A Comprehensive Review of Polymeric Wastewater Purification Membranes
Abstract
:1. Introduction
2. Dendritic Polymers
2.1. Titanium Supported Dendrimers
2.2. Magnetic Supports for Dendrimers
2.3. Dendrimers Supported on Nantural Materials
2.4. Carbon-Based Supports for Dendrimers
2.5. Miscellaneous Dendrimers
2.6. Outlook
3. Carbon-Based Polymeric Membranes
3.1. Carbon Nanotubes-Based Membranes
3.2. Graphene and Graphene Oxide-Based Membranes
4. Metal and Metal Oxide Nanoparticles
4.1. Membranes Infused with Aluminium Nanoparticles
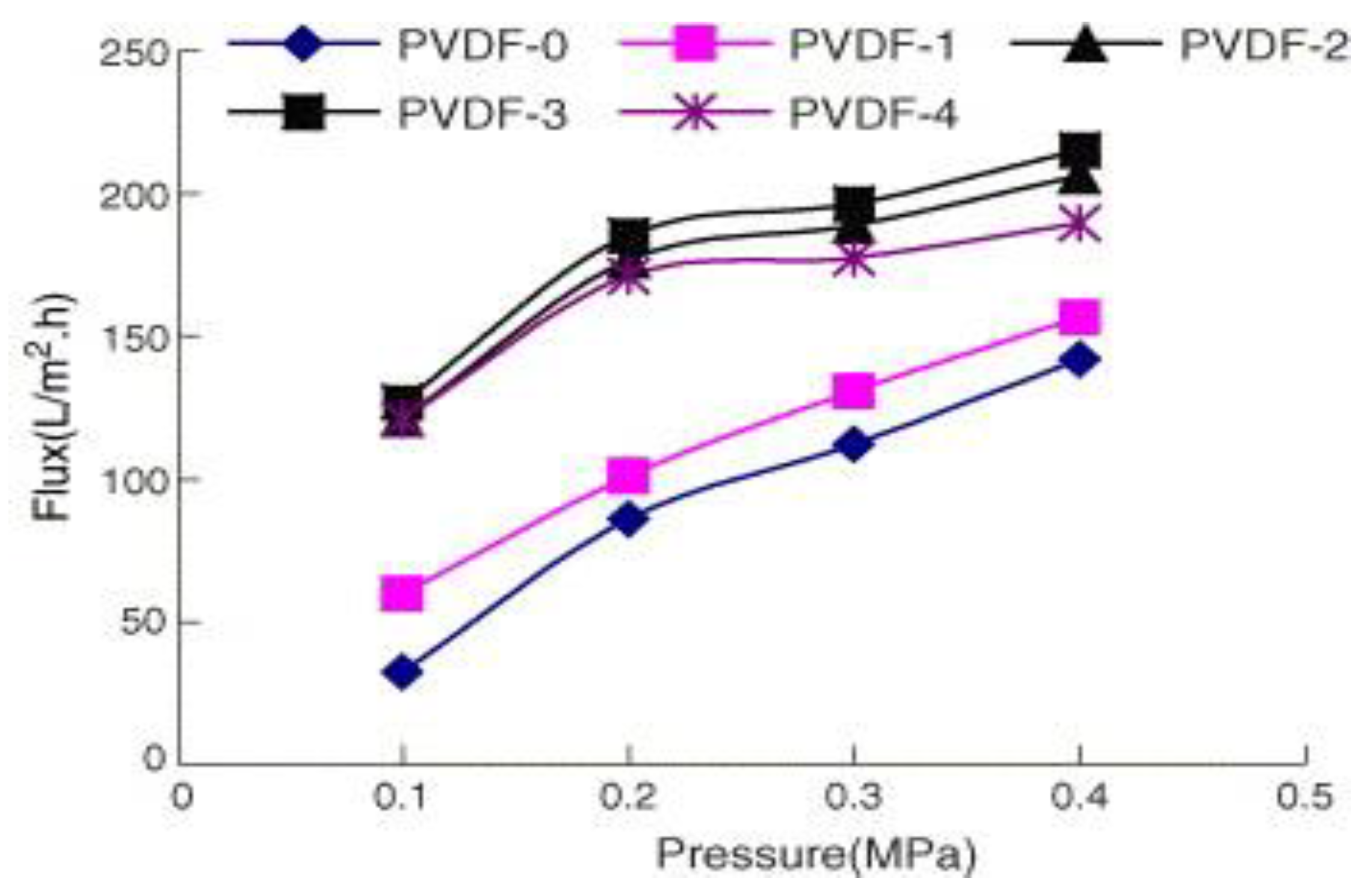
- The permeate flux constantly increased with the addition of Al2O3 NPs; however, the trend reversed after the quantity of Al2O3 exceeded a certain limit. The trend of flux is represented in Figure 3.
- Further, the SEM micrographs of PVDF-0 and PVDF-2 membranes were studied. Micropores were found to be distributed on either side of the membrane without any difference among modified and unmodified membranes. Both membranes displayed finger-like pores along with sponge wall linkage. Thus it was concluded that Al2O3 NPs do not influence the surface structures, inner pores, and cross-section.
- It was also observed that, with an increase in Al2O3 particle concentration, the contact angle subsequently decreased. Other values such as porosity, rejection, and other cut-off value were not affected. This observation concludes that Al2O3 particles can improve the hydrophilicity of the PVDF membrane but have no influence on pore size and amount in the membrane.
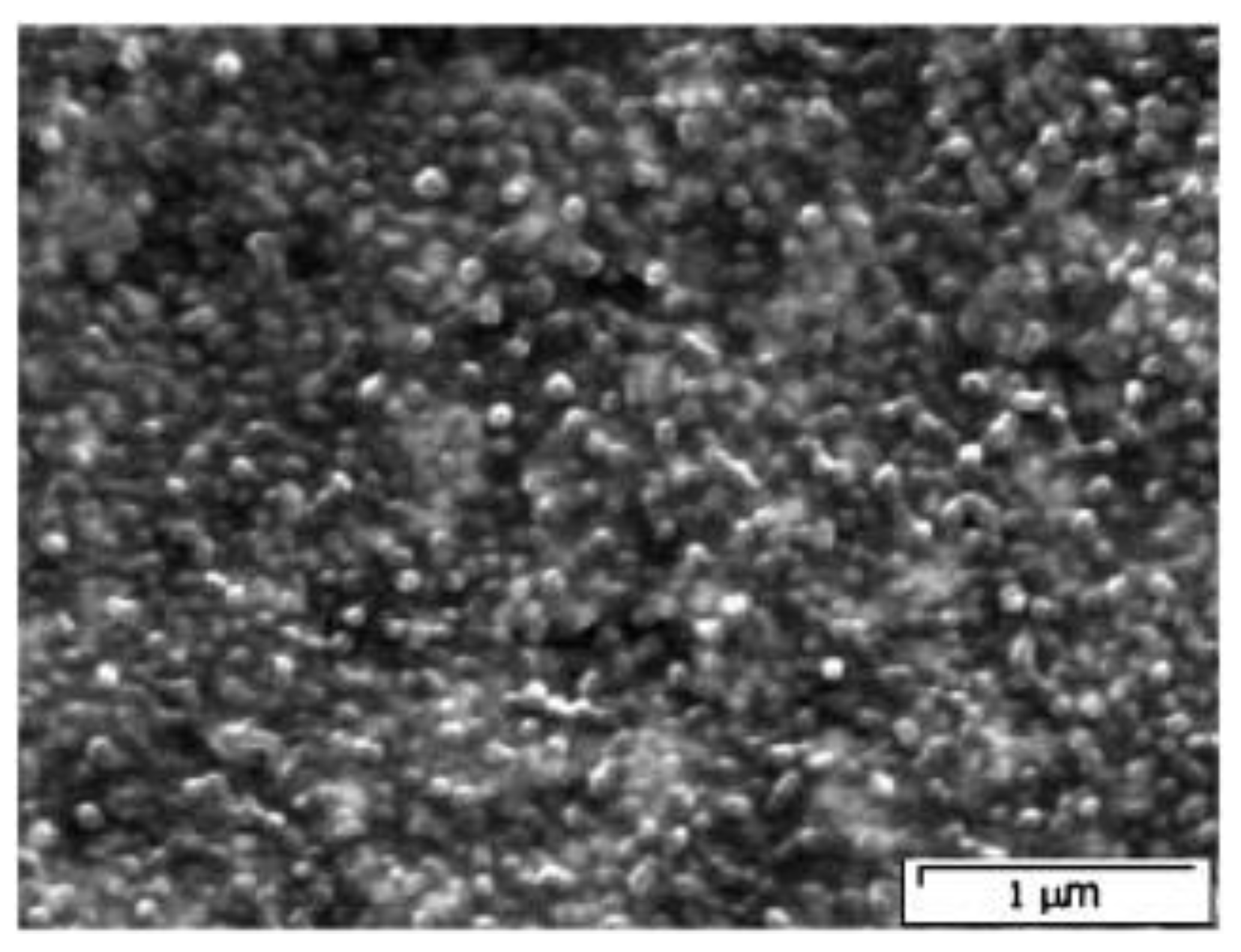
- Figure 4 shows that Al2O3 NPs were mostly dispersed uniformly, with a few large bundles. These might be due to the overlapping or coalescing of particles in the membrane.
4.2. Membranes Infused with Zirconium Nanoparticles
4.3. Membranes Infused with Silver Nanoparticles
4.4. Membranes Infused with Magnesium Nanoparticles
4.5. Membranes Infused with Copper Nanoparticles
4.6. Outlook
5. Zwitter-Ion Based Polymeric Membranes
6. Zeolite-Based Polymeric Membranes
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nanosci. Technol. 2009, 452, 337–346. [Google Scholar] [CrossRef]
- Babbar, R.; Arya, D.; Joshi, H. A Proposed Decision Support System for River Water Quality Management in India. J. Decis. Syst. 2009, 18, 411–427. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Ma, Y.; Xu, M.; Qin, S.; Liu, X.; Feng, H.; Hou, L. Purification of pickling wastewater from the steel industry using membrane filters: Performance and membrane fouling. Environ. Eng. Res. 2020, 27, 106–115. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Goh, P.S.; Wong, K.C.; Zulhairun, A.K.; Ismail, A.F. Enhancing desalination performance of thin film composite membrane through layer by layer assembly of oppositely charged titania nanosheet. Desalination 2020, 476, 114167. [Google Scholar] [CrossRef]
- Han, K.N.; Yu, B.Y.; Kwak, S.-Y. Hyperbranched poly(amidoamine)/polysulfone composite membranes for Cd(II) removal from water. J. Membr. Sci. 2012, 396, 83–91. [Google Scholar] [CrossRef]
- Park, S.; Yang, E.; Park, H.; Choi, H. Fabrication of functionalized halloysite nanotube blended ultrafiltration membranes for high flux and fouling resistance. Environ. Eng. Res. 2019, 25, 771–778. [Google Scholar] [CrossRef] [Green Version]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Misdan, N.; Ismail, A.F.; Hilal, N. Recent advances in the development of (bio)fouling resistant thin film composite membranes for desalination. Desalination 2016, 380, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Zhu, J.; Cong, S.; Wang, J.; Van der Bruggen, B.; Liu, J.; Zhang, Y. High flux thin film nanocomposite membranes based on porous organic polymers for nanofiltration. J. Membr. Sci. 2019, 585, 19–28. [Google Scholar] [CrossRef]
- Ortega, M.; Merino, A.G.; Fraile-Martínez, O.; Recio-Ruiz, J.; Pekarek, L.; Guijarro, L.G.; García-Honduvilla, N.; Álvarez-Mon, M.; Buján, J.; García-Gallego, S. Dendrimers and Dendritic Materials: From Laboratory to Medical Practice in Infectious Diseases. Pharmaceutics 2020, 12, 874. [Google Scholar] [CrossRef]
- Bethi, B.; Sonawane, S.H.; Bhanvase, B.A.; Gumfekar, S.P. Nanomaterials-based advanced oxidation processes for wastewater treatment: A review. Chem. Eng. Process. Process. Intensif. 2016, 109, 178–189. [Google Scholar] [CrossRef]
- Barakat, M.A.; Ramadan, M.H.; Alghamdi, M.A.; Algarny, S.S.; Woodcock, H.L.; Kuhn, J.N. Remediation of Cu (II), Ni (II), and Cr (III) ions from simulated wastewater by dendrimer/titania composites. J. Environ. Manag. 2013, 117, 50–57. [Google Scholar] [CrossRef]
- Barakat, M.; Ramadan, M.; Kuhn, J.; Woodcock, H. Equilibrium and kinetics of Pb2+adsorption from aqueous solution by dendrimer/titania composites. Desalination Water Treat. 2014, 52, 5869–5875. [Google Scholar] [CrossRef]
- Castillo, V.A.; Barakat, M.A.; Ramadan, M.H.; Woodcock, H.L.; Kuhn, J.N. Metal ion remediation by polyamidoamine dendrimers: A comparison of metal ion, oxidation state, and titania immobilization. Int. J. Environ. Sci. Technol. 2014, 11, 1497–1502. [Google Scholar] [CrossRef] [Green Version]
- Chou, C.-M.; Lien, H.-L. Dendrimer-conjugated magnetic nanoparticles for removal of zinc (II) from aqueous solutions. J. Nanoparticle Res. 2011, 13, 2099–2107. [Google Scholar] [CrossRef]
- Yen, C.-H.; Lien, H.-L.; Chung, J.-S.; Yeh, H.-D. Adsorption of precious metals in water by dendrimer modified magnetic nanoparticles. J. Hazard. Mater. 2017, 322, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Ge, Y.; Wu, Z.; Qin, W. DNA fragments assembled on polyamidoamine-grafted core-shell magnetic silica nanoparticles for removal of mercury(II) and methylmercury(I). J. Chem. Technol. Biotechnol. 2017, 92, 819–826. [Google Scholar] [CrossRef]
- Kim, K.-J.; Park, J.-W. Stability and reusability of amine-functionalized magnetic-cored dendrimer for heavy metal adsorption. J. Mater. Sci. 2017, 52, 843–857. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Abedin-Moghanaki, A.; Hosseini, S.H. Synthesis of poly(amidoamine)-graft-poly(methyl acrylate) magnetic nanocomposite for removal of lead contaminant from aqueous media. Int. J. Environ. Sci. Technol. 2016, 13, 2437–2448. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.L.; Li, J.; Hong, G.-B.; Chang, C.-T. Dendrimer Modified Magnetic Nanoparticles as Adsorbents for Removal of Dyes. J. Nanosci. Nanotechnol. 2013, 13, 6814–6819. [Google Scholar] [CrossRef]
- Zarghami, Z.; Akbari, A.; Latifi, A.M.; Amani, M.A. Design of a new integrated chitosan-PAMAM dendrimer biosorbent for heavy metals removing and study of its adsorption kinetics and thermodynamics. Bioresour. Technol. 2016, 205, 230–238. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; He, X.; Xiao, M.; Zhang, W.; Lu, C. A super biosorbent from dendrimer poly(amidoamine)-grafted cellulose nanofibril aerogels for effective removal of Cr(vi). J. Mater. Chem. A 2015, 3, 14703–14711. [Google Scholar] [CrossRef]
- Wang, P.; Ma, Q.; Hu, D.; Wang, L. Removal of Reactive Blue 21 onto magnetic chitosan microparticles functionalized with polyamidoamine dendrimers. React. Funct. Polym. 2015, 91, 43–50. [Google Scholar] [CrossRef]
- Algarra, M.; Vázquez, M.I.; Alonso, B.; Casado, C.M.; Casado, J.; Benavente, J. Characterization of an engineered cellulose based membrane by thiol dendrimer for heavy metals removal. Chem. Eng. J. 2014, 253, 472–477. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Zhang, G.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Poly(amidoamine) modified graphene oxide as an efficient adsorbent for heavy metal ions. Polym. Chem. 2013, 4, 2164–2167. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, B.; He, S.; Man, R. Preparation of Graphene-Oxide/Polyamidoamine Dendrimers and Their Adsorption Properties toward Some Heavy Metal Ions. J. Chem. Eng. Data 2014, 59, 1719–1726. [Google Scholar] [CrossRef]
- Xiao, W.; Yan, B.; Zeng, H.; Liu, Q. Dendrimer functionalized graphene oxide for selenium removal. Carbon 2016, 105, 655–664. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Ziccarelli, I.; Espro, C.; Galvagno, S.; Giofrè, S.; Romeo, R.; Cicero, N.; Bua, G.D.; Lanza, G.; et al. Removal of heavy metal ions from wastewaters using dendrimer-functionalized multi-walled carbon nanotubes. Environ. Sci. Pollut. Res. 2017, 24, 14735–14747. [Google Scholar] [CrossRef]
- Hayati, B.; Maleki, A.; Najafi, F.; Daraei, H.; Gharibi, F.; McKay, G. Super high removal capacities of heavy metals (Pb 2+ and Cu 2+) using CNT dendrimer. J. Hazard. Mater. 2017, 336, 146–157. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, D. Removal of Copper from Contaminated Soil by Use of Poly(amidoamine) Dendrimers. Environ. Sci. Technol. 2005, 39, 2369–2375. [Google Scholar] [CrossRef] [PubMed]
- Gajjar, D.; Patel, R.; Patel, H.; Patel, P.M. Designing of Triazine Based Dendrimer and its Application in Removal of Heavy Metal Ions from Water. Chem. Sci. Trans. 2014, 3, 897–908. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhao, D. Removal of Lead from Contaminated Soils Using Poly(amidoamine) Dendrimers. Ind. Eng. Chem. Res. 2006, 45, 1758–1765. [Google Scholar] [CrossRef]
- Taleb, M.A.; Elsigeny, S.M.; Ibrahim, M.M. Radiation synthesis and characterization of polyamidoamine dendrimer macromolecules with different loads of nickel salt for adsorption of some metal ion. Radiat. Phys. Chem. 2007, 76, 1612–1618. [Google Scholar] [CrossRef]
- Beraa, A.; Hajjaji, M.; Laurent, R.; Delavaux-Nicot, B.; Caminade, A.-M. Removal of chromate from aqueous solutions by dendrimers-clay nanocomposites. Desalination Water Treat. 2016, 57, 14290–14303. [Google Scholar] [CrossRef]
- Rao, N.; Singh, R.; Bashambu, L. Carbon-based nanomaterials: Synthesis and prospective applications. Mater. Today Proc. 2021, 44, 608–614. [Google Scholar] [CrossRef]
- Geng, B.; Jin, Z.; Li, T.; Qi, X. Kinetics of hexavalent chromium removal from water by chitosan-Fe0 nanoparticles. Chemosphere 2009, 75, 825–830. [Google Scholar] [CrossRef]
- Cheung, W.; Szeto, Y.; McKay, G. Enhancing the adsorption capacities of acid dyes by chitosan nano particles. Bioresour. Technol. 2009, 100, 1143–1148. [Google Scholar] [CrossRef]
- Hinds, B.J.; Chopra, N.; Rantell, T.; Andrews, R.; Gavalas, V.; Bachas, L. Aligned Multiwalled Carbon Nanotube Membranes. Science 2004, 303, 62–65. [Google Scholar] [CrossRef] [Green Version]
- Hummer, G.; Rasaiah, J.C.; Noworyta, J.P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 2001, 414, 188–190. [Google Scholar] [CrossRef]
- Thomas, J.A.; McGaughey, A.J.H. Water Flow in Carbon Nanotubes: Transition to Subcontinuum Transport. Phys. Rev. Lett. 2009, 102, 184502. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, J.; Zhang, L.; Xing, R.; Jiao, T.; Gao, F.-M.; Peng, Q. Facile synthesis of self-assembled carbon nanotubes/dye composite films for sensitive electrochemical determination of Cd(II) ions. Nanotechnology 2018, 29, 445603. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Hill, J.M. Modeling on ion rejection using membranes comprising ultra-small radii carbon nanotubes. Eur. Phys. J. B 2012, 85, 1–7. [Google Scholar] [CrossRef]
- Farid, M.U.; Khanzada, N.K.; An, A.K. Understanding fouling dynamics on functionalized CNT-based membranes: Mechanisms and reversibility. Desalination 2019, 456, 74–84. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, D.; Yan, T.; Wen, X.; Shi, L.; Zhang, J. Graphene prepared via a novel pyridine–thermal strategy for capacitive deionization. J. Mater. Chem. 2012, 22, 23745–23748. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Compton, O.C.; Dommett, G.H.B.; Ruoff, R.S.; Nguyen, S.T. Systematic Post-assembly Modification of Graphene Oxide Paper with Primary Alkylamines. Chem. Mater. 2010, 22, 4153–4157. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ying, Y.; Peng, X. Graphene oxide nanosheet: An emerging star material for novel separation membranes. J. Mater. Chem. A 2014, 2, 13772–13782. [Google Scholar] [CrossRef]
- Lu, J.; Yang, J.-X.; Wang, J.; Lim, A.; Wang, S.; Loh, K.P. One-Pot Synthesis of Fluorescent Carbon Nanoribbons, Nanoparticles, and Graphene by the Exfoliation of Graphite in Ionic Liquids. ACS Nano 2009, 3, 2367–2375. [Google Scholar] [CrossRef]
- Peng, L.; Xu, Z.; Liu, Z.; Wei, Y.; Sun, H.; Zhao, X.; Gao, C. An iron-based green approach to 1-h production of single-layer graphene oxide. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bao, C.; Song, L.; Xing, W.; Yuan, B.; Wilkie, C.A.; Huang, J.; Guo, Y.; Hu, Y. Preparation of graphene by pressurized oxidation and multiplex reduction and its polymer nanocomposites by masterbatch-based melt blending. J. Mater. Chem. 2012, 22, 6088–6096. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, Z.; Liu, Z.; Zhang, T. Exfoliation at the Liquid/Air Interface to Assemble Reduced Graphene Oxide Ultrathin Films for a Flexible Noncontact Sensing Device. Adv. Mater. 2015, 27, 1370–1375. [Google Scholar] [CrossRef]
- Chen, X.; Liu, G.; Zhang, H.; Fan, Y. Fabrication of graphene oxide composite membranes and their application for pervaporation dehydration of butanol. Chin. J. Chem. Eng. 2015, 23, 1102–1109. [Google Scholar] [CrossRef]
- Tsou, C.-H.; An, Q.-F.; Lo, S.-C.; De Guzman, M.; Hung, W.-S.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Effect of microstructure of graphene oxide fabricated through different self-assembly techniques on 1-butanol dehydration. J. Membr. Sci. 2015, 477, 93–100. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, Y.; Gao, C. High-Flux Graphene Oxide Nanofiltration Membrane Intercalated by Carbon Nanotubes. ACS Appl. Mater. Interfaces 2015, 7, 8147–8155. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chen, G.; Huang, R.; Gao, C. A novel composite nanofiltration (NF) membrane prepared from glycolchitin/poly(acrylonitrile) (PAN) by epichlorohydrin cross-linking. J. Membr. Sci. 2007, 297, 51–58. [Google Scholar] [CrossRef]
- Yang, L.; Tang, B.; Wu, P. UF membrane with highly improved flux by hydrophilic network between graphene oxide and brominated poly(2,6-dimethyl-1,4-phenylene oxide). J. Mater. Chem. A 2014, 2, 18562–18573. [Google Scholar] [CrossRef]
- Kou, L.; Gao, C. Making silicananoparticle-covered graphene oxide nanohybrids as general building blocks for large-area superhydrophilic coatings. Nanoscale 2011, 3, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hu, M.; Mi, B. Membrane surface modification with TiO2–graphene oxide for enhanced photocatalytic performance. J. Membr. Sci. 2014, 455, 349–356. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Namiesnik, J. Abiotic degradation of chlorinated ethanes and ethenes in water. Environ. Sci. Pollut. Res. 2012, 19, 1994–2006. [Google Scholar] [CrossRef] [Green Version]
- Jain, P.; Pradeep, T. Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnol. Bioeng. 2005, 90, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Bashambu, L.; Singh, R.; Verma, J. Metal/metal oxide nanocomposite membranes for water purification. Mater. Today Proc. 2021, 44, 538–545. [Google Scholar] [CrossRef]
- Chang, J.-H.; Yang, T.-J.; Tung, C.-H. Performance of nano- and nonnano-catalytic electrodes for decontaminating municipal wastewater. J. Hazard. Mater. 2009, 163, 152–157. [Google Scholar] [CrossRef]
- Mei, N.; Xuguang, L.; Jinming, D.; Husheng, J.; Liqiao, W.; Bingshe, X. Antibacterial activity of chitosan coated Ag-loaded nano-SiO2 composites. Carbohydr. Polym. 2009, 78, 54–59. [Google Scholar] [CrossRef]
- Babu, A.T.; Antony, R. Green synthesis of silver doped nano metal oxides of zinc & copper for antibacterial properties, adsorption, catalytic hydrogenation & photodegradation of aromatics. J. Environ. Chem. Eng. 2019, 7, 102840. [Google Scholar] [CrossRef]
- Hildebrand, H.; Mackenzie, K.; Kopinke, F.-D. Pd/Fe3O4 nano-catalysts for selective dehalogenation in wastewater treatment processes—Influence of water constituents. Appl. Catal. B Environ. 2009, 91, 389–396. [Google Scholar] [CrossRef]
- Malekizadeh, A.; Schenk, P.M. High flux water purification using aluminium hydroxide hydrate gels. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Jung, B. Preparation of hydrophilic polyacrylonitrile blend membranes for ultrafiltration. J. Membr. Sci. 2004, 229, 129–136. [Google Scholar] [CrossRef]
- Nunes, S.P.; Peinemann, K.V. Ultrafiltration membranes from PVDF/PMMA blends. J. Membr. Sci. 1992, 73, 25–35. [Google Scholar] [CrossRef]
- Yan, L.; Li, Y.S.; Xiang, C.B.; Xianda, S. Effect of nano-sized Al2O3-particle addition on PVDF ultrafiltration membrane performance. J. Membr. Sci. 2006, 276, 162–167. [Google Scholar] [CrossRef]
- Xu, Z.-K.; Xiao, L.; Wang, J.-L.; Springer, J. Gas separation properties of PMDA/ODA polyimide membranes filling with polymeric nanoparticles. J. Membr. Sci. 2002, 202, 27–34. [Google Scholar] [CrossRef]
- Singh, R.; Singh, M.; Gautam, S. Hydrogen economy, energy, and liquid organic carriers for its mobility. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Nunes, S. Inorganic modification of proton conductive polymer membranes for direct methanol fuel cells. J. Membr. Sci. 2002, 203, 215–225. [Google Scholar] [CrossRef]
- Vallejo, E.; Pourcelly, G.; Gavach, C.; Mercier, R.; Pineri, M. Sulfonated polyimides as proton conductor exchange membranes. Physicochemical properties and separation H+/Mz+ by electrodialysis comparison with a perfluorosulfonic membrane. J. Membr. Sci. 1999, 160, 127–137. [Google Scholar] [CrossRef]
- Jalani, N.H.; Dunn, K.; Datta, R. Synthesis and characterization of Nafion®-MO2 (M= Zr, Si, Ti) nanocomposite membranes for higher temperature PEM fuel cells. Electrochim. Acta 2005, 51, 553–560. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, H.; Chen, W.; Pan, M. Nafion–zirconia nanocomposite membranes formed via in situ sol–gel process. Int. J. Hydrog. Energy 2010, 35, 2796–2801. [Google Scholar] [CrossRef]
- Koók, L.; Bakonyi, P.; Harnisch, F.; Kretzschmar, J.; Chae, K.-J.; Zhen, G.; Kumar, G.; Rózsenberszki, T.; Tóth, G.; Nemestothy, N.; et al. Biofouling of membranes in microbial electrochemical technologies: Causes, characterization methods and mitigation strategies. Bioresour. Technol. 2019, 279, 327–338. [Google Scholar] [CrossRef]
- Handok, C.T.; Huda, A.; Gulo, F. Synthesis Pathway and Powerful Antimicrobial Properties of Silver Nanoparticle: A Critical Review. Asian J. Sci. Res. 2019, 12, 1–17. [Google Scholar] [CrossRef]
- Basri, H.; Ismail, A.; Aziz, M. Polyethersulfone (PES)–silver composite UF membrane: Effect of silver loading and PVP molecular weight on membrane morphology and antibacterial activity. Desalination 2011, 273, 72–80. [Google Scholar] [CrossRef]
- Liu, X.; Foo, L.-X.; Li, Y.; Lee, J.-Y.; Cao, B.; Tang, C.Y. Fabrication and characterization of nanocomposite pressure retarded osmosis (PRO) membranes with excellent anti-biofouling property and enhanced water permeability. Desalination 2016, 389, 137–148. [Google Scholar] [CrossRef]
- Davies, R.L.; Etris, S.F. The development and functions of silver in water purification and disease control. Catal. Today 1997, 36, 107–114. [Google Scholar] [CrossRef]
- Trevors, J. Silver resistance and accumulation in bacteria. Enzym. Microb. Technol. 1987, 9, 331–333. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Esche-richia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Coleman, M.; Koros, W. Isomeric polyimides based on fluorinated dianhydrides and diamines for gas separation applications. J. Membr. Sci. 1990, 50, 285–297. [Google Scholar] [CrossRef]
- Hess, S.; Staudt-Bickel, C.; Lichtenthaler, R. Propene/propane separation with copolyimide membranes containing silver ions. J. Membr. Sci. 2006, 275, 52–60. [Google Scholar] [CrossRef]
- Ahamed, M.; Karns, M.; Goodson, M.; Rowe, J.; Hussain, S.M.; Schlager, J.J.; Hong, Y. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol. Appl. Pharmacol. 2008, 233, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Li, Y.; Chung, T.-S.; Liu, Y. Enhanced gas separation performance of nanocomposite membranes using MgO nanoparticles. J. Membr. Sci. 2007, 302, 207–217. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Copper, An Ancient Remedy Returning to Fight Microbial, Fungal and Viral Infections. Curr. Chem. Biol. 2009, 3, 272–278. [Google Scholar] [CrossRef]
- Ingle, A.P.; Durán, N.; Rai, M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: A review. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1009. [Google Scholar] [CrossRef]
- Kanninen, P.; Johans, C.; Merta, J.; Kontturi, K. Influence of ligand structure on the stability and oxidation of copper nanoparticles. J. Colloid Interface Sci. 2008, 318, 88–95. [Google Scholar] [CrossRef]
- Bassyouni, M.; Abdel-Aziz, M.; Zoromba, M.S.; Abdel-Hamid, S.; Drioli, E. A review of polymeric nanocomposite membranes for water purification. J. Ind. Eng. Chem. 2019, 73, 19–46. [Google Scholar] [CrossRef]
- Palza, H.; Quijada, R.; Delgado, K. Antimicrobial polymer composites with copper micro- and nanoparticles: Effect of particle size and polymer matrix. J. Bioact. Compat. Polym. 2015, 30, 366–380. [Google Scholar] [CrossRef]
- Ben-Sasson, M.; Zodrow, K.; Genggeng, Q.; Kang, Y.; Giannelis, E.P.; Elimelech, M. Surface Functionalization of Thin-Film Composite Membranes with Copper Nanoparticles for Antimicrobial Surface Properties. Environ. Sci. Technol. 2014, 48, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Soroush, A.; Luong, T.V.A.; Brennan, G.; Rahaman, S.; Asadishad, B.; Tufenkji, N. Spray- and spin-assisted layer-by-layer assembly of copper nanoparticles on thin-film composite reverse osmosis membrane for biofouling mitigation. Water Res. 2016, 99, 188–199. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, J.; Uliana, A.A.; Tian, M.; Zhang, Y.; Zhang, Y.; Volodin, A.; Simoens, K.; Yuan, S.; Li, J.; et al. Mussel-Inspired Architecture of High-Flux Loose Nanofiltration Membrane Functionalized with Antibacterial Reduced Graphene Oxide–Copper Nanocomposites. ACS Appl. Mater. Interfaces 2017, 9, 28990–29001. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, J.; Li, L.; Jiang, S. Strong Resistance of Phosphorylcholine Self-Assembled Monolayers to Protein Adsorption: Insights into Nonfouling Properties of Zwitterionic Materials. J. Am. Chem. Soc. 2005, 127, 14473–14478. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polym. 2010, 51, 5283–5293. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Shih, Y.-J.; Lai, C.-J.; Kung, H.-H.; Jiang, S. Blood-Inert Surfaces via Ion-Pair Anchoring of Zwitterionic Copolymer Brushes in Human Whole Blood. Adv. Funct. Mater. 2013, 23, 1100–1110. [Google Scholar] [CrossRef]
- Huang, C.-J.; Brault, N.D.; Li, Y.; Yu, Q.; Jiang, S. Controlled Hierarchical Architecture in Surface-initiated Zwitterionic Polymer Brushes with Structurally Regulated Functionalities. Adv. Mater. 2012, 24, 1834–1837. [Google Scholar] [CrossRef]
- Shaoyi, J.; Jiang, S. Molecular Understanding and Design of Zwitterionic Materials. Adv. Mater. 2015, 27, 15–26. [Google Scholar] [CrossRef]
- Choi, H.; Jung, Y.; Han, S.; Tak, T.; Kwon, Y.-N. Surface modification of SWRO membranes using hydroxyl poly(oxyethylene) methacrylate and zwitterionic carboxylated polyethyleneimine. J. Membr. Sci. 2015, 486, 97–105. [Google Scholar] [CrossRef]
- Liu, F.; Ma, B.-R.; Zhou, D.; Xiang, Y.-H.; Xue, L.-X. Breaking through tradeoff of Polysulfone ultrafiltration membranes by zeolite 4A. Microporous Mesoporous Mater. 2014, 186, 113–120. [Google Scholar] [CrossRef]
- Han, R.; Zhang, S.; Liu, C.; Wang, Y.; Jian, X. Effect of NaA zeolite particle addition on poly(phthalazinone ether sulfone ketone) composite ultrafiltration (UF) membrane performance. J. Membr. Sci. 2009, 345, 5–12. [Google Scholar] [CrossRef]
- He, T.; Zhou, W.; Bahi, A.; Yang, H.; Ko, F. High permeability of ultrafiltration membranes based on electrospun PVDF modified by nanosized zeolite hybrid membrane scaffolds under low pressure. Chem. Eng. J. 2014, 252, 327–336. [Google Scholar] [CrossRef]
- Leo, C.; Kamil, N.A.; Junaidi, M.; Kamal, S.; Ahmad, A.L. The potential of SAPO-44 zeolite filler in fouling mitigation of polysulfone ultrafiltration membrane. Sep. Purif. Technol. 2013, 103, 84–91. [Google Scholar] [CrossRef]
- Wee, S.-L.; Tye, C.-T.; Bhatia, S. Membrane separation process—Pervaporation through zeolite membrane. Sep. Purif. Technol. 2008, 63, 500–516. [Google Scholar] [CrossRef]
- Kunnakorn, D.; Rirksomboon, T.; Aungkavattana, P.; Kuanchertchoo, N.; Atong, D.; Kulprathipanja, S.; Wongkasemjit, S. Performance of sodium A zeolite membranes synthesized via microwave and autoclave techniques for water–ethanol separation: Recycle-continuous pervaporation process. Desalination 2011, 269, 78–83. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, R.; Singh, M.; Kumari, N.; Janak; Maharana, S.; Maharana, P. A Comprehensive Review of Polymeric Wastewater Purification Membranes. J. Compos. Sci. 2021, 5, 162. https://doi.org/10.3390/jcs5060162
Singh R, Singh M, Kumari N, Janak, Maharana S, Maharana P. A Comprehensive Review of Polymeric Wastewater Purification Membranes. Journal of Composites Science. 2021; 5(6):162. https://doi.org/10.3390/jcs5060162
Chicago/Turabian StyleSingh, Rasmeet, Mandeep Singh, Nisha Kumari, Janak, Sthitapragyan Maharana, and Pragyansu Maharana. 2021. "A Comprehensive Review of Polymeric Wastewater Purification Membranes" Journal of Composites Science 5, no. 6: 162. https://doi.org/10.3390/jcs5060162
APA StyleSingh, R., Singh, M., Kumari, N., Janak, Maharana, S., & Maharana, P. (2021). A Comprehensive Review of Polymeric Wastewater Purification Membranes. Journal of Composites Science, 5(6), 162. https://doi.org/10.3390/jcs5060162





