Negative Thermal Expansion Properties of Sm0.85Sr0.15MnO3-δ
Abstract
:1. Introduction
2. Experimental Procedures
3. Results and Discussion
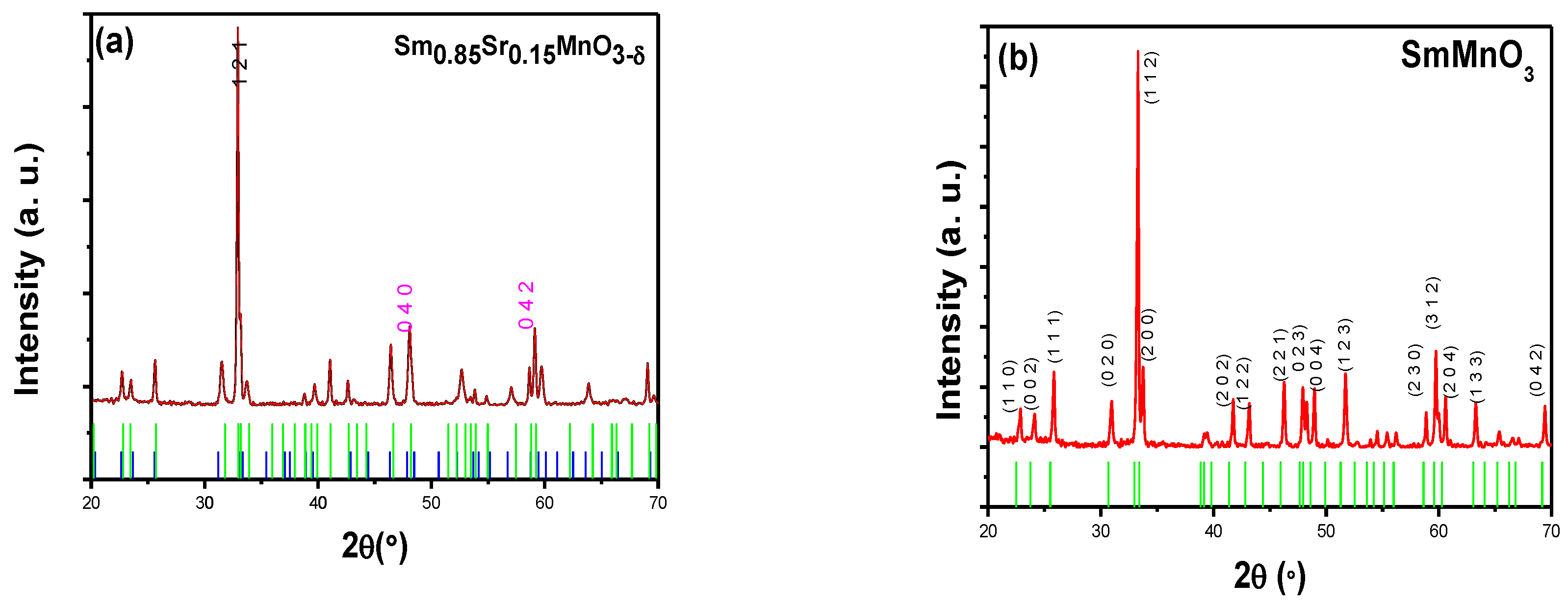
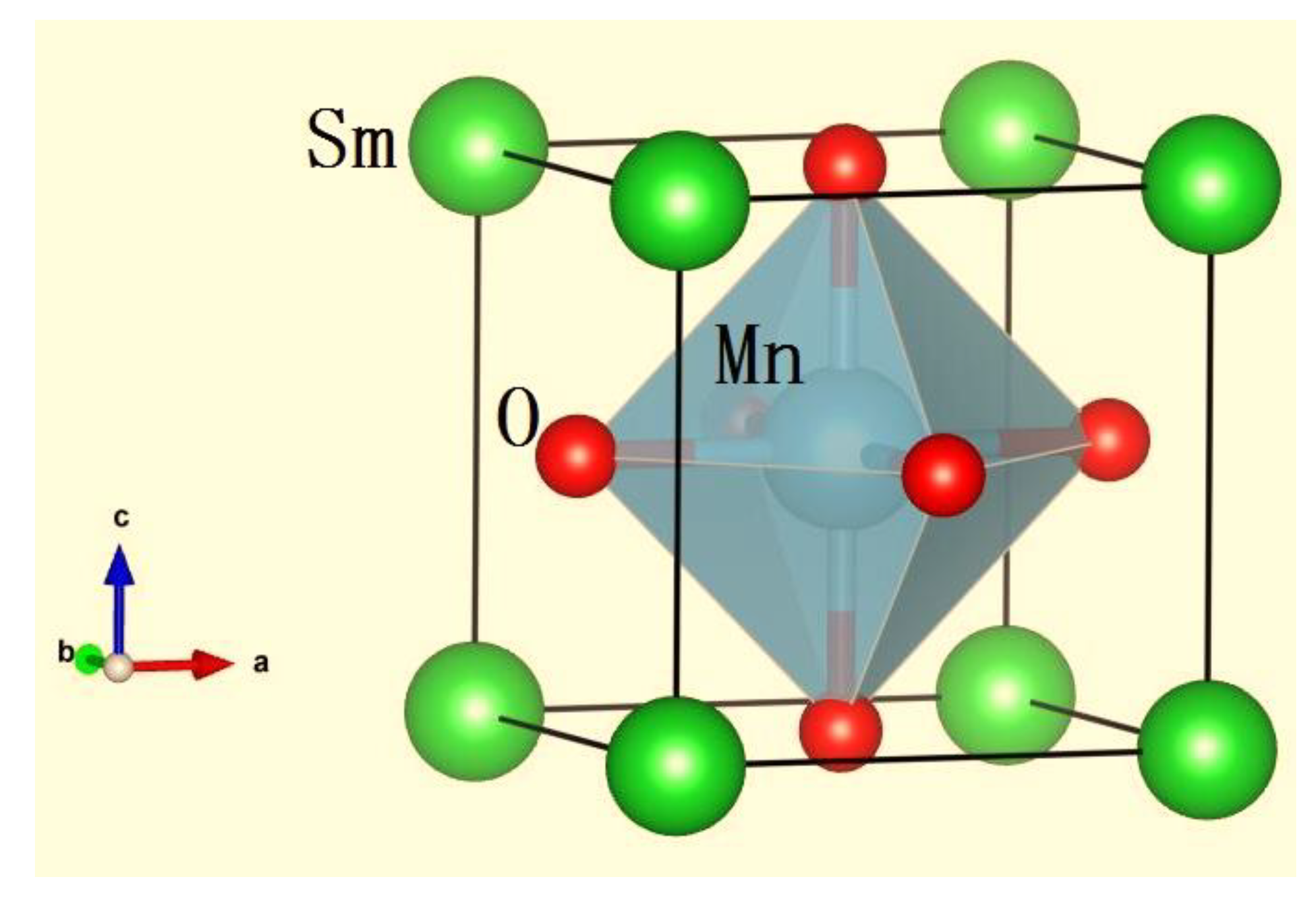
3.1. Phase Analysis
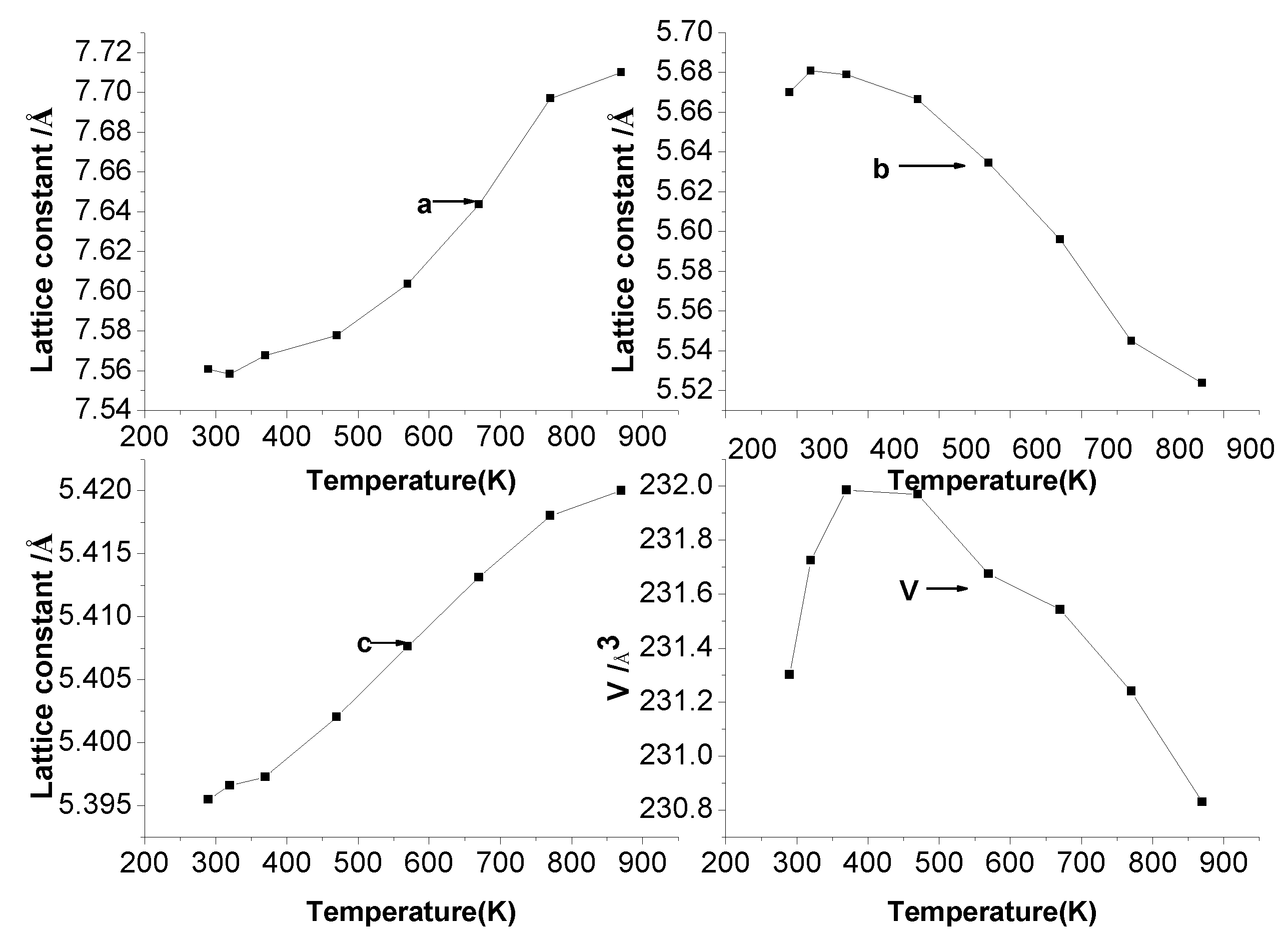
3.2. Thermal Expansion Property
3.3. Discussion
4. Conclusions
- (1)
- A novel negative thermal expansion material composed of Sm0.85Sr0.15MnO3-δ was synthesized using the solid-state method with an NTE coefficient of −10.08 × 10−6/K from 360 to 873 K.
- (2)
- The particles were homogenous spherical or elliptic–spherical particles with a uniform particle size of about 1~2 μm.
- (3)
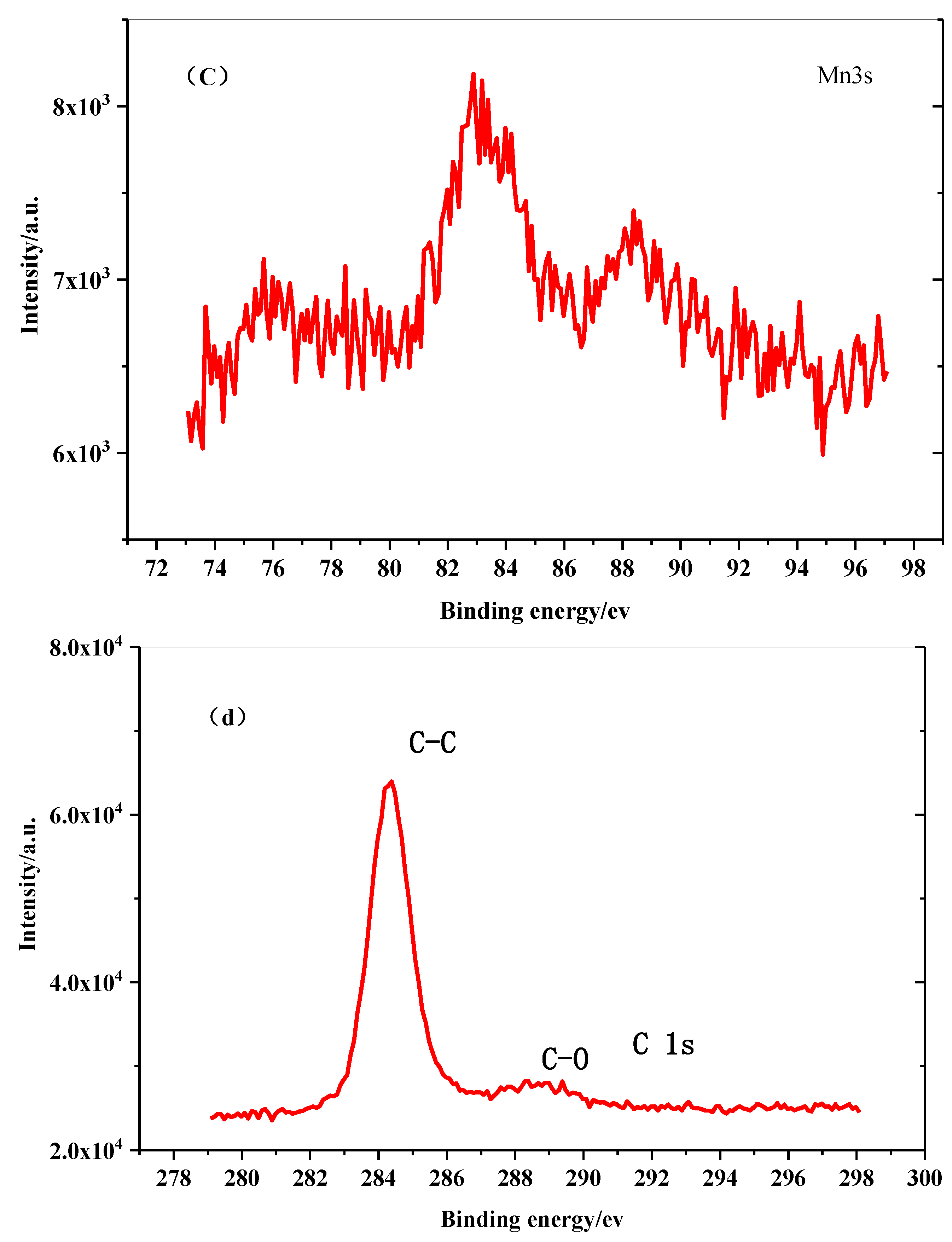
- The ceramic Sm0.85Sr0.15MnO3-δ crystallized in an orthorhombic structure with the space group Pbnm. When Sr2+ substituted the Sm3+ in SmMnO3, Sr2+ occupied the position of Sm3+. To maintain the valence balance, electronic transfer occurred in the Mn3+, converting into Mn4+ in Sm0.85Sr0.15MnO3-δ. The Mn3+-O2−-Mn4+ structure formed in the process.
- (4)
- The thermal property of Sm0.85Sr0.15MnO3-δ is considered to be related to the interaction of the lattice vibration and electron transfer between Mn ions. As the temperature rise, the lattice vibrated dramatically and more Mn3+ converted into Mn4+. Additionally, the electron transfer rate increased between the Mn3+ and Mn4+ ions as the temperatures increased. The number of Mn3+ ions that can cause the Jahn–Teller effect increasesd. The oxygen ions in the Mn3+O6 octahedron became slant or even produced oxygen defects. The contributions of the lattice vibrations and electron transfer between Mn3+ and Mn4+ to the thermal expansion changed with the increasing temperature.
- (5)
- The pore energy in the sintered body partially absorbed the expansion of the a-axis a and the c-axis; the negative expansion phenomenon can be explained from the perspective of the contraction of the b-axis. The abnormal thermal expansion behavior of the Sm0.85Sr0.15MnO3-δ perovskite system is caused by the presence of pores in the sintered body combined with the negative expansion of the b-axis in the perovskite system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, J.S.O.; Mary, T.A.; Vogt, T.; Subramanian, M.A. Sleight, Negative Thermal Expansion in ZrW2O8 and HfW2O8. Mater. Chem. 1996, 8, 2809–2823. [Google Scholar] [CrossRef]
- Sahoo, P.P.; Sumithra, S.; Madras, G. Row, Synthesis, Structure, Negative Thermal Expansion, and Photocatalytic Property of Mo Doped ZrV2O7. Inorg. Chem. 2011, 50, 8774–8781. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Sanson, A.; Sun, Q.; Fan, L.; Venier, A.; de Souza, D.O.; Xing, X.; Chen, J. Strong Negative Thermal Expansion of Cu2PVO7 in a Wide Temperature Range. Chem. Mater. 2021, 33, 1321–1329. [Google Scholar] [CrossRef]
- Shi, N.; Sanso, A.; Venier, A.; Fan, L.; Sun, C.; Xing, X.; Chen, J. Negative and zero thermal expansion in α-(Cu2−xZnx)V2O7 solid solutions. Chem Commun. 2020, 56, 10666–10669. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.S.; Li, F.; Song, W.B.; Yuan, B.H.; Cheng, Y.G.; Liang, E.J.; Chao, M.J. Control of reaction processes for rapid synthesis of low-thermal-expansion Ca1−xSrxZr4P6O24 ceramics. Ceram. Int. 2014, 40, 6013–6020. [Google Scholar] [CrossRef]
- Oba, Y.; Tadano, T.; Akashi, R.; Tsuneyuki, S. First-principles study of phonon anharmonicity and negative thermal expansion in ScF3. Phys. Rev. Mater. 2019, 3, 033601. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, A.L.; Calleja, M.; Conterio, M.J.; Dove, M.T.; Evans, J.S.O.; Keen, D.A.; Peters, L.; Tucker, M.G. Colossal positive and negative thermal expansion in the framework material Ag3(Co(CN)6). Science 2008, 319, 794–797. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.J.; Xu, W.; Xu, X.D.; Li, L.F.; Pan, X.Q.; Evans, D. Negative thermal expansion and electrical properties of Mn3Cu0.6NbxGe0.4−xN (x = 0.05–0.25) compounds. Mater. Lett. 2008, 62, 2381–2384. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Chao, M.; Zhang, Y.; Li, Y.; Wu, Y. Negative thermal expansion property of Eu0.8Sr0·2MnO3−δ. Results Mater. 2020, 8, 100154. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Chang, D.; Sun, Q.; Chao, M.; Jia, Y. Charge transfer induced negative thermal expansion in perovskite BiNiO3. Comput. Mater. Sci. 2016, 113, 198–202. [Google Scholar] [CrossRef]
- Long, Y.W.; Hayashi, N.; Saito, T.; Azuma, M.; Muranaka, S.; Shimakawa, Y. Temperature-induced A–B intersite charge transfer in an A-site-ordered LaCu3Fe4O12 perovskite. Nature 2009, 458, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Yuan, B.H.; Cheng, Y.G.; Liang, E.J.; Ge, X.H.; Yuan, H.L.; Zhang, Y.; Guo, J.; Chao, M.J. Phase transition and negative thermal expansion of HfMnMo3O12. Mater. Res. Bull. 2018, 99, 255–259. [Google Scholar] [CrossRef]
- Wang, H.; Yang, M.; Chao, M.; Guo, J.; Tang, X.; Jiao, Y.; Liang, E. Negative thermal expansion properties of Cu1.5Mg0.5V2O7. Ceram. Int. 2019, 45, 9814–9819. [Google Scholar] [CrossRef]
- Wang, H.; Yang, M.; Chao, M.; Guo, J.; Gao, Q.; Jiao, Y.; Tang, X.; Liang, E. Negative thermal expansion property of β-Cu2V2O7. Solid State Ion. 2019, 343, 115086. [Google Scholar] [CrossRef]
- Warne-Lang, V.; Sato, M.; Ozeki, M.; Kadowaki, Y.; Yokoyama, Y.; Katayama, N.; Okamoto, Y.; Takenaka, K. Annealing effects on negative thermal expansion properties of ball-milled β-Cu1.8Zn0.2V2O7 fine particles. Ceram. Int. 2020, 46, 27655–27659. [Google Scholar] [CrossRef]
- Rotermel, M.V.; Krasnenko, T.I.; Titova, S.G.; Praynichnikov, S.V. Negative volume thermal expansion of monoclinic Cu2−2xZn2xV2O7 in the temperature range from 93 to 673 K. J. Solid State Chem. 2020, 285, 121221. [Google Scholar] [CrossRef]
- Azuma, M.; Chen, W.T.; Seki, H.; Czapski, M.; Olga, S.; Oka, K. Colossal negative thermal expansion in BiNiO3 induced by intermetallic charge transfer. Nat. Commun. 2011, 2, 347. [Google Scholar] [CrossRef] [Green Version]
- Hirano, A.; Hirano, F.; Matsumura, T.; Imanishi, N.; Takeda, Y. An anomalous thermal expansion in the perovskite system Gd1−xSrxMnO3 (0 ≤ x ≤ 0.3). Solid State Ion. 2007, 177, 749–755. [Google Scholar] [CrossRef]
- Fu, L.J.; Chao, M.J.; Chen, H.; Liu, X.S.; Liu, Y.M.; Yu, J.M.; Liang, E.J.; Li, Y.C.; Xiao, X. Negative thermal expansion property of Er0.7Sr0.3NiO3−δ. Phys. Lett. A 2014, 378, 1909–1912. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Zhang, N.; Li, Y.; Wu, Y. Negative thermal expansion property of Sm1−xCuxMnO3−δ. J. Mater. Res. Technol. 2021, 12, 2267–2272. [Google Scholar] [CrossRef]
- Yamamoto, H.; Imai, T.; Sakai, Y.; Azuma, M. Colossal Negative Thermal Expansion in Electron-Doped PbVO3 Perovskites. Angew. Chem. Int. Ed. 2018, 57, 8170–8173. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, H.; Sakai, Y.; Nishikubo, T.; Pan, Z.; Oka, K.; Yamamoto, H.; Azuma, M. Negative Thermal Expansion in Lead-Free La-Substituted Bi0.5Na0.5VO3. Chem. Mater. 2020, 32, 4832–4837. [Google Scholar] [CrossRef]
- Wang, J.; Xu, P.; Yuan, H.; Gao, Q.; Sun, Q.; Liang, E. Negative thermal expansion driven by acoustic phonon modes in rhombohedral Zn2GeO4. Results Phys. 2020, 19, 103531. [Google Scholar] [CrossRef]
- Wei, S.; Kong, X.; Wang, H.; Mao, Y.; Chao, M.; Guo, J.; Liang, E. Negative thermal expansion property of CuMoO4. Optik 2018, 160, 61–67. [Google Scholar] [CrossRef]
- Mohamed, Z.; Tka, E.; Dhahri, J.; Hlil, E.K. Critical behavior near the paramagnetic to ferromagnetic phase transition temperature in La0.67Sr0.1Ca0.23MnO3 compound. J. Alloys Compd. 2016, 688, 1260–1267. [Google Scholar] [CrossRef]
- Khiem, N.V.; Bau, L.V.; Son, L.H.; Phuc, N.X.; Nam, D.N.H. Influence of A-site cation size on the magnetic and transport properties of (Nd1−yYy)0.7Sr0.3MnO3 (0 < y < 0.42). J. Magn. Magn. Mater. 2003, 262, 490–495. [Google Scholar]
- Abdulvagidov, B.; Djabrailov, Z.; Abdulvagidov, B.; Kurbakov, A.I. New Universality Class Associated with Jahn-Teller Distortion and Double Exchange. J. Exp. Theor. Phys. 2020, 130, 528–542. [Google Scholar] [CrossRef]
- Wu, F.; Ping, Y. Combining Landau Zener Theory and Kinetic Monte Carlo Sampling for Small Polaron Mobility of Doped BiVO4. arXiv 2018, arXiv:1808.02507. [Google Scholar]
- Mohanty, S.; Mukherjee, S. Effect of Jahn-Teller distortion on microstructural and dielectric properties of La based double perovskites. J. Alloys Compd. 2022, 892, 162204. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Sun, W.; Zhang, X.; Liu, H.; Chen, X.; Zeng, X. Phase transition temperature and negative thermal expansion of Sc-substituted In2(MoO4)3 ceramics. J. Mater. Sci. 2020, 55, 5730–5740. [Google Scholar] [CrossRef]
- Wei, X.S.; Zhou, Q.; Yan, X.; Li, X.; Zhu, B. Preparation and properties of Zr2WP2O12 with negative thermal expansion without sintering additives. Process. Appl. Ceram. 2020, 14, 173–180. [Google Scholar]
- Sakthipandi, K.; Rajendran, V. Phase transitions of bulk and nanocrystalline La1−xPbxMnO3 (x = 0.3, 0.4, and 0.5) perovskite manganite materials using ultrasonic measurements. Mater. Chem. Phys. 2013, 138, 581–592. [Google Scholar] [CrossRef]






| Element | Sm | Sr | Mn | O |
|---|---|---|---|---|
| (at.%) | 14.46 | 2.39 | 16.20 | 66.95 |
| Sample | Pore Size (nm) | Pore Volume (cm3/g) | BET Surface Area (m2/g) |
|---|---|---|---|
| Sm0.85Sr0.15MnO3-δ | 15.7842 | 0.002563 | 0.6351 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, Y.; Li, Y.; Wu, Y. Negative Thermal Expansion Properties of Sm0.85Sr0.15MnO3-δ. J. Compos. Sci. 2022, 6, 156. https://doi.org/10.3390/jcs6060156
Li Y, Zhang Y, Li Y, Wu Y. Negative Thermal Expansion Properties of Sm0.85Sr0.15MnO3-δ. Journal of Composites Science. 2022; 6(6):156. https://doi.org/10.3390/jcs6060156
Chicago/Turabian StyleLi, Yucheng, Yang Zhang, Yongtian Li, and Yifeng Wu. 2022. "Negative Thermal Expansion Properties of Sm0.85Sr0.15MnO3-δ" Journal of Composites Science 6, no. 6: 156. https://doi.org/10.3390/jcs6060156
APA StyleLi, Y., Zhang, Y., Li, Y., & Wu, Y. (2022). Negative Thermal Expansion Properties of Sm0.85Sr0.15MnO3-δ. Journal of Composites Science, 6(6), 156. https://doi.org/10.3390/jcs6060156






