First-Principles Study of Electronic and Optical Properties of Tri-Layered van der Waals Heterostructures Based on Blue Phosphorus and Zinc Oxide
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
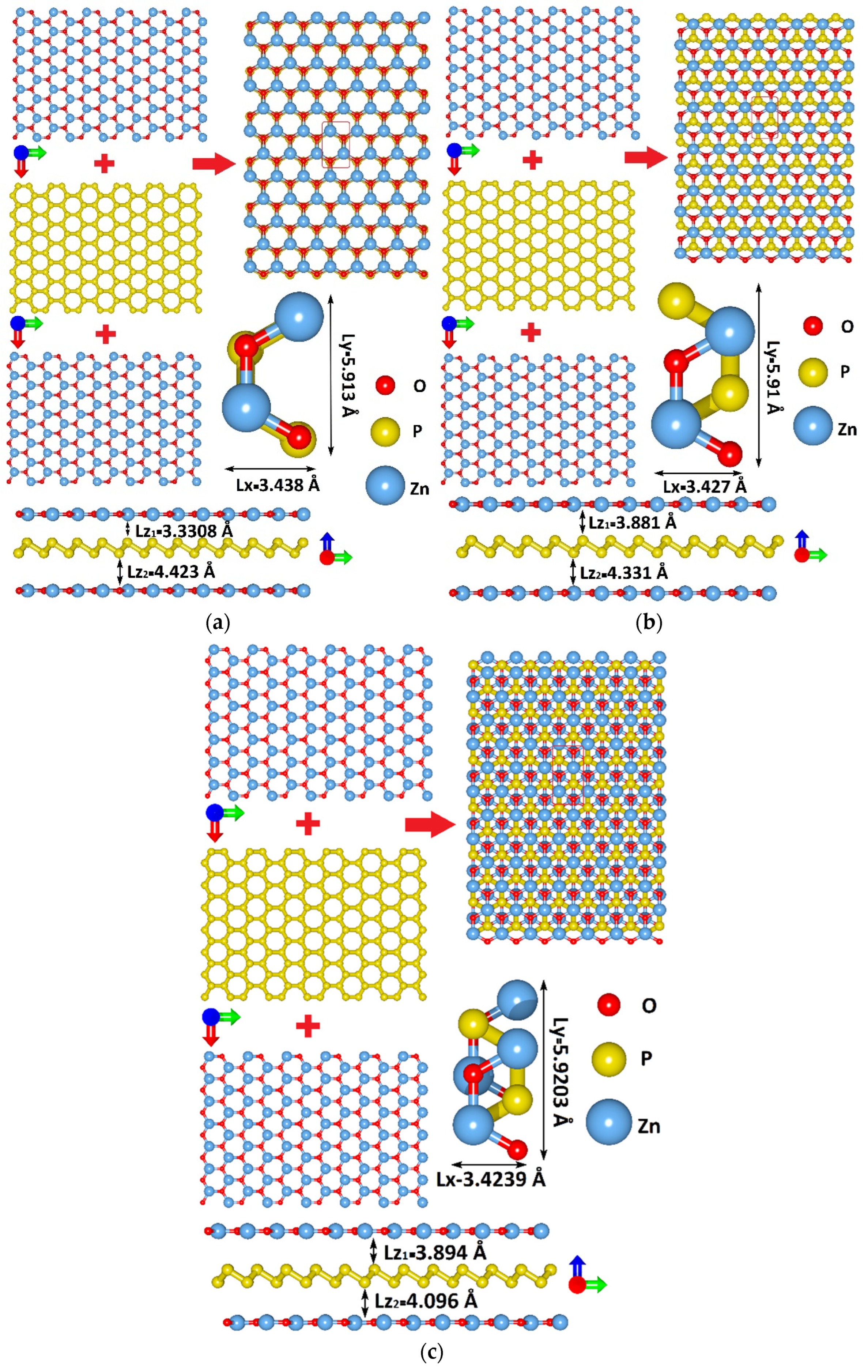
3.1. Atomistic Models of van der Waals ZnO/BlueP/ZnO Heterostructure
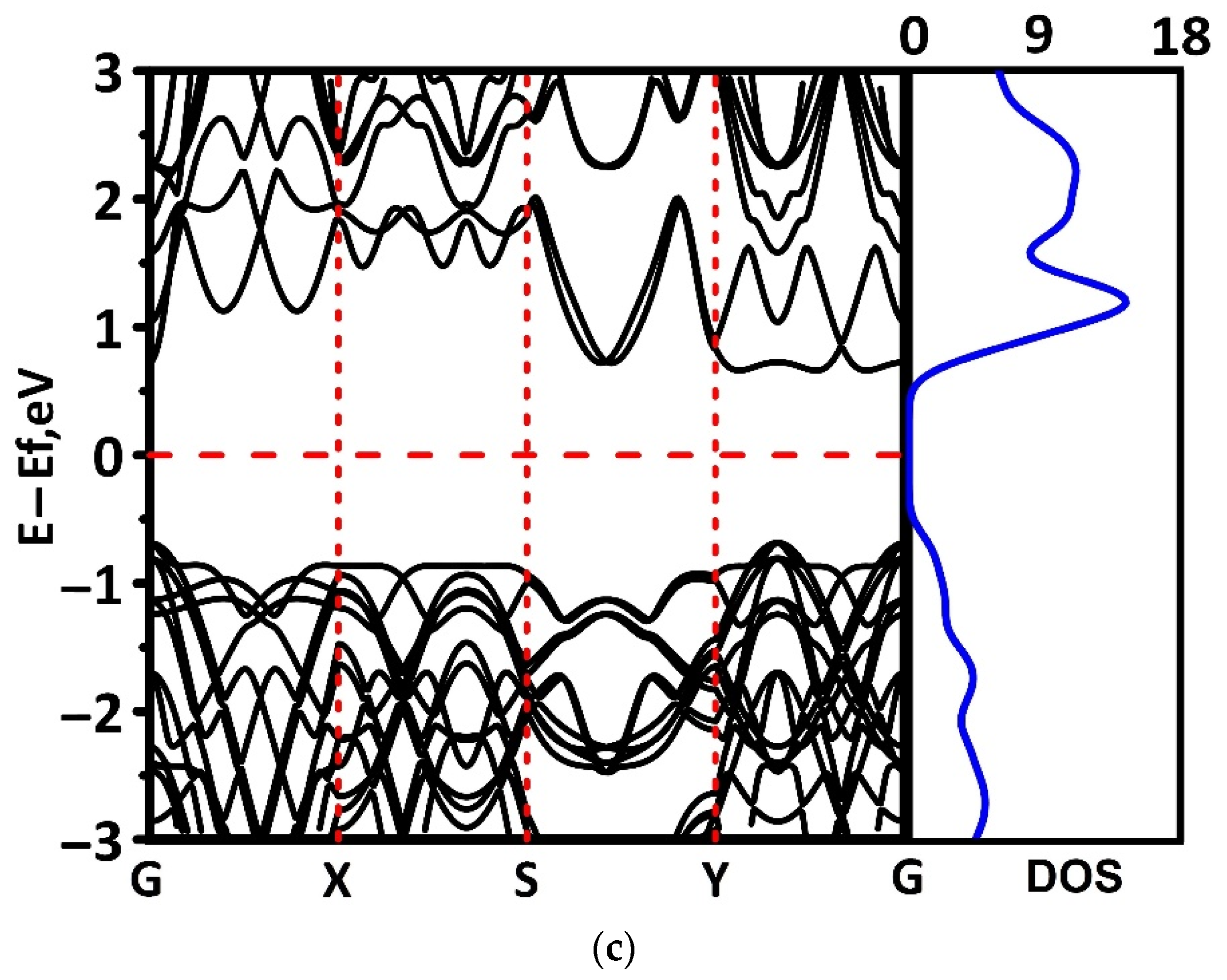
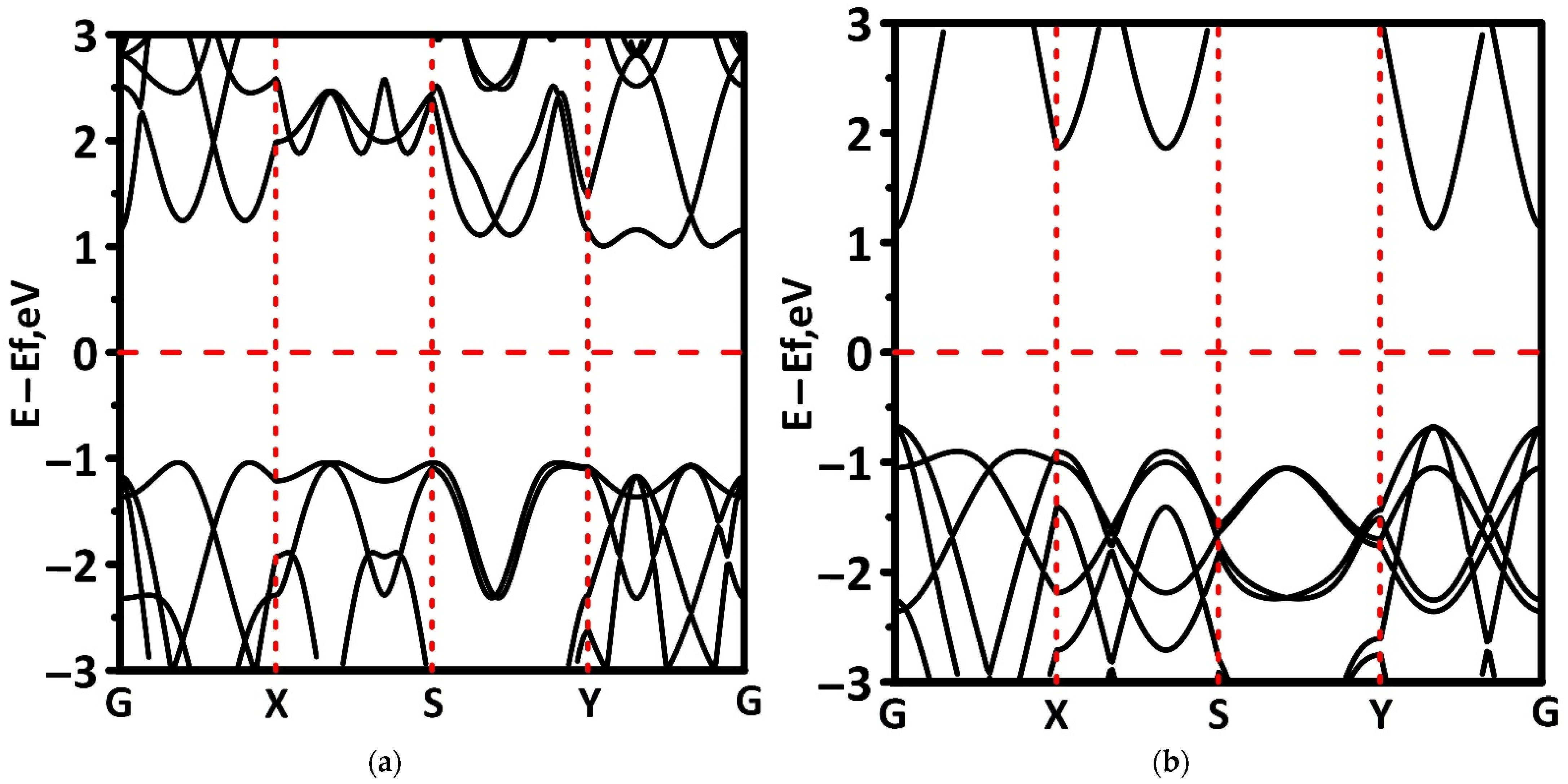
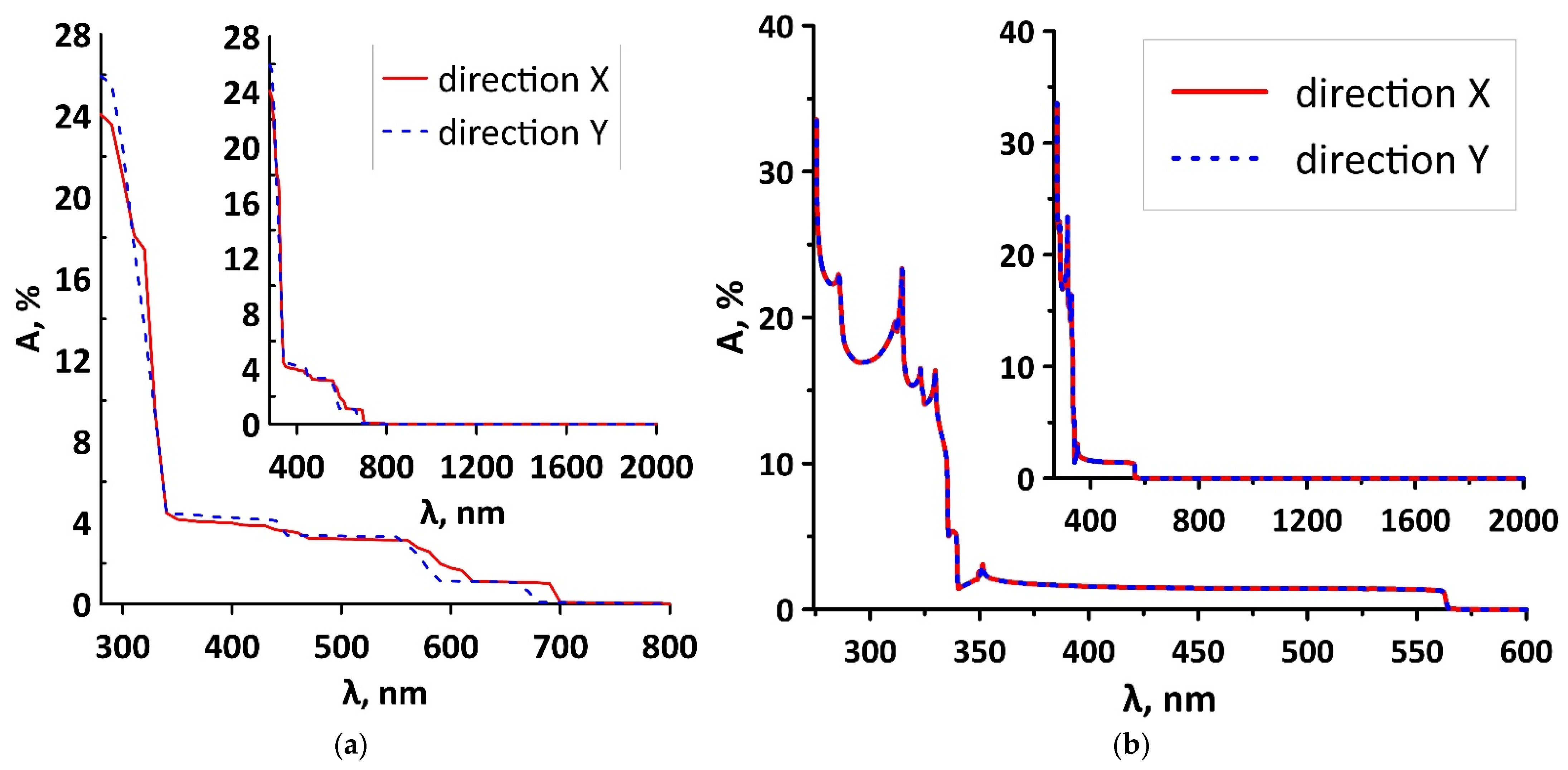
3.2. Electronic and Optical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Castro Neto, A.H. 2D materials and van der Waals heterostructures. Science 2016, 353, aac9439. [Google Scholar] [CrossRef] [Green Version]
- Castellanos-Gomez, A. Why all the fuss about 2D semiconductors. Nat. Photonics 2016, 10, 202–204. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Yin, X.; Ulin-Avila, E.; Geng, B.; Zentgraf, T.; Ju, L.; Wang, F.; Zhang, X. A graphene-based broadband optical modulator. Nature 2011, 474, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Furchi, M.; Urich, A.; Pospischil, A.; Lilley, G.; Unterrainer, K.; Detz, H.; Klang, P.; Andrews, A.M.; Schrenk, W.; Strasser, G.; et al. Microcavity-integrated graphene photodetector. Nano Lett. 2012, 12, 2773–2777. [Google Scholar] [CrossRef]
- Eda, G.; Maier, S.A. Two-dimensional crystals: Managing light for optoelectronics. ACS Nano 2013, 7, 5660–5665. [Google Scholar] [CrossRef]
- Xia, F.; Wang, H.; Xiao, D.; Ramasubramaniam, A. Two-dimensional material nanophotonics. Nat. Photonics 2014, 8, 899–907. [Google Scholar] [CrossRef]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef]
- Duong, D.L.; Yun, S.J.; Lee, Y.H. van der Waals Layered Materials: Opportunities and Challenges. ACS Nano 2017, 11, 11803–11830. [Google Scholar] [CrossRef]
- Yao, J.; Yang, G. Van der Waals heterostructures based on 2D layered materials: Fabrication, characterization, and application in photodetection. J. Appl. Phys. 2022, 131, 161101. [Google Scholar] [CrossRef]
- Nguyen, B.H.; Nguyen, V.H. Two-dimensional hexagonal semiconductors beyond graphene. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 043001. [Google Scholar] [CrossRef]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Bellus, M.Z.; Li, M.; Lane, S.D.; Ceballos, F.; Cui, Q.; Zeng, X.C.; Zhao, H. Type-I van der Waals heterostructure formed by MoS2 and ReS2 monolayers. Nanoscale Horiz. 2017, 2, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Kim, J.; Shi, S.F.; Zhang, Y.; Jin, C.; Sun, Y.; Tongay, S.; Wu, J.; Zhang, Y.; Wang, F. Ultrafast charge transfer in atomically thin MoS2/WS2 heterostructures. Nat. Nanotechnol. 2014, 9, 682–686. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Lin, J.; Wang, X.; Shi, G.; Lei, S.; Lin, Z.; Zou, X.; Ye, G.; Vajtai, R.; Yakobson, B.I.; et al. Vertical and in-plane heterostructures from WS2/MoS2 monolayers. Nat. Mater. 2014, 13, 1135–1142. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Hu, S.; Su, L.; Huang, L.; Liu, Y.; Jin, Z.; Purezky, A.A.; Geohegan, D.B.; Kim, K.W.; Zhang, Y.; et al. Equally efficient interlayer exciton relaxation and improved absorption in epitaxial and nonepitaxial MoS2/WS2 heterostructures. Nano Lett. 2015, 15, 486–491. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Chen, F.; Zhao, S.; Su, W. Recent progress in the CVD growth of 2D vertical heterostructures based on transition-metal dichalcogenides. CrystEngComm 2021, 23, 8239–8254. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, B.; Guan, X.; Han, L.; Wu, L.; Guan, P.; Lu, P. Design and analysis of III-V two-dimensional van der Waals heterostructures for ultra-thin solar cells. Appl. Surf. Sci. 2022, 586, 152799. [Google Scholar] [CrossRef]
- Britnell, L.; Gorbachev, R.V.; Jalil, R.; Belle, B.D.; Schedin, F.; Mishchenko, A.; Georgiou, T.; Katsnelson, M.I.; Eaves, L.; Morozov, S.V.; et al. Field-effect tunneling transistor based on vertical graphene heterostructures. Science 2012, 335, 947–950. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.C.; Li, Z.; Shi, L.; Cronin, S.B. Thermoelectric transport across graphene/hexagonal boron nitride/graphene heterostructures. Nano Res. 2015, 8, 666–672. [Google Scholar] [CrossRef]
- Withers, F.; Del Pozo-Zamudio, O.; Mishchenko, A.; Rooney, A.P.; Gholinia, A.; Watanabe, K.; Taniguchi, T.; Haigh, S.J.; Geim, A.K.; Tartakovskii, A.I.; et al. Light-emitting diodes by band-structure engineering in van der Waals heterostructures. Nat. Mater. 2015, 14, 301–306. [Google Scholar] [CrossRef]
- Li, X.; Lin, S.; Lin, X.; Xu, Z.; Wang, P.; Zhang, S.; Zhong, H.; Xu, W.; Wu, Z.; Fang, W. Graphene/h-BN/GaAs sandwich diode as solar cell and photodetector. Opt. Express 2016, 24, 134–145. [Google Scholar] [CrossRef]
- Mawwa, J.; Shamim, S.; Khanom, S.; Hossain, M.K.; Ahmed, F. In-plane graphene/boron nitride heterostructures and their potential application as toxic gas sensors. RSC Adv. 2021, 11, 32810–32823. [Google Scholar] [CrossRef]
- Yu, L.; Lee, Y.H.; Ling, X.; Santos, E.J.; Shin, Y.C.; Lin, Y.; Dubey, M.; Kaxiras, E.; Kong, J.; Wang, H.; et al. Graphene/MoS2 hybrid technology for large-scale two-dimensional electronics. Nano Lett. 2014, 14, 3055–3063. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ma, F.; Sun, M. Graphene, hexagonal boron nitride, and their heterostructures: Properties and applications. RSC Adv. 2017, 7, 16801–16822. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Zhang, L.; Xu, W.; Pan, J.; Song, S.; Zhang, Y.; Zhou, H.; Wang, Y.; Bao, L.; Zhang, Y.Y.; et al. Stable Silicene in Graphene/Silicene Van der Waals Heterostructures. Adv. Mater. 2018, 30, e1804650. [Google Scholar] [CrossRef]
- Mudd, G.W.; Svatek, S.A.; Hague, L.; Makarovsky, O.; Kudrynskyi, Z.R.; Mellor, C.J.; Beton, P.H.; Eaves, L.; Novoselov, K.S.; Kovalyuk, Z.D.; et al. High broad-band photoresponsivity of mechanically formed InSe-graphene van der Waals heterostructures. Adv. Mater. 2015, 27, 3760–3776. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Zhang, T.; Cheng, G.; Li, T.; Wang, S.; Wei, W.; Zhou, X.; Yu, W.; Sun, Y.; Wang, P.; et al. Interlayer Transition and Infrared Photodetection in Atomically Thin Type-II MoTe2/MoS2 van der Waals Heterostructures. ACS Nano 2016, 10, 3852–3858. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kumar, A.; Srivastava, S.; Tankeshwar, K. van der Waals heterostructures based on allotropes of phosphorene and MoSe2. Phys. Chem. Chem. Phys. 2017, 19, 22023–22032. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Chin, M.L.; Najmaei, S.; Guo, Q.; Xia, F.; Wang, H.; Dube, M. Optoelectronic devices based on two-dimensional transition metal dichalcogenides. Nano Res. 2016, 9, 1543–1560. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Wang, Z.; Gu, Y.; Ye, Y.; Chai, X.; Ye, J.; Chen, Y.; Xie, R.; Zhou, Y.; et al. Unipolar barrier photodetectors based on van der Waals heterostructures. Nat. Electron. 2021, 4, 357–363. [Google Scholar] [CrossRef]
- Li, Q.; Meng, J.; Li, Z. Recent progress on Schottky sensors based on two-dimensional transition metal dichalcogenides. J. Mater. Chem. A 2022, 10, 8107–8128. [Google Scholar] [CrossRef]
- Jia, Z.; Li, S.; Xiang, J.; Wen, F.; Bao, X.; Feng, S.; Yang, R.; Liu, Z. Highly sensitive and fast monolayer WS2 phototransistors realized by SnS nanosheet decoration. Nanoscale 2017, 9, 1916–1924. [Google Scholar] [CrossRef]
- Yoo, G.; Choi, S.; Park, S.; Lee, K.T.; Lee, S.; Oh, M.S.; Heo, J.; Park, H.J. Flexible and Wavelength-Selective MoS2 Phototransistors with Monolithically Integrated Transmission Color Filters. Sci. Rep. 2017, 7, 40945. [Google Scholar] [CrossRef] [Green Version]
- Huo, N.J.; Yang, Y.J.; Li, J.B. Optoelectronics based on 2D TMDs and heterostructures. J. Semicond. 2017, 38, 031002. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Z.; Yin, L.; Cheng, R.; Wang, J.; Wen, Y.; Shifa, T.A.; Wang, F.; Zhang, Y.; Zhan, X.; et al. 2D library beyond graphene and transition metal dichalcogenides: A focus on photodetection. Chem. Soc. Rev. 2018, 47, 6296–6341. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Y.; Hu, Y.; Gu, H. Ultraviolet Detectors Based on Wide Bandgap Semiconductor Nanowire: A Review. Sensors 2018, 18, 2072. [Google Scholar] [CrossRef] [Green Version]
- Mannix, A.J.; Zhou, X.F.; Kiraly, B.; Wood, J.D.; Alducin, D.; Myers, B.D.; Liu, X.; Fisher, B.L.; Santiago, U.; Guest, J.R.; et al. Synthesis of borophenes: Anisotropic, two-dimensional boron polymorphs. Science 2015, 350, 1513–1516. [Google Scholar] [CrossRef] [Green Version]
- Feng, B.; Zhang, J.; Zhong, Q.; Li, W.; Li, S.; Li, H.; Cheng, P.; Meng, S.; Chen, L.; Wu, K. Experimental realization of two-dimensional boron sheets. Nat. Chem. 2016, 8, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Golias, E.; Krivenkov, M.; Varykhalov, A.; Sánchez-Barriga, J.; Rader, O. Band Renormalization of Blue Phosphorus on Au(111). Nano Lett. 2018, 18, 6672–6678. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wu, X.; Zhu, Z.; Li, G.; Mi, C. On the elasticity and piezoelectricity of black(blue) phosphorus/ZnO van der Waals heterostructures. Comput. Mater. Sci. 2019, 169, 109134. [Google Scholar] [CrossRef]
- Li, B.; Zhang, S.F.; Ji, W.X.; Zhang, C.W.; Li, P.; Wang, P.J. The electric-field and strain inducing electronic and optical properties of the blue phosphorene/ZnO heterostructures. Phys. E Low-Dimens. Syst. Nanostructures 2020, 115, 113650. [Google Scholar] [CrossRef]
- Niu, X.; Li, Y.; Shu, H.; Yao, X.; Wang, J. Efficient Carrier Separation in Graphitic Zinc Oxide and Blue Phosphorus van der Waals Heterostructure. J. Phys. Chem. C 2017, 121, 3648–3653. [Google Scholar] [CrossRef]
- Momeni, K.; Ji, Y.; Wang, Y.; Paul, S.; Neshani, S.; Yilmaz, D.E.; Shin, Y.K.; Zhang, D.; Jiang, J.W.; Park, H.S.; et al. Multiscale computational understanding and growth of 2D materials: A review. Npj Comput. Mater. 2020, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Soler, J.M.; Artacho, E.; Gale, J.D.; García, A.; Junquera, J.; Ordejón, P.; Sánchez-Portal, D. The SIESTA method for ab-initio order-N materials simulation. J. Phys. Condens. Matt. 2002, 14, 2745–2779. [Google Scholar] [CrossRef] [Green Version]
- The SIESTA Group. Available online: Departments.icmab.es/leem/siesta/ (accessed on 10 April 2021).
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Berland, K.; Hyldgaard, P. Exchange functional that tests the robustness of the plasmon description of the van der Waals density functional. Phys. Rev. B 2014, 89, 035412. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Economou, E.N. Green’s Functions in Quantum Physics, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 55–75. [Google Scholar]
- Chen, L.L.; Cui, Y.; Xiong, Z.; Zhou, M.; Gao, Y. Chemical functionalization of the ZnO monolayer: Structural and electronic properties. RSC Adv. 2019, 9, 21831–21843. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Ren, C.; Zhang, S.; Ji, W.; Zhang, C.; Li, P.; Wang, P. Electronic Structural and Optical Properties of Multilayer Blue Phosphorus: A First-Principle Study. J. Nanomater. 2019, 2019, 4020762. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slepchenkov, M.M.; Kolosov, D.A.; Glukhova, O.E. First-Principles Study of Electronic and Optical Properties of Tri-Layered van der Waals Heterostructures Based on Blue Phosphorus and Zinc Oxide. J. Compos. Sci. 2022, 6, 163. https://doi.org/10.3390/jcs6060163
Slepchenkov MM, Kolosov DA, Glukhova OE. First-Principles Study of Electronic and Optical Properties of Tri-Layered van der Waals Heterostructures Based on Blue Phosphorus and Zinc Oxide. Journal of Composites Science. 2022; 6(6):163. https://doi.org/10.3390/jcs6060163
Chicago/Turabian StyleSlepchenkov, Michael M., Dmitry A. Kolosov, and Olga E. Glukhova. 2022. "First-Principles Study of Electronic and Optical Properties of Tri-Layered van der Waals Heterostructures Based on Blue Phosphorus and Zinc Oxide" Journal of Composites Science 6, no. 6: 163. https://doi.org/10.3390/jcs6060163
APA StyleSlepchenkov, M. M., Kolosov, D. A., & Glukhova, O. E. (2022). First-Principles Study of Electronic and Optical Properties of Tri-Layered van der Waals Heterostructures Based on Blue Phosphorus and Zinc Oxide. Journal of Composites Science, 6(6), 163. https://doi.org/10.3390/jcs6060163






