Cutting-Edge Green Polymer/Nanocarbon Nanocomposite for Supercapacitor—State-of-the-Art
Abstract
1. Introduction
2. Polymer-Based Materials for Energy Storage
3. Green Nanocomposite for Energy Storage
3.1. Green Nanocomposite Derived from Conducting Polymer/Nanocarbon for Energy Storage
3.1.1. Overview of Conjugated Polymer-Based Supercapacitive Nanomaterials
3.1.2. Use of Green Approaches to Form Conducting Polymer Nanocomposite for Supercapacitors
3.2. Green Cellulose/Nanocarbon Nanocomposites for Energy Storage
| Material | Nanofiller | Specific Capacitance | Capacitance Retention (%) | Ref. |
|---|---|---|---|---|
| Bacterial cellulose | Graphene | 1274.2 Fg−1 | 96.4 | [104] |
| Bacterial cellulose | Graphene oxide | 160 Fg−1 | 90.3 | [105] |
| Bacterial cellulose | Carbon nanotube | 50.5 Fg−1 | 99.5 | [106] |
| Bacterial cellulose | Polypyrrole | 153 Fg−1 | 93.0 | [107] |
| Bacterial cellulose | Carbonization | 216 Fg−1 | 97.6 | [108] |
| Bacterial cellulose | Carbonization | 204.9 Fg−1 | 90.0 | [109] |
| Bacterial cellulose | Carbonization | 422 Fg−1 | 113 | [110] |
| Bacterial cellulose | Polypyrrole; Carbon nanotube | 228 Fg−1 | 88.0 | [103] |
| Bacterial cellulose | Polypyrrole; Graphene | 1.93 Fcm−2 | 56.3 | [111] |
| Bacterial cellulose | Polypyrrole; Graphene | 4.16 Fcm−2 | 91.5 | [112] |
| Bacterial cellulose | Polypyrrole; reduced graphene oxide | 3.66 Fcm−2 | 73.5 | [113] |
| Bacterial cellulose | Reduced graphene oxide | 2106–2544 mFcm−2 | 100 | [114] |
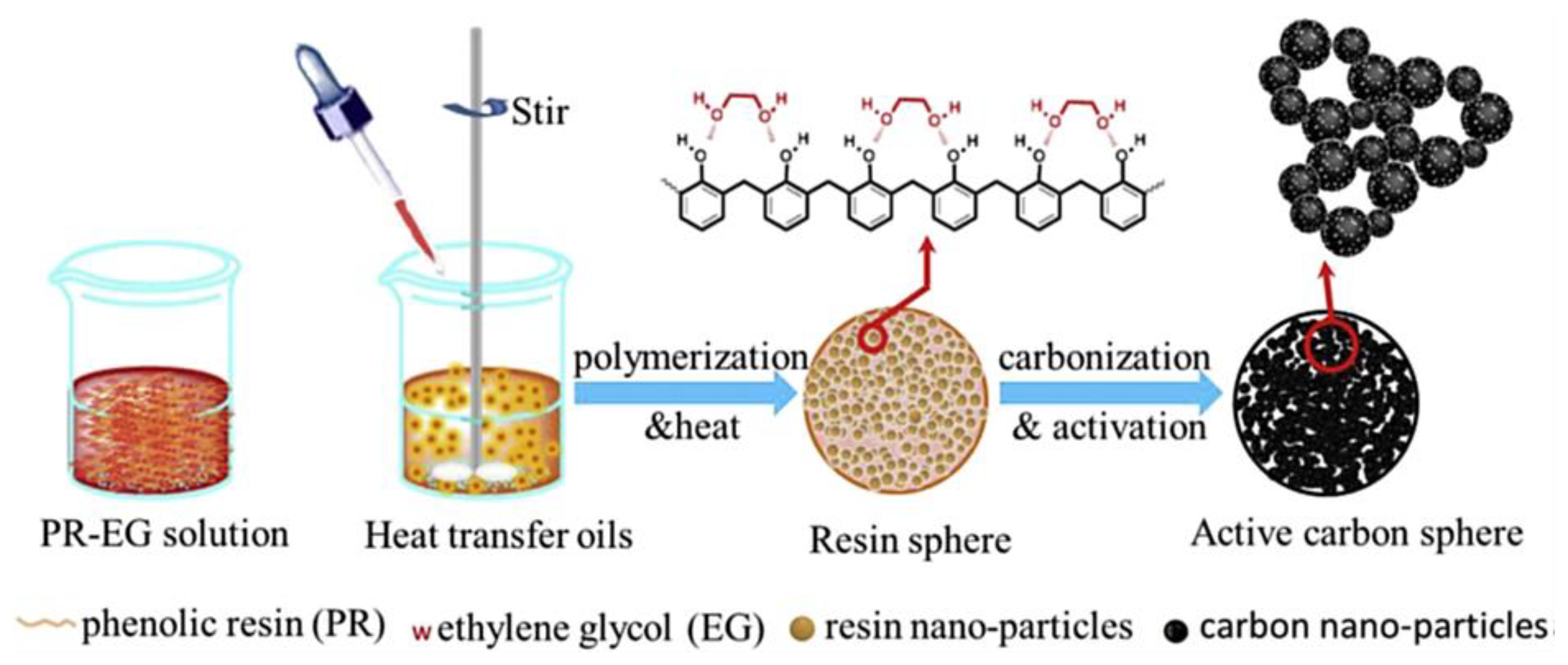
3.3. Green Nanocomposite Derivative of Thermosetting Polymer/Nanocarbon for Energy Storage
4. Opportunities, Challenges, and Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, M.; Huang, Y.; Chen, R.; Huang, Q.; Yang, Y.; Zhong, L.; Ren, J.; Wang, X. Green conversion of ganoderma lucidum residues to electrode materials for supercapacitors. Adv. Compos. Hybrid Mater. 2021, 4, 1270–1280. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Edo, M.G.; Alemán, C. Powering the future: Application of cellulose-based materials for supercapacitors. Green Chem. 2016, 18, 5930–5956. [Google Scholar] [CrossRef]
- Alam, M.F.; Laskar, A.A.; Zubair, M.; Baig, U.; Younus, H. Immobilization of yeast alcohol dehydrogenase on polyaniline coated silver nanoparticles formed by green synthesis. J. Mol. Catal. B Enzym. 2015, 119, 78–84. [Google Scholar] [CrossRef]
- Mehta, S.; Jha, S.; Huang, D.; Arole, K.; Liang, H. Microwave Synthesis of MnO2-Lignin Composite Electrodes for Supercapacitors. J. Compos. Sci. 2021, 5, 216. [Google Scholar] [CrossRef]
- Kausar, A. Conjugated Polymer/Graphene Oxide Nanocomposites—State-of-the-Art. J. Compos. Sci. 2021, 5, 292. [Google Scholar] [CrossRef]
- Koronis, G.; Silva, A.; Ong, M. Comparison of Structural Performance and Environmental Impact of Epoxy Composites Modified by Glass and Flax Fabrics. J. Compos. Sci. 2022, 6, 284. [Google Scholar] [CrossRef]
- Blatsi, C.; Patsidis, A.C.; Psarras, G.C. Dielectric Properties and Energy Storage of Hybrid/Boron Nitride/Titanium Carbide/Epoxy Nanocomposites. J. Compos. Sci. 2022, 6, 259. [Google Scholar] [CrossRef]
- Kausar, A.; Bocchetta, P. Poly (methyl methacrylate) Nanocomposite Foams Reinforced with Carbon and Inorganic Nanoparticles—State-of-the-Art. J. Compos. Sci. 2022, 6, 129. [Google Scholar] [CrossRef]
- Shrestha, R.G.; Shrestha, L.K.; Ariga, K. Carbon Nanoarchitectonics for Energy and Related Applications. C 2021, 7, 73. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, B.; Zhang, J. Recent development of repairable, malleable and recyclable thermosetting polymers through dynamic transesterification. Polymer 2020, 194, 122392. [Google Scholar] [CrossRef]
- Thakur, S.; Martínez-Alonso, C.; Lopez-Hernandez, E.; Lopez-Manchado, M.A.; Verdejo, R. Melt and solution processable novel photoluminescent polymer blends for multifaceted advanced applications. Polymer 2021, 215, 123378. [Google Scholar] [CrossRef]
- Kausar, A.; Bocchetta, P. Polymer/Graphene Nanocomposite Membranes: Status and Emerging Prospects. J. Compos. Sci. 2022, 6, 76. [Google Scholar] [CrossRef]
- Kausar, A. Green nanocomposites for energy storage. J. Compos. Sci. 2021, 5, 202. [Google Scholar] [CrossRef]
- Ning, N.; Wang, M.; Zhou, G.; Qiu, Y.; Wei, Y. Effect of polymer nanoparticle morphology on fracture toughness enhancement of carbon fiber reinforced epoxy composites. Compos. Part B Eng. 2022, 234, 109749. [Google Scholar] [CrossRef]
- Alsaad, A.M.; Aljarrah, I.A.; Ahmad, A.; Al-Bataineh, Q.M.; Shariah, A.; Al-Akhras, M.A.; Telfah, A.D. The structural, optical, thermal, and electrical properties of synthesized PEO/GO thin films. Appl. Phys. A 2022, 128, 676. [Google Scholar] [CrossRef]
- Singh, J.; Dhaliwal, A.; Sharma, K.; Sehgal, R.; Kumar, V. Conductive polymer-based composite photocatalysts for environment and energy applications. In Conjugated Polymers for Next-Generation Applications; Woodhead Publishing: Cambridge, UK, 2022; pp. 505–538. [Google Scholar]
- Albarqouni, Y.M.; Lee, S.P.; Ali, G.A.; Ethiraj, A.S.; Algarni, H.; Chong, K.F. Facile synthesis of reduced graphene oxide aerogel in soft drink as supercapacitor electrode. J. Nanostruct. Chem. 2022, 12, 417–427. [Google Scholar] [CrossRef]
- Sun, K.; Li, J.; Wu, D.; Jiang, J. Green synthesis of porous honeycomblike carbon materials for supercapacitor electrodes. Ind. Eng. Chem. Res. 2020, 59, 14288–14295. [Google Scholar] [CrossRef]
- Liu, S.; Yu, T.; Wu, Y.; Li, W.; Li, B. Evolution of cellulose into flexible conductive green electronics: A smart strategy to fabricate sustainable electrodes for supercapacitors. RSC Adv. 2014, 4, 34134–34143. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.-M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19. [Google Scholar] [CrossRef]
- Chen, G.Z. Supercapacitor and supercapattery as emerging electrochemical energy stores. Int. Mater. Rev. 2017, 62, 173–202. [Google Scholar] [CrossRef]
- Sen, P.; De, A.; Chowdhury, A.D.; Bandyopadhyay, S.; Agnihotri, N.; Mukherjee, M. Conducting polymer based manganese dioxide nanocomposite as supercapacitor. Electrochim. Acta 2013, 108, 265–273. [Google Scholar] [CrossRef]
- Libich, J.; Máca, J.; Vondrák, J.; Čech, O.; Sedlaříková, M. Supercapacitors: Properties and applications. J. Energy Storage 2018, 17, 224–227. [Google Scholar] [CrossRef]
- Pothu, R.; Gaddam, S.K.; Vadivel, S.; Boddula, R. Graphene-Based Materials for Self-Healable Supercapacitors. Mater. Res. Found. 2020, 64, 167–180. [Google Scholar]
- Naoi, K.; Naoi, W.; Aoyagi, S.; Miyamoto, J.-i.; Kamino, T. New generation “nanohybrid supercapacitor”. Acc. Chem. Res. 2013, 46, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar]
- Li, Y.; Wang, B.; Chen, H.; Feng, W. Improvement of the electrochemical properties via poly (3, 4-ethylenedioxythiophene) oriented micro/nanorods. J. Power Sources 2010, 195, 3025–3030. [Google Scholar] [CrossRef]
- Poizot, P.; Dolhem, F. Clean energy new deal for a sustainable world: From non-CO2 generating energy sources to greener electrochemical storage devices. Energy Environ. Sci. 2011, 4, 2003–2019. [Google Scholar] [CrossRef]
- Snook, G.A.; Peng, C.; Fray, D.J.; Chen, G.Z. Achieving high electrode specific capacitance with materials of low mass specific capacitance: Potentiostatically grown thick micro-nanoporous PEDOT films. Electrochem. Commun. 2007, 9, 83–88. [Google Scholar] [CrossRef]
- Liu, K.; Hu, Z.; Xue, R.; Zhang, J.; Zhu, J. Electropolymerization of high stable poly (3, 4-ethylenedioxythiophene) in ionic liquids and its potential applications in electrochemical capacitor. J. Power Sources 2008, 179, 858–862. [Google Scholar] [CrossRef]
- Khomenko, V.; Frackowiak, E.; Beguin, F. Determination of the specific capacitance of conducting polymer/nanotubes composite electrodes using different cell configurations. Electrochim. Acta 2005, 50, 2499–2506. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.L.; Zhao, X.; Wu, J. Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar] [CrossRef]
- Nayak, S.R.; Mohana, K.N.S.; Hegde, M.B.; Rajitha, K.; Madhusudhana, A.M.; Naik, S.R. Functionalized multi-walled carbon nanotube/polyindole incorporated epoxy: An effective anti-corrosion coating material for mild steel. J. Alloy. Compd. 2021, 856, 158057. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; pp. 320–329. [Google Scholar]
- Kebede, A.A.; Kalogiannis, T.; Van Mierlo, J.; Berecibar, M. A comprehensive review of stationary energy storage devices for large scale renewable energy sources grid integration. Renew. Sustain. Energy Rev. 2022, 159, 112213. [Google Scholar] [CrossRef]
- Xiao, J.; Han, J.; Zhang, C.; Ling, G.; Kang, F.; Yang, Q.H. Dimensionality, function and performance of carbon materials in energy storage devices. Adv. Energy Mater. 2022, 12, 2100775. [Google Scholar] [CrossRef]
- Chen, H.-W.; Hsu, C.-Y.; Chen, J.-G.; Lee, K.-M.; Wang, C.-C.; Huang, K.-C.; Ho, K.-C. Plastic dye-sensitized photo-supercapacitor using electrophoretic deposition and compression methods. J. Power Sources 2010, 195, 6225–6231. [Google Scholar] [CrossRef]
- Mousty, C.; Leroux, F. LDHs as electrode materials for electrochemical detection and energy storage: Supercapacitor, battery and (bio)-sensor. Recent Pat. Nanotechnol. 2012, 6, 174–192. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Aziz, U. Supercapattery: Merging of battery-supercapacitor electrodes for hybrid energy storage devices. J. Energy Storage 2022, 46, 103823. [Google Scholar] [CrossRef]
- Kausar, A. Epitome of Fullerene in Conducting Polymeric Nanocomposite—Fundamentals and Beyond. Polym. Plast. Technol. Mater. 2022, 1–14. [Google Scholar] [CrossRef]
- Dhilip Kumar, R.; Nagarani, S.; Sethuraman, V.; Andra, S.; Dhinakaran, V. Investigations of conducting polymers, carbon materials, oxide and sulfide materials for supercapacitor applications: A review. Chem. Pap. 2022, 76, 3371–3385. [Google Scholar] [CrossRef]
- Thakur, A.K.; Majumder, M.; Choudhary, R.B. π-Conjugated polymeric materials for cutting-edge electrochemical energy storage devices. In Conjugated Polymers for Next-Generation Applications; Woodhead Publishing: Cambridge, UK, 2022; pp. 145–173. [Google Scholar]
- Chen, Z.; Augustyn, V.; Wen, J.; Zhang, Y.; Shen, M.; Dunn, B.; Lu, Y. High-performance supercapacitors based on intertwined CNT/V2O5 nanowire nanocomposites. Adv. Mater. 2011, 23, 791–795. [Google Scholar] [CrossRef]
- Kim, Y.-T.; Tadai, K.; Mitani, T. Highly dispersed ruthenium oxide nanoparticles on carboxylated carbon nanotubes for supercapacitor electrode materials. J. Mater. Chem. 2005, 15, 4914–4921. [Google Scholar] [CrossRef]
- Senthilkumar, B.; Selvan, R.K.; Meyrick, D.; Minakshi, M. Synthesis and characterization of manganese molybdate for symmetric capacitor applications. Int. J. Electrochem. Sci. 2015, 10, 185–193. [Google Scholar]
- Amoura, D.; Sánchez-Jiménez, M.; Estrany, F.; Makhloufi, L.; Alemán, C. Clay incorporation at the dielectric layer of multilayer polymer films for electrochemical activation. Eur. Polym. J. 2015, 69, 296–307. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.P.; Cheng, H.M. Advanced materials for energy storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef]
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.-M.; Van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; World Scientific: Singapore, 2011; pp. 148–159. [Google Scholar]
- Salahdin, O.D.; Sayadi, H.; Solanki, R.; Parra, R.M.R.; Al-Thamir, M.; Jalil, A.T.; Izzat, S.E.; Hammid, A.T.; Arenas, L.A.B.; Kianfar, E. Graphene and carbon structures and nanomaterials for energy storage. Appl. Phys. A 2022, 128, 1–23. [Google Scholar]
- Wu, G.; Wu, X.; Zhu, X.; Xu, J.; Bao, N. Two-dimensional hybrid nanosheet-based supercapacitors: From building block architecture, fiber assembly, and fabric construction to wearable applications. ACS Nano 2022, 16, 10130–10155. [Google Scholar] [CrossRef]
- Sivasankarapillai, V.S.; Keertheeswari, N.V.; Chahal, P.; Wabaidur, S.M.; Ponnusamy, V.K.; Dhanusuraman, R. Facile electrodeposition fabrication of raspberry-like gold microspheres decorated polydiphenylamine nanohybrid coated electrode for efficient direct methanol fuel cell application. Fuel 2022, 330, 125530. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Li, H.; Huang, Y.; Zhi, C. Polymers for supercapacitors: Boosting the development of the flexible and wearable energy storage. Mater. Sci. Eng. R Rep. 2020, 139, 100520. [Google Scholar]
- Choudhary, R.B.; Ansari, S.; Purty, B. Robust electrochemical performance of polypyrrole (PPy) and polyindole (PIn) based hybrid electrode materials for supercapacitor application: A review. J. Energy Storage 2020, 29, 101302. [Google Scholar] [CrossRef]
- Pandolfo, T.; Ruiz, V.; Sivakkumar, S.; Nerkar, J. General properties of electrochemical capacitors. In Supercapacitors: Materials, Systems, and Applications; Béguin, F., Frąckowiak, E., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 69–109. [Google Scholar]
- Abdelhamid, M.E.; O’Mullane, A.P.; Snook, G.A. Storing energy in plastics: A review on conducting polymers & their role in electrochemical energy storage. RSC Adv. 2015, 5, 11611–11626. [Google Scholar]
- Kim, I.T.; Tannenbaum, A.; Tannenbaum, R. Anisotropic conductivity of magnetic carbon nanotubes embedded in epoxy matrices. Carbon 2011, 49, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Nam, I.; Lee, H.-K.; Jang, J. Electromagnetic interference shielding/absorbing characteristics of CNT-embedded epoxy composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1110–1118. [Google Scholar] [CrossRef]
- Al-Osaimi, J.; Alhosiny, N.; Badawi, A.; Abdallah, S. The effects of CNTs types on the structural and electrical properties of CNTs/PMMA nanocomposite films. Int. J. Eng. Technol. 2013, 13, 77–79. [Google Scholar]
- Jayalakshmi, M.; Balasubramanian, K. Simple capacitors to supercapacitors-an overview. Int. J. Electrochem. Sci. 2008, 3, 1196–1217. [Google Scholar]
- Liu, A.; Li, C.; Bai, H.; Shi, G. Electrochemical deposition of polypyrrole/sulfonated graphene composite films. J. Phys. Chem. C 2010, 114, 22783–22789. [Google Scholar] [CrossRef]
- Gao, Y. Graphene and polymer composites for supercapacitor applications: A review. Nanoscale Res. Lett. 2017, 12, 387. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, S.; Xu, K.; Zhang, Y.; Zhang, L.; Lou, G.; Wu, Y.; Zhu, E.; Chen, H.; Shen, Z. Sustainable activated carbons from dead ginkgo leaves for supercapacitor electrode active materials. Chem. Eng. Sci. 2018, 181, 36–45. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X. Conducting polymers directly coated on reduced graphene oxide sheets as high-performance supercapacitor electrodes. J. Phys. Chem. C 2012, 116, 5420–5426. [Google Scholar] [CrossRef]
- Chandra, S.; Patel, M.D.; Lang, H.; Bahadur, D. Dendrimer-functionalized magnetic nanoparticles: A new electrode material for electrochemical energy storage devices. J. Power Sources 2015, 280, 217–226. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Selvaganesh, S.V.; Mathiyarasu, J.; Phani, K.; Yegnaraman, V. Chemical synthesis of PEDOT–Au nanocomposite. Nanoscale Res. Lett. 2007, 2, 546. [Google Scholar] [CrossRef]
- Alvi, F.; Ram, M.K.; Basnayaka, P.A.; Stefanakos, E.; Goswami, Y.; Kumar, A. Graphene–polyethylenedioxythiophene conducting polymer nanocomposite based supercapacitor. Electrochim. Acta 2011, 56, 9406–9412. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, V.; Awasthi, K. Polyaniline–carbon nanotube composites: Preparation methods, properties, and applications. Polym. Plast. Technol. Eng. 2018, 57, 70–97. [Google Scholar] [CrossRef]
- Oueiny, C.; Berlioz, S.; Perrin, F.-X. Carbon nanotube–polyaniline composites. Prog. Polym. Sci. 2014, 39, 707–748. [Google Scholar]
- Alvarez de Cienfuegos, L.; Robles, R.; Miguel, D.; Justicia, J.; Cuerva, J.M. Reduction reactions in green solvents: Water, supercritical carbon dioxide, and ionic liquids. ChemSusChem 2011, 4, 1035–1048. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Shim, J.-J. Green synthesis and characterization of carbon nanotubes/polyaniline nanocomposites. J. Spectrosc. 2015, 2015, 297804. [Google Scholar] [CrossRef]
- Zhao, X.; Gnanaseelan, M.; Jehnichen, D.; Simon, F.; Pionteck, J. Green and facile synthesis of polyaniline/tannic acid/rGO composites for supercapacitor purpose. J. Mater. Sci. 2019, 54, 10809–10824. [Google Scholar] [CrossRef]
- Ur Rahman, S.; Röse, P.; ul Haq Ali Shah, A.; Krewer, U.; Bilal, S. An amazingly simple, fast and green synthesis route to polyaniline nanofibers for efficient energy storage. Polymers 2020, 12, 2212. [Google Scholar] [CrossRef]
- Simotwo, S.K.; DelRe, C.; Kalra, V. Supercapacitor electrodes based on high-purity electrospun polyaniline and polyaniline–carbon nanotube nanofibers. ACS Appl. Mater. Interfaces 2016, 8, 21261–21269. [Google Scholar] [CrossRef]
- Sekhar, S.C.; Nagaraju, G.; Yu, J.S. Conductive silver nanowires-fenced carbon cloth fibers-supported layered double hydroxide nanosheets as a flexible and binder-free electrode for high-performance asymmetric supercapacitors. Nano Energy 2017, 36, 58–67. [Google Scholar] [CrossRef]
- Song, B.; Tuan, C.-C.; Huang, X.; Li, L.; Moon, K.-S.; Wong, C.-P. Sulfonated polyaniline decorated graphene nanocomposites as supercapacitor electrodes. Mater. Lett. 2016, 166, 12–15. [Google Scholar] [CrossRef]
- Ghosh, S.; Yadav, S.; Devi, A.; Thomas, T. Techno-economic understanding of Indian energy-storage market: A perspective on green materials-based supercapacitor technologies. Renew. Sustain. Energy Rev. 2022, 161, 112412. [Google Scholar] [CrossRef]
- Siva, V.; Murugan, A.; Shameem, A.; Thangarasu, S.; Bahadur, S.A. A facile microwave-assisted combustion synthesis of NiCoFe2O4 anchored polymer nanocomposites as an efficient electrode material for asymmetric supercapacitor application. J. Energy Storage 2022, 48, 103965. [Google Scholar] [CrossRef]
- Daniel, S. Carbon Nanotube (CNT)-Based Polymeric Supercapacitors. In Polymer Nanocomposites in Supercapacitors; CRC Press: Boca Raton, FL, USA, 2022; pp. 61–76. [Google Scholar]
- Sarno, M.; Casa, M. Green and one-step synthesis for Ag/graphene hybrid supercapacitor with remarkable performance. J. Phys. Chem. Solids 2018, 120, 241–249. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G. Green synthesis and electrochemical performances of ZnO/graphene nanocomposites. Ionics 2022, 28, 3547–3555. [Google Scholar] [CrossRef]
- Mou, Z.; Yang, Q.; Peng, J.; Yan, R.; Zhao, B.; Ge, Y.; Xiao, D. One-step green synthesis of oil-dispersible carbonized polymer dots as eco-friendly lubricant additives with superior dispersibility, lubricity, and durability. J. Colloid Interface Sci. 2022, 623, 762–774. [Google Scholar] [CrossRef]
- Lin, X.; Xu, Q.; Gan, L.; Owens, G.; Chen, Z. Cyclodextrin modified green synthesized graphene oxide@ iron nanoparticle composites for enhanced removal of oxytetracycline. J. Colloid Interface Sci. 2022, 608, 3159–3167. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.K.; Das, A.K.; Pradhan, D. High performance solid-state asymmetric supercapacitor using green synthesized graphene–WO3 nanowires nanocomposite. ACS Sustain. Chem. Eng. 2017, 5, 10128–10138. [Google Scholar] [CrossRef]
- Arthisree, D.; Madhuri, W. Optically active polymer nanocomposite composed of polyaniline, polyacrylonitrile and green-synthesized graphene quantum dot for supercapacitor application. Int. J. Hydrog. Energy 2020, 45, 9317–9327. [Google Scholar] [CrossRef]
- Çıplak, Z.; Yıldız, A.; Yıldız, N. Green preparation of ternary reduced graphene oxide-au@ polyaniline nanocomposite for supercapacitor application. J. Energy Storage 2020, 32, 101846. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Zhou, J.; Bian, L.; Zhu, E.; Hai, J.; Tang, J.; Tang, W. Graphene/polypyrrole intercalating nanocomposites as supercapacitors electrode. Electrochim. Acta 2013, 112, 44–52. [Google Scholar] [CrossRef]
- Smith, J.; Wren, J.; Odziemkowski, M.; Shoesmith, D. The electrochemical response of preoxidized copper in aqueous sulfide solutions. J. Electrochem. Soc. 2007, 154, C431–C438. [Google Scholar] [CrossRef]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO). Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, L.; Liu, B.; Wang, Y.; Cui, Y.; Bao, Z.; Yu, G. Nanostructured conductive polypyrrole hydrogels as high-performance, flexible supercapacitor electrodes. J. Mater. Chem. A 2014, 2, 6086–6091. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, F.; Dou, H.; Yuan, C.; Yang, S.; Hao, L.; Shen, L.; Zhang, L.; Zhang, X. Preparation and electrochemical capacitance of hierarchical graphene/polypyrrole/carbon nanotube ternary composites. Electrochim. Acta 2012, 69, 160–166. [Google Scholar] [CrossRef]
- Mini, P.; Balakrishnan, A.; Nair, S.V.; Subramanian, K. Highly super capacitive electrodes made of graphene/poly (pyrrole). Chem. Commun. 2011, 47, 5753–5755. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wang, G.; Yang, C.; Jiang, H.; Li, C. A graphene/carbon nanotube@ π-conjugated polymer nanocomposite for high-performance organic supercapacitor electrodes. J. Mater. Chem. A 2015, 3, 3880–3890. [Google Scholar] [CrossRef]
- Brakat, A.; Zhu, H. Nanocellulose-graphene hybrids: Advanced functional materials as multifunctional sensing platform. Nano-Micro Lett. 2021, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- Brakat, A.; Zhu, H. Nanocellulose-Graphene Derivative Hybrids: Advanced Structure-Based Functionality from Top-down Synthesis to Bottom-up Assembly. ACS Appl. Bio Mater. 2021, 4, 7366–7401. [Google Scholar] [CrossRef] [PubMed]
- Malho, J.-M.; Laaksonen, P.i.; Walther, A.; Ikkala, O.; Linder, M.B. Facile method for stiff, tough, and strong nanocomposites by direct exfoliation of multilayered graphene into native nanocellulose matrix. Biomacromolecules 2012, 13, 1093–1099. [Google Scholar] [CrossRef]
- Song, L.; Li, Y.; Xiong, Z.; Pan, L.; Xu, X.; Lu, S. Water-Induced shape memory effect of nanocellulose papers from sisal cellulose nanofibers with graphene oxide. Carbohydr. Polym. 2018, 179, 110–117. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Haafiz, M.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, H.; Wang, Q.; Lu, Y.; Yan, J.; Cheng, W.; Rojas, O.J.; Han, G. Composite membranes of polyacrylonitrile cross-linked with cellulose nanocrystals for emulsion separation and regeneration. Compos. Part A Appl. Sci. Manuf. 2022, 164, 107300. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, D.; Lee, Y.H.; Chen, W.; Lee, S.Y. Nanocellulose for energy storage systems: Beyond the limits of synthetic materials. Adv. Mater. 2019, 31, 1804826. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, J.; Rafiq, M.I.; Dai, S.; Tang, J.; Tang, W. Growth of NiMn layered double hydroxide and polypyrrole on bacterial cellulose nanofibers for efficient supercapacitors. Electrochim. Acta 2019, 295, 82–91. [Google Scholar] [CrossRef]
- Song, N.; Tan, H.; Zhao, Y. Carbon fiber-bridged polyaniline/graphene paper electrode for a highly foldable all-solid-state supercapacitor. J. Solid State Electrochem. 2019, 23, 9–17. [Google Scholar] [CrossRef]
- Yao, J.; Ji, P.; Sheng, N.; Guan, F.; Zhang, M.; Wang, B.; Chen, S.; Wang, H. Hierarchical core-sheath polypyrrole@ carbon nanotube/bacterial cellulose macrofibers with high electrochemical performance for all-solid-state supercapacitors. Electrochim. Acta 2018, 283, 1578–1588. [Google Scholar] [CrossRef]
- Liu, R.; Ma, L.; Huang, S.; Mei, J.; Li, E.; Yuan, G. Large areal mass and high scalable and flexible cobalt oxide/graphene/bacterial cellulose electrode for supercapacitors. J. Phys. Chem. C 2016, 120, 28480–28488. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Zhu, E.; Tang, J.; Liu, X.; Tang, W. Facile synthesis of bacterial cellulose fibres covalently intercalated with graphene oxide by one-step cross-linking for robust supercapacitors. J. Mater. Chem. C 2015, 3, 1011–1017. [Google Scholar] [CrossRef]
- Kang, Y.J.; Chun, S.-J.; Lee, S.-S.; Kim, B.-Y.; Kim, J.H.; Chung, H.; Lee, S.-Y.; Kim, W. All-solid-state flexible supercapacitors fabricated with bacterial nanocellulose papers, carbon nanotubes, and triblock-copolymer ion gels. ACS Nano 2012, 6, 6400–6406. [Google Scholar] [CrossRef]
- Wang, F.; Kim, H.-J.; Park, S.; Kee, C.-D.; Kim, S.-J.; Oh, I.-K. Bendable and flexible supercapacitor based on polypyrrole-coated bacterial cellulose core-shell composite network. Compos. Sci. Technol. 2016, 128, 33–40. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, J.; Wu, X.; Shan, D.; Zhou, Q.; Jiang, L.; Yang, D.; Fan, Z. Facile synthesis of carbon nanofibers-bridged porous carbon nanosheets for high-performance supercapacitors. J. Power Sources 2016, 307, 190–198. [Google Scholar] [CrossRef]
- Chen, L.F.; Huang, Z.H.; Liang, H.W.; Gao, H.L.; Yu, S.H. Three-dimensional heteroatom-doped carbon nanofiber networks derived from bacterial cellulose for supercapacitors. Adv. Funct. Mater. 2014, 24, 5104–5111. [Google Scholar] [CrossRef]
- Shan, D.; Yang, J.; Liu, W.; Yan, J.; Fan, Z. Biomass-derived three-dimensional honeycomb-like hierarchical structured carbon for ultrahigh energy density asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 13589–13602. [Google Scholar] [CrossRef]
- Liu, R.; Ma, L.; Huang, S.; Mei, J.; Xu, J.; Yuan, G. Large areal mass, flexible and freestanding polyaniline/bacterial cellulose/graphene film for high-performance supercapacitors. RSC Adv. 2016, 6, 107426–107432. [Google Scholar] [CrossRef]
- Walton, I.M.; Cox, J.M.; Benson, C.A.; Patel, D.D.G.; Chen, Y.-S.; Benedict, J.B. The role of atropisomers on the photo-reactivity and fatigue of diarylethene-based metal–organic frameworks. New J. Chem. 2016, 40, 101–106. [Google Scholar] [CrossRef]
- Ma, L.; Liu, R.; Niu, H.; Wang, F.; Liu, L.; Huang, Y. Freestanding conductive film based on polypyrrole/bacterial cellulose/graphene paper for flexible supercapacitor: Large areal mass exhibits excellent areal capacitance. Electrochim. Acta 2016, 222, 429–437. [Google Scholar] [CrossRef]
- Ma, L.; Liu, R.; Niu, H.; Xing, L.; Liu, L.; Huang, Y. Flexible and freestanding supercapacitor electrodes based on nitrogen-doped carbon networks/graphene/bacterial cellulose with ultrahigh areal capacitance. ACS Appl. Mater. Interfaces 2016, 8, 33608–33618. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, A.; Dev, C.; Meem, M.T.; Gafur, M.A.; Hoque, M.A. Preparation and mechanical properties of green epoxy/chitosan/silver nanocomposite. Green Mater. 2022, 10, 110–118. [Google Scholar] [CrossRef]
- Shaik, S.; Franchetti, M.J. Recycling and Sustainability of Nanocomposites. In Applications of Nanocomposites; CRC Press: Boca Raton, FL, USA, 2022; pp. 236–259. [Google Scholar]
- Norizan, M.; Shazleen, S.; Alias, A.; Sabaruddin, F.; Rizal, M.; Zainudin, E.; Abdullah, N.; Samsudin, M.; Kamarudin, S.; Norrrahim, M. Nanocellulose-Based Nanocomposites for Sustainable Applications: A Review. Nanomaterials 2022, 12, 3483. [Google Scholar] [CrossRef]
- Guadagno, L.; Raimondo, M.; Vittoria, V.; Vertuccio, L.; Naddeo, C.; Russo, S.; De Vivo, B.; Lamberti, P.; Spinelli, G.; Tucci, V. Development of epoxy mixtures for application in aeronautics and aerospace. RSC Adv. 2014, 4, 15474–15488. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Tang, L.-C.; Yan, D.; Zhao, L.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. Improved dispersion and interface in the graphene/epoxy composites via a facile surfactant-assisted process. Compos. Sci. Technol. 2013, 82, 60–68. [Google Scholar] [CrossRef]
- Khare, K.S.; Khabaz, F.; Khare, R. Effect of carbon nanotube functionalization on mechanical and thermal properties of cross-linked epoxy–carbon nanotube nanocomposites: Role of strengthening the interfacial interactions. ACS Appl. Mater. Interfaces 2014, 6, 6098–6110. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhou, S. Facile fabrication of highly conductive polystyrene/nanocarbon composites with robust interconnected network via electrostatic attraction strategy. J. Mater. Chem. C 2018, 6, 550–557. [Google Scholar] [CrossRef]
- Lee, S.; Suh, D.; Kim, W.; Xu, C.; Kim, T.; Song, C.; Yoo, C.; Kim, Y.; Kim, J.; Baik, S. Carbon nanotube covalent bonding mediates extraordinary electron and phonon transports in soft epoxy matrix interface materials. Carbon 2020, 157, 12–21. [Google Scholar] [CrossRef]
- Guo, Y.; Ruan, K.; Shi, X.; Yang, X.; Gu, J. Factors affecting thermal conductivities of the polymers and polymer composites: A review. Compos. Sci. Technol. 2020, 193, 108134. [Google Scholar]
- Zhang, J.; Jiang, D. Interconnected multi-walled carbon nanotubes reinforced polymer-matrix composites. Compos. Sci. Technol. 2011, 71, 466–470. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Ma, C.-C.M.; Teng, C.-C.; Huang, Y.-W.; Liao, S.-H.; Huang, Y.-L.; Tien, H.-W.; Lee, T.-M.; Chiou, K.-C. Effect of functionalized carbon nanotubes on the thermal conductivity of epoxy composites. Carbon 2010, 48, 592–603. [Google Scholar] [CrossRef]
- Zabihi, O.; Khodabandeh, A.; Mostafavi, S.M. Preparation, optimization and thermal characterization of a novel conductive thermoset nanocomposite containing polythiophene nanoparticles using dynamic thermal analysis. Polym. Degrad. Stab. 2012, 97, 3–13. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, J.; Chen, S.; Wu, L.; Zhuo, D.; Cheng, X. Green fabrication of graphene oxide/epoxy nanocomposite and its application in diamond abrasive tools. Compos. Part B Eng. 2019, 177, 107383. [Google Scholar] [CrossRef]
- Seok, H.; Kim, D.S. Preparation and mechanical properties of green epoxy nanocomposites with cellulose nanocrystals. Polym. Eng. Sci. 2020, 60, 439–445. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, Y.; Peng, M. Green preparation of epoxy/graphene oxide nanocomposites using a glycidylamine epoxy resin as the surface modifier and phase transfer agent of graphene oxide. ACS Appl. Mater. Interfaces 2016, 8, 1854–1866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhao, J.; Feng, C.; Zhao, R.; Sun, Y.; Guan, T.; Han, B.; Tang, N.; Wang, J.; Li, K. Scalable synthesis of hierarchical macropore-rich activated carbon microspheres assembled by carbon nanoparticles for high rate performance supercapacitors. J. Power Sources 2017, 342, 363–370. [Google Scholar] [CrossRef]
- Ma, C.; Fan, Q.; Dirican, M.; Subjalearndee, N.; Cheng, H.; Li, J.; Song, Y.; Shi, J.; Zhang, X. Rational design of meso-/micro-pores for enhancing ion transportation in highly-porous carbon nanofibers used as electrode for supercapacitors. Appl. Surf. Sci. 2021, 545, 148933. [Google Scholar] [CrossRef]
- Ma, C.; Song, Y.; Shi, J.; Zhang, D.; Zhong, M.; Guo, Q.; Liu, L. Phenolic-based carbon nanofiber webs prepared by electrospinning for supercapacitors. Mater. Lett. 2012, 76, 211–214. [Google Scholar] [CrossRef]
- Duran, B.; Unver, I.C.; Bereket, G. Investigation of Supporting Electrolyte Effect on Supercapacitor Properties of Poly (Carbazole) Films. J. Electrochem. Sci. Technol. 2020, 11, 41–49. [Google Scholar] [CrossRef]



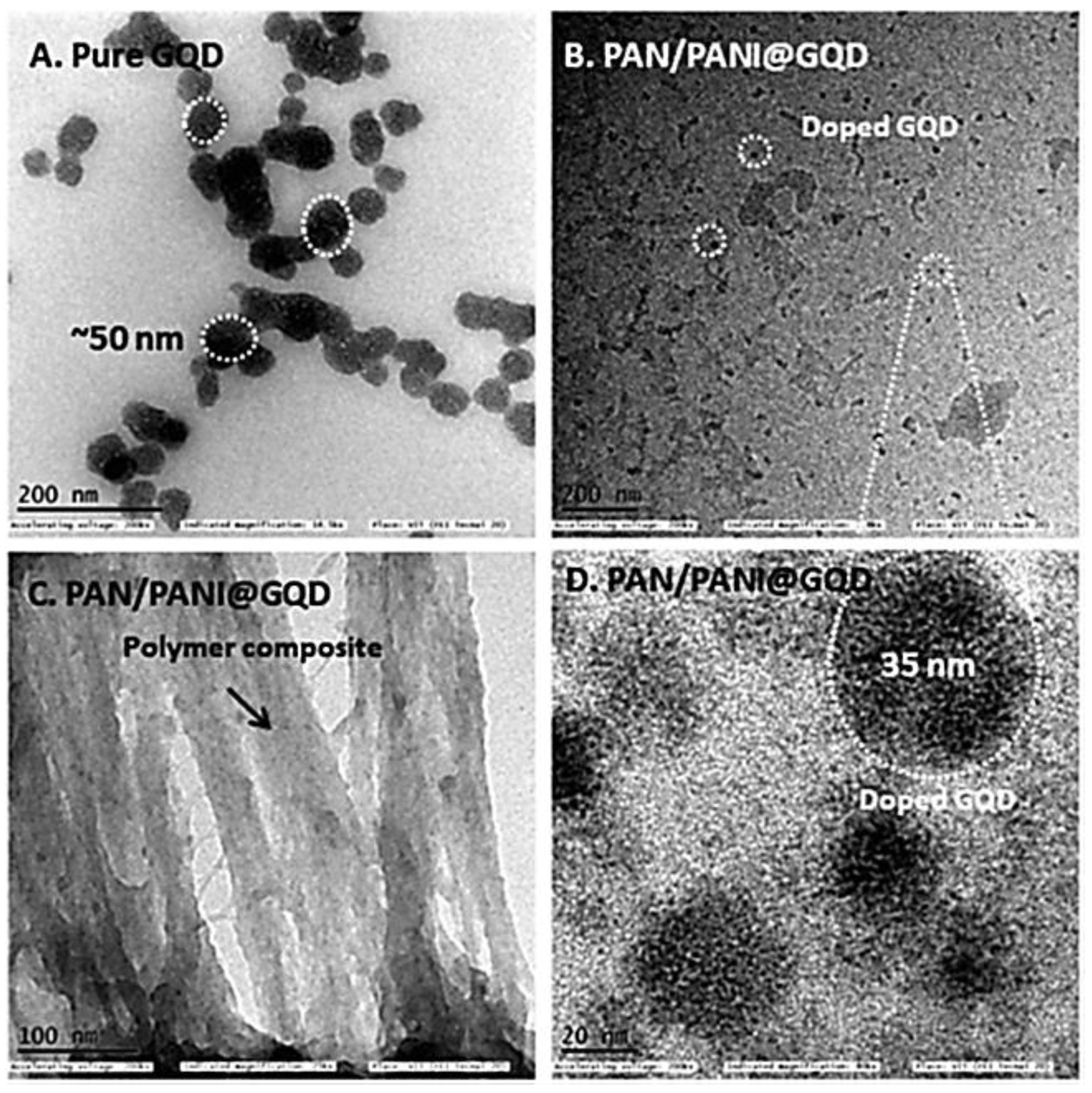
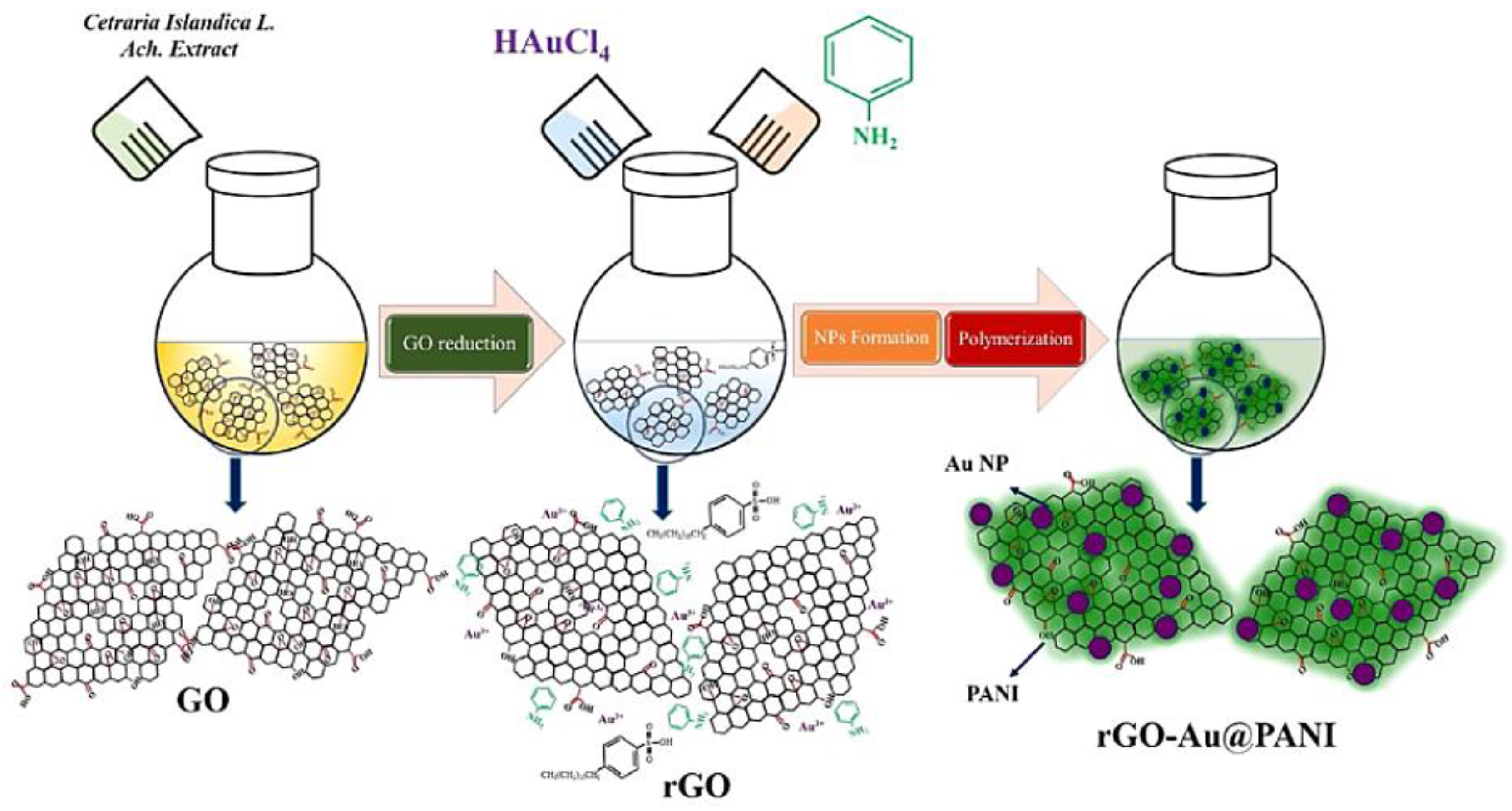

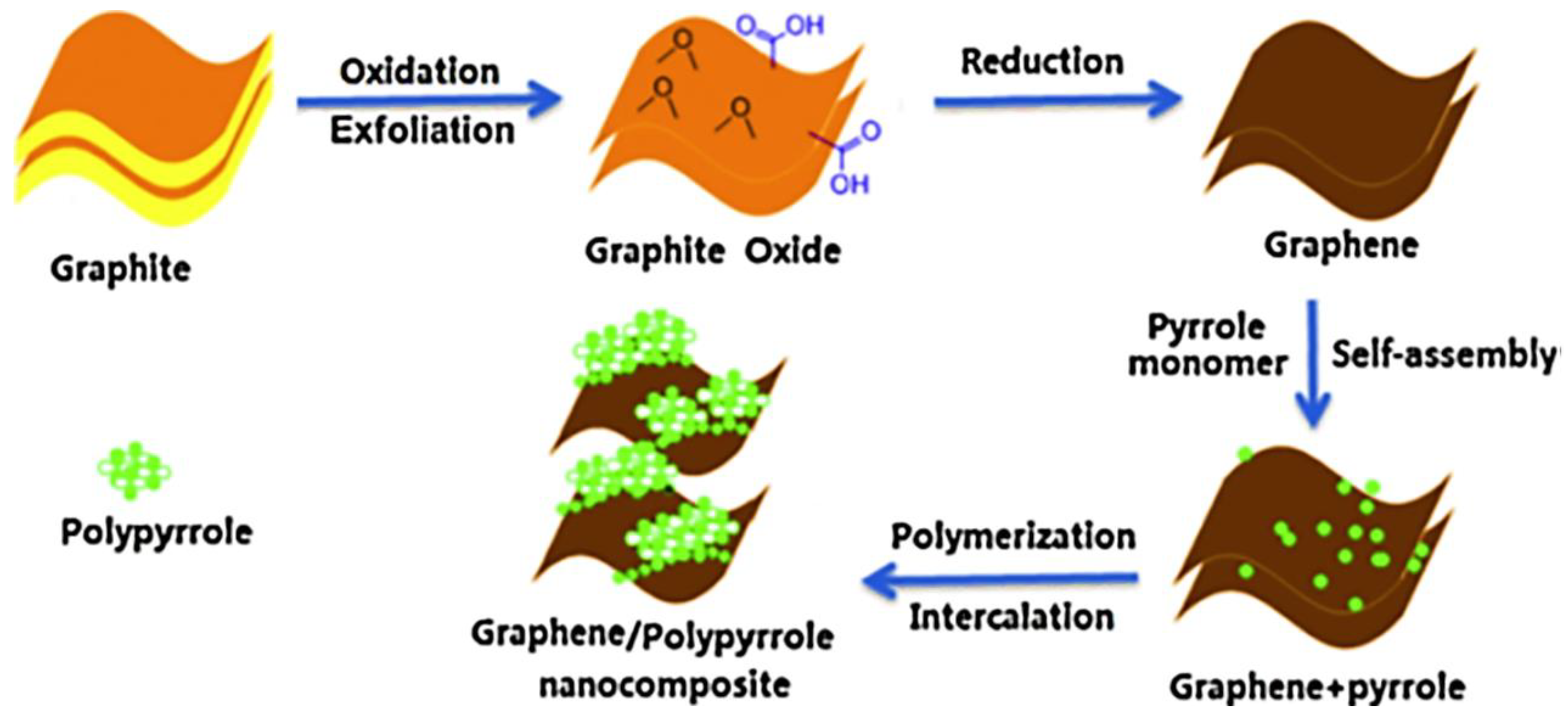


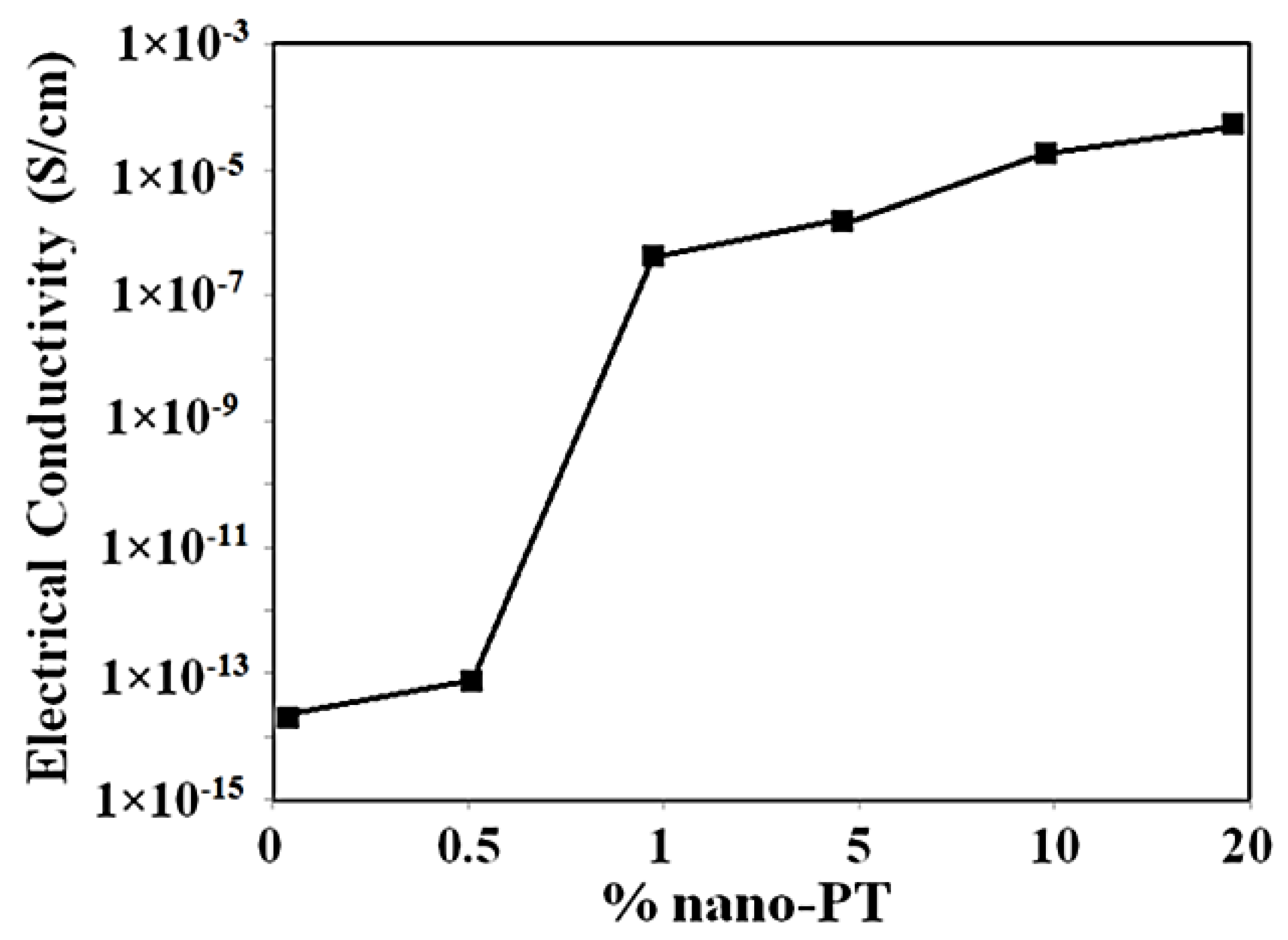
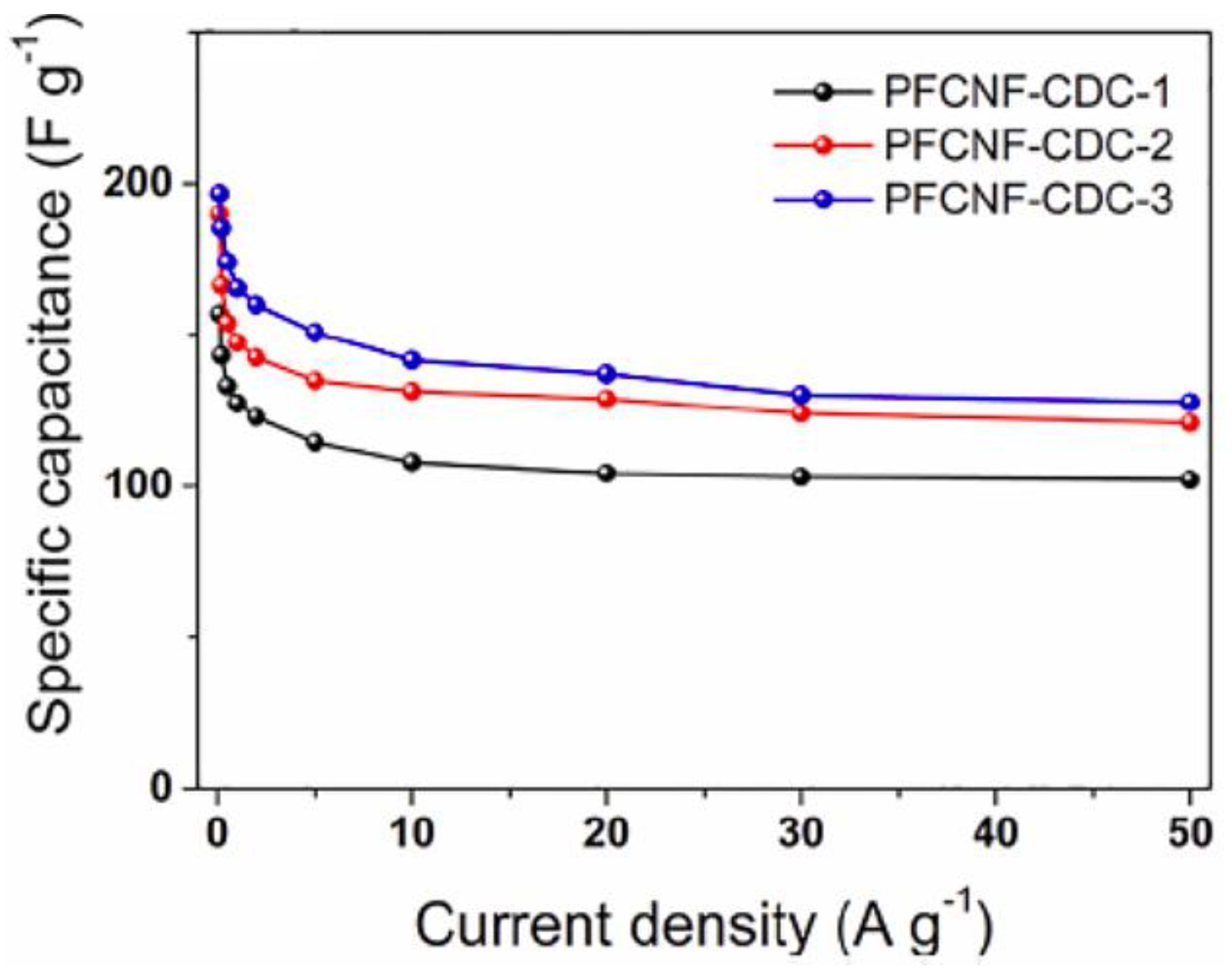
| Scan Rate (mVs−1) | Specific Capacitance (Fg−1) | |
|---|---|---|
| 2MHCl | 10 | 374 |
| 20 | 285 | |
| 50 | 177 | |
| 100 | 116 | |
| 2MH2SO4 | 10 | 261 |
| 20 | 246 | |
| 50 | 151 | |
| 100 | 125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kausar, A.; Ahmad, I.; Maaza, M.; Eisa, M.H.; Bocchetta, P. Cutting-Edge Green Polymer/Nanocarbon Nanocomposite for Supercapacitor—State-of-the-Art. J. Compos. Sci. 2022, 6, 376. https://doi.org/10.3390/jcs6120376
Kausar A, Ahmad I, Maaza M, Eisa MH, Bocchetta P. Cutting-Edge Green Polymer/Nanocarbon Nanocomposite for Supercapacitor—State-of-the-Art. Journal of Composites Science. 2022; 6(12):376. https://doi.org/10.3390/jcs6120376
Chicago/Turabian StyleKausar, Ayesha, Ishaq Ahmad, Malik Maaza, M. H. Eisa, and Patrizia Bocchetta. 2022. "Cutting-Edge Green Polymer/Nanocarbon Nanocomposite for Supercapacitor—State-of-the-Art" Journal of Composites Science 6, no. 12: 376. https://doi.org/10.3390/jcs6120376
APA StyleKausar, A., Ahmad, I., Maaza, M., Eisa, M. H., & Bocchetta, P. (2022). Cutting-Edge Green Polymer/Nanocarbon Nanocomposite for Supercapacitor—State-of-the-Art. Journal of Composites Science, 6(12), 376. https://doi.org/10.3390/jcs6120376








