Abstract
An on-package colorimetric label was fabricated using Hibiscus sabdariffa L. anthocyanin as a freshness indicator because its color depends on pH. The anthocyanins were embedded within a chitosan matrix. The colorimetric labels were applied to estimate the spoilage of fish food during storage at 25 °C for 3 days. According to scanning electron microscopy results, the inclusion of the anthocyanins in chitosan matrix resulted in formation dense and uniform film. The chitosan colorimetric labels had acceptable thicknesses (78–85 µm), moisture contents (14–16%), swelling indices (84–102%), water vapor permeabilities (3.0–3.2 × 10−11 g m/m2 s Pa), tensile strengths (11.3–12.3 MPa), and elongation at breaks (14–39%). It is noteworthy that the label can distinguish fish spoilage by color turn from light brown (fresh) to grayish (spoiled) by the naked-eye, due to alterations in the pH content and formation of volatile basic nitrogen during storage. Our results indicate that all-natural color labels can be an effective method to monitor the fish spoilage during storage, which may improve food quality and sustainability.
1. Introduction
Natural film-forming materials, like many proteins and polysaccharides, are being explored for their ability to construct more sustainable and green packaging materials to replace synthetic plastics [1,2]. The functional performance of these biopolymer-based packaging materials is often enhanced by incorporating additives that provide specific desirable functions, such as product monitoring (smart packaging) or product preservation (active packaging) [3,4]. Smart packaging materials include sensors that can monitor the food and/or environment inside the package and provide information about its freshness, quality, or safety [5,6]. The information provided by these sensors can be used by the food industry, retailers, and consumers to determine when a food should be sold, consumed, and discarded. Three major systems exist for intelligent packaging: data carriers, sensors, and indicators. There are many types of indicators that are categorized into three groups: freshness, Time-Temperature, and gas indicators. Colorimetric indicators based on pH changes are one of the most widely explored sensors in food packaging research because of their simplicity and non-invasive nature [7,8]. Color changes can easily be visually detected by food retailers and consumers [6,9]. Natural pigments isolated from botanical sources are being utilized for this purpose because they are sustainable, safe, and label friendly, as well as exhibiting additional functional attributes, such as antioxidant and antimicrobial activity [10]. Plants contain a variety of pigments with the potential of being utilized as pH-sensitive sensors, especially anthocyanins [11]. Anthocyanins are extracted from many different types of spices, fruits, and vegetables, including saffron, rich black plum peel, red cabbage, red barberry, blueberry, purple sweet potato, and cinnamomum camphora fruit [12,13,14,15,16]. Anthocyanins with antimicrobial and antioxidant effect can improve the shelf life of foods by acting as natural preservatives in packaging materials [17]. Anthocyanins are water-soluble pigments whose color is strongly dependent on the pH of their surroundings [18], which is due to changes in their chemical structure [2].
One of the most widely used film-forming biopolymers for constructing sustainable packaging materials is chitosan, which is a cationic polysaccharide obtained from crab shells and some mushrooms [6,19]. In this study, we explored the possibility of using chitosan to construct biopolymer films that were loaded with anthocyanin-based color sensors. Several researchers have reported that chitosan can form films that are thin, flexible, translucent, and capable of incorporating functional additives [19,20,21]. The fact that chitosan can form clear films is important as it enables any color changes to be detected more easily [8].
Hibiscus sabdariffa L. (Roselle plant) is an annual herbaceous plant that belongs to the Malvaceae family [22]. It is widely cultivated around the world, including Asia, Central America, and Africa. H. sabdariffa has been shown to exhibit antioxidant, antibacterial, anti-inflammatory, cholesterol lowering, and hepatoprotective properties, which has been ascribed to the presence of anthocyanins and other bioactive phytochemicals [23]. The flowers of H. sabdariffa have a strong reddish color, which is due to the presence of a variety of anthocyanins, including delphinidin-3-sambubioside, cyanidin-3-sambubioside, cyanidin-3-glucoside, and delphinidin-3-glucoside [24]. In a recently published article, Toro-Márquez, Merino and Gutiérrez [25] developed composite films based on corn starch, montmorillonite, and anthocyanin-Jamaica (Hibiscus sabdariffa) flower extract. They studied the structural, thermal, and physicomechanical properties and color change of films at different pH conditions (1, 7, and 13). The objective of our study was to determine whether H. sabdariffa pigments could be used as natural labels to detect changes in food quality. Our hypothesis was that H. sabdariffa pigments in packaging materials can detect quality changes in fresh foods during storage because their deterioration leads to changes in pH.
In this study, H. sabdariffa anthocyanins (HSAs) were incorporated into chitosan-based on-package colorimetric labels to monitor fish spoilage. Initially, the structural, mechanical, optical, and barrier characteristics of the HSA-chitosan labels were characterized. Then, the response of these labels to changes in pH and ammonia levels was characterized. Finally, the potential of the HSA-chitosan labels for monitoring fish spoilage during room storage was evaluated.
2. Materials and Methods
2.1. Materials
Dried Hibiscus (Hibiscus sabdariffa) calyces were procured from the local market (Tabriz, Republic of Iran). Chitosan (low molecular weight, 50,000–190,000 Da (based on viscosity), 20–300 cP, 1 wt% in 1% acetic acid at 25 °C) and degree of deacetylation = 75–85%), ammonia solution, and sodium hydroxide (NaOH) pellets were bought from Sigma-Aldrich (St. Louis, MI, USA). Glycerol, hydrochloric acid (HCL), acetic acid, and anhydrous CaCl2 were obtained from the Merck Co (Darmstadt, Germany). Ethanol (99.9%) was supplied by the Kimia Shimi Company (Tabriz, Iran). Fish meat was bought from the Fish Store (Tabriz, Iran). Analytical-grade reagents were used for all studies.
2.2. Anthocyanin Extraction
Anthocyanins were extracted from the H. sabdariffa calyces using a soaking method. Initially, the dried powder of H. sabdariffa was mixed with a blend of 70 mL distilled water and 30 mL ethanol and the mixture was gently agitated using a mechanical shaker (KS 130 Basic, IKA, Germany) at 50 rpm for 24 h. After filtration, the ethanol was evaporated from the resulting HSA solution at 50 °C. The color change and UV–visible spectra of the HSA solutions obtained were then characterized after their pH values were adjusted [26].
2.3. Fabrication of Colorimetric Label
The colorimetric pH-sensitivity label was prepared by a casting method. Initially, a film-forming solution containing 3% chitosan (w/v) and 30 wt% glycerol (w/v) (on chitosan basis) in 100 mL of 1% acetic acid was prepared by vigorously stirring at 25 °C for 5 h. Following this, 3% HSAs (w/v) was added to the chitosan gel matrix, and the resulting mixture was stirred for 15 min and then degassed for 3 min to remove any air bubbles. Finally, the resulting solution was decanted into a plastic petri dish (8 cm diameter) and dried at 37 °C in a drying oven to obtain the colorimetric labels. Each components concentration was calculated according to the chitosan weight. For comparison, a control chitosan label was prepared without H. sabdariffa.
2.4. Structural Characterization of Label
The surface microstructure of the labels was monitored by the SEM instrument (TeScan-Mira 3xmu SEM, Czech Republic) at 25 kV accelerating voltage. The molecular structures of the chitosan and HAS-loaded chitosan labels were characterized using a Fourier transform infrared (FTIR) spectrometer (Tensor 27, Bruker, Billerica, MA, USA) over the wavenumber range from 4000 to 400 cm−1 with 4 cm−1 resolutions. The tensile strength (TS) and elongation at break (EAB) of the labels were measured using a tensile testing machine (Model DBBP-20, Republic of Korea). The sample dimensions used were 10 cm × 1 cm, the maximum stretching distance was 30 mm, and the stretching speed was 10 mm/min.
2.5. Dimensions and Physical Properties of Labels
The thickness of the labels was measured at five randomly selected locations using a micrometer (Mitutoyo, Japan) and the average was calculated. The moisture content (MC) of the labels was computed by the difference in weight of the labels before and after drying at 110 °C for 24 h:
The swelling index (SI) of the labels was determined by measuring the weight of the label before (WDry) and after (WWet) they were soaked in 20 mL of distilled water for 24 h:
The water vapor permeability (WVP) was determined using a standardized gravimetric method (ASTM-E96-1995). First, the open tops of cylindrical cups containing 5 g anhydrous CaCl2 (0% RH, 25 °C) were sealed using the label material. The cups were then placed within a desiccator containing distilled water (100% RH, 25 °C) and weighed every 3 h 6 times.
The water contact angle (WCA) was measured using a contact angle analyzer (Phoneix 150, Republic of Korea): the sample size was 3 cm × 6 cm, the test liquid was water, the drop size was 5 μL, and the equilibrium time was 20 s.
2.6. Color Characterization of Label
The transparency of the labels was determined by dividing the film transmission measured at 600 nm using a spectrophotometer by the label thickness measured by a digital micrometer. The optical properties (absorption and transmittance) of the film samples were also recorded from 200 to 800 nm using a UV–vis spectrophotometer (Unico, UV-2100, Dayton, USA).
A colorimeter (Hunter lab, Konica Minolta, Japan) was used to analyze the tristimulus color coordinates (L, a, b) of the labels. The color difference (ΔE) was also calculated from the color coordinates:
The values from the white screen used as a back plate were: Ls (98.87), as (−2.54), and bs (3.95).
2.7. Ammonia Vapor Sensitivity Test
The sensitivity of the labels to ammonia vapor was analyzed using a method described previously with slight modifications. Briefly, labels were cut into a circle (2 cm diameter) and then placed in 80 mL of NH3 solution (0.8 M). Digital photographs of the samples were recorded every 5 min for 30 min. The sensitivity (SRGB) of the labels was then calculated:
2.8. Color Release Test
A color release test was used to evaluate the affinity for the anthocyanins for the chitosan-based labels. Each label was immersed in 30 mL of food simulants with different polarities (0%, 10%, 50%, or 95% ethanol in distilled water) and then gently shaken for 4 h. A UV–vis spectrophotometer was then used to measure the absorbance of the label solutions after predetermined times (15, 30, 60, 90, 120, 180, and 240 min). The pigment release rate was then calculated as anthocyanin/mm2.
2.9. Fish Spoilage Test
Fish slices were placed in a plastic container and an HSA-loaded label was affixed to the inner side of the top of each container. The containers were then stored at 25 °C for three days. The pH values, TVB-N levels, and color of the labels were recorded every 12 h at 25 °C. The color changes of the labels were documented using digital photography.
2.10. Statistical Analysis
Means and standard deviations (SD) were calculated from repeated experiments. The experimental results were tested using a one-way analysis of variance (ANOVA) by Microsoft Excel 2016. A statistically significant result was defined as p < 0.05.
3. Results and Discussion
3.1. pH Response of Anthocyanin Solutions
The halochromic behavior of the HSA solutions were characterized by measuring the color and UV–visible spectra at different pH values (2–12) (Figure 1a,b). With increasing pH, the color of the HSA solutions changed from reddish to pink to olive to yellow. This color change can be ascribed to pH-induced changes in the molecular characteristics of the anthocyanins, from flavylium cation (pH ≤ 3) to quinonoidal anhydro-base (pH 6–7) to chalcone (pH ≥ 8) (Figure 1c) [15]. The color change pattern of HAS was similar to onion peel extract [27].
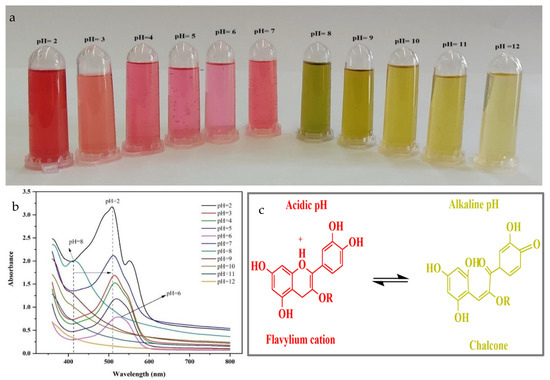
Figure 1.
(a) The appearance of the HSA solutions; (b) UV–vis spectra of HSA solutions at different pH values; (c) Chemical structure of anthocyanins at different pH values.
The maximum absorbance in the UV–visible spectra was around ~510 nm under highly acidic conditions, which corresponds to the flavylium cations. With increasing pH, the maximum absorbance gradually decreased and there was a slight bathochromic shift to higher wavelengths, which can be ascribed to the transition to a chalcone structure [28,29]. Similar findings have been reported for anthocyanins extracted from black plum peels [15] and blueberries [30]. The observed change in color of the HSA solutions with pH suggests that these anthocyanins might be suitable for application as natural halochromic indicators in the labels.
3.2. Structural Characterization of Labels
SEM was used to provide insights into the morphology of the chitosan-based labels (Figure 2a,b). The labels formed from chitosan had smooth, homogeneous, and compact surfaces in both the presence and absence of the HSAs. There was no evidence of cracks or other irregularities in the films containing the HSAs, suggesting that the anthocyanins were well dispersed within the chitosan matrix. This phenomenon can be attributed to the existence of OH groups in the anthocyanins, which can form intramolecular H-bonds with the chitosan molecules [31,32].
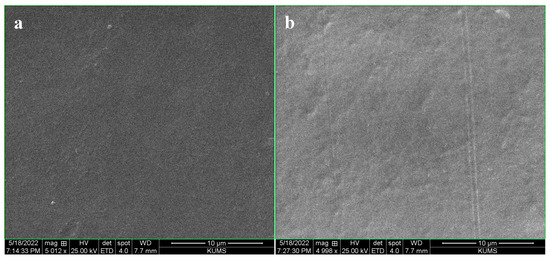
Figure 2.
SEM images of (a) pure chitosan and (b) HSA-loaded chitosan labels.
FTIR analysis was conducted to provide information about the molecular interactions of the chitosan-based labels (Figure 3). The FTIR spectra show that there was a peak at 3240 cm−1, which can be ascribed to O-H stretching vibrations caused by intramolecular and intermolecular hydrogen bonding, as well as N-H stretching vibrations caused by amino groups in the chitosan. The peak observed at 2895 cm−1 can be ascribed to the stretching vibration of C-H bonds in –CH2 and –CH3 groups. The peak observed at 1640 cm−1 refer to C=O stretching vibrations of Amide I groups. The absorption peak at 1010 cm−1 can be ascribed to C-O and C-N stretching vibrations molecules [1].
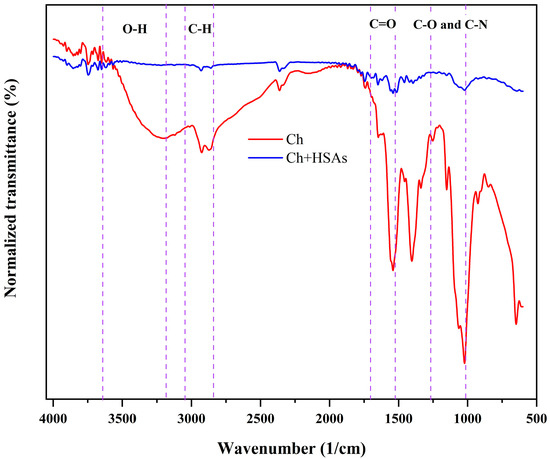
Figure 3.
FTIR spectra of pure chitosan and HSA-loaded chitosan labels.
A comparison of the FTIR spectra of the pure chitosan and the HSA-loaded chitosan labels indicated that the immobilization of anthocyanins results in noteworthy changes in the spectra of the label, which is consistent with changes in the composition and molecular interactions of the labels. The increase in intensity of the peaks after incorporation of the HSA into the chitosan labels can be attributed to the formation of H-bonds between the O-H groups in the anthocyanins and the O-H groups in the chitosan. Overall, these observations suggest that the HSA were compatible with the chitosan matrix.
Compared with the pure chitosan labels, the ones containing the HSAs had higher tensile strength and lower elongation at break values (Table 1). This effect can be ascribed to the strong binding of anthocyanin molecules to multiple chitosan chains leading to increased crosslinking. [33]. As a result, the labels became stronger and less flexible, which is in agreement with other studies on the impact of anthocyanins on the mechanical properties of biopolymer films [34].

Table 1.
Dimensions and physical properties of chitosan and HSA-loaded chitosan labels.
3.3. Dimensions and Physical Properties of Labels
The HAS-loaded chitosan label (85.4 µm) was significantly thicker than that of the pure chitosan label (79.8 µm) (Table 1), even though they were prepared under the same conditions. This effect can mainly be attributed to the increased solids content of the colorimetric labels caused by the presence of the anthocyanin additives. However, the crosslinking of the chitosan molecules by anthocyanin may also have altered the molecular organization of the biopolymer molecules in the labels, thereby rising their thickness. Other studies have also shown that adding anthocyanins to chitosan films increases their thickness [35].
The pure chitosan labels had a higher moisture content amount (16.3%) than the HSA-loaded ones (14.3%). This effect might have occurred because the interactions between the anthocyanin and chitosan molecules within the biopolymer matrix reduced the number of free hydroxyl groups available for water molecules to interact with [36,37]. The swelling index of the pure chitosan labels (102%) was significantly higher than that of the HSA-loaded ones (83.6%), which can be accredited to the fact that the crosslinking of the chitosan molecules in the biopolymer matrix by anthocyanins restricted their ability to swell in the presence of water [38].
Measurements of the WVP of packaging materials provide insights into their ability to resist the movement of water from inside to outside the packaging or vice versa, which has an important impact on food quality and shelf life. The WVP of the pure chitosan label (3.2 × 10−11 g m/m2 s Pa) was slightly more than the one that contained the HSAs (2.99 × 10−11 g m/m2 s Pa). This effect may have been for the reason that crosslinking of the chitosan molecules altered the microstructure of the biopolymer network (such as its porosity), which declined the ability of the water vapor molecules to travel through. Similar results have been found when another kind of anthocyanin was incorporated into polycaprolactone films [39].
The wettability of the labels was studied using a water contact angle test (Figure 4a,b). The wettability of the pure chitosan film (WCA = 63.7°) was greater than that of the one that contained the HSAs (57.8°). This result proposes that the presence of the anthocyanins in the labels reduced their hydrophobicity. This is probably because the anthocyanins are more hydrophilic than chitosan [40,41]. Other studies have also revealed that inclosing anthocyanins to biopolymer-based packaging materials reduces their contact angle [19,41,42].
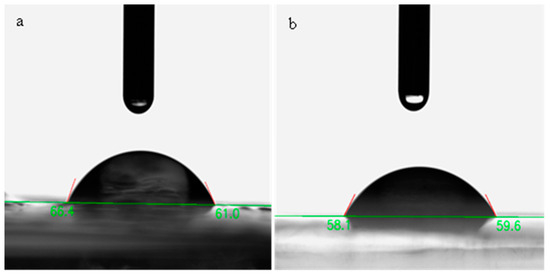
Figure 4.
Schematic diagram of WCA of pure Ch (a) and Ch/HSAs label (b).
3.4. Color Characterization of Labels
The appearance and transparency of packaging materials is important because it impacts consumer perception of food products, as well as the susceptibility of foods to photodegradation reactions that can cause discoloration and nutritional loss [43]. Visually, the pure chitosan labels appeared colorless and highly transparent, whereas the ones containing the HSAs appeared light brown and slightly cloudy, which suggests that the existence of the anthocyanins caused selective light absorption and scattering.
The UV–visible light transmittance and absorbance spectra of the labels were recorded via a UV–visible spectrophotometer to provide further insights into their optical properties (Figure 5a,b). The HSA-loaded chitosan labels exhibited greater UV light barrier characteristics and declined light transmittance (especially around ~280 nm) than the pure chitosan ones. This effect can be ascribed to the capability of anthocyanins to absorb UV light, as well as to their ability to change the microstructure of the chitosan network in a way that increases light scattering [28,44]. For instance, crosslinking of the chitosan molecules by the anthocyanins may have led to the formation of heterogeneities with dimensions close to the wavelength of UV light. The fact that the transmittance of the labels containing the anthocyanins was less in the visible wavelength range is consistent with the visible observations that they were less clear and browner than the pure chitosan labels. Reduction in clarity and changes in color of biopolymer-based films has been reported after incorporating anthocyanins by [8,43].
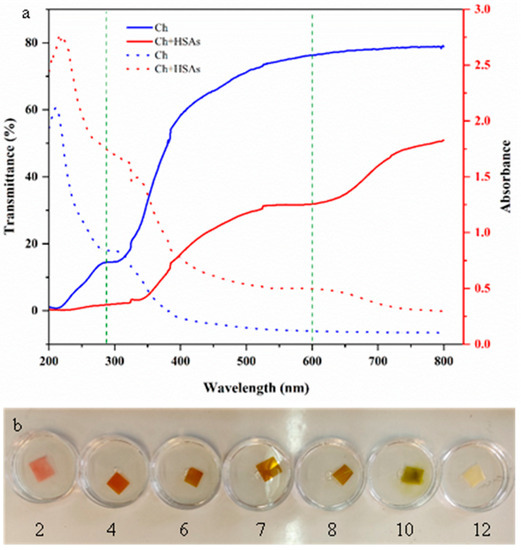
Figure 5.
(a) Impact of wavelength on the UV–visible transmittance and absorbance of pure chitosan and HSA-loaded chitosan labels; (b) Photographs of the HSA-loaded chitosan labels at pH values ranging from 2 to 12.
3.5. Ammonia Test
Seafood products release volatile ammonia when they deteriorate due to the generation of nitrogenous products by microorganisms when they digest proteins. The release of these gases can cause color changes in anthocyanins, which can therefore be used as a halochromic sensor of food quality. In this study, the sensitivity of the HSA-loaded chitosan films to ammonia compounds released from fish samples was evaluated using a sensitivity test (SRGB). The sensitivity of the labels to volatile NH3 was measured at 5-min intervals for 25 min. The color change and SRGB values of the labels are shown in Figure 6. Based on sensitivity results, the color of the labels changed from light brown to green and their SRGB value increased to 37.5% in the first 5 min. This color change can be ascribed to the fact that NH3 reacts with H2O in the labels, which results in the production of NH4+ and OH−. The presence of the NH4+ changes the pH inside the labels, which promotes a structural transformation of the anthocyanins, leading to a color change from light brown to green. The color of the labels changed from green to dark green after about 10 min of exposure to the ammonia atmosphere, and the SRGB value increased to 42.3%. Upon further storage, the labels became darker green and the SRGB values increased. These results indicate that the HSA-loaded chitosan labels responded significantly to the presence of the volatile ammonia within the first 5 min, which indicates they had a relatively fast response time. Other researchers have also reported that biopolymer films containing other kinds of anthocyanins also exhibit this kind of behavior [7].
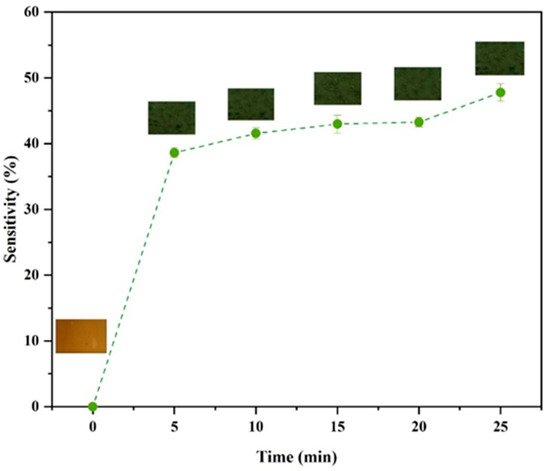
Figure 6.
Impact of incubation time on the color and color sensitivity of HSA-loaded chitosan labels in the presence of ammonia gas.
3.6. Release of HSA from Labels into Food Simulants
The release of anthocyanins from chitosan films into four model food simulants with different polarities is shown in Figure 7. All samples exhibited a relatively fast release during the first 30 min (1.2–5%) followed by a slower release over the next 60 min, until a relatively constant value was reached. The release of the anthocyanins depended on the alcohol concentration in the food simulants. The release of HSAs from the labels was fairly similar when they were exposed to contact with food simulants containing relatively low ethanol concentrations (0, 10, or 50%). However, the release rate was much smaller for the sample containing the highest ethanol concentration (95%). This effect may have been because high ethanol levels reduced the swelling of the labels, thereby inhibiting the diffusion of the anthocyanins out of the chitosan network. Similar findings have been reported by other researchers using different types of anthocyanins [6,45].
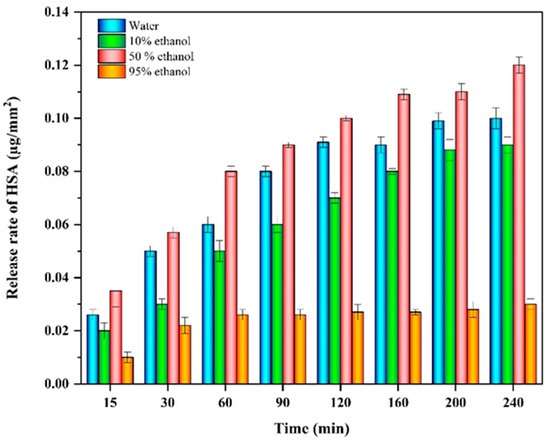
Figure 7.
Release rate of anthocyanins from HSA-loaded chitosan labels when brought into contact with food simulants with different polarities.
3.7. Monitoring of Fish Spoilage
Finally, the HSA-loaded chitosan labels were used to monitor the spoilage of fish during storage at 25 °C (Figure 8a). Visibly, the color of the labels changed from light brown before storage to grayish after storage. This phenomenon can be attributed to the increase in TVB-N level and pH due to decomposition of the fish protein tissue by enzymes and microorganisms, leading to ammonia production (Figure 8b). Indeed, the pH of the samples increased from 6.8 to 8.4 and the TVB-N concentration increased from 6.7 to 53.3 mg/100 g after 72 h storage. These results suggest that the fish samples would be inedible after three days storage because they exceeded the safe consumption threshold [46]. Trials on fish show that HSA-loaded chitosan labels may be applied for spoilage monitoring of fish samples, due to their significant color change (ΔE) at storage time. There were good linear correlations between the pH value of the fish and the color change (R2 = 0.990; Figure 8c), and between the TVB-N levels of the fish and the color change (R2= 0.9909; Figure 8d). Several other studies have shown the potential of anthocyanins as natural colorimetric sensors to monitor the freshness of foods. For instance, Wang et al. used a chitosan/chitin ester nanofiber film matrix and eggplant peel anthocyanins to distinguish pork freshness [47]. Their indicator films were also sensitive to NH3 and pH and provided information about changes in the freshness of the pork after storage of 48 h. In another study, Alnadari et al. reported similar results for freshness monitoring of beef using a carboxymethyl cellulose based indicator imbued with Cinnamomum camphora fruit peel waste anthocyanins [16].
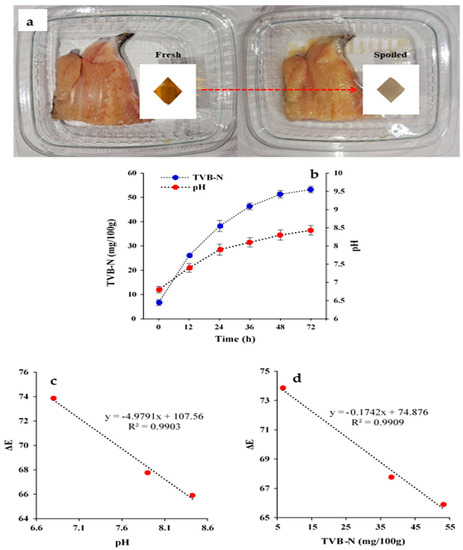
Figure 8.
(a) Color changes of on-packaging HSA-loaded chitosan labels during fish storage; (b) Change in TVB-N and pH values of fish sample during storage; (c,d) correlation analysis of quality parameters (pH, and TVB-N) of fish and ΔE of the labels.
4. Conclusions
This study has shown that on-package colorimetric labels can be assembled from natural pigments (anthocyanins) and polymers (chitosan). As observed, these labels changed color, owing to the presence of ammonia gas inside packaging and alterations in pH and could therefore be used to monitor changes in the fish quality at duration of storage. Microscopy and spectroscopy analysis suggested that the HSAs were successfully integrated into the chitosan matrix within the labels. The incorporation of the anthocyanins increased their mechanical strength but made them more brittle, which was attributed to their ability to crosslink the chitosan molecules. Moreover, the anthocyanins reduced the swelling and WVP of the chitosan films, which was again attributed to their crosslinking effects. The color change of the anthocyanins are pH and volatile nitrogenous gas contents dependent, which suggested they would be suitable for monitoring the spoilage of meat products. This was confirmed by using the anthocyanin-loaded chitosan labels to monitor the freshness of fish during the duration of storage. The labels turned from light brown (fresh) to grey (spoiled) after storage, with the magnitude of the color change being closely correlated to the pH and TVB-N levels.
These natural sensors could be utilized within the food industry to provide producers, retailers, and consumers with information about the freshness of foodstuffs in real time. In the food industry, the utilization of these smart packaging materials may boost sustainability and reduce environmental concerns.
Author Contributions
A.K.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, visualization, writing—original draft; M.T.: conceptualization, data curation, Formal analysis, funding acquisition, investigation, methodology, project administration, visualization, writing—original draft; M.A.S.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, visualization, writing—original draft; A.E.: conceptualization, funding acquisition, investigation, methodology, project administration, supervision, visualization, writing—original draft; D.J.M.: investigation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The study was approved and supported by the Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran [project no. 70155].
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no competing interest.
References
- Alizadeh Sani, M.; Khezerlou, A.; Tavassoli, M.; Mohammadi, K.; Hassani, S.; Ehsani, A.; McClements, D.J. Bionanocomposite active packaging material based on soy protein Isolate/Persian Gum/Silver nanoparticles; fabrication and characteristics. Colloids Interfaces 2022, 6, 57. [Google Scholar] [CrossRef]
- Abedi-Firoozjah, R.; Salim, S.A.; Hasanvand, S.; Assadpour, E.; Azizi-Lalabadi, M.; Prieto, M.A.; Jafari, S.M. Application of smart packaging for seafood: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1438–1461. [Google Scholar] [CrossRef]
- Kaur, G.; Sharma, S.; Mir, S.A.; Dar, B.N. Nanobiocomposite Films: A “Greener Alternate” for Food Packaging. Food Bioprocess Technol. 2021, 14, 1013–1027. [Google Scholar] [CrossRef]
- Milad, T.; Khezerlou, A.; Moghaddam, T.N.; Firoozy, S.; Bakhshizadeh, M.; Sani, M.A.; Hashemi, M.; Ehsani, A.; Lorenzo, J.M. Sumac (Rhus coriaria L.) anthocyanin loaded-pectin and chitosan nanofiber matrices for real-time monitoring of shrimp freshness. Int. J. Biol. Macromol. 2023, 242, 125044. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Costa, M.J.; Fuciños, C.; Pastrana, L.M.; Vicente, A.A. Development of Active and Nanotechnology-based Smart Edible Packaging Systems: Physical–chemical Characterization. Food Bioprocess Technol. 2014, 7, 1472–1482. [Google Scholar] [CrossRef]
- Arezou, K.; Tavassoli, M.; Alizadeh-Sani, M.; Hashemi, M.; Ehsani, A.; Bangar, S.P. Multifunctional food packaging materials: Lactoferrin loaded Cr-MOF in films-based gelatin/κ-carrageenan for food packaging applications. Int. J. Biol. Macromol. 2023, 251, 126334. [Google Scholar] [CrossRef]
- Tavassoli, M.; Alizadeh Sani, M.; Khezerlou, A.; Ehsani, A.; Jahed-Khaniki, G.; McClements, D.J. Smart Biopolymer-Based Nanocomposite Materials Containing pH-Sensing Colorimetric Indicators for Food Freshness Monitoring. Molecules 2022, 27, 3168. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W. pH-responsive chitosan-based film incorporated with alizarin for intelligent packaging applications. Food Hydrocoll. 2020, 102, 105629. [Google Scholar] [CrossRef]
- Becerril, R.; Nerín, C.; Silva, F. Bring some colour to your package: Freshness indicators based on anthocyanin extracts. Trends Food Sci. Technol. 2021, 111, 495–505. [Google Scholar] [CrossRef]
- López-Cruz, R.; Sandoval-Contreras, T.; Iñiguez-Moreno, M. Plant Pigments: Classification, Extraction, and Challenge of Their Application in the Food Industry. Food Bioprocess Technol. 2023, 1–17. [Google Scholar] [CrossRef]
- Qi, D.; Xiao, Y.; Xia, L.; Li, L.; Jiang, S.; Jiang, S.; Wang, H. Colorimetric films incorporated with nisin and anthocyanins of pomegranate/Clitoria ternatea for shrimp freshness monitoring and retaining. Food Packag. Shelf Life 2022, 33, 100898. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, X.; Zhai, X.; Huang, X.; Jiang, C.; Holmes, M. Preparation of an intelligent pH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cui, Z.; Shang, M.; Zhong, Y. A colorimetric film based on polyvinyl alcohol/sodium carboxymethyl cellulose incorporated with red cabbage anthocyanin for monitoring pork freshness. Food Packag. Shelf Life 2021, 28, 100641. [Google Scholar] [CrossRef]
- Sohany, M.; Tawakkal, I.S.; Ariffin, S.H.; Shah, N.N.; Yusof, Y.A. Characterization of Anthocyanin Associated Purple Sweet Potato Starch and Peel-Based pH Indicator Films. Foods 2021, 10, 2005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Yong, H.; Qin, Y.; Liu, J.; Liu, J. Development of multifunctional food packaging films based on chitosan, TiO2 nanoparticles and anthocyanin-rich black plum peel extract. Food Hydrocoll. 2019, 94, 80–92. [Google Scholar] [CrossRef]
- Alnadari, F.; Al-Dalali, S.; Pan, F.; Abdin, M.; Frimpong, E.B.; Dai, Z.; Al-Dherasi, A.; Zeng, X. Physicochemical Characterization, Molecular Modeling, and Applications of Carboxymethyl Chitosan-Based Multifunctional Films Combined with Gum Arabic and Anthocyanins. Food Bioprocess Technol. 2023, 1–19. [Google Scholar] [CrossRef]
- Fang, Z.; Zhao, Y.; Warner, R.D.; Johnson, S.K. Active and intelligent packaging in meat industry. Trends Food Sci. Technol. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, S.; Chen, X. A visual pH sensing film using natural dyes from Bauhinia blakeana Dunn. Sens. Actuators B Chem. 2014, 198, 268–273. [Google Scholar] [CrossRef]
- Gasti, T.; Dixit, S.; D’Souza, O.J.; Hiremani, V.D.; Vootla, S.K.; Masti, S.P.; Chougale, R.B.; Malabadi, R.B. Smart biodegradable films based on chitosan/methylcellulose containing Phyllanthus reticulatus anthocyanin for monitoring the freshness of fish fillet. Int. J. Biol. Macromol. 2021, 187, 451–461. [Google Scholar] [CrossRef]
- Chen, M.; Yan, T.; Huang, J.; Zhou, Y.; Hu, Y. Fabrication of halochromic smart films by immobilizing red cabbage anthocyanins into chitosan/oxidized-chitin nanocrystals composites for real-time hairtail and shrimp freshness monitoring. Int. J. Biol. Macromol. 2021, 179, 90–100. [Google Scholar] [CrossRef]
- Tavassoli, M.; Khezerlou, A.; Firoozy, S.; Ehsani, A.; Punia Bangar, S. Chitosan-based film incorporated with anthocyanins of red poppy (Papaver rhoeas L.) as a colorimetric sensor for the detection of shrimp freshness. Int. J. Food Sci. Technol. 2023, 58, 3050–3057. [Google Scholar] [CrossRef]
- Riaz, G.; Chopra, R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed. Pharmacother. 2018, 102, 575–586. [Google Scholar] [CrossRef]
- Nguyen, Q.-D.; Dang, T.-T.; Nguyen, T.-V.-L.; Nguyen, T.-T.-D.; Nguyen, N.-N. Microencapsulation of roselle (Hibiscus sabdariffa L.) anthocyanins: Effects of different carriers on selected physicochemical properties and antioxidant activities of spray-dried and freeze-dried powder. Int. J. Food Prop. 2022, 25, 359–374. [Google Scholar] [CrossRef]
- Jabeur, I.; Pereira, E.; Barros, L.; Calhelha, R.C.; Soković, M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Hibiscus sabdariffa L. as a source of nutrients, bioactive compounds and colouring agents. Food Res. Int. 2017, 100, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Toro-Márquez, L.A.; Merino, D.; Gutiérrez, T.J. Bionanocomposite Films Prepared from Corn Starch with and Without Nanopackaged Jamaica (Hibiscus sabdariffa) Flower Extract. Food Bioprocess Technol. 2018, 11, 1955–1973. [Google Scholar] [CrossRef]
- Huang, H.-L.; Tsai, I.L.; Lin, C.; Hang, Y.-H.; Ho, Y.-C.; Tsai, M.-L.; Mi, F.-L. Intelligent films of marine polysaccharides and purple cauliflower extract for food packaging and spoilage monitoring. Carbohydr. Polym. 2023, 299, 120133. [Google Scholar] [CrossRef]
- Gulati, M.; Keshav Murthy, P.S.; Reddy, J.P. Effect of Onion Peel Extract on Structural, Mechanical, Thermal, and Antioxidant Properties of Methylcellulose Films. Food Bioprocess Technol. 2023, 16, 1–15. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Zheng, P.; Kang, X.; Chen, M.; Li, Y.; Ge, Y.; Hu, Y.; Pang, J. Preparation of an intelligent film based on chitosan/oxidized chitin nanocrystals incorporating black rice bran anthocyanins for seafood spoilage monitoring. Carbohydr. Polym. 2019, 222, 115006. [Google Scholar] [CrossRef]
- Luchese, C.L.; Sperotto, N.; Spada, J.C.; Tessaro, I.C. Effect of blueberry agro-industrial waste addition to corn starch-based films for the production of a pH-indicator film. Int. J. Biol. Macromol. 2017, 104, 11–18. [Google Scholar] [CrossRef]
- Luchese, C.L.; Abdalla, V.F.; Spada, J.C.; Tessaro, I.C. Evaluation of blueberry residue incorporated cassava starch film as pH indicator in different simulants and foodstuffs. Food Hydrocoll. 2018, 82, 209–218. [Google Scholar] [CrossRef]
- Amaregouda, Y.; Kamanna, K.; Gasti, T. Fabrication of intelligent/active films based on chitosan/polyvinyl alcohol matrices containing Jacaranda cuspidifolia anthocyanin for real-time monitoring of fish freshness. Int. J. Biol. Macromol. 2022, 218, 799–815. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, Q.; Yu, H.; Chen, X.; Yuan, G. Colorimetric indicator based on chitosan/gelatin with nano-ZnO and black peanut seed coat anthocyanins for application in intelligent packaging. Food Res. Int. 2022, 160, 111664. [Google Scholar] [CrossRef]
- Jiang, G.; Hou, X.; Zeng, X.; Zhang, C.; Wu, H.; Shen, G.; Li, S.; Luo, Q.; Li, M.; Liu, X.; et al. Preparation and characterization of indicator films from carboxymethyl-cellulose/starch and purple sweet potato (Ipomoea batatas (L.) lam) anthocyanins for monitoring fish freshness. Int. J. Biol. Macromol. 2020, 143, 359–372. [Google Scholar] [CrossRef]
- Halász, K.; Csóka, L. Black chokeberry (Aronia melanocarpa) pomace extract immobilized in chitosan for colorimetric pH indicator film application. Food Packag. Shelf Life 2018, 16, 185–193. [Google Scholar] [CrossRef]
- Lan, W.; Wang, S.; Zhang, Z.; Liang, X.; Liu, X.; Zhang, J. Development of red apple pomace extract/chitosan-based films reinforced by TiO2 nanoparticles as a multifunctional packaging material. Int. J. Biol. Macromol. 2021, 168, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and characterization of active and intelligent packaging films based on cassava starch and anthocyanins from Lycium ruthenicum Murr. Int. J. Biol. Macromol. 2019, 134, 80–90. [Google Scholar] [CrossRef]
- You, P.; Wang, L.; Zhou, N.; Yang, Y.; Pang, J. A pH-intelligent response fish packaging film: Konjac glucomannan/carboxymethyl cellulose/blackcurrant anthocyanin antibacterial composite film. Int. J. Biol. Macromol. 2022, 204, 386–396. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Pu, Y.; Chen, S.; Li, H.; Zhong, Y. Novel colorimetric films based on polyvinyl alcohol/sodium carboxymethyl cellulose doped with anthocyanins and betacyanins to monitor pork freshness. Food Chem. 2023, 404, 134426. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Zou, X.; Arslan, M.; Shi, J.; Zhai, X.; Xiao, J.; Wang, X.; Huang, X.; Li, Z.; et al. A high-stable and sensitive colorimetric nanofiber sensor based on PCL incorporating anthocyanins for shrimp freshness. Food Chem. 2022, 377, 131909. [Google Scholar] [CrossRef]
- Lavrič, G.; Oberlintner, A.; Filipova, I.; Novak, U.; Likozar, B.; Vrabič-Brodnjak, U. Functional Nanocellulose, Alginate and Chitosan Nanocomposites Designed as Active Film Packaging Materials. Polymers 2021, 13, 2523. [Google Scholar] [CrossRef]
- Fernández-Marín, R.; Fernandes, S.C.M.; Sánchez, M.Á.A.; Labidi, J. Halochromic and antioxidant capacity of smart films of chitosan/chitin nanocrystals with curcuma oil and anthocyanins. Food Hydrocoll. 2022, 123, 107119. [Google Scholar] [CrossRef]
- Sani, M.A.; Dabbagh-Moghaddam, A.; Jahed-Khaniki, G.; Ehsani, A.; Sharifan, A.; Khezerlou, A.; Tavassoli, M.; Maleki, M. Biopolymers-based multifunctional nanocomposite active packaging material loaded with zinc oxide nanoparticles, quercetin and natamycin; development and characterization. J. Food Meas. Charact. 2023, 17, 2488–2504. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Bai, R.; Miao, Z.; Zhang, X.; Liu, J. Development of antioxidant and intelligent pH-sensing packaging films by incorporating purple-fleshed sweet potato extract into chitosan matrix. Food Hydrocoll. 2019, 90, 216–224. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Zhang, X.; Liu, Y.; Qin, Y.; Liu, J. Effects of anthocyanin-rich purple and black eggplant extracts on the physical, antioxidant and pH-sensitive properties of chitosan film. Food Hydrocoll. 2019, 94, 93–104. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of gelatin/carrageenan-based color-indicator film integrated with shikonin and propolis for smart food packaging applications. ACS Appl. Bio Mater. 2020, 4, 770–779. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, T.-S.; Yan, H.; Hu, X.; Ren, T. Novel pH-sensitive films based on starch/polyvinyl alcohol and food anthocyanins as a visual indicator of shrimp deterioration. Int. J. Biol. Macromol. 2020, 145, 768–776. [Google Scholar] [CrossRef]
- Wang, F.; Zhan, J.; Ma, R.; Tian, Y. Simultaneous improvement of the physical and biological properties of starch films by incorporating steviol glycoside-based solid dispersion. Carbohydr. Polym. 2023, 311, 120766. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).