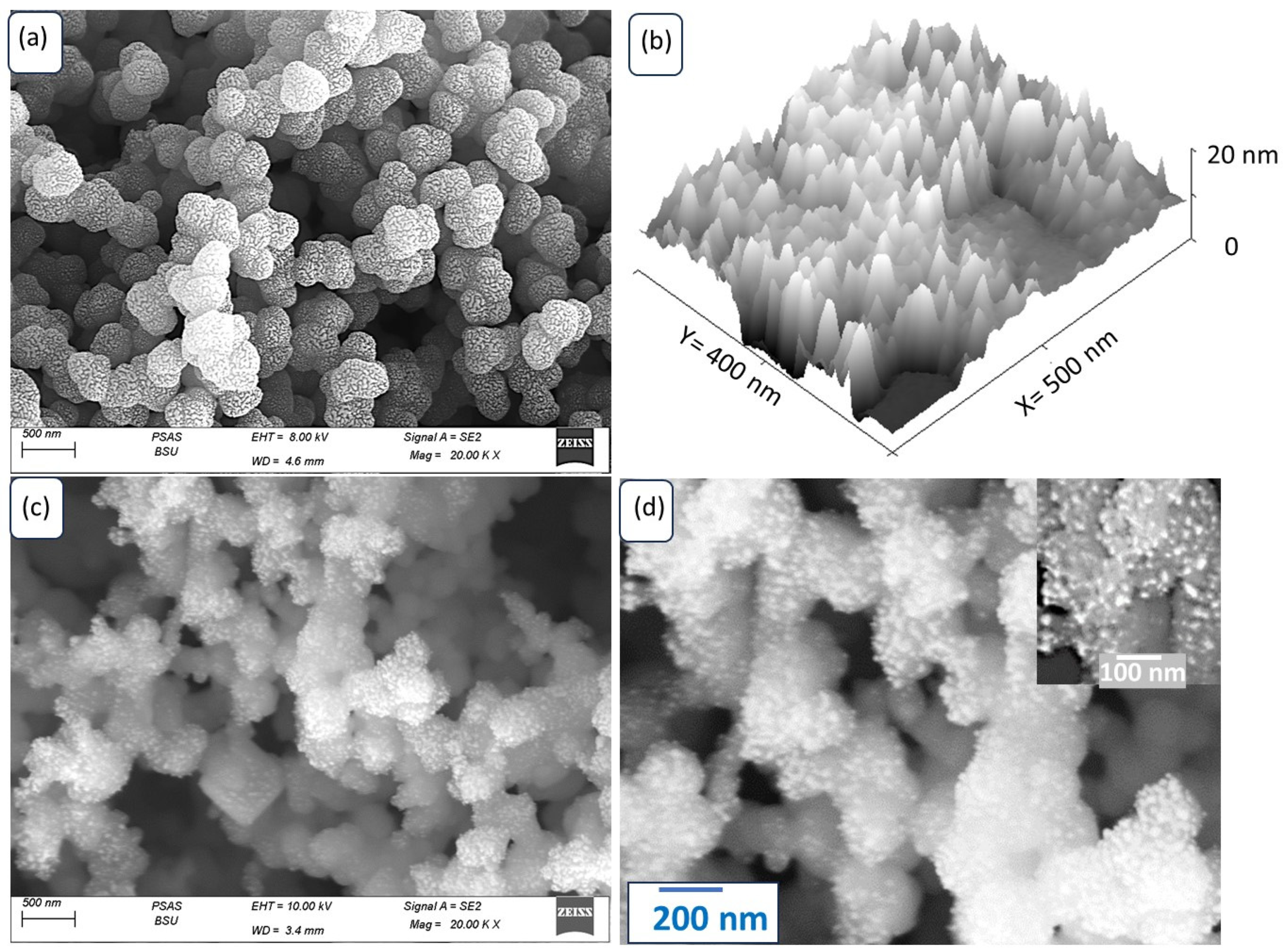
3.1. Analyses
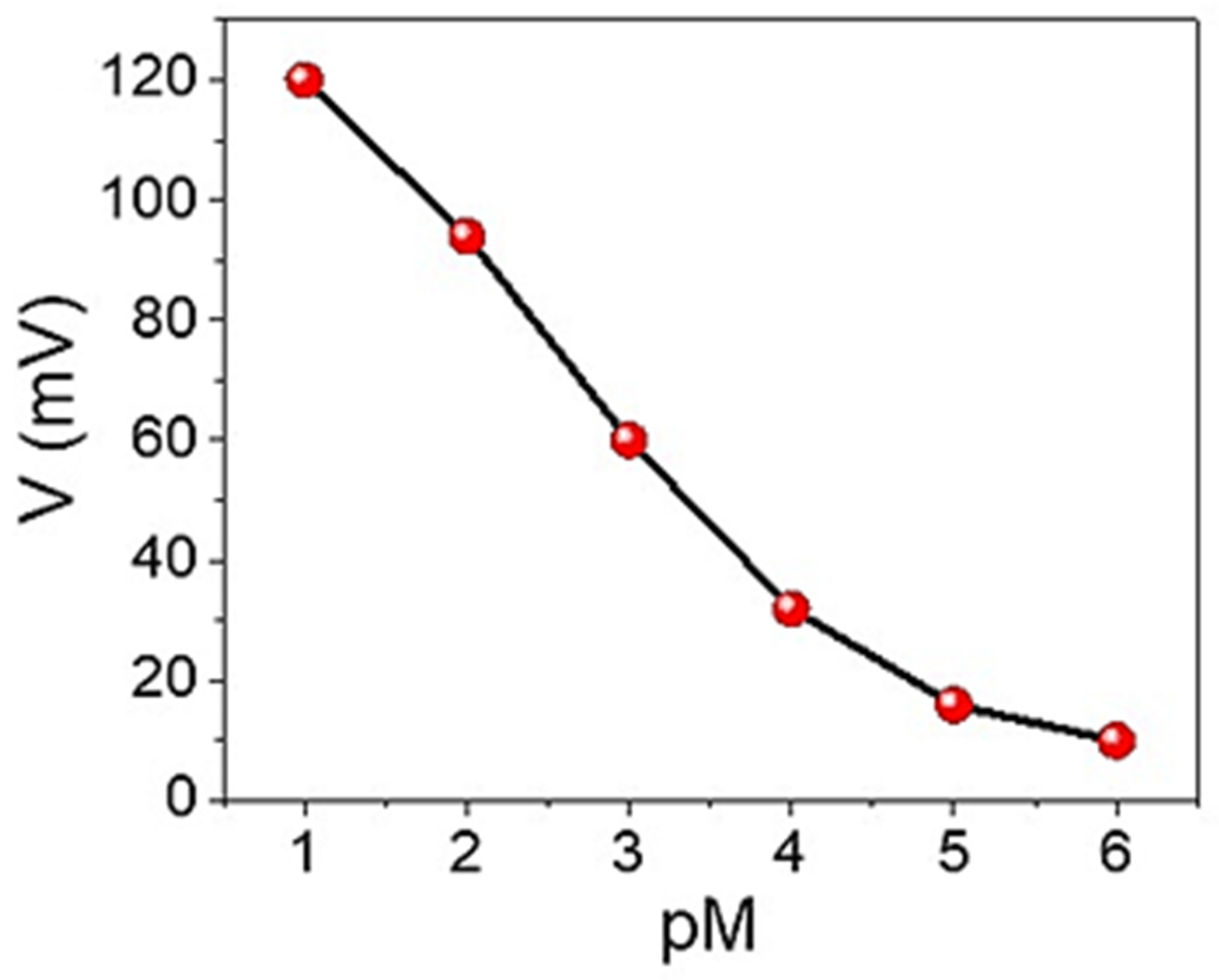
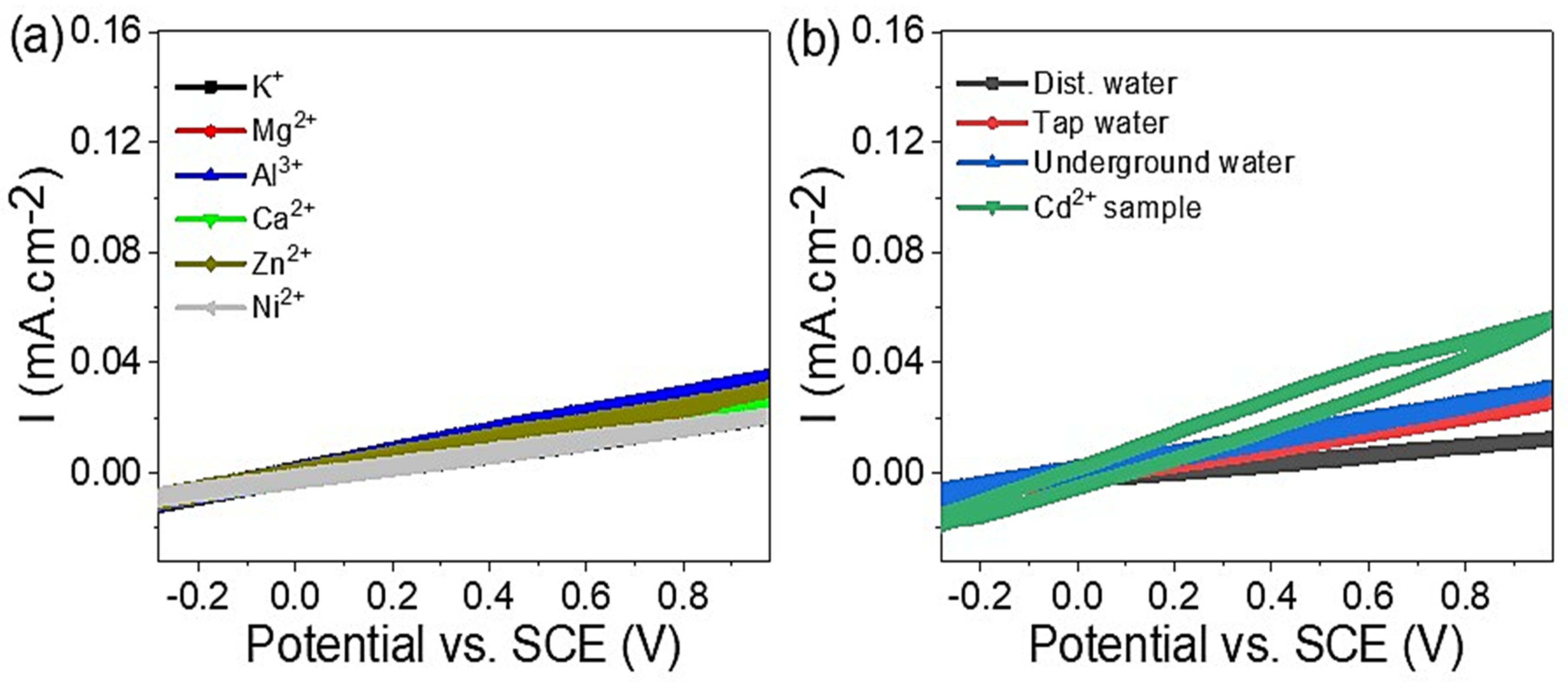
3.2. Sensing Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Çelebi, H.; Gök, G.; Gök, O. Adsorption Capability of Brewed Tea Waste in Waters Containing Toxic Lead(II), Cadmium (II), Nickel (II), and Zinc(II) Heavy Metal Ions. Sci. Rep. 2020, 10, 17570. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; He, Y.; He, S.; Luo, J.; Zeng, Y.; Zhang, X.; Huo, Y.; Jie, Y.; Xing, H. Exogenous Plant Growth Regulator and Foliar Fertilizers for Phytoextraction of Cadmium with Boehmeria Nivea [L.] Gaudich from Contaminated Field Soil. Sci. Rep. 2023, 13, 11019. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Tefsen, B.; Wells, M. From Speciation to Toxicity: Using a “Two-in-One” Whole-Cell Bioreporter Approach to Assess Harmful Effects of Cd and Pb. Water Res. 2022, 217, 118384. [Google Scholar] [CrossRef]
- Forghani Tehrani, G.; Rubinos, D.A.; Kelm, U.; Ghadimi, S. Environmental and Human Health Risks of Potentially Harmful Elements in Mining-Impacted Soils: A Case Study of the Angouran Zn–Pb Mine, Iran. J. Environ. Manag. 2023, 334, 117470. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, P.; Gu, M.; Xue, J. Trace Heavy Metals and Harmful Elements in Roots and Rhizomes of Herbs: Screening Level Analysis and Health Risk Assessment. Chin. Herb. Med. 2022, 14, 622–629. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Shaban, M. Nanoporous Chromium Thin Film for Active Detection of Toxic Heavy Metals Traces Using Surface-Enhanced Raman Spectroscopy. Mater. Res. Express 2020, 7, 015084. [Google Scholar] [CrossRef]
- Rabia, M.; Elsayed, A.M.; Alnuwaiser, M.A. Decoration of Poly-3-methyl Aniline with As(III) Oxide and Hydroxide as an Effective Photoelectrode for Electroanalytical Photon Sensing with Photodiode-like Behavior. Micromachines 2023, 14, 1573. [Google Scholar] [CrossRef]
- Karbalaee Hosseini, A.; Tadjarodi, A. Luminescent Cd Coordination Polymer Based on Thiazole as a Dual-Responsive Chemosensor for 4-Nitroaniline and CrO42− in Water. Sci. Rep. 2023, 13, 269. [Google Scholar] [CrossRef]
- Zhan, W.; Chen, Z.; Hu, J.; Chen, X. Vertical CuO Nanowires Array Electrodes: Visible Light Sensitive Photoelectrochemical Biosensor of Ethanol Detection. Mater. Sci. Semicond. Process. 2018, 85, 90–97. [Google Scholar] [CrossRef]
- Shoaie, N.; Daneshpour, M.; Azimzadeh, M.; Mahshid, S.; Khoshfetrat, S.M.; Jahanpeyma, F.; Gholaminejad, A.; Omidfar, K.; Foruzandeh, M. Electrochemical Sensors and Biosensors Based on the Use of Polyaniline and Its Nanocomposites: A Review on Recent Advances. Mikrochim. Acta 2019, 186, 465. [Google Scholar] [CrossRef]
- Lan, T.; Fallatah, A.; Suiter, E.; Padalkar, S. Size Controlled Copper (I) Oxide Nanoparticles Influence Sensitivity of Glucose Biosensor. Sensors 2017, 17, 1944. [Google Scholar] [CrossRef]
- Khalil, M.M.; Issa, Y.M.; Zayed, S.I.M.; Ali, F.Q. New Potentiometric Membrane Sensors for Determination of Alverine Citrate in Pharmaceutical Compounds and Biological Fluids. Int. J. Adv. Res. 2014, 2, 1096–1109. [Google Scholar]
- El Nady, J.; Shokry, A.; Khalil, M.; Ebrahim, S.; Elshaer, A.M.; Anas, M. One-Step Electrodeposition of a Polypyrrole/NiO Nanocomposite as a Supercapacitor Electrode. Sci. Rep. 2022, 12, 3611. [Google Scholar] [CrossRef]
- Ebrahim, S.; Shokry, A.; Khalil, M.M.A.; Ibrahim, H.; Soliman, M. Polyaniline/Ag Nanoparticles/Graphene Oxide Nanocomposite Fluorescent Sensor for Recognition of Chromium (VI) Ions. Sci. Rep. 2020, 10, 13617. [Google Scholar] [CrossRef]
- Xing, Y.; Liu, Z.; Li, B.; Li, L.; Yang, X.; Zhang, G. The Contrastive Study of Two Thiophene-Derived Symmetrical Schiff Bases as Fluorescence Sensors for Ga3+ Detection. Sens. Actuators B Chem. 2021, 347, 130497. [Google Scholar] [CrossRef]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. A Label-Free Electrochemical Biosensor for Highly Sensitive Detection of GM2A Based on Gold Nanoparticles/Conducting Amino-Functionalized Thiophene Polymer Layer. Sens. Actuators B Chem. 2023, 392, 134025. [Google Scholar] [CrossRef]
- Sadia, M.; Khan, J.; Khan, R.; Kamran, A.W.; Zahoor, M.; Ullah, R.; Bari, A.; Ali, E.A. Rapid Detection of Cd2+ Ions in the Aqueous Medium Using a Highly Sensitive and Selective Turn-On Fluorescent Chemosensor. Molecules 2023, 28, 3635. [Google Scholar] [CrossRef] [PubMed]
- Gudadur, K.S.; Veluswamy, P. Flexible Reversible Polymer Nano-Composite Thin Film Patch for Wearable Temperature Sensor. Surf. Interfaces 2023, 39, 102975. [Google Scholar] [CrossRef]
- Ganesha, H.; Veeresh, S.; Nagaraju, Y.; Devendrappa, H. MoS2/Polymer Nanotube Composite Material Used for the Electrochemical Sensor Detection of Biologically Active Compounds. Inorg. Chem. Commun. 2023, 156, 111228. [Google Scholar] [CrossRef]
- Rabia, M.; Trabelsi, A.B.G.; Elsayed, A.M.; Alkallas, F.H. Porous-Spherical Cr2O3-Cr(OH)3-Polypyrrole/Polypyrrole Nanocomposite Thin-Film Photodetector and Solar Cell Applications. Coatings 2023, 13, 1240. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. Electropolymerization of m -Toluidin on Platinum Electrode from Aqueous Acidic Solution and Character of the Obtained Polymer. Adv. Polym. Technol. 2018, 37, 126–136. [Google Scholar] [CrossRef]
- Sayyah, E.-S.M.; Shaban, M.; Rabia, M. A Sensor of m -Cresol Nanopolymer/Pt-Electrode Film for Detection of Lead Ions by Potentiometric Methods. Adv. Polym. Technol. 2018, 37, 1296–1304. [Google Scholar] [CrossRef]
- Coşkun, S.; Koziol, K.K.K. Synthesis of High Aspect Ratio WO2 Nanostructures. J. Nanoparticle Res. 2016, 18, 51. [Google Scholar] [CrossRef]
- Dinh, T.D.; Zhang, D.; Tuan, V.N. High Iodine Adsorption Performances under Off-Gas Conditions by Bismuth-Modified ZnAl-LDH Layered Double Hydroxide. RSC Adv. 2020, 10, 14360–14367. [Google Scholar] [CrossRef]
- Srinivasan, P.; Madhu, D.K.; Pedugu Sivaraman, S.; Nagarajan, S.; Rao, C.V.S.B.; Chinaraga, P.K.; Mohan, A.M.; Deivasigamani, P. Chromoionophoric Probe Imbued Porous Polymer Monolith as a Three-in-One Solid-State Naked-Eye Sensor for the Selective Sensing and Recovery of Ultra-Trace Lead, Mercury, and Cadmium Ions from Industrial/Environmental Samples. Chem. Eng. J. 2023, 471, 144627. [Google Scholar] [CrossRef]
- Anju, P.V.; Deivasigamani, P. Structurally Engineered Ion-Receptor Probe Immobilized Porous Polymer Platform as Reusable Solid-State Chromogenic Sensor for the Ultra-Trace Sensing and Recovery of Mercury Ions. J. Hazard. Mater. 2023, 454, 131431. [Google Scholar] [CrossRef]
- Bukhari, A.; Ijaz, I.; Gilani, E.; Nazir, A.; Zain, H.; Shaheen, A.; Hussain, S.; Imtiaz, A. Highly Rapid and Efficient Removal of Heavy Metals, Heavy Rare Earth Elements, and Phenolic Compounds Using EDTA-Cross-Linked MXene Polymer Composite: Adsorption Characteristics and Mechanisms. Chem. Eng. Res. Des. 2023, 194, 497–513. [Google Scholar] [CrossRef]
- Deligonul, N.; Yildiz, I.; Bilgin, S.; Gokce, I.; Isildak, O. Green Fluorescent Protein-Multi Walled Carbon Nanotube Based Polymeric Membrane Electrode for Bismuth Ion Detection. Microchem. J. 2023, 190, 108710. [Google Scholar] [CrossRef]
- Shirzadmehr, A.; Afkhami, A.; Madrakian, T. A New Nano-Composite Potentiometric Sensor Containing an Hg2+-Ion Imprinted Polymer for the Trace Determination of Mercury Ions in Different Matrices. J. Mol. Liq. 2015, 204, 227–235. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Das, N.C. Characterization of Various Polymer Composite Sensors. In Polymeric Nanocomposite Materials for Sensor Applications; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Sawston, UK, 2023; pp. 121–140. [Google Scholar] [CrossRef]
- Verma, A.; Gupta, R.; Verma, A.S.; Kumar, T. A Review of Composite Conducting Polymer-Based Sensors for Detection of Industrial Waste Gases. Sens. Actuators Rep. 2023, 5, 100143. [Google Scholar] [CrossRef]
- Ngoensawat, U.; Pisuchpen, T.; Sritana-anant, Y.; Rodthongkum, N.; Hoven, V.P. Conductive Electrospun Composite Fibers Based on Solid-State Polymerized Poly(3,4-Ethylenedioxythiophene) for Simultaneous Electrochemical Detection of Metal Ions. Talanta 2022, 241, 123253. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnuwaiser, M.A.; Rabia, M. A Highly Promising Flower-Shaped WO2I2/Poly(1H-Pyrrole) Nanocomposite Thin Film as a Potentiometric Sensor for the Detection of Cd2+ Ions in Water. J. Compos. Sci. 2023, 7, 439. https://doi.org/10.3390/jcs7100439
Alnuwaiser MA, Rabia M. A Highly Promising Flower-Shaped WO2I2/Poly(1H-Pyrrole) Nanocomposite Thin Film as a Potentiometric Sensor for the Detection of Cd2+ Ions in Water. Journal of Composites Science. 2023; 7(10):439. https://doi.org/10.3390/jcs7100439
Chicago/Turabian StyleAlnuwaiser, Maha Abdallah, and Mohamed Rabia. 2023. "A Highly Promising Flower-Shaped WO2I2/Poly(1H-Pyrrole) Nanocomposite Thin Film as a Potentiometric Sensor for the Detection of Cd2+ Ions in Water" Journal of Composites Science 7, no. 10: 439. https://doi.org/10.3390/jcs7100439
APA StyleAlnuwaiser, M. A., & Rabia, M. (2023). A Highly Promising Flower-Shaped WO2I2/Poly(1H-Pyrrole) Nanocomposite Thin Film as a Potentiometric Sensor for the Detection of Cd2+ Ions in Water. Journal of Composites Science, 7(10), 439. https://doi.org/10.3390/jcs7100439






