Synthesis of Vanadia-Mayenite Nanocomposites and Characterization of Their Structure, Morphology and Surface Sites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Materials
2.2. Characterization of the Samples
3. Results
3.1. Textural Properties of the Materials
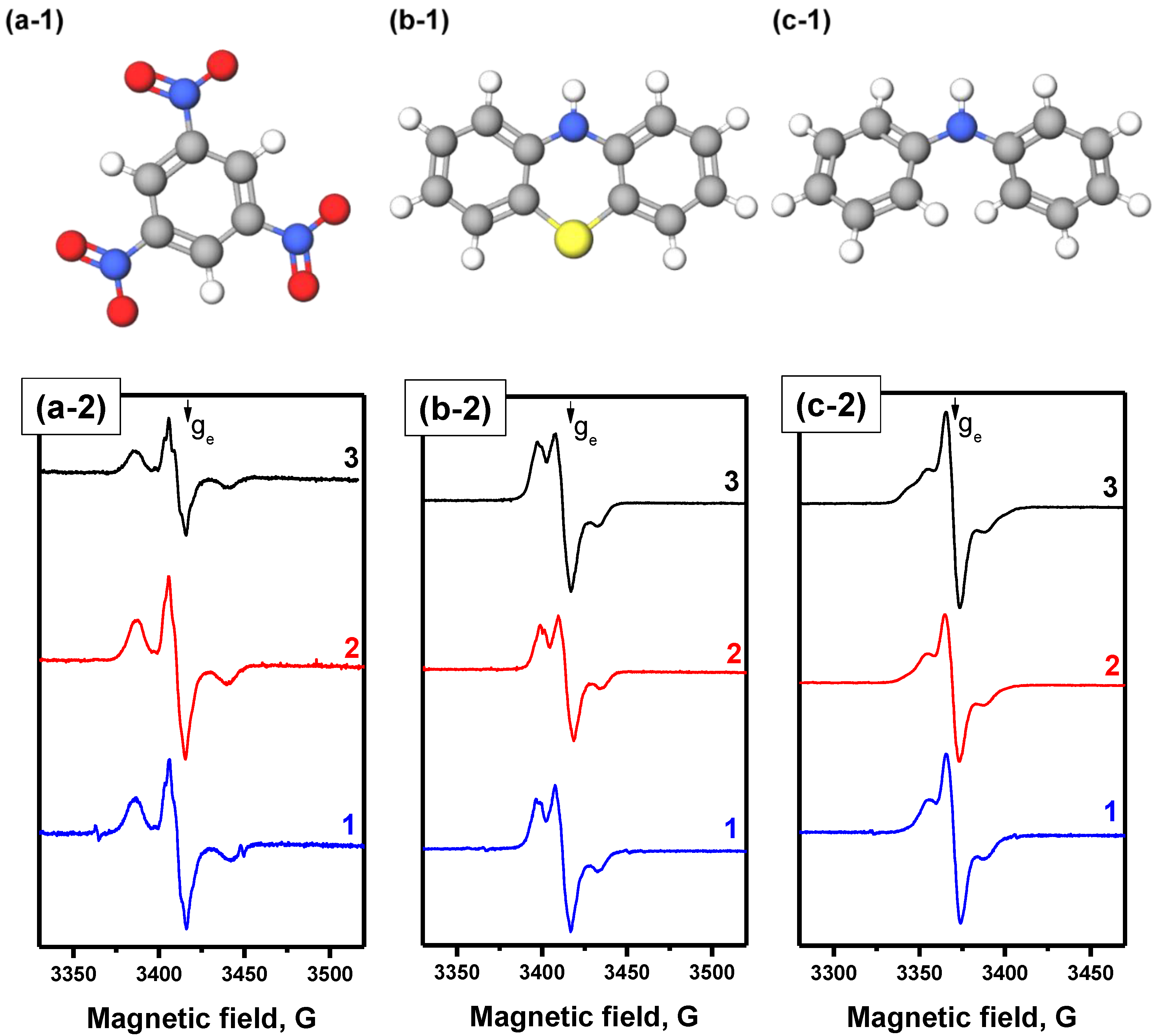
3.2. EPR Study
3.3. XRD Study
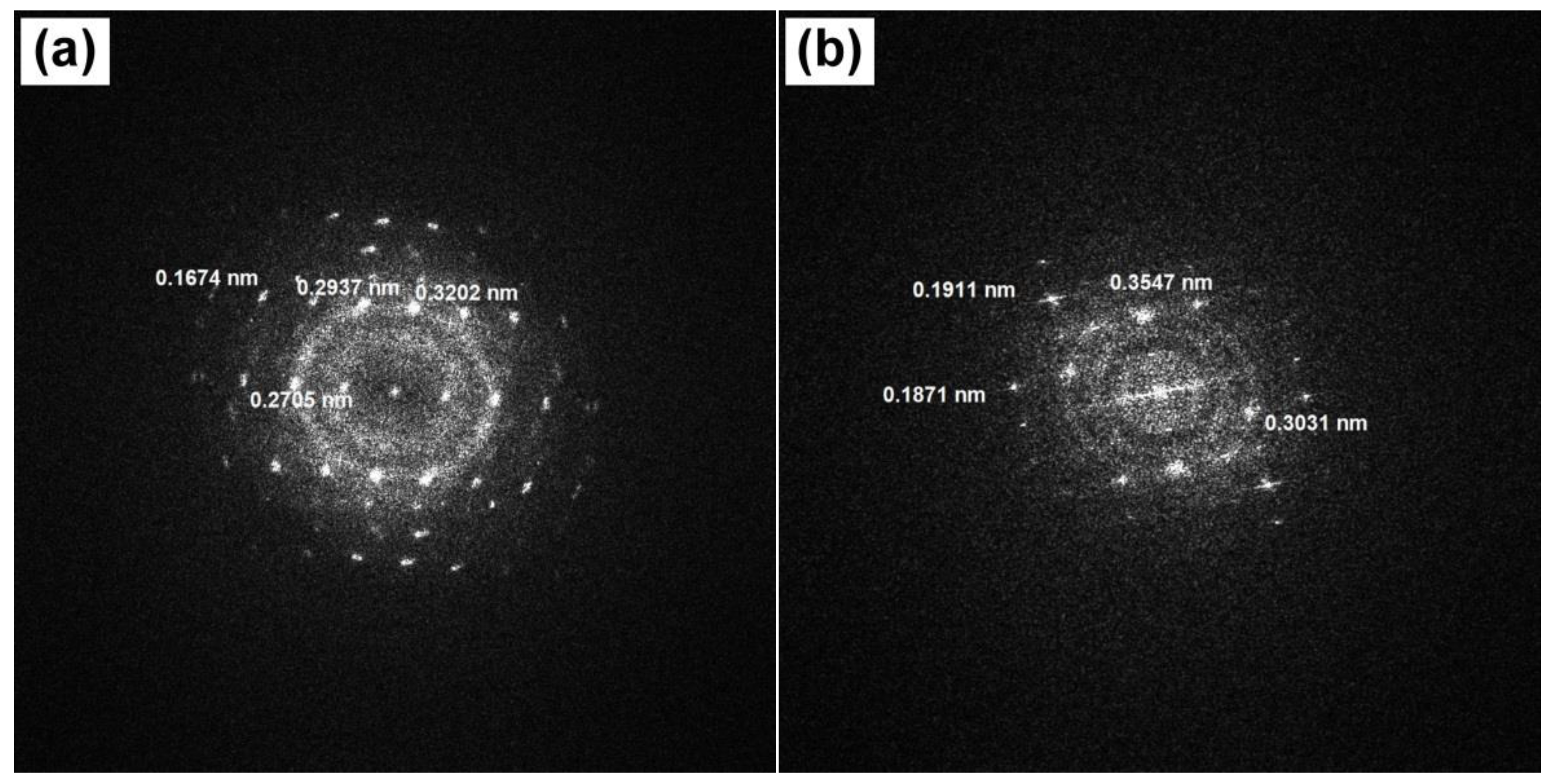
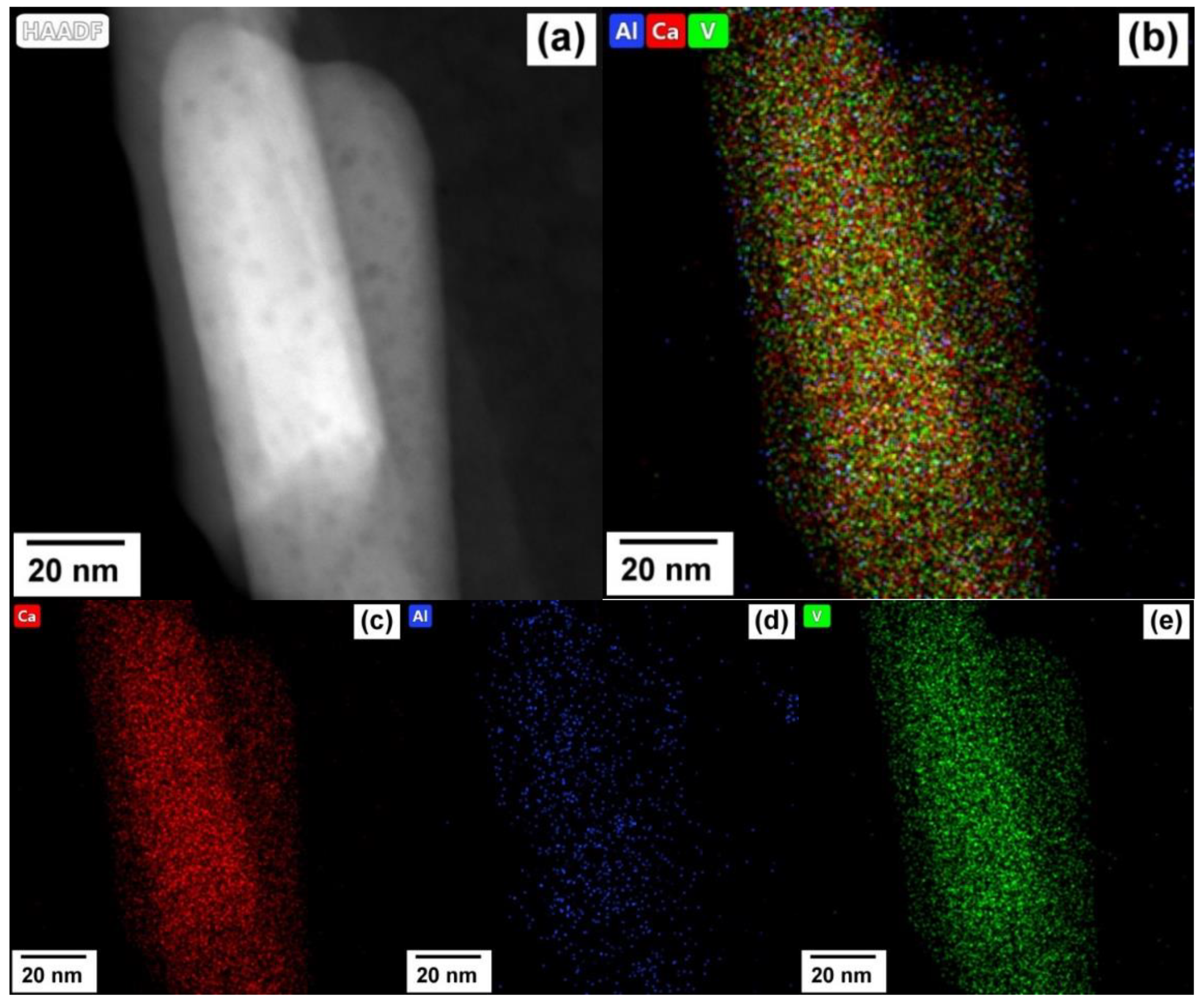
3.4. TEM Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ideker, J.H.; Scrivener, K.L.; Fryda, H.; Touzo, B. Calcium Aluminate Cements. In Lea’s Chemistry of Cement and Concrete; Butterworth-Heinemann: Oxford, UK, 2019; pp. 537–584. [Google Scholar]
- Hayashi, K.; Matsuishi, S.; Kamiya, T.; Hirano, M.; Hosono, H. Light-induced conversion of an insulating refractory oxide into a persistent electronic conductor. Nature 2002, 419, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hosono, H.; Hirano, M.; Hayashi, K.; Nishioka, M.; Kashiwagi, H.; Torimoto, Y.; Sadakata, M. High-intensity atomic oxygen radical anion emission mechanism from 12CaO·7Al2O3 crystal surface. Surf. Sci. 2003, 527, 100–112. [Google Scholar] [CrossRef]
- Yang, S.; Kondo, J.N.; Hayashi, K.; Hirano, M.; Domen, K.; Hosono, H. Partial oxidation of methane to syngas over promoted C12A7. Appl. Catal. A Gen. 2004, 277, 239–246. [Google Scholar] [CrossRef]
- Kitano, M.; Inoue, Y.; Yamazaki, Y.; Hayashi, F.; Kanbara, S.; Matsuishi, S.; Yokoyama, T.; Kim, S.-W.; Hara, M.; Hosono, H. Ammonia synthesis using a stable electride as an electron donor and reversible hydrogen store. Nat. Chem. 2012, 4, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Kuehster, A.E.; DeBoer, B.A.; Preston, A.D.; Ma, K. Enhanced thermionic emission of mayenite electride composites in an Ar glow discharge plasma. Ceram. Int. 2021, 47, 16614–16631. [Google Scholar] [CrossRef]
- Miyakawa, M.; Murata, H.; Imai, M. Direct synthesis of H−-encaged 12CaO·7Al2O3 crystals by high-pressure processes. Ceram. Int. 2019, 45, 16028–16031. [Google Scholar] [CrossRef]
- Meza-Trujillo, I.; Devred, F.; Gaigneaux, E.M. Production of high surface area mayenite (C12A7) via an assisted solution combustion synthesis (SCS) toward catalytic soot oxidation. Mater. Res. Bull. 2019, 119, 110621. [Google Scholar] [CrossRef]
- Volodin, A.M.; Zaikovskii, V.I.; Kenzhin, R.M.; Bedilo, A.F.; Mishakov, I.V.; Vedyagin, A.A. Synthesis of nanocrystalline calcium aluminate C12A7 under carbon nanoreactor conditions. Mater. Lett. 2017, 189, 210–212. [Google Scholar] [CrossRef]
- Volodin, A.M.; Bedilo, A.F.; Stoyanovskii, V.O.; Zaikovskii, V.I. High-temperature synthesis of finely dispersed oxide materials and C12A7:e electrides in carbon nanoreactor conditions. Nanosyst. Phys. Chem. Math. 2018, 9, 558–567. [Google Scholar] [CrossRef]
- Kapishnikov, A.V.; Kenzhin, R.M.; Koskin, A.P.; Volodin, A.M.; Geydt, P.V. Mayenite synthesis from hydroxide precursors: Structure formation and active sites on its surface. Materials 2022, 15, 778. [Google Scholar] [CrossRef]
- Khan, K.; Tareen, A.K.; Aslam, M.; Thebo, K.H.; Khan, U.; Wang, R.; Shams, S.S.; Han, Z.; Ouyang, Z. A comprehensive review on synthesis of pristine and doped inorganic room temperature stable mayenite electride, [Ca24Al28O64]4+(e−)4 and its applications as a catalyst. Prog. Solid State Chem. 2019, 54, 1–19. [Google Scholar] [CrossRef]
- Huang, J.; Valenzano, L.; Sant, G. Framework and channel modifications in mayenite (12CaO·7Al2O3) nanocages By cationic doping. Chem. Mater. 2015, 27, 4731–4741. [Google Scholar] [CrossRef]
- Kumamoto, N.; Nakauchi, D.; Kato, T.; Okada, G.; Kawaguchi, N.; Yanagida, T. Photoluminescence, scintillation and thermally-stimulated luminescence properties of Tb-doped 12CaO·7Al2O3 single crystals grown by FZ method. J. Rare Earths 2017, 35, 957–963. [Google Scholar] [CrossRef]
- Kumaresh, T.; Awin, E.W.; Bhaskar, L.K.; Djordjevic, M.P.; Matović, B.; Kumar, R. Combustion synthesis of luminescent Eu-doped single phase Mayenite. J. Solid State Chem. 2021, 302, 122420. [Google Scholar] [CrossRef]
- Duan, J.; Liu, Y.; Pan, X.; Gu, Y.; Zheng, X.; Li, W.; Wang, W.; Wang, C.; Yu, J. Transparency, photoluminescence and X-ray luminescence study of Eu3+ doped mayenite glass. Mater. Lett. 2016, 173, 102–106. [Google Scholar] [CrossRef]
- Teusner, M.; De Souza, R.A.; Krause, H.; Ebbinghaus, S.G.; Martin, M. Oxygen transport in undoped and doped mayenite. Solid State Ion. 2016, 284, 25–27. [Google Scholar] [CrossRef]
- Ruttanapun, C.; Srepusharawoot, P.; Maensiri, S. Effect of Fe3+-doped Ca12Al14O33 cement on optical and thermal properties. Chin. J. Phys. 2018, 56, 252–260. [Google Scholar] [CrossRef]
- Savuto, E.; Di Carlo, A.; Gallucci, K.; Natali, S.; Bocci, E. Characterization and performance analysis of an innovative Ni/Mayenite catalyst for the steam reforming of raw syngas. Fuel 2017, 194, 348–356. [Google Scholar] [CrossRef]
- Salasin, J.R.; Schwerzler, S.E.A.; Koehler, M.R.; Keffer, D.J.; Rawn, C.J. The effect of process parameters on the amorphous citrate sol-gel synthesis of Cu-doped Ca12Al14O33. Materialia 2018, 4, 466–477. [Google Scholar] [CrossRef]
- Meza-Trujillo, I.; Mary, A.; Pietrzyk, P.; Sojka, Z.; Gaigneaux, E.M. Nature and role of Cu(II) species in doped C12A7 catalysts for soot oxidation. Appl. Catal. B Environ. 2022, 316, 121604. [Google Scholar] [CrossRef]
- Balcaen, V.; Sack, I.; Olea, M.; Marin, G.B. Transient kinetic modeling of the oxidative dehydrogenation of propane over a vanadia-based catalyst in the absence of O2. Appl. Catal. A Gen. 2009, 371, 31–42. [Google Scholar] [CrossRef]
- Rodemerck, U.; Sokolov, S.; Stoyanova, M.; Bentrup, U.; Linke, D.; Kondratenko, E.V. Influence of support and kind of VO species on isobutene selectivity and coke deposition in non-oxidative dehydrogenation of isobutane. J. Catal. 2016, 338, 174–183. [Google Scholar] [CrossRef]
- Rodemerck, U.; Stoyanova, M.; Kondratenko, E.V.; Linke, D. Influence of the kind of VOx structures in VOx/MCM-41 on activity, selectivity and stability in dehydrogenation of propane and isobutane. J. Catal. 2017, 352, 256–263. [Google Scholar] [CrossRef]
- Hu, P.; Lang, W.-Z.; Yan, X.; Chen, X.-F.; Guo, Y.-J. Vanadium-doped porous silica materials with high catalytic activity and stability for propane dehydrogenation reaction. Appl. Catal. A Gen. 2018, 553, 65–73. [Google Scholar] [CrossRef]
- Shan, Y.-L.; Zhao, W.-T.; Zhao, S.-L.; Wang, X.-X.; Sun, H.-L.; Yu, W.-L.; Ding, J.-W.; Feng, X.; Chen, D. Effects of alumina phases on the structure and performance of VOx/Al2O3 catalysts in non-oxidative propane dehydrogenation. Mol. Catal. 2021, 504, 111466. [Google Scholar] [CrossRef]
- García, H.; Roth, H.D. Generation and reactions of organic radical cations in zeolites. Chem. Rev. 2002, 102, 3947–4008. [Google Scholar] [CrossRef]
- Chiesa, M.; Giamello, E.; Che, M. EPR Characterization and reactivity of surface-localized inorganic radicals and radical ions. Chem. Rev. 2009, 110, 1320–1347. [Google Scholar] [CrossRef]
- Golubeva, E.N.; Chumakova, N.A. Spin probe method for diagnostics of polyester porous matrixes formed in supercritical carbon dioxide (Review). Russ. J. Phys. Chem. B 2020, 13, 1088–1094. [Google Scholar] [CrossRef]
- Anpo, M.; Costentin, G.; Giamello, E.; Lauron-Pernot, H.; Sojka, Z. Characterisation and reactivity of oxygen species at the surface of metal oxides. J. Catal. 2021, 393, 259–280. [Google Scholar] [CrossRef]
- Malykhin, S.E.; Volodin, A.M.; Bedilo, A.F.; Zhidomirov, G.M. Generation of O− radical anions on MgO surface: Long-distance charge separation or homolytic dissociation of chemisorbed water? J. Phys. Chem. C 2009, 113, 10350–10353. [Google Scholar] [CrossRef]
- Bedilo, A.F.; Volodin, A.M. Radical cations of aromatic molecules with high ionization potentials on the surfaces of oxide catalysts: Formation, properties, and reactivity. Kinet. Catal. 2009, 50, 314–324. [Google Scholar] [CrossRef]
- Bedilo, A.F.; Shuvarakova, E.I.; Rybinskaya, A.A.; Medvedev, D.A. Characterization of electron-donor and electron-acceptor sites on the surface of sulfated alumina using spin probes. J. Phys. Chem. C 2014, 118, 15779–15794. [Google Scholar] [CrossRef]
- Shuvarakova, E.I.; Bedilo, A.F.; Chesnokov, V.V.; Kenzhin, R.M. Dehydrochlorination of 1-chlorobutane over nanocrystalline MgO: The role of electron-acceptor sites. Top. Catal. 2018, 61, 2035–2041. [Google Scholar] [CrossRef]
- Medvedev, D.A.; Rybinskaya, A.A.; Kenzhin, R.M.; Volodin, A.M.; Bedilo, A.F. Characterization of electron donor sites on Al2O3 surface. Phys. Chem. Chem. Phys. 2012, 14, 2587–2598. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Chen, S.; Pan, D.; Cui, X.; Qiao, Y.; Li, R. Ordered mesoporous alumina-supported vanadium oxides as an efficient catalyst for ethylbenzene dehydrogenation to styrene with CO2. Catal. Commun. 2018, 115, 12–16. [Google Scholar] [CrossRef]
- Held, A.; Kowalska-Kuś, J.; Janiszewska, E.; Jankowska, A.; Nowińska, K. Epoxidation of propane with oxygen and/or nitrous oxide over silica-supported vanadium oxide. J. Catal. 2021, 404, 231–243. [Google Scholar] [CrossRef]
- Huang, Z.; Ma, S.; Qu, B.; Li, D.; Zhou, R. Migration and oxidation of vanadium atom and dimer supported on anatase TiO2(1 0 1) surface. Appl. Surf. Sci. 2021, 565, 150517. [Google Scholar] [CrossRef]
- Rajitha, P.; Mahesh Kumar, P.; Mallesh, D.; Lingaiah, N. Understanding the role of Al2O3-ZrO2 mixed oxides as support for vanadium catalysts for the selective ammoxidation of o-chlorotoluene to o-chlorobenzonitrile. Mol. Catal. 2021, 515, 111885. [Google Scholar] [CrossRef]
- Fernandes, A.; Ribeiro, M.F.; Lourenço, J.P. Conversion of glycerol over vanadium supported beta zeolite: Role of acidity and alkali cations. Microporous Mesoporous Mater. 2022, 329, 111536. [Google Scholar] [CrossRef]
- Di Carlo, A.; Borello, D.; Sisinni, M.; Savuto, E.; Venturini, P.; Bocci, E.; Kuramoto, K. Reforming of tar contained in a raw fuel gas from biomass gasification using nickelmayenite catalyst. Int. J. Hydrogen Energy 2015, 40, 9088–9095. [Google Scholar] [CrossRef]
- Thommes, M. Physical adsorption characterization of nanoporous materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Thommes, M. Progress in the physisorption characterization of nanoporous gas storage materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption hysteresis loops and the characterization of nanoporous materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Volodin, A.M.; Kenzhin, R.M.; Stoyanovskii, V.O.; Rogov, V.A.; Kriventsov, V.V.; Mishakov, I.V. The role of chemisorbed water in formation and stabilization of active sites on Pd/Alumina oxidation catalysts. Catal. Today 2018, 307, 102–110. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Volodin, A.M.; Stoyanovskii, V.O.; Kenzhin, R.M.; Plyusnin, P.E.; Shubin, Y.V.; Mishakov, I.V. Effect of alumina phase transformation on stability of low-loaded Pd-Rh catalysts for CO oxidation. Top. Catal. 2016, 60, 152–161. [Google Scholar] [CrossRef]
- Luchez, F.; Carré, S.; Moissette, A.; Poizat, O. Sorption and spontaneous ionization of phenothiazine within channel type zeolites: Effect of the confinement on the electron transfers. RSC Adv. 2011, 1, 341–350. [Google Scholar] [CrossRef]
- Shaydyuk, Y.; Turrell, S.; Moissette, A.; Hureau, M.; Gomza, Y.; Klepko, V.; Lebovka, N. New phenothiazine–laponite hybrid systems: Adsorption and ionization. J. Mol. Struct. 2014, 1056–1057, 1–6. [Google Scholar] [CrossRef]
- Freitas, V.L.S.; Gomes, J.R.B.; Liebman, J.F.; Ribeiro da Silva, M.D.M.C. Energetic and reactivity properties of 9,10-dihydroacridine and diphenylamine: A comparative overview. J. Chem. Thermodyn. 2017, 115, 276–284. [Google Scholar] [CrossRef]
- Ilyina, E.V.; Gerus, Y.Y.; Cherepanova, S.V.; Bedilo, A.F. Synthesis of C12A7 calcium aluminate aerogels. Mater. Lett. 2021, 293, 129699. [Google Scholar] [CrossRef]
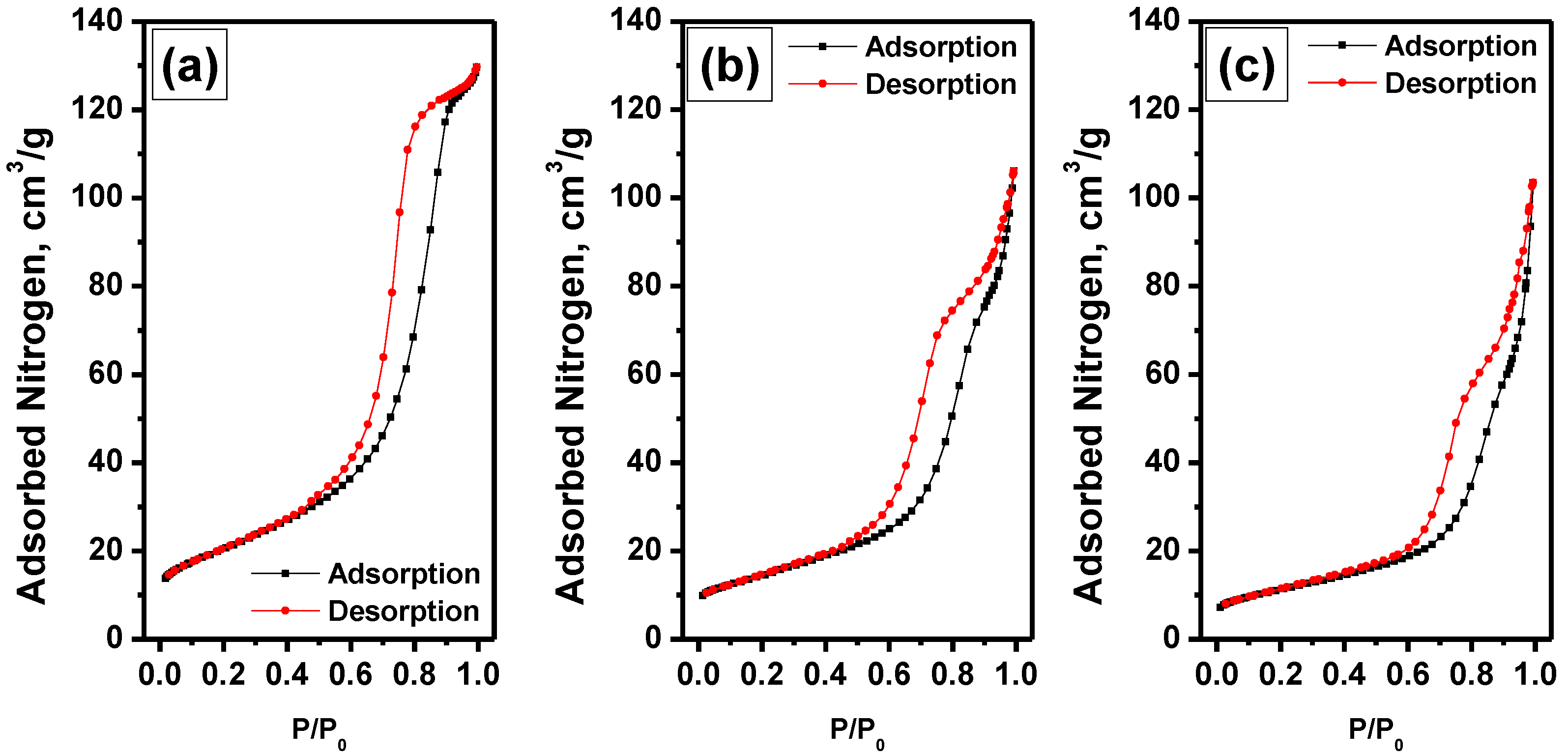
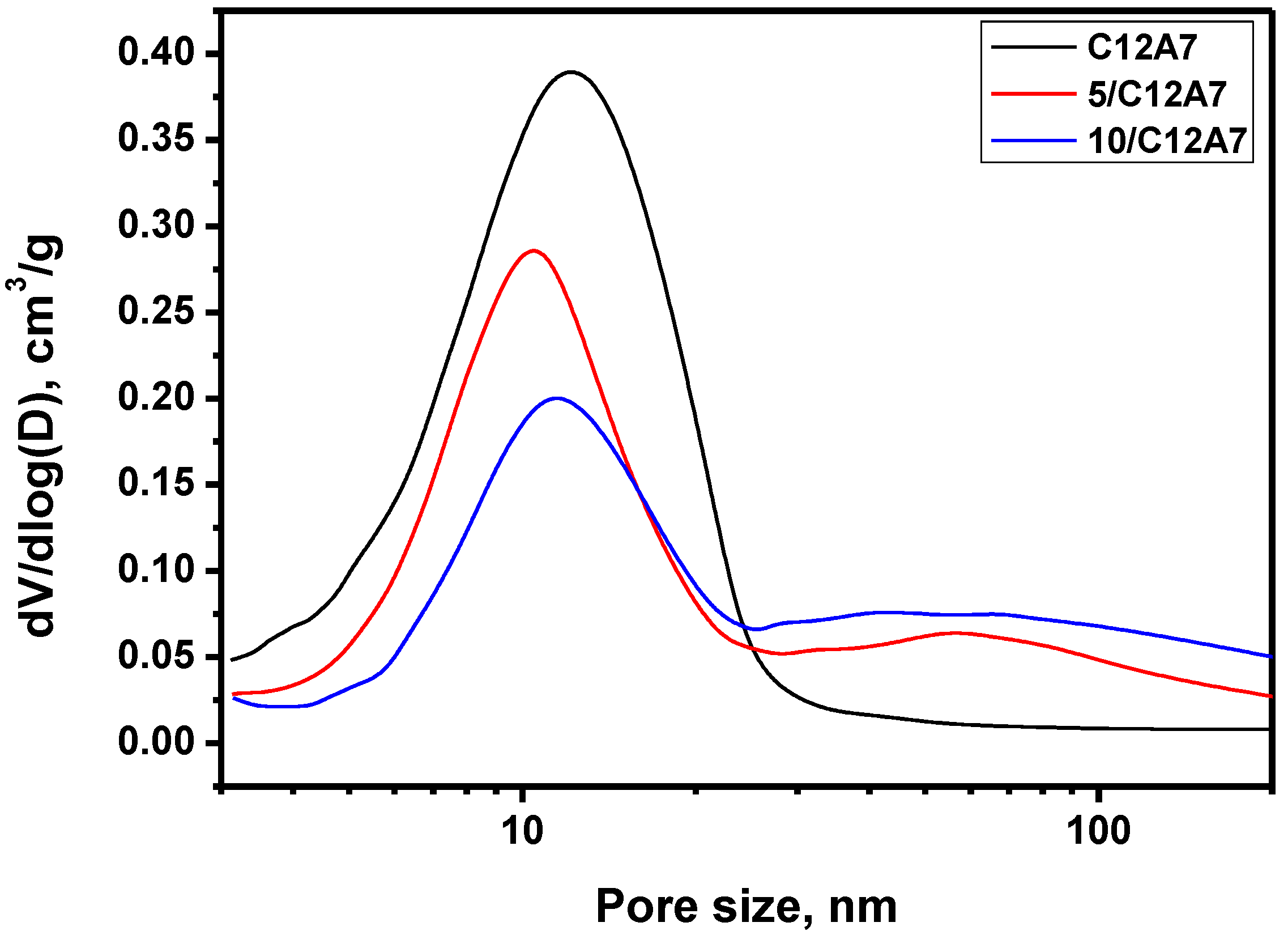


| Sample | SSA, m2∙g−1 | Vpore, cm3∙g−1 | Dav, nm |
|---|---|---|---|
| C12A7 | 73 ± 4 | 0.20 ± 0.01 | 11 ± 0.6 |
| 5%V/C12A7 | 52 ± 3 | 0.16 ± 0.01 | 13 ± 0.7 |
| 10%V/C12A7 | 40 ± 2 | 0.16 ± 0.01 | 16 ± 0.8 |
| Spin Probe | Sample | ||
|---|---|---|---|
| C12A7 | 5%V/C12A7 | 10% V/C12A7 | |
| Diphenylamine | 0.71 ± 0.14 | 0.64 ± 0.13 | 0.97 ± 0.20 |
| Phenothiazine | 0.93 ± 0.19 | 0.92 ± 0.18 | 1.33 ± 0.27 |
| 1,3,5-Trinitrobenzene | 0.68 ± 0.14 | 0.69 ± 0.12 | 0.58 ± 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuvarakova, E.I.; Ilyina, E.V.; Cherepanova, S.V.; Gerasimov, E.Y.; Bedilo, A.F.; Vedyagin, A.A. Synthesis of Vanadia-Mayenite Nanocomposites and Characterization of Their Structure, Morphology and Surface Sites. J. Compos. Sci. 2022, 6, 254. https://doi.org/10.3390/jcs6090254
Shuvarakova EI, Ilyina EV, Cherepanova SV, Gerasimov EY, Bedilo AF, Vedyagin AA. Synthesis of Vanadia-Mayenite Nanocomposites and Characterization of Their Structure, Morphology and Surface Sites. Journal of Composites Science. 2022; 6(9):254. https://doi.org/10.3390/jcs6090254
Chicago/Turabian StyleShuvarakova, Ekaterina I., Ekaterina V. Ilyina, Svetlana V. Cherepanova, Evgeny Y. Gerasimov, Alexander F. Bedilo, and Aleksey A. Vedyagin. 2022. "Synthesis of Vanadia-Mayenite Nanocomposites and Characterization of Their Structure, Morphology and Surface Sites" Journal of Composites Science 6, no. 9: 254. https://doi.org/10.3390/jcs6090254
APA StyleShuvarakova, E. I., Ilyina, E. V., Cherepanova, S. V., Gerasimov, E. Y., Bedilo, A. F., & Vedyagin, A. A. (2022). Synthesis of Vanadia-Mayenite Nanocomposites and Characterization of Their Structure, Morphology and Surface Sites. Journal of Composites Science, 6(9), 254. https://doi.org/10.3390/jcs6090254










