Dependence of the Dynamic Mechanical Properties and Structure of Polyurethane-Clay Nanocomposites on the Weight Fraction of Clay
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Synthesis of Crosslinked Polyurethane (Neat XPU)
2.3. In Situ Preparation of XPU–Clay Nanocomposites
2.4. Characterization
3. Results and Discussion
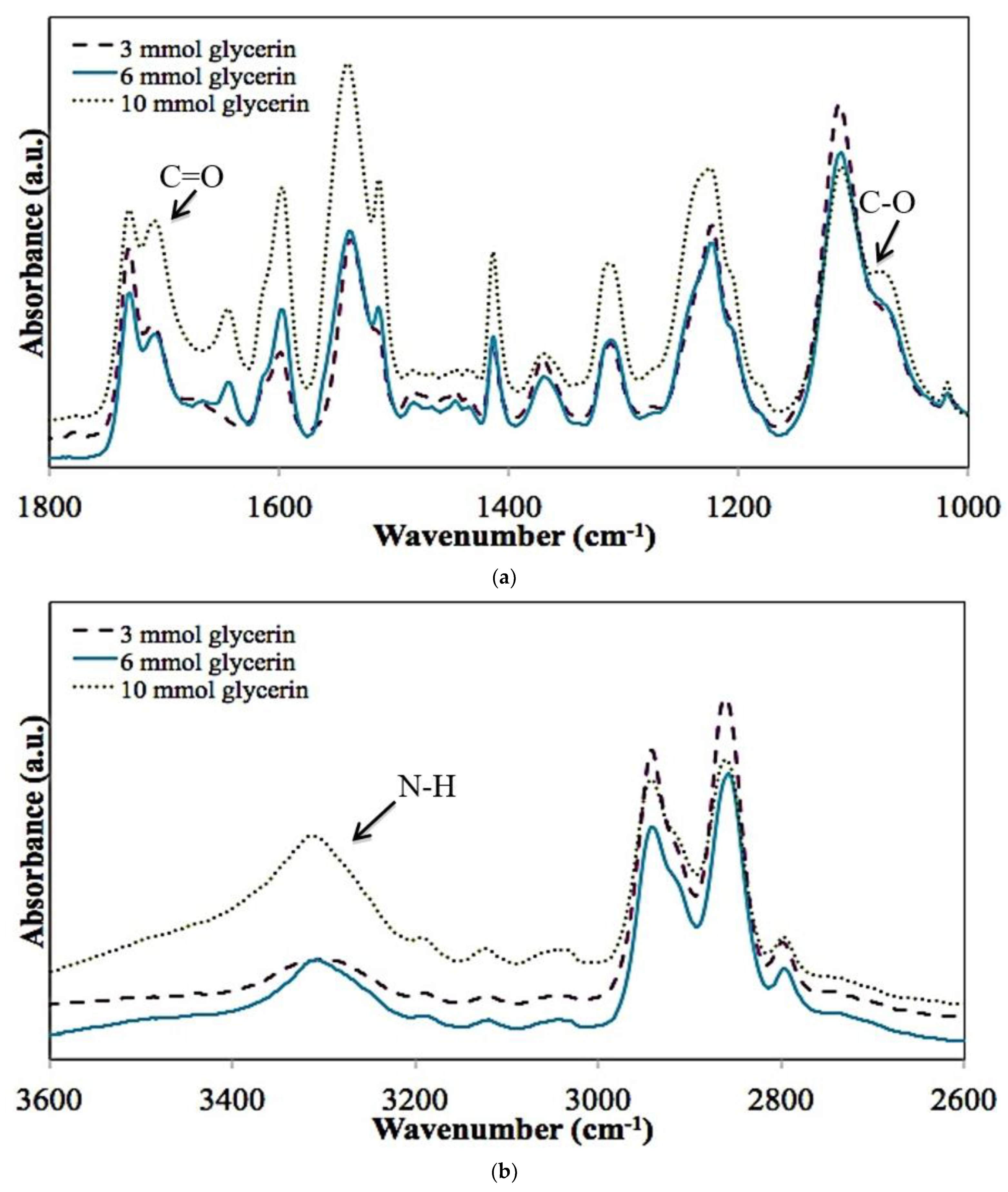
3.1. Chemical Characterization
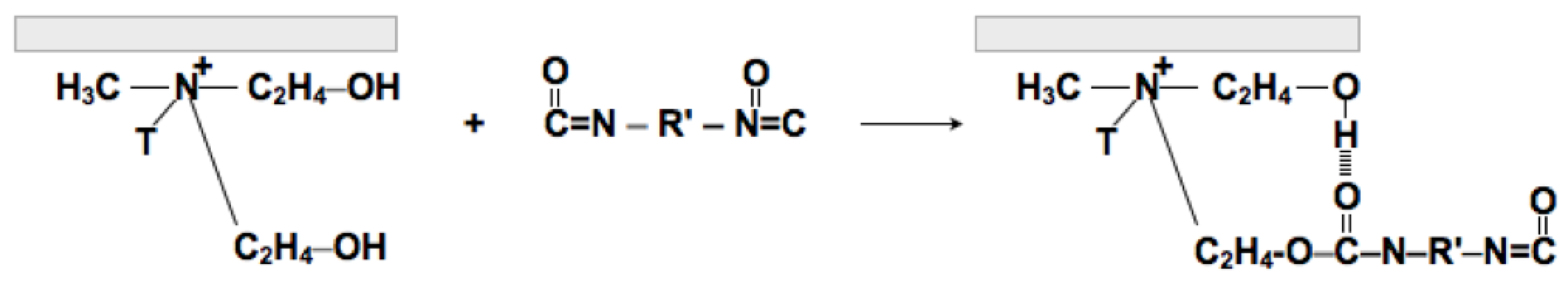
3.2. Analysis of Chemical Crosslinking Process
3.3. Clay Dispersion
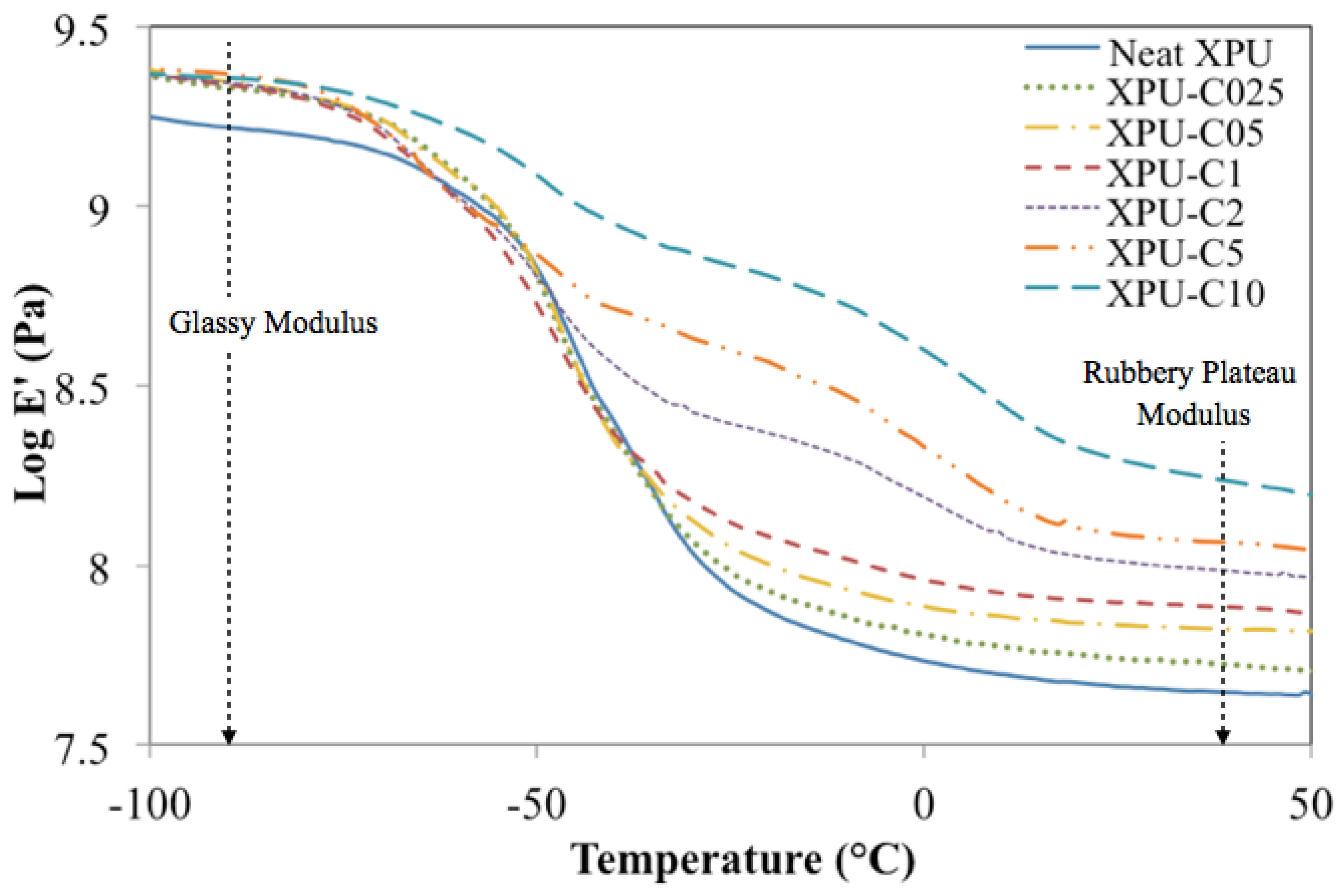
3.4. Storage Modulus
3.5. Glass-Transition Region
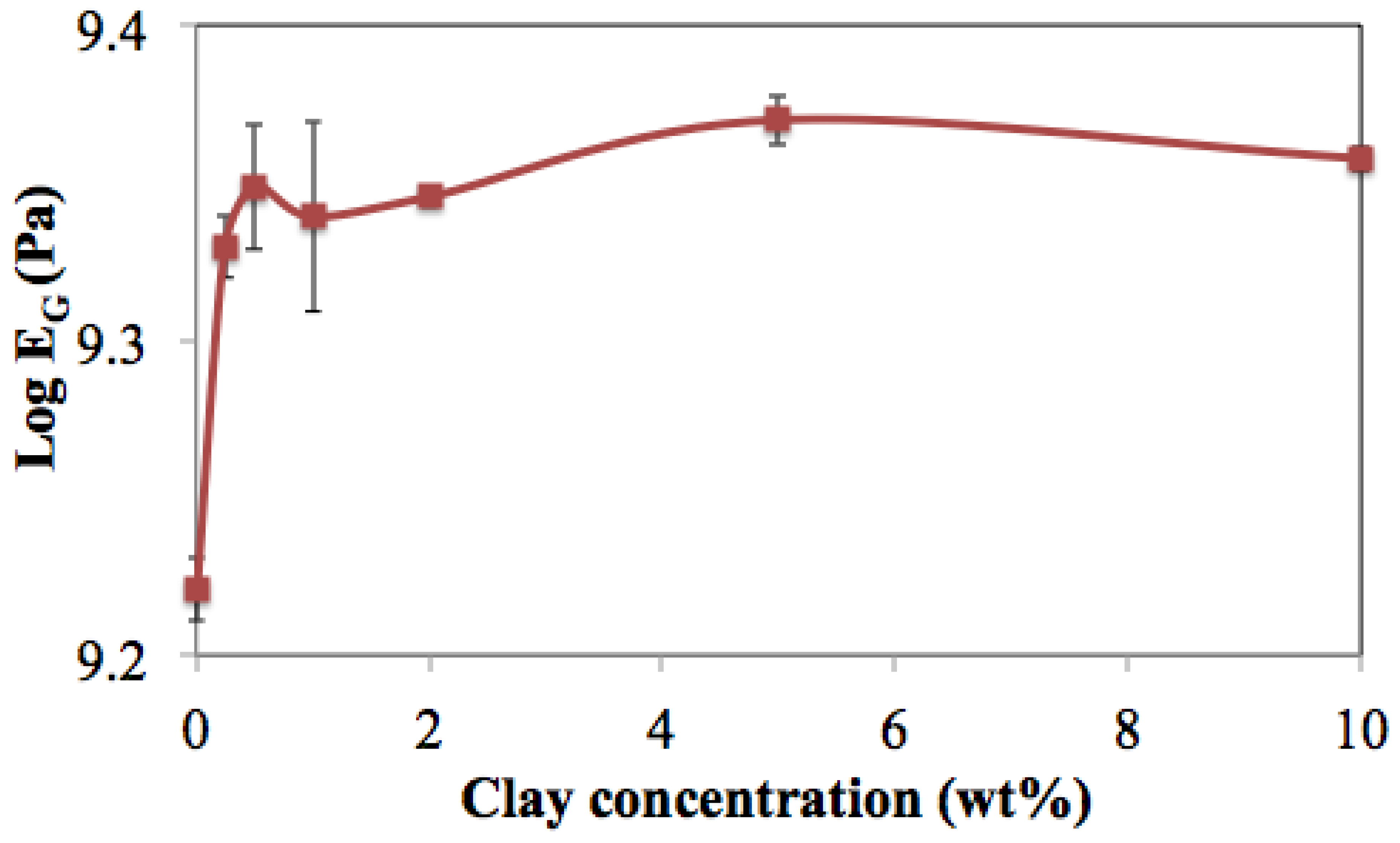
3.6. Rubbery Plateau Modulus
3.7. Tan δ Curve
3.8. Crosslink Density
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joshi, M.; Banerjee, K.; Prasanth, R.; Thakare, V. Polymer/clay nanocomposite based coatings for enhanced gas barrier property. Indian J. Fibre Text. Res. 2006, 31, 202. [Google Scholar]
- Kornmann, X.; Lindberg, H.; Berglund, L.A. Synthesis of epoxy–clay nanocomposites: Influence of the nature of the clay on structure. Polymer 2001, 42, 1303–1310. [Google Scholar] [CrossRef]
- Shelley, J.S.; Mather, P.T.; DeVries, K.L. Reinforcement and environmental degradation of nylon-6/clay nanocomposites. Polymer 2001, 42, 5849–5858. [Google Scholar] [CrossRef]
- Fu, X.; Qutubuddin, S. Synthesis of polystyrene–clay nanocomposites. Mater. Lett. 2000, 42, 12–15. [Google Scholar] [CrossRef]
- Kato, M.; Okamoto, H.; Hasegawa, N.; Tsukigase, A.; Usuki, A. Preparation and properties of polyethylene-clay hybrids. Polym. Eng. Sci. 2004, 43, 1312. [Google Scholar] [CrossRef]
- Suin, S.; Maiti, S.; Shrivastava, N.K.; Khatua, B.B. Mechanically improved and optically transparent polycarbonate/clay nanocomposites using phosphonium modified organoclay. Mater. Des. 2014, 54, 553–563. [Google Scholar] [CrossRef]
- Hu, Y.; Song, L.; Xu, J.; Yang, L.; Chen, Z.; Fan, W. Synthesis of polyurethane/clay intercalated nanocomposites. Colloid Polym. Sci. 2001, 279, 819–822. [Google Scholar] [CrossRef]
- Motawie, A.M.; Madani, M.; Esmail, E.A.; Dacrorry, A.Z.; Othman, H.M.; Badr, M.M.; Abulyazied, D.E. Electrophysical characteristics of polyurethane/organo-bentonite nanocomposites. Egypt. J. Petrol. 2014, 23, 379–387. [Google Scholar] [CrossRef]
- Pradhan, K.C.; Nayak, P.L. Synthesis and characterization of polyurethane nanocomposite from castor oil-hexamethylene diisocyanate (HMDI). Adv. Appl. Sci. Res. 2012, 3, 3045–3052. [Google Scholar]
- Stranskowski, M.; Strankowska, J.; Gazda, M.; Pisczyk, L.; Nowaczyk, G.; Jurga, S. Thermoplastic polyurethane/(organically modified montmorillonite) nanocomposites produced by in situ polymerization. Express Polym. Lett. 2012, 6, 610. [Google Scholar] [CrossRef]
- Rehab, A.; Salahuddin, N. Nanocomposite materials based on polyurethane intercalated into montmorillonite clay. Mater. Sci. Eng. A 2005, 399, 368–376. [Google Scholar] [CrossRef]
- Solarski, S.; Benali, S.; Rochery, M.; Devaux, E.; Alexandre, M.; Monteverde, F.; Dubois, P. Synthesis of a polyurethane/clay nanocomposite used as coating: Interactions between the counterions of clay and the isocyanate and incidence on the nanocomposite structure. J. Appl. Polym. Sci. 2005, 95, 238–244. [Google Scholar] [CrossRef]
- Yao, K.J.; Song, M.; Hourston, D.J.; Luo, D.Z. Polymer/layered clay nanocomposites: 2 polyurethane nanocomposites. Polymer 2002, 43, 1017–1020. [Google Scholar] [CrossRef]
- Verma, G.; Kaushik, A.; Ghosh, A.K. Preparation, characterization and properties of organoclay reinforced polyurethane nanocomposite coatings. J. Plast. Film Sheeting 2012, 29, 3. [Google Scholar] [CrossRef]
- Sengwa, R.J.; Choudhary, S. Structural characterization of hydrophilic polymer blends/montmorillonite clay nanocomposites. J. Appl. Polym. Sci. 2014, 131, 40617. [Google Scholar] [CrossRef]
- Wang, J.; Iroh, J.O.; Long, A. Controlling the structure and rheology of polyimide/nanoclay composites by condensation polymerization. J. Appl. Polym. Sci. 2012, 125, E486–E494. [Google Scholar] [CrossRef]
- Cervantes-Uc, J.M.; Cauich-Rodriguez, J.V.; Vazquez-Torres, H.; Garfias-Mesias, L.F.; Paul, D.R. Thermal degradation of commercially available organoclays studied by TGA–FTIR. Thermochim. Acta 2007, 457, 92–102. [Google Scholar] [CrossRef]
- Morgan, A.B.; Gilman, J.W. Characterization of polymer-layered silicate (clay) nanocomposites by transmission electron microscopy and X-ray diffraction: A comparative study. J. Appl. Polym. Sci. 2003, 87, 1329–1338. [Google Scholar] [CrossRef]
- Wang, Z.; Pinnavaia, T.J. Nanolayer reinforcement of elastomeric polyurethane. Chem. Mater. 1998, 10, 3769–3771. [Google Scholar] [CrossRef]
- Joulazadeh, M.; Navarchian, A.H. Study on elastic modulus of crosslinked polyurethane/organoclay nanocomposites. Polym. Adv. Technol. 2011, 22, 2022–2031. [Google Scholar] [CrossRef]
- Giroto, A.S.; doValle, S.F.; Ribeira, T.; Ribeira, C.; Mattoso, L.H.C. Towards Urea and Glycerol Utilization as Building Block for Polyurethane Production: A Detailed Study about Reactivity, and Structure for Environmentally Friendly Polymer Synthesis. React. Funct. Polym. 2020, 153, 104629. [Google Scholar] [CrossRef]
- Zhu, Y.; Iroh, J.O.; Rajagopolan, R.; Aykanat, A.; Vaia, R. Optimizing the synthesis and thermal properties of conducting polymer–montmorillonite clay nanocomposites. Energies 2022, 15, 1291. [Google Scholar] [CrossRef]
- Peng, S.; Iroh, J.O. Synthesis and characterization of crosslinked polyurethane/clay nanocomposites. J. Appl. Polym. Sci. 2016, 133, 43346. [Google Scholar] [CrossRef]
- Barick, A.K.; Tripathy, D.K. Effect of organically modified layered silicate nanoclay on the dynamic viscoelastic properties of thermoplastic polyurethane nanocomposites. Appl. Clay Sci. 2011, 52, 312–321. [Google Scholar] [CrossRef]
- Behera, A.K.; Avancha, S.; Manna, S.; Sen, R.; Adhikari, B. Effect of nanoclay on physical, mechanical, and microbial degradation of jute-reinforced, soy milk-based nano-biocomposites. Polym. Eng. Sci. 2014, 54, 345–354. [Google Scholar] [CrossRef]
- Samaniuk, J.; Litchfield, D.; Baird, D. Improving the exfoliation of layered silicate in a poly (ethylene terephthalate) matrix using supercritical carbon dioxide. Polym. Eng. Sci. 2009, 49, 2329–2341. [Google Scholar] [CrossRef]
- Iroh, J.O.; Longun, J. Viscoelastic properties of montmorillonite clay/polyimide composite membranes and thin films. J. Inorg. Organomet. Polym. 2012, 22, 653–661. [Google Scholar] [CrossRef]
- Rooj, S.; Das, A.; Stockelhuber, K.W.; Wang, D.-Y.; Galiatsatos, V.; Heinrich, G. Understanding the reinforcing behavior of expanded clay particles in natural rubber compounds. Soft Matter 2013, 9, 3798–3808. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Panda, S.S.; Raju, K.V.S.N. Properties of diamine chain extended polyurethane-urea coatings. J. Jpn. Soc. Colour Mater. 2004, 77, 540–547. [Google Scholar] [CrossRef][Green Version]
- Hill, L.W. Calculation of crosslink density in short chain networks. Prog. Org. Coat. 1997, 31, 235–243. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, S.; Iroh, J.O. Dependence of the Dynamic Mechanical Properties and Structure of Polyurethane-Clay Nanocomposites on the Weight Fraction of Clay. J. Compos. Sci. 2022, 6, 173. https://doi.org/10.3390/jcs6060173
Peng S, Iroh JO. Dependence of the Dynamic Mechanical Properties and Structure of Polyurethane-Clay Nanocomposites on the Weight Fraction of Clay. Journal of Composites Science. 2022; 6(6):173. https://doi.org/10.3390/jcs6060173
Chicago/Turabian StylePeng, Shirley, and Jude O. Iroh. 2022. "Dependence of the Dynamic Mechanical Properties and Structure of Polyurethane-Clay Nanocomposites on the Weight Fraction of Clay" Journal of Composites Science 6, no. 6: 173. https://doi.org/10.3390/jcs6060173
APA StylePeng, S., & Iroh, J. O. (2022). Dependence of the Dynamic Mechanical Properties and Structure of Polyurethane-Clay Nanocomposites on the Weight Fraction of Clay. Journal of Composites Science, 6(6), 173. https://doi.org/10.3390/jcs6060173






