Optimization of Electrical Intensity for Electrochemical Anodic Oxidation to Modify the Surface of Carbon Fibers and Preparation of Carbon Nanotubes/Carbon Fiber Multi-Scale Reinforcements
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
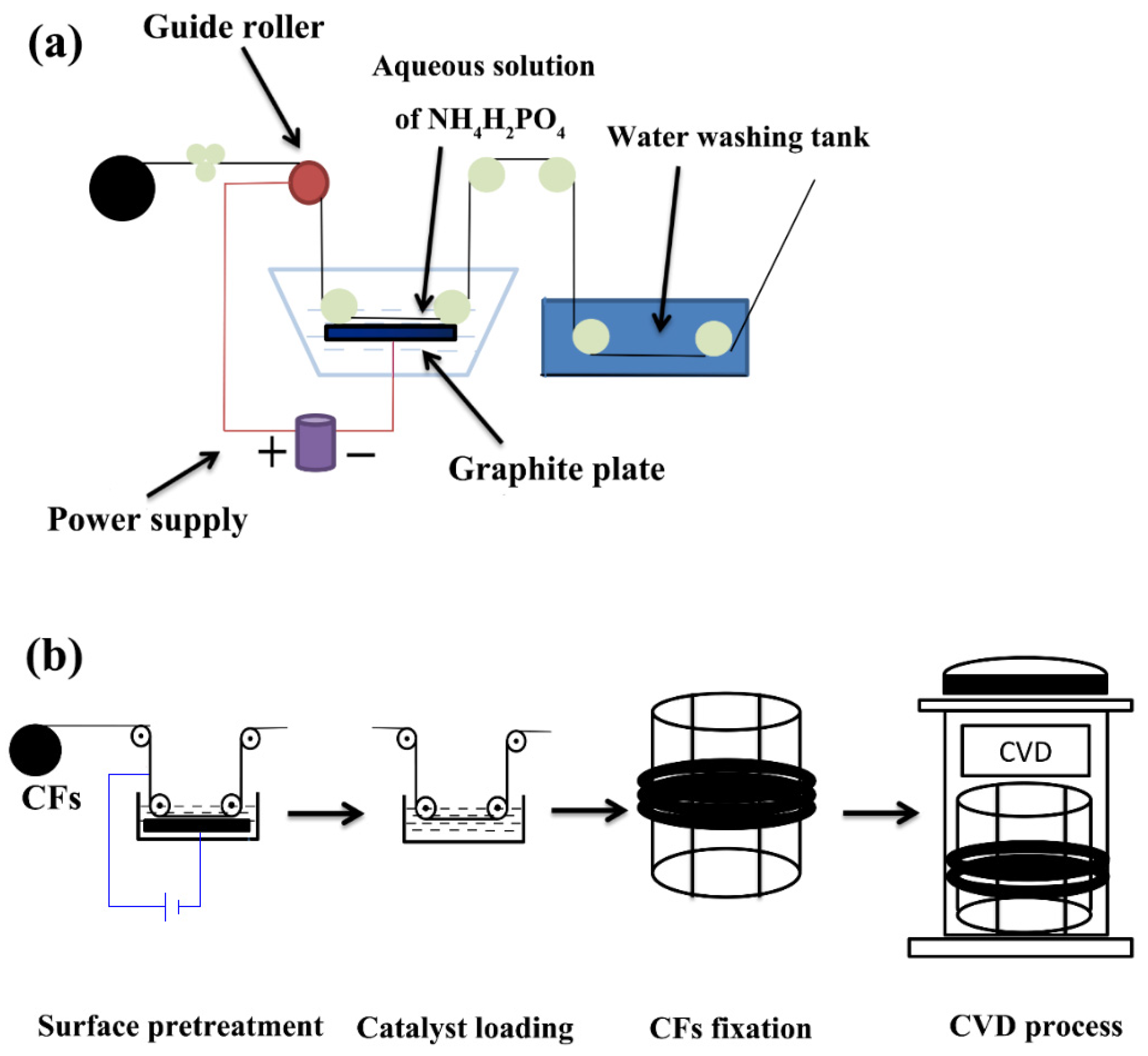
2.2. Preparation of CNTs/CF Multi-Scale Reinforcements
- As for HNO3 treatment, the desized CFs were fully immersed in a solution tank filled with HNO3 aqueous solution (10 wt%), which kept in a 60 °C oven for 1 h.
- With regard to H2O2 oxidation, we fully submerged the desized CFs into a solution tank filled with H2O2 aqueous solution (30 wt%) and kept them in a 60 °C oven for 1.5 h.
- Regarding EAO, this study adopted a laboratory-made EAO treatment device, as shown in Figure 1a. The desized CFs entered the electrolytic tank through the guide roller, and the electrolyte was an aqueous solution of NH4H2PO4 (5 wt%). The two variables affecting the etching degree of the CF surface, i.e., the wire speed and the electrolytic intensity, were integrated into the electrical intensity per unit mass of CFs in order to quantify the intensity of the EAO treatment.
2.3. Characterization
3. Results and Discussion
3.1. Effect of Surface Modification on CF Surface Morphology
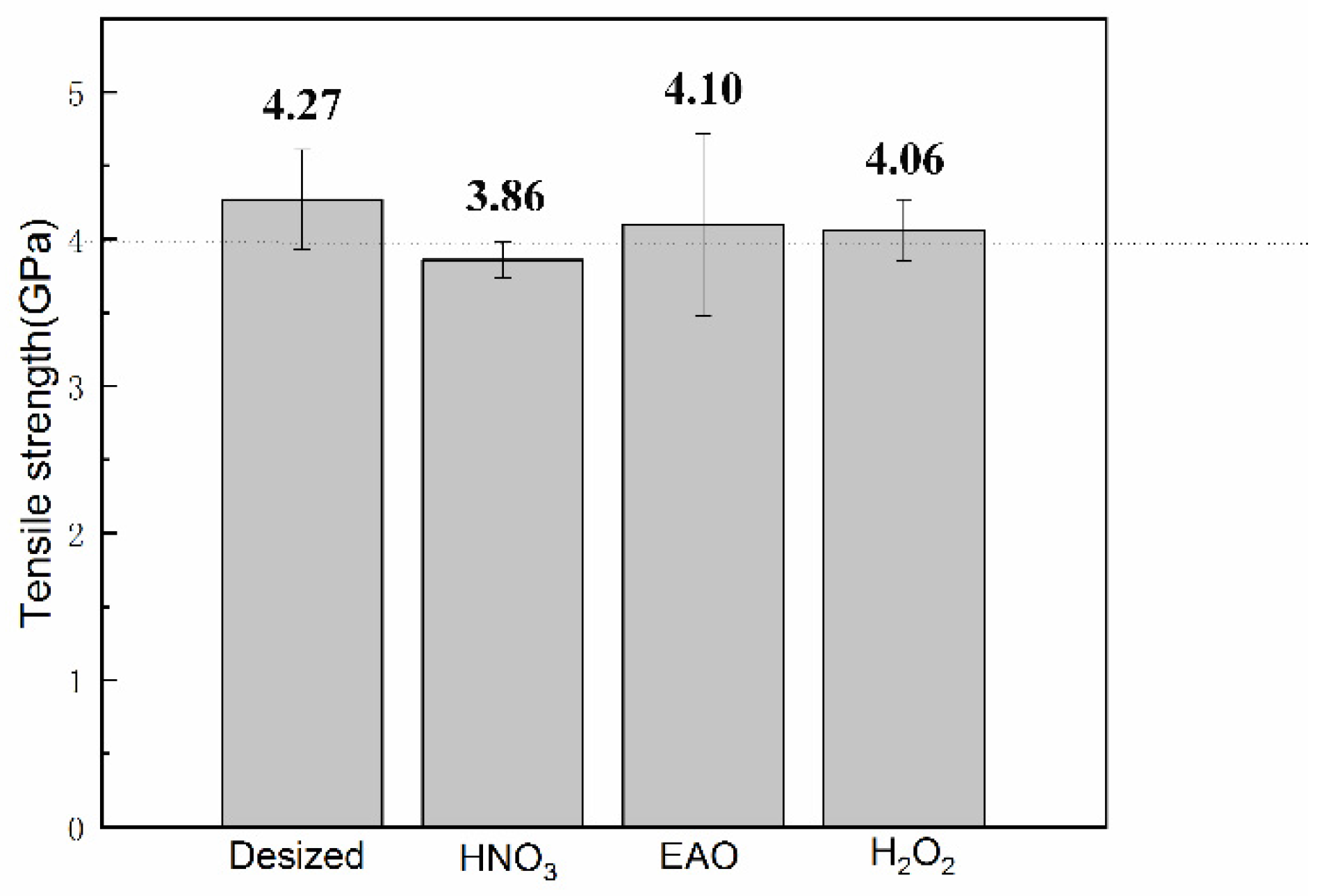
3.2. Effect of Surface Modification on Single-Filament Tensile Strength
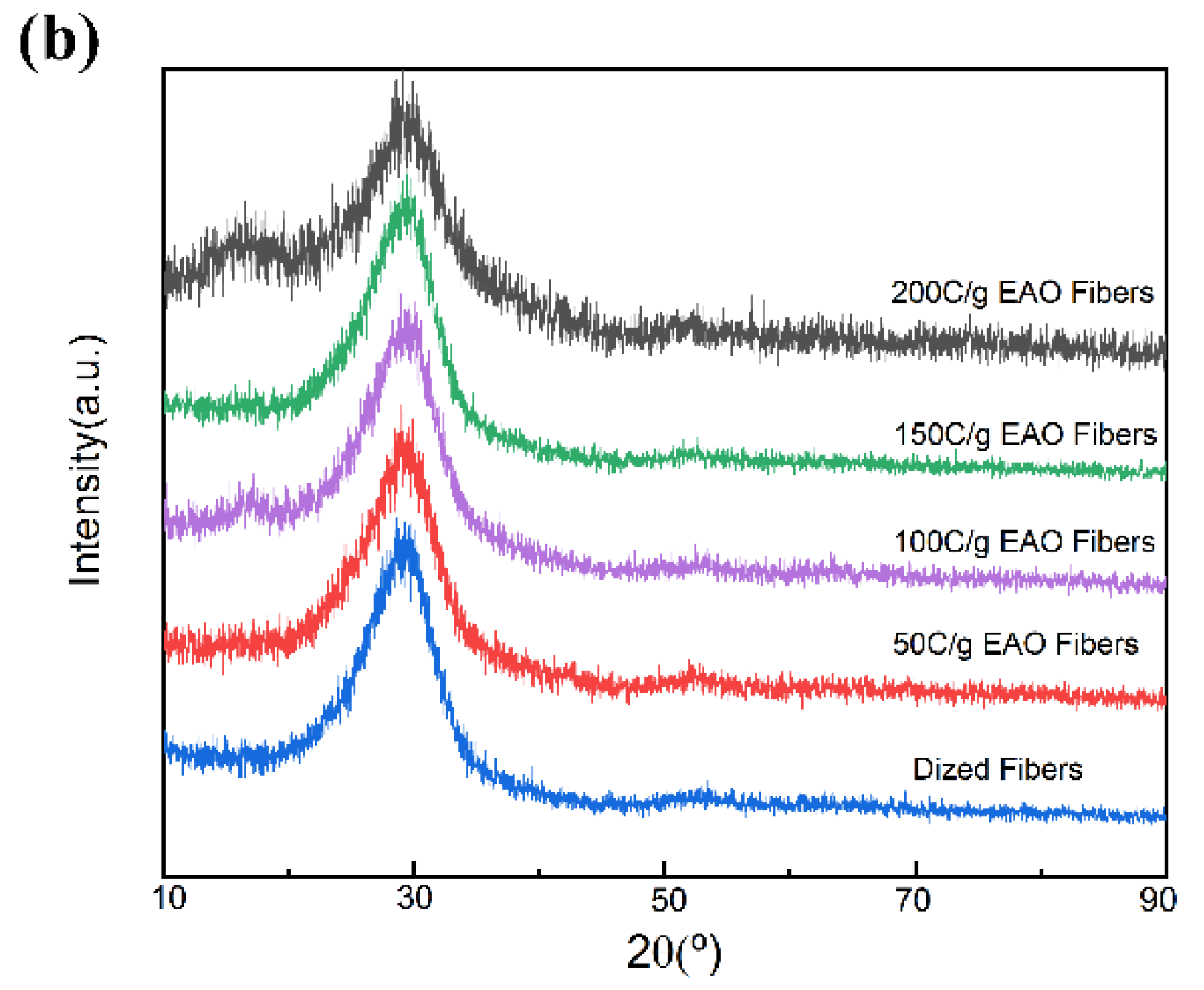
3.3. Effect of EAO Treatment on Surface Chemical Composition of CFs
3.4. Optimization of Process Parameters of EAO
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Kumar, S. Recent Progress in Fabrication, Structure, and Properties of Carbon Fibers. Polym. Rev. 2012, 52, 234–258. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, C.; Wang, Y.; Qin, J.; Ma, Z.; Cui, X.; Wang, Q.; Wei, H. Effect of CNTs deposition on carbon fiber followed by amination on the interfacial properties of epoxy composites. Compos. Struct. 2022, 292, 115665. [Google Scholar] [CrossRef]
- Gangineni, P.K.; Yandrapu, S.; Ghosh, S.K.; Anand, A.; Prusty, R.K.; Ray, B.C. Mechanical behavior of Graphene decorated carbon fiber reinforced polymer composites: An assessment of the influence of functional groups. Compos. Part A Appl. Sci. Manuf. 2019, 122, 36–44. [Google Scholar] [CrossRef]
- Nie, H.-J.; Shen, X.-J.; Tang, B.-L.; Dang, C.-Y.; Yang, S.; Fu, S.-Y. Effectively enhanced interlaminar shear strength of carbon fiber fabric/epoxy composites by oxidized short carbon fibers at an extremely low content. Compos. Sci. Technol. 2019, 183, 107803. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Liu, Y.; Wang, X.; Yang, B. A novel surface modification of carbon fiber for high-performance thermoplastic polyurethane composites. Appl. Surf. Sci. 2016, 382, 144–154. [Google Scholar] [CrossRef]
- Dong, J.; Jia, C.; Wang, M.; Fang, X.; Wei, H.; Xie, H.; Zhang, T.; He, J.; Jiang, Z.; Huang, Y. Improved mechanical properties of carbon fiber-reinforced epoxy composites by growing carbon black on carbon fiber surface. Compos. Sci. Technol. 2017, 149, 75–80. [Google Scholar] [CrossRef]
- Wu, Q.; Li, M.; Gu, Y.; Li, Y.; Zhang, Z. Nano-analysis on the structure and chemical composition of the interphase region in carbon fiber composite. Compos. Part A Appl. Sci. Manuf. 2014, 56, 143–149. [Google Scholar] [CrossRef]
- Adstedt, K.; Stojcevski, F.; Newman, B.; Hayne, D.J.; Henderson, L.C.; Mollenhauer, D.; Nepal, D.; Tsukruk, V. Carbon Fiber Surface Functional Landscapes: Nanoscale Topography and Property Distribution. ACS Appl. Mater. Interfaces 2022, 14, 4699–4713. [Google Scholar] [CrossRef]
- Hu, J.; Li, F.; Wang, B.; Zhang, H.; Ji, C.; Wang, S.; Zhou, Z. A two-step combination strategy for significantly enhancing the interfacial adhesion of CF/PPS composites: The liquid-phase oxidation followed by grafting of silane coupling agent. Compos. Part B Eng. 2020, 191, 107966. [Google Scholar] [CrossRef]
- Yongqiang, L.; Chunzheng, P. Chemically grafting carbon nanotubes onto carbon fibers for enhancing interfacial strength in carbon fiber/HDPE composites. Surf. Interface Anal. 2018, 50, 552–557. [Google Scholar] [CrossRef]
- Qin, J.; Wang, C.; Wang, Y.; Su, S.; Lu, R.; Yao, Z.; Wang, Q.; Gao, Q. Communication—A Technique for Online Continuous Manufacture of Carbon Nanotubes-Grown Carbon Fibers. ECS J. Solid State Sci. Technol. 2019, 8, M23–M25. [Google Scholar] [CrossRef]
- Russello, M.; Diamanti, E.K.; Catalanotti, G.; Ohlsson, F.; Hawkins, S.C.; Falzon, B.G. Enhancing the electrical conductivity of carbon fibre thin-ply laminates with directly grown aligned carbon nanotubes. Compos. Struct. 2018, 206, 272–278. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Wang, Z.; Wang, Y.; Lu, R.; Qin, J. Colossal permittivity of carbon nanotubes grafted carbon fiber-reinforced epoxy composites. Mater. Lett. 2018, 211, 273–276. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, C.; Qin, J.; Wang, Y.; Wang, Q.; Wei, H. Fex-Co1-x bimetallic catalysts for highly efficient growth of carbon nanotubes on carbon fibers. Ceram. Int. 2020, 46, 27158–27162. [Google Scholar] [CrossRef]
- Su, S.; Wang, Y.; Qin, J.; Wang, C.; Yao, Z.; Lu, R.; Wang, Q. Continuous method for grafting CNTs on the surface of carbon fibers based on cobalt catalyst assisted by thiourea. J. Mater. Sci. 2019, 54, 12498–12508. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, Y.; Qin, J.; Wang, X.; Lu, R.; Qu, C.; Wang, C. Scalable manufacturing of carbon nanotubes on continuous carbon fibers surface from chemical vapor deposition. Vacuum 2018, 152, 84–90. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Su, S. Optimization of Process Conditions for Continuous Growth of CNTs on the Surface of Carbon Fibers. J. Compos. Sci. 2021, 5, 111. [Google Scholar] [CrossRef]
- Guo, M.; Liu, J.; Yuan, Y.; Zhang, Z.; Yin, S.; Leng, J.; Huang, N. CNTs/Cf based counter electrode for highly efficient hole-transport-material-free perovskite solar cells. J. Photochem. Photobiol. A Chem. 2020, 403, 112843. [Google Scholar] [CrossRef]
- Rong, H.; Dahmen, K.-H.; Garmestani, H.; Yu, M.; Jacob, K.I. Comparison of chemical vapor deposition and chemical grafting for improving the mechanical properties of carbon fiber/epoxy composites with multi-wall carbon nanotubes. J. Mater. Sci. 2013, 48, 4834–4842. [Google Scholar] [CrossRef]
- Qin, J.; Wang, C.; Lu, R.; Su, S.; Yao, Z.; Zheng, L.; Gao, Q.; Wang, Y.; Wang, Q.; Wei, H. Uniform growth of carbon nanotubes on carbon fiber cloth after surface oxidation treatment to enhance interfacial strength of composites. Compos. Sci. Technol. 2020, 195, 108198. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, R.; He, M.; Sun, L.; Wang, C.; Liu, L.; Zhao, L.; Cui, H.; Cao, A. Effect of a multiscale reinforcement by carbon fiber surface treatment with graphene oxide/carbon nanotubes on the mechanical properties of reinforced carbon/carbon composites. Compos. Part A Appl. Sci. Manuf. 2016, 90, 433–440. [Google Scholar] [CrossRef]
- Zhang, W.; Deng, X.; Sui, G.; Yang, X. Improving interfacial and mechanical properties of carbon nanotube-sized carbon fiber/epoxy composites. Carbon 2019, 145, 629–639. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Jiang, H.; Tan, H.; Liu, D. Continuous in-situ growth of carbon nanotubes on carbon fibers at various temperatures for efficient electromagnetic wave absorption. Carbon 2022, 200, 94–107. [Google Scholar] [CrossRef]
- Fan, W.; Wang, Y.; Wang, C.; Chen, J.; Wang, Q.; Yuan, Y.; Niu, F. High efficient preparation of carbon nanotube-grafted carbon fibers with the improved tensile strength. Appl. Surf. Sci. 2016, 364, 539–551. [Google Scholar] [CrossRef]
- Wang, B.; Fu, Q.; Sun, L.; Lu, Y.; Liu, Y. Improving the tribological performance of carbon fiber reinforced resin composite by grafting MWCNT and GNPs on fiber surface. Mater. Lett. 2022, 306, 130953. [Google Scholar] [CrossRef]
- Xu, S.; Jiang, Q. Surface modification of carbon fiber support by ferrous oxalate for biofilm wastewater treatment system. J. Clean. Prod. 2018, 194, 416–424. [Google Scholar] [CrossRef]
- Fan, W.; Wang, Y.; Chen, J.; Yuan, Y.; Li, A.; Wang, Q.; Wang, C. Controllable growth of uniform carbon nanotubes/carbon nanofibers on the surface of carbon fibers. RSC Adv. 2015, 5, 75735–75745. [Google Scholar] [CrossRef]
- Qin, R.; Tian, Y.; Zheng, L.; Qin, J.; Tao, Y.; Song, C.; Fan, W.; Wang, Y. Studying the growth of carbon nanotubes on carbon fibers surface under different catalysts and electrochemical treatment conditions. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 156–162. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, Y.; Xu, X.; Cui, B.; Yao, Z.; Wang, Y.; Wang, C.; Wang, Y.; Wang, Y. Continuous growth of carbon nanotubes on the surface of carbon fibers for enhanced electromagnetic wave absorption properties. Ceram. Int. 2022, 48, 1869–1878. [Google Scholar] [CrossRef]
- Chang, Q.; Zhao, H.; He, R. The mechanical properties of plasma-treated carbon fiber reinforced PA6 composites with CNT. Surf. Interface Anal. 2017, 49, 1244–1248. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, W.; Liu, P.; Tian, J.; Xu, M.; Sun, S.; Wang, Y. Study on atmospheric air glow discharge plasma generation and surface modification of carbon fiber fabric. Plasma Process. Polym. 2020, 17, 1900148. [Google Scholar] [CrossRef]
- Liang, Y.; Tuo, Z.; Zhao, Q.; Lin, S.; Lin, Z.; Han, Z.; Ren, L. Study on preparation and mechanical properties of bionic carbon fiber reinforced epoxy resin composite with eagle feather structure. Mater. Res. Express 2021, 8, 065301. [Google Scholar] [CrossRef]
- Li, H.; Liebscher, M.; Ranjbarian, M.; Hempel, S.; Tzounis, L.; Schröfl, C.; Mechtcherine, V. Electrochemical modification of carbon fiber yarns in cementitious pore solution for an enhanced interaction towards concrete matrices. Appl. Surf. Sci. 2019, 487, 52–58. [Google Scholar] [CrossRef]
- Fu, Y.; Li, H.; Cao, W. Enhancing the interfacial properties of high-modulus carbon fiber reinforced polymer matrix composites via electrochemical surface oxidation and grafting. Compos. Part A Appl. Sci. Manuf. 2020, 130, 105719. [Google Scholar] [CrossRef]
- Qian, X.; Zhong, J.; Zhi, J.; Heng, F.; Wang, X.; Zhang, Y.; Song, S. Electrochemical surface modification of polyacrylonitrile-based ultrahigh modulus carbon fibers and its effect on the interfacial properties of UHMCF/EP composites. Compos. Part B Eng. 2019, 164, 476–484. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Y.; Wang, C.; Xu, X.; Li, M.; Xu, Z.; Tan, H.; Wang, Y. Effect of electrochemical anodization and growth time on continuous growth of carbon nanotubes on carbon fiber surface. Ceram. Int. 2022, 48, 29695–29704. [Google Scholar] [CrossRef]
- Wen, Z.; Xu, C.; Qian, X.; Zhang, Y.; Wang, X.; Song, S.; Dai, M.; Zhang, C. A two-step carbon fiber surface treatment and its effect on the interfacial properties of CF/EP composites: The electrochemical oxidation followed by grafting of silane coupling agent. Appl. Surf. Sci. 2019, 486, 546–554. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, Y.; Qin, J.; Yao, Z.; Cui, X.; Cui, B.; Yue, Y.; Wang, Y.; Wang, C. Growth of carbon nanotubes on the surface of carbon fiber using Fe–Ni bimetallic catalyst at low temperature. Ceram. Int. 2021, 47, 1625–1631. [Google Scholar] [CrossRef]




| Main Element | Desized (At%) | EAO (At%) |
|---|---|---|
| C | 92.36 | 63.52 |
| O | 7.64 | 33.83 |
| N | 0 | 2.65 |
| Sample | 2θ (°) | d(002) (Å) |
|---|---|---|
| Desized | 25.693 | 3.465 |
| 50 C/g | 25.537 | 3.490 |
| 100 C/g | 25.236 | 3.504 |
| 150 C/g | 25.429 | 3.496 |
| 200 C/g | 25.512 | 3.533 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Wang, Y.; Cui, B.; Wang, C.; Tan, H.; Jiang, H.; Xu, Z.; Wang, C.; Zhuang, G. Optimization of Electrical Intensity for Electrochemical Anodic Oxidation to Modify the Surface of Carbon Fibers and Preparation of Carbon Nanotubes/Carbon Fiber Multi-Scale Reinforcements. J. Compos. Sci. 2022, 6, 395. https://doi.org/10.3390/jcs6120395
Li M, Wang Y, Cui B, Wang C, Tan H, Jiang H, Xu Z, Wang C, Zhuang G. Optimization of Electrical Intensity for Electrochemical Anodic Oxidation to Modify the Surface of Carbon Fibers and Preparation of Carbon Nanotubes/Carbon Fiber Multi-Scale Reinforcements. Journal of Composites Science. 2022; 6(12):395. https://doi.org/10.3390/jcs6120395
Chicago/Turabian StyleLi, Mengfan, Yanxiang Wang, Bowen Cui, Chengjuan Wang, Hongxue Tan, Haotian Jiang, Zhenhao Xu, Chengguo Wang, and Guangshan Zhuang. 2022. "Optimization of Electrical Intensity for Electrochemical Anodic Oxidation to Modify the Surface of Carbon Fibers and Preparation of Carbon Nanotubes/Carbon Fiber Multi-Scale Reinforcements" Journal of Composites Science 6, no. 12: 395. https://doi.org/10.3390/jcs6120395
APA StyleLi, M., Wang, Y., Cui, B., Wang, C., Tan, H., Jiang, H., Xu, Z., Wang, C., & Zhuang, G. (2022). Optimization of Electrical Intensity for Electrochemical Anodic Oxidation to Modify the Surface of Carbon Fibers and Preparation of Carbon Nanotubes/Carbon Fiber Multi-Scale Reinforcements. Journal of Composites Science, 6(12), 395. https://doi.org/10.3390/jcs6120395





