Materials and Manufacturing Techniques for Polymeric and Ceramic Scaffolds Used in Implant Dentistry
Abstract
1. Introduction
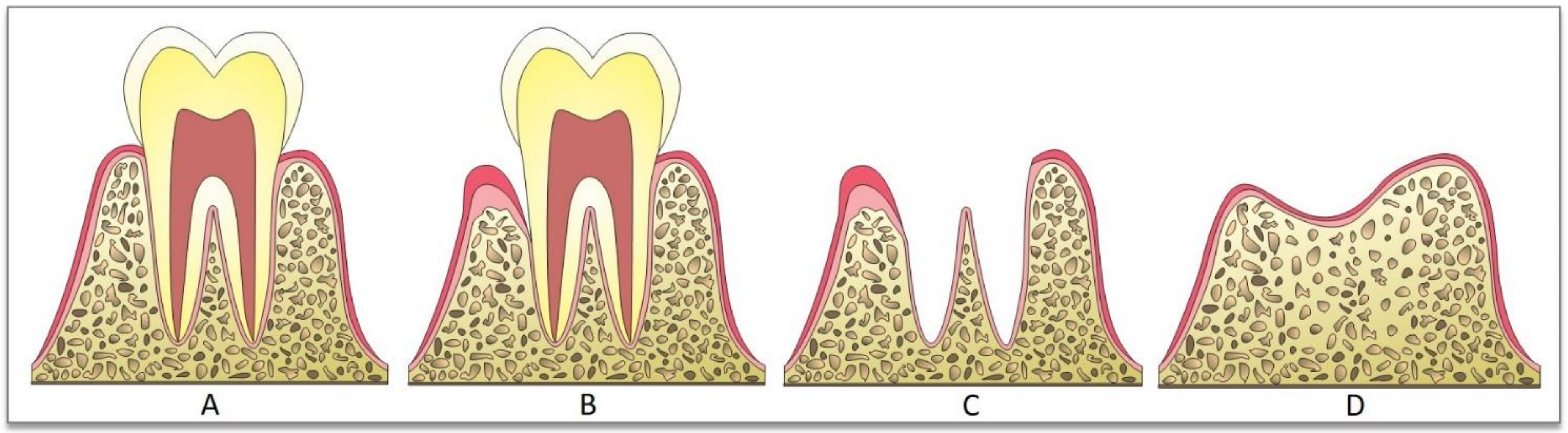
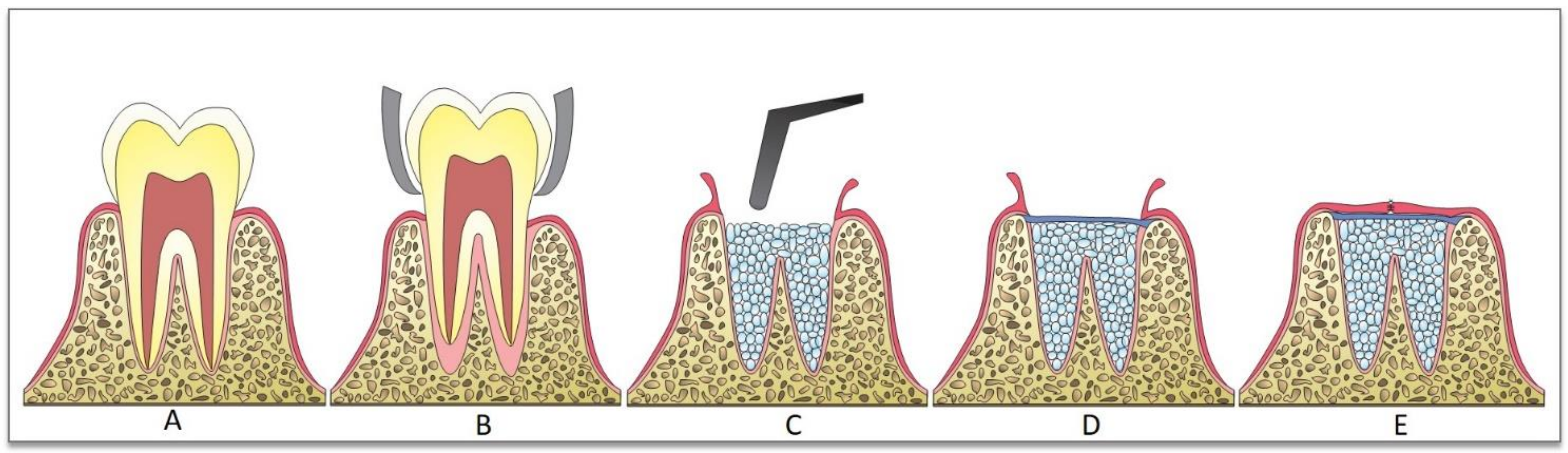
2. Bone-Grafting Techniques
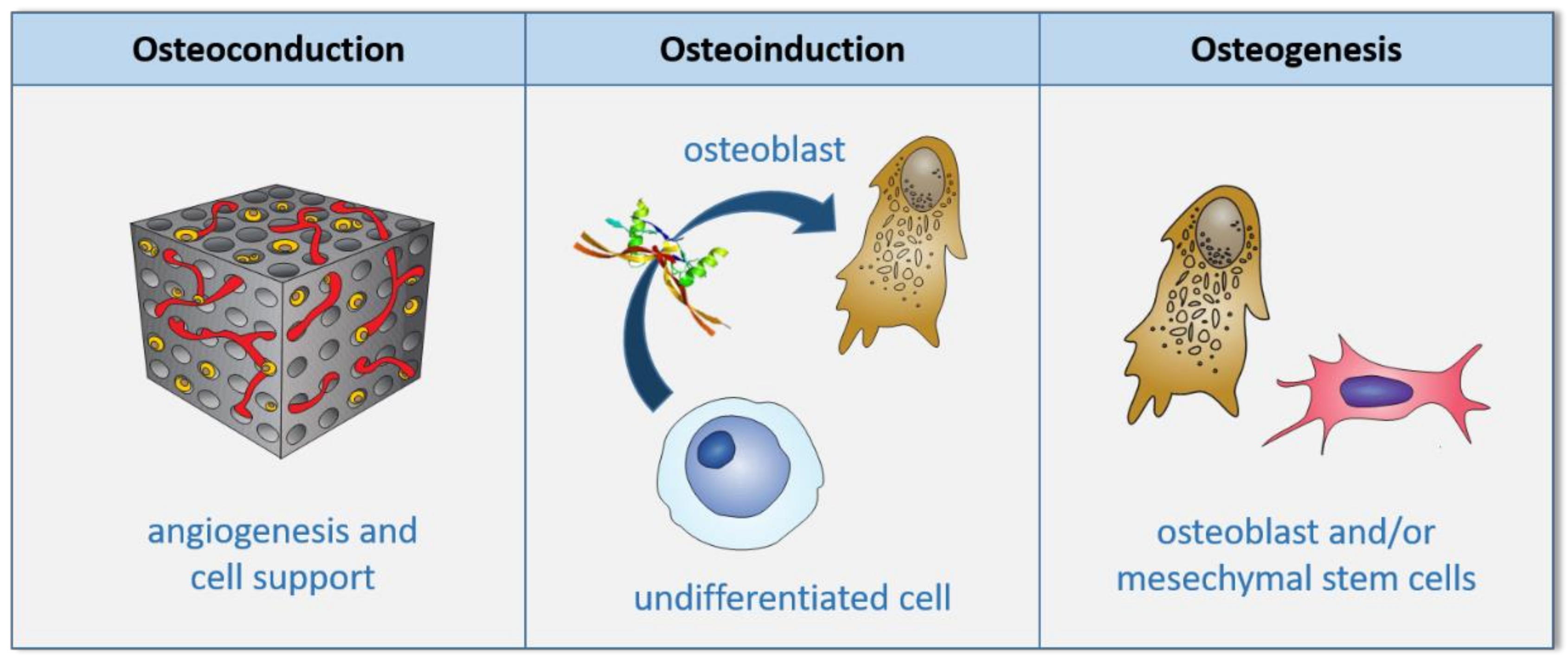
3. Scaffolds
4. Expected Properties for a Scaffold
5. Polymeric Biomaterials Applied to Fabricate Scaffolds Used in Implant Dentistry
5.1. Poly-Lactic Acid (PLA)
5.2. Poly-Glycolic Acid (PGA)
5.3. Polylactic-Co-Glycolic Acid (PLGA)
5.4. Polycaprolactone (PCL)
5.5. Polyvinyl Alcohol (PVA)
5.6. Polypropylene Fumarate (PPF)
6. Ceramic Biomaterials Used in Scaffolds Applied in Implant Dentistry
6.1. Hydroxyapatite (HAp)
6.2. Tricalcium Phosphates (TCP)
6.3. Bioactive Glass (BG)
6.4. Zirconia (ZrO2)
7. Techniques for Manufacturing Scaffolds
7.1. Conventional Techniques
7.2. Additive Manufacturing Techniques
8. Future Studies
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brånemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindström, J.; Hallén, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scan. J. Plast. Rec. Surg. 1977, 16, 1–132. [Google Scholar]
- Adell, R.; Lekholm, U.; Rockler, B.; Brånemark, P.I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int. J. Oral Surg. 1981, 10, 387–416. [Google Scholar]
- Adell, R.; Eriksson, B.; Lekholm, U.; I Brånemark, P.; Jemt, T. Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int. J. Oral Maxillofac. Implant. 1990, 5, 347–359. [Google Scholar]
- Howe, M.-S.; Keys, W.; Richards, D. Long-term (10-year) dental implant survival: A systematic review and sensitivity meta-analysis. J. Dent. 2019, 84, 9–21. [Google Scholar] [CrossRef]
- Srinivasan, M.; Meyer, S.; Mombelli, A.; Müller, F. Dental implants in the elderly population: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2017, 28, 920–930. [Google Scholar] [CrossRef]
- Van Der Weijden, F.; Dell’Acqua, F.; Slot, D.E. Alveolar bone dimensional changes of post-extraction sockets in humans: A systematic review. J. Clin. Periodontol. 2009, 36, 1048–1058. [Google Scholar] [CrossRef]
- Tallgren, A. The continuing reduction of the residual alveolar ridges in complete denture wearers: A mixed-longitudinal study covering 25 years. J. Prosthet. Dent. 2003, 89, 427–435. [Google Scholar] [CrossRef]
- Araujo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef]
- Shokri, T.; Wang, W.; Vincent, A.; Cohn, J.E.; Kadakia, S.; Ducic, Y. Osteoradionecrosis of the Maxilla: Conservative Management and Reconstructive Considerations. Semin. Plast. Surg. 2020, 34, 106–113. [Google Scholar] [CrossRef]
- Wu, C.; Pan, W.; Feng, C.; Su, Z.; Duan, Z.; Zheng, Q.; Hua, C.; Li, C. Grafting materials for alveolar cleft reconstruction: A systematic review and best-evidence synthesis. Int. J. Oral. Maxillofac. Surg. 2018, 47, 345–356. [Google Scholar] [CrossRef]
- Chiapasco, M.; Casentini, P.; Zaniboni, M. Bone augmentation procedures in implant dentistry. Int. J. Oral Maxillofac. Implant. 2009, 24, 237–259. [Google Scholar]
- Cardaropoli, D.; Cardaropolli, G. Preservation of the postextraction alveolar ridge: A clinical and histologic study. Int. J. Periodontics Restor. Dent. 2008, 5, 469–477. [Google Scholar]
- Perelman-Karmon, M.; Kozlovsky, A.; Lilov, R.; Artzi, Z. Socket site preservation using bovine bone mineral with and without a bioabsorbable collagen membrane. Int. J. Periodontics Restor. Dent. 2012, 32, 459–465. [Google Scholar]
- Esposito, M.; Grusovin, M.G.; Kwan, S.; Worthington, H.V.; Coulthard, P. Interventions for replacing missing teeth: Bone augmentation techniques for dental implant treatment. Cochrane Database Syst. Rev. 2003, 16, CD003607. [Google Scholar] [CrossRef]
- Simion, M.; Jovanovic, A.S.; Trisi, P.; Scarano, A.; Piattelli, A. Vertical ridge augmentation around dental implants using a mebrane technique and autogenous bone or allografts in humans. Int. J. Periodontics Restor. Dent. 1998, 18, 8–23. [Google Scholar]
- Buser, D.; Dula, K.; Hirt, H.P.; Schenk, R.K. Lateral ridge augmentation using autografts and barrier membranes: A clinical study with 40 partially edentulous patients. J. Oral Maxillofac. Surg. 1996, 54, 420–432. [Google Scholar] [CrossRef]
- Simion, M.; Fontana, F.; Rasperini, G.; Maiorana, C. Long-term evaluation of osseointegrated implants placed in sites augmented with sinus floor elevation associated with vertical ridge augmentation: A retrospective study of 38 consecutive implants with 1- to 7-year follow-up. Int. J. Periodontics Restor. Dent. 2004, 24, 208–221. [Google Scholar]
- Elsalanty, M.E.; Genecov, D.G. Bone Grafts in Craniofacial Surgery. Craniomaxillofacial Trauma Reconstr. 2009, 2, 125–134. [Google Scholar] [CrossRef]
- Sheikh, Z.; Sima, C.; Glogauer, M. Bone Replacement Materials and Techniques Used for Achieving Vertical Alveolar Bone Augmentation. Materials 2015, 8, 2953–2993. [Google Scholar] [CrossRef]
- Tessier, P.; Kawamoto, H.; Matthews, D.; Posnick, J.; Raulo, Y.; Tulasne, J.F.; Wolfe, S.A. Autogenous Bone Grafts and Bone Substitutes—Tools and Techniques: I. A 20,000-Case Experience in Maxillofacial and Craniofacial Surgery. Plast. Reconstr. Surg. 2005, 116, 6S–24S. [Google Scholar] [CrossRef]
- Al Ruhaimi, K.A. Bone graft substitutes: A comparative qualitative histologic review of current osteoconductive grafting materials. Int. J. Oral Maxillofac. Implant. 2001, 16, 105–114. [Google Scholar]
- Acocella, A.; Bertolai, R.; Colafranceschi, M.; Sacco, R. Clinical, histological and histomorphometric evaluation of the healing of mandibular ramus bone block grafts for alveolar ridge augmentation before implant placement. J. Cranio-Maxillofac. Surg. 2010, 38, 222–230. [Google Scholar] [CrossRef]
- Scarano, A.; Degidi, M.; Iezzi, G.; Pecora, G.; Piattelli, M.; Orsini, G.; Caputi, S.; Perrotti, V.; Mangano, C.; Piattelli, A. Maxillary Sinus Augmentation with Different Biomaterials: A Comparative Histologic and Histomorphometric Study in Man. Implant. Dent. 2006, 15, 197–207. [Google Scholar] [CrossRef]
- Keles, G.C.; Sumer, M.; Cetinkaya, B.O.; Tutkun, F.; Simsek, S.B. Effect of Autogenous Cortical Bone Grafting in Conjunction with Guided Tissue Regeneration in the Treatment of Intraosseous Periodontal Defects. Eur. J. Dent. 2010, 4, 403–411. [Google Scholar] [CrossRef]
- Iasella, J.M.; Greenwell, H.; Miller, R.L.; Hill, M.; Drisko, C.; Bohra, A.A.; Scheetz, J.P. Ridge Preservation with Freeze-Dried Bone Allograft and a Collagen Membrane Compared to Extraction Alone for Implant Site Development: A Clinical and Histologic Study in Humans. J. Periodontol. 2003, 74, 990–999. [Google Scholar] [CrossRef]
- Araújo, M.G.; Lindhe, J. Socket grafting with the use of autologous bone: An experimental study in the dog. Clin. Oral Implant. Res. 2011, 22, 9–13. [Google Scholar] [CrossRef]
- Carvalho, P.H.D.A.; Trento, G.D.S.; Moura, L.B.; Cunha, G.; Gabrielli, M.A.C.; Pereira-Filho, V.A. Horizontal ridge augmentation using xenogenous bone graft—Systematic review. Oral Maxillofac. Surg. 2019, 23, 271–279. [Google Scholar] [CrossRef]
- Goulet, J.A.; Senunas, L.E.; De Silva, G.L.; Greenfield, M.L.V.H. Autogenous Iliac Crest Bone Graft: Complications and Functional Assessment. Clin. Orthop. Relat. Res. 1997, 339, 76–81. [Google Scholar] [CrossRef]
- Coquelin, L.; Fialaire-Legendre, A.; Roux, S.; Poignard, A.; Bierling, P.; Hernigou, P.; Chevallier, N.; Rouard, H. In Vivo and In Vitro Comparison of Three Different Allografts Vitalized with Human Mesenchymal Stromal Cells. Tissue Eng. Part A 2012, 18, 1921–1931. [Google Scholar] [CrossRef]
- Wenz, B.; Oesch, B.; Horst, M. Analysis of the risk of transmitting bovine spongiform encephalopathy through bone grafts derived from bovine bone. Biomaterials 2001, 22, 1599–1606. [Google Scholar] [CrossRef]
- Kim, Y.; Nowzari, H.; Rich, S.K. Risk of Prion Disease Transmission through Bovine-Derived Bone Substitutes: A Systematic Review. Clin. Implant. Dent. Relat. Res. 2013, 15, 645–653. [Google Scholar] [CrossRef]
- Chavda, S.; Levin, L. Human Studies of Vertical and Horizontal Alveolar Ridge Augmentation Comparing Different Types of Bone Graft Materials: A Systematic Review. J. Oral Implant. 2018, 44, 74–84. [Google Scholar] [CrossRef]
- Papageorgiou, S.N.; Papageorgiou, P.N.; Deschner, J.; Götz, W. Comparative effectiveness of natural and synthetic bone grafts in oral and maxillofacial surgery prior to insertion of dental implants: Systematic review and network meta-analysis of parallel and cluster randomized controlled trials. J. Dent. 2016, 48, 1–8. [Google Scholar] [CrossRef]
- Shetty, V.; Han, T.J. Alloplastic materials in reconstructive periodontal surgery. Dent. Clin. N. Am. 1991, 35, 521–530. [Google Scholar]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef]
- Wang, E.; Han, J.; Zhang, X.; Wu, Y.; Deng, X.-L. Efficacy of a mineralized collagen bone-grafting material for peri-implant bone defect reconstruction in mini pigs. Regen. Biomater. 2019, 6, 107–111. [Google Scholar] [CrossRef]
- Teng, F.; Zhang, Q.; Wu, M.; Rachana, S.; Ou, G. Clinical use of ridge-splitting combined with ridge expansion osteotomy, sandwich bone augmentation, and simultaneous implantation. Br. J. Oral Maxillofac. Surg. 2014, 52, 703–708. [Google Scholar] [CrossRef]
- Galarraga-Vinueza, M.; Magini, R.; Henriques, B.; Teughels, W.; Fredel, M.; Hotza, D.; Souza, J.; Boccaccini, A.R. Nanostructured biomaterials embedding bioactive molecules. In Nanostructured Biomaterials for Cranio-Maxillofacial and Oral Applications; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 143–158. [Google Scholar]
- Braddock, M.; Houston, P.; Campbell, C.; Ashcroft, P. Born again bone: Tissue engineering for bone repair. News Physiol. Sci. 2001, 16, 208–213. [Google Scholar] [CrossRef]
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef]
- Tutak, W.; Sarkar, S.; Lin-Gibson, S.; Farooque, T.M.; Jyotsnendu, G.; Wang, D.; Kohn, J.; Bolikal, D.; Simon, C.G. The support of bone marrow stromal cell differentiation by airbrushed nanofiber scaffolds. Biomaterials 2013, 34, 2389–2398. [Google Scholar] [CrossRef]
- Ramay, H.R.; Zhang, M. Preparation of porous hydroxyapatite scaffolds by combination of the gel-casting and polymer sponge methods. Biomaterials 2003, 24, 3293–3302. [Google Scholar] [CrossRef]
- Zhao, J.; Xiao, S.; Lu, X.; Wang, J.; Weng, J. A study on improving mechanical properties of porous HA tissue engineering scaffolds by hot isostatic pressing. Biomed. Mater. 2006, 1, 188–192. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Shuai, C.; Li, Y.; Feng, P.; Guo, W.; Yang, W.; Peng, S. Positive feedback effects of Mg on the hydrolysis of poly-l-lactic acid (PLLA): Promoted degradation of PLLA scaffolds. Polym. Test. 2018, 68, 27–33. [Google Scholar] [CrossRef]
- Saffar, J.-L.; Colombier, M.; Detienville, R. Bone Formation in Tricalcium Phosphate-Filled Periodontal Intrabony Lesions. Histological Observations in Humans. J. Periodontol. 1990, 61, 209–216. [Google Scholar] [CrossRef]
- Schepers, E.; De Clercq, M.; Ducheyne, P.; Kempeneers, R. Bioactive glass particulate material as a filler for bone lesions. J. Oral Rehabil. 1991, 18, 439–452. [Google Scholar] [CrossRef]
- Pereira, H.F.; Cengiz, I.F.; Silva, F.S.; Reis, R.L.; Oliveira, J.M. Scaffolds and coatings for bone regeneration. J. Mater. Sci. Mater. Med. 2020, 31, 27. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, V.; Kumar, R.; Kumar, R.; Pruncu, C.I. Fabrication and characterization of ZrO2 incorporated SiO2–CaO–P2O5 bioactive glass scaffolds. J. Mech. Behav. Biomed. Mater. 2020, 109, 103854. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2001, 21, 2335–2346. [Google Scholar] [CrossRef]
- Erbetta, C.D.C.; Alves, R.J.; Resende, J.M.; Freitas, R.; de Sousa, R.G. Synthesis and Characterization of Poly(D,L-Lactide-co-Glycolide) Copolymer. J. Biomater. Nanobiotechnol. 2012, 3, 208–225. [Google Scholar] [CrossRef]
- Miao, X.; Tan, D.M.; Li, J.; Xiao, Y.; Crawford, R.W. Mechanical and biological properties of hydroxyapatite/tricalcium phosphate scaffolds coated with poly(lactic-co-glycolic acid). Acta Biomater. 2008, 4, 638–645. [Google Scholar] [CrossRef]
- Liu, X.; Okada, M.; Maeda, H.; Fujii, S.; Furuzono, T. Hydroxyapatite/biodegradable poly(l-lactide–co-ε-caprolactone) composite microparticles as injectable scaffolds by a Pickering emulsion route. Acta Biomater. 2011, 7, 821–828. [Google Scholar] [CrossRef]
- Chandra, R. Biodegradable polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Dibbern-Brunelli, D.; Atvars, T.D.Z.; Joekes, I.; Barbosa, V.C. Mapping phases of poly(vinyl alcohol) and poly(vinyl acetate) blends by FTIR microspectroscopy and optical fluorescence microscopy. J. Appl. Polym. Sci. 1998, 69, 645–655. [Google Scholar] [CrossRef]
- Yaszemski, M.J.; Payne, R.G.; Hayes, W.C.; Langer, R.; Mikos, A.G. In vitro degradation of a poly(propylene fumarate)-based composite material. Biomaterials 1996, 17, 2127–2130. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- De Lima, I.R.; Alves, G.G.; Fernandes, G.V.D.O.; Dias, E.P.; Soares, G.D.A.; Granjeiro, J.M. Evaluation of the in vivo biocompatibility of hydroxyapatite granules incorporated with zinc ions. Mater. Res. 2010, 13, 563–568. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- Aboushelib, M.N.; Shawky, R. Osteogenesis ability of CAD/CAM porous zirconia scaffolds enriched with nano-hydroxyapatite particles. Int. J. Implant. Dent. 2017, 3, 21. [Google Scholar] [CrossRef]
- Alizadeh, A.; Moztarzadeh, F.; Ostad, S.N.; Azami, M.; Geramizadeh, B.; Hatam, G.; Bizari, D.; Tavangar, S.M.; Vasei, M.; Ai, J. Synthesis of calcium phosphate-zirconia scaffold and human endometrial adult stem cells for bone tissue engineering. Artif. Cells Nanomed. Biotechnol. 2014, 44, 66–73. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef]
- Que, R.A.; Chan, S.W.P.; Jabaiah, A.M.; Lathrop, R.H.; Da Silva, N.A.; Wang, S.-W. Tuning cellular response by modular design of bioactive domains in collagen. Biomaterials 2015, 53, 309–317. [Google Scholar] [CrossRef]
- Cicciù, M.; Scott, A.; Cicciù, D.; Tandon, R.; Maiorana, C. Recombinant Human Bone Morphogenetic Protein-2 Promote and Stabilize Hard and Soft Tissue Healing for Large Mandibular New Bone Reconstruction Defects. J. Craniofacial Surg. 2014, 25, 860–862. [Google Scholar] [CrossRef]
- Okamoto, M.; John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Liu, X.; Ma, P.X. Polymeric Scaffolds for Bone Tissue Engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef]
- Hollister, S.J. Scaffold engineering: A bridge to where? Biofabrication 2009, 1, 012001. [Google Scholar] [CrossRef]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Costa-Pinto, A.R.; Reis, R.L.; Neves, N.M. Scaffolds Based Bone Tissue Engineering: The Role of Chitosan. Tissue Eng. Part B Rev. 2011, 17, 331–347. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D.L. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Thein-Han, W.; Misra, R. Biomimetic chitosan–nanohydroxyapatite composite scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 1182–1197. [Google Scholar] [CrossRef]
- Akay, G.; Birch, M.; Bokhari, M. Microcellular polyHIPE polymer supports osteoblast growth and bone formation in vitro. Biomaterials 2004, 25, 3991–4000. [Google Scholar] [CrossRef]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef]
- Prananingrum, W.; Naito, Y.; Galli, S.; Bae, J.; Sekine, K.; Hamada, K.; Tomotake, Y.; Wennerberg, A.; Jimbo, R.; Ichikawa, T. Bone ingrowth of various porous titanium scaffolds produced by a moldless and space holder technique: An in vivo study in rabbits. Biomed. Mater. 2016, 11, 015012. [Google Scholar] [CrossRef]
- Yang, S.; Leong, K.-F.; Du, Z.; Chua, C.-K. The Design of Scaffolds for Use in Tissue Engineering. Part I. Traditional Factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, J.; Zhao, L.; Zhang, F.; Liang, X.-J.; Guo, Y.; Weir, M.D.; Reynolds, M.A.; Gu, N.; Xu, H.H. Magnetic field and nano-scaffolds with stem cells to enhance bone regeneration. Biomaterials 2018, 183, 151–170. [Google Scholar] [CrossRef]
- Funda, G.; Taschieri, S.; Bruno, G.A.; Grecchi, E.; Paolo, S.; Girolamo, D.; Del Fabbro, M. Nanotechnology Scaffolds for Alveolar Bone Regeneration. Materials 2020, 13, 201. [Google Scholar] [CrossRef]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Johnson, A.J.W. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Thomson, R.C.; Yaszemski, M.J.; Powers, J.M.; Mikos, A.G. Fabrication of biodegradable polymer scaffolds to engineer trabecular bone. J. Biomater. Sci. Polym. Ed. 1995, 7, 23–38. [Google Scholar] [CrossRef]
- Davison, N.L.; Groot, F.B.-D.; Grijpma, D.W. Degradation of Biomaterials. Tissue Eng. 2015, 177–215. [Google Scholar] [CrossRef]
- Wang, L.; Wu, S.; Cao, G.; Fan, Y.; Dunne, N.; Li, X. Biomechanical studies on biomaterial degradation and co-cultured cells: Mechanisms, potential applications, challenges and prospects. J. Mater. Chem. B 2019, 7, 7439–7459. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cells Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 1–19. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.; Maspoch, M. Processing of poly(lactic acid): Characterization of chemical structure, thermal stability and mechanical properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Park, K.; Xanthos, M. A study on the degradation of polylactic acid in the presence of phosphonium ionic liquids. Polym. Degrad. Stab. 2009, 94, 834–844. [Google Scholar] [CrossRef]
- Carothers, W.H.; Dorough, G.L.; Van Natta, F.J. Studies of polymerization and ring formation. X. the reversible polymerization of six-membered cyclic esters. J. Am. Chem. Soc. 1932, 54, 761–772. [Google Scholar] [CrossRef]
- Puelacher, W.; Vacanti, J.; Ferraro, N.; Schloo, B.; Vacanti, C. Femoral shaft reconstruction using tissue-engineered growth of bone. Int. J. Oral Maxillofac. Surg. 1996, 25, 223–228. [Google Scholar] [CrossRef]
- Mooney, D.J.; Mazzoni, C.L.; Breuer, C.; McNamara, K.; Hern, D.; Vacanti, J.P.; Langer, R. Stabilized polyglycolic acid fibre-based tubes for tissue engineering. Biomaterials 1996, 17, 115–124. [Google Scholar] [CrossRef]
- Amiryaghoubi, N.; Fathi, M.; Pesyan, N.N.; Samiei, M.; Barar, J.; Omidi, Y. Bioactive polymeric scaffolds for osteogenic repair and bone regenerative medicine. Med. Res. Rev. 2020, 40, 1833–1870. [Google Scholar] [CrossRef]
- Mano, J.F.; A Sousa, R.; Boesel, L.F.; Neves, N.M.; Reis, R.L. Bioinert, biodegradable and injectable polymeric matrix composites for hard tissue replacement: State of the art and recent developments. Compos. Sci. Technol. 2004, 64, 789–817. [Google Scholar] [CrossRef]
- Astete, C.E.; Sabliov, C.M. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. 2006, 17, 247–289. [Google Scholar] [CrossRef]
- Sordi, M.B.; Da Cruz, A.C.C.; Aragones, Á.; Cordeiro, M.M.R.; Magini, R.D.S. PLGA+HA/βTCP scaffold incorporating simvastatin: A promising biomaterial for bone tissue engineering. J. Oral Implant. 2020. [Google Scholar] [CrossRef]
- Shen, H.; Hu, X.; Yang, F.; Bei, J.; Wang, S. Cell affinity for bFGF immobilized heparin-containing poly(lactide-co-glycolide) scaffolds. Biomaterials 2011, 32, 3404–3412. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Fu, S.; Ni, P.; Wang, B.; Chu, B.; Peng, J.; Zheng, L.; Zhao, X.; Luo, F.; Wei, Y.; Qian, Z. In vivo biocompatibility and osteogenesis of electrospun poly(ε-caprolactone)–poly(ethylene glycol)–poly(ε-caprolactone)/nano-hydroxyapatite composite scaffold. Biomaterials 2012, 33, 8363–8371. [Google Scholar] [CrossRef]
- Supaphol, P.; Chuangchote, S. On the electrospinning of poly(vinyl alcohol) nanofiber mats: A revisit. J. Appl. Polym. Sci. 2008, 108, 969–978. [Google Scholar] [CrossRef]
- Ding, B.; Kim, H.-Y.; Lee, S.-C.; Shao, C.-L.; Lee, D.-R.; Park, S.-J.; Kwag, G.-B.; Choi, K.-J. Preparation and characterization of a nanoscale PVA fibers aggregate produced by electro spinning method. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1261–1268. [Google Scholar] [CrossRef]
- Jeong, B.-H.; Hoek, E.M.; Yan, Y.; Subramani, A.; Huang, X.; Hurwitz, G.; Ghosh, A.K.; Jawor, A. Interfacial polymerization of thin film nanocomposites: A new concept for reverse osmosis membranes. Compos. Sci. Technol. 2007, 294, 1–7. [Google Scholar] [CrossRef]
- Wong, K.K.H.; Hutter, J.L.; Zinke-Allmang, M.; Wan, W. Physical properties of ion beam treated electrospun poly(vinyl alcohol) nanofibers. Eur. Polym. J. 2009, 45, 1349–1358. [Google Scholar] [CrossRef]
- Dom, A.J.; Manor, N.; Elmalak, O. Biodegradable bone cement compositions based on acrylate and epoxide terminated poly(propylene fumarate) oligomers and calcium salt compositions. Biomaterials 1996, 17, 411–417. [Google Scholar] [CrossRef]
- He, S.; Timmer, M.; Yaszemski, M.; Yasko, A.; Engel, P.; Mikos, A. Synthesis of biodegradable poly(propylene fumarate) networks with poly(propylene fumarate)–diacrylate macromers as crosslinking agents and characterization of their degradation products. Polymer 2001, 42, 1251–1260. [Google Scholar] [CrossRef]
- Timmer, M.D.; Shin, H.; Horch, R.A.; Ambrose, C.G.; Mikos, A.G. In Vitro Cytotoxicity of Injectable and Biodegradable Poly(propylene fumarate)-Based Networks: Unreacted Macromers, Cross-Linked Networks, and Degradation Products. Biomacromolecules 2003, 4, 1026–1033. [Google Scholar] [CrossRef]
- Walker, J.M.; Bodamer, E.; Krebs, O.; Luo, Y.; Kleinfehn, A.; Becker, M.L.; Dean, D. Effect of Chemical and Physical Properties on the In Vitro Degradation of 3D Printed High Resolution Poly(propylene fumarate) Scaffolds. Biomacromolecules 2017, 18, 1419–1425. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; González-Calbet, J.M. Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Tuukkanen, J.; Nakamura, M. Hydroxyapatite as a Nanomaterial for Advanced Tissue Engineering and Drug Therapy. Curr. Pharm. Des. 2017, 23, 3786–3793. [Google Scholar] [CrossRef]
- Daculsi, G.; LeGeros, R. Biphasic calcium phosphate (BCP) bioceramics: Chemical, physical and biological properties. In Encyclopedia of Biomaterials and Biomedical Engineering; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–9. [Google Scholar]
- Matassi, F.; Nistri, L.; Paez, D.C.; Innocenti, M. New biomaterials for bone regeneration. Clin. Cases Miner. Bone Metab. 2011, 8, 21–24. [Google Scholar]
- Leong, K.; Cheah, C.; Chua, C. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterial 2003, 24, 2363–2378. [Google Scholar] [CrossRef]
- Gaihre, B.; Jayasuriya, A.C. Comparative investigation of porous nano-hydroxyapaptite/chitosan, nano-zirconia/chitosan and novel nano-calcium zirconate/chitosan composite scaffolds for their potential applications in bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 330–339. [Google Scholar] [CrossRef]
- Best, S.M.; Porter, A.E.; Thian, E.S.; Huang, J. Bioceramics: Past, present and for the future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Properties of Osteoconductive Biomaterials: Calcium Phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Abukawa, H.; Papadaki, M.; Abulikemu, M.; Leaf, J.; Vacanti, J.P.; Kaban, L.B.; Troulis, M.J. The Engineering of Craniofacial Tissues in the Laboratory: A Review of Biomaterials for Scaffolds and Implant Coatings. Dent. Clin. N. Am. 2006, 50, 205–216. [Google Scholar] [CrossRef]
- Bohner, M. Calcium orthophosphates in medicine: From ceramics to calcium phosphate cements. Injury 2000, 31, D37–D47. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Biphasic, triphasic and multiphasic calcium orthophosphates. Acta Biomater. 2012, 8, 963–977. [Google Scholar] [CrossRef]
- Montazeri, L.; Javadpour, J.; Shokrgozar, M.A.; Bonakdar, S.; Javadian, S. Hydrothermal synthesis and characterization of hydroxyapatite and fluorhydroxyapatite nano-size powders. Biomed. Mater. 2010, 5, 045004. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Ceramics for medical applications. J. Chem. Soc. Dalton Trans. 2001, 97–108. [Google Scholar] [CrossRef]
- Kroese-Deutman, H.C.; Ruhé, P.; Spauwen, P.H.; Jansen, J.A. Bone inductive properties of rhBMP-2 loaded porous calcium phosphate cement implants inserted at an ectopic site in rabbits. Biomaterials 2005, 26, 1131–1138. [Google Scholar] [CrossRef]
- Boden, S.D. Bioactive Factors for Bone Tissue Engineering. Clin. Orthop. Relat. Res. 1999, 367, S84–S94. [Google Scholar] [CrossRef]
- Yuan, H.; Zou, P.; Yang, Z.; Zhang, X.; De Bruijn, J.D.; De Groot, K. Bone morphogenetic protein and ceramic-induced osteogenesis. J. Mater. Sci. Mater. Med. 1998, 9, 717–721. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef]
- Pawar, A.M.; Sawant, K. Bioactive glass in dentistry: A systematic review. Saudi J. Oral Sci. 2020, 7, 3. [Google Scholar] [CrossRef]
- Da Cruz, A.C.C.; Pochapski, M.T.; Tramonti, R.; da Silva, J.C.Z.; Antunes, A.C.; Pilatti, G.L.; Santos, F.A. Evaluation of physical–chemical properties and biocompatibility of a microrough and smooth bioactive glass particles. J. Mater. Sci. Mater. Med. 2008, 19, 2809–2817. [Google Scholar] [CrossRef]
- Vogel, M.; Voigt, C.; Gross, U.M.; Müller-Mai, C.M. In vivo comparison of bioactive glass particles in rabbits. Biomaterials 2001, 22, 357–362. [Google Scholar] [CrossRef]
- Bosetti, M.; Hench, L.; Cannas, M. Interaction of bioactive glasses with peritoneal macrophages and monocytesin vitro. J. Biomed. Mater. Res. 2002, 60, 79–85. [Google Scholar] [CrossRef]
- Wilson, T.; Parikka, V.; Holmbom, J.; Ylänen, H.; Penttinen, R. Intact surface of bioactive glass S53P4 is resistant to osteoclastic activity. J. Biomed. Mater. Res. Part A 2006, 77, 67–74. [Google Scholar] [CrossRef]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive Glass Applications in Dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef]
- Mesquita-Guimarães, J.; Leite, M.A.; Souza, J.C.M.; Henriques, B.; Silva, F.S.; Hotza, D.; Boccaccini, A.R.; Fredel, M.C. Processing and strengthening of 58S bioactive glass-infiltrated titania scaffolds. J. Biomed. Mater. Res. Part A 2017, 105, 590–600. [Google Scholar] [CrossRef]
- Galarraga-Vinueza, M.E.; Mesquita-Guimarães, J.; Magini, R.S.; Souza, J.C.M.; Fredel, M.C.; Boccaccini, A.R. Mesoporous bioactive glass embedding propolis and cranberry antibiofilm compounds. J. Biomed. Mater. Res. Part A 2018, 106, 1614–1625. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L. Ceramics for medical applications: A picture for the next 20 years. J. Eur. Ceram. Soc. 2009, 29, 1245–1255. [Google Scholar] [CrossRef]
- Kelly, J.R.; Denry, I. Stabilized zirconia as a structural ceramic: An overview. Dent. Mater. 2008, 24, 289–298. [Google Scholar] [CrossRef]
- De Bortoli, L.S.; Schabbach, L.M.; Fredel, M.C.; Hotza, D.; Henriques, B. Ecological footprint of biomaterials for implant dentistry: Is the metal-free practice an eco-friendly shift? J. Clean. Prod. 2018, 213, 723–732. [Google Scholar] [CrossRef]
- Kelly, P.M.; Rose, L.F. The martensitic transformation in ceramics—Its role in transformation toughening. Prog. Mater. Sci. 2002, 47, 463–557. [Google Scholar] [CrossRef]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Garvie, R.C.; Hannink, R.H.; Pascoe, R.T. Ceramic steel? Nature 1975, 258, 703–704. [Google Scholar]
- Mondal, D.; So-Ra, S.; Sarkar, S.K.; Min, Y.K.; Yang, H.M.; Lee, B.T. Fabrication of multilayer ZrO2–biphasic calcium phosphate–poly-caprolactone unidirectional channeled scaffold for bone tissue formation. J. Biomater. Appl. 2012, 28, 462–472. [Google Scholar] [CrossRef]
- Malmström, J.; Slotte, C.; Adolfsson, E.; Norderyd, O.; Thomsen, P. Bone response to free form-fabricated hydroxyapatite and zirconia scaffolds: A histological study in the human maxilla. Clin. Oral Implant. Res. 2009, 20, 379–385. [Google Scholar] [CrossRef]
- An, S.-H.; Matsumoto, T.; Miyajima, H.; Nakahira, A.; Kim, K.-H.; Imazato, S. Porous zirconia/hydroxyapatite scaffolds for bone reconstruction. Dent. Mater. 2012, 28, 1221–1231. [Google Scholar] [CrossRef]
- Pattnaik, S.; Nethala, S.; Tripathi, A.; Saravanan, S.; Moorthi, A.; Selvamurugan, N. Chitosan scaffolds containing silicon dioxide and zirconia nano particles for bone tissue engineering. Int. J. Biol. Macromol. 2011, 49, 1167–1172. [Google Scholar] [CrossRef]
- Mesquita-Guimarães, J.; Ramos, L.; Detsch, R.; Henriques, B.; Fredel, M.; Silva, F.; Boccaccini, A. Evaluation of in vitro properties of 3D micro-macro porous zirconia scaffolds coated with 58S bioactive glass using MG-63 osteoblast-like cells. J. Eur. Ceram. Soc. 2019, 39, 2545–2558. [Google Scholar] [CrossRef]
- Kumar, A.; Nune, K.C.; Murr, L.E.; Misra, R.D.K. Biocompatibility and mechanical behaviour of three-dimensional scaffolds for biomedical devices: Process–structure–property paradigm. Int. Mater. Rev. 2016, 61, 20–45. [Google Scholar] [CrossRef]
- Tamay, D.G.; Usal, T.D.; Alagoz, A.S.; Yucel, D.; Hasirci, N.; Hasirci, V. 3D and 4D Printing of Polymers for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2009, 7, 164. [Google Scholar] [CrossRef]
- Castro, V.O.; Fredel, M.C.; Aragones, Á.; Barra, G.M.D.O.; Cesca, K.; Merlini, C. Electrospun fibrous membranes of poly (lactic-co-glycolic acid) with β-tricalcium phosphate for guided bone regeneration application. Polym. Test. 2020, 86, 106489. [Google Scholar] [CrossRef]
- Dos Santos, V.I.; Merlini, C.; Aragones, Á.; Cesca, K.; Fredel, M.C. In vitro evaluation of bilayer membranes of PLGA/hydroxyapatite/β-tricalcium phosphate for guided bone regeneration. Mater. Sci. Eng. C 2020, 112, 110849. [Google Scholar] [CrossRef]
- Kumar, P.; Dehiya, B.S.; Sindhu, A.; Kumar, R.; Pruncu, C.I.; Yadav, A. Fabrication and characterization of silver nanorods incorporated calcium silicate scaffold using polymeric sponge replica technique. Mater. Des. 2020, 195, 109026. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E.; Barberi, J.; Kargozar, S.; Marchi, J.; Massera, J.; Verné, E. Processing methods for making porous bioactive glass-based scaffolds—A state-of-the-art review. Int. J. Appl. Ceram. Technol. 2019, 16, 1762–1796. [Google Scholar] [CrossRef]
- Carlier, A.; Skvortsov, G.A.; Hafezi, F.; Ferraris, E.; Patterson, J.; Koc, B.; Van Oosterwyck, H. Computational model-informed design and bioprinting of cell-patterned constructs for bone tissue engineering. Biofabrication 2016, 8, 025009. [Google Scholar] [CrossRef]
- Cheung, H.-Y.; Lau, K.-T.; Lu, T.-P.; Hui, D. A critical review on polymer-based bio-engineered materials for scaffold development. Compos. Part B Eng. 2007, 38, 291–300. [Google Scholar] [CrossRef]
- Coelho, P.G.; Hollister, S.J.; Flanagan, C.L.; Fernandes, P.R. Bioresorbable scaffolds for bone tissue engineering: Optimal design, fabrication, mechanical testing and scale-size effects analysis. Med. Eng. Phys. 2015, 37, 287–296. [Google Scholar] [CrossRef]
- Prasad, A.; Sankar, M.; Katiyar, V. State of Art on Solvent Casting Particulate Leaching Method for Orthopedic ScaffoldsFabrication. Mater. Today Proc. 2017, 4, 898–907. [Google Scholar] [CrossRef]
- Brougham, C.M.; Levingstone, T.J.; Shen, N.; Cooney, G.M.; Jockenhoevel, S.; Flanagan, T.C.; O’Brien, F.J. Freeze-Drying as a Novel Biofabrication Method for Achieving a Controlled Microarchitecture within Large, Complex Natural Biomaterial Scaffolds. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef]
- Kumar, P.; Saini, M.; Dehiya, B.S.; Umar, A.; Sindhu, A.; Mohammed, H.; Al-Hadeethi, Y.; Guo, Z. Fabrication and in-vitro biocompatibility of freeze-dried CTS-nHA and CTS-nBG scaffolds for bone regeneration applications. Int. J. Biol. Macromol. 2020, 149, 1–10. [Google Scholar] [CrossRef]
- Conoscenti, G.; Schneider, T.; Stoelzel, K.; Pavia, F.C.; Brucato, V.; Goegele, C.; La Carrubba, V.; Schulze-Tanzil, G. PLLA scaffolds produced by thermally induced phase separation (TIPS) allow human chondrocyte growth and extracellular matrix formation dependent on pore size. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 449–459. [Google Scholar] [CrossRef]
- Moghadam, M.Z.; Hassanajili, S.; Esmaeilzadeh, F.; Ayatollahi, M.; Ahmadi, M. Formation of porous HPCL/LPCL/HA scaffolds with supercritical CO2 gas foaming method. J. Mech. Behav. Biomed. Mater. 2017, 69, 115–127. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, L.; Wang, N.; Gong, S.; Wang, L.; Li, Q.; Shen, C.; Turng, L.-S. Fabrication of polycaprolactone electrospun fibers with different hierarchical structures mimicking collagen fibrils for tissue engineering scaffolds. Appl. Surf. Sci. 2018, 427, 311–325. [Google Scholar] [CrossRef]
- Lin, W.; Chen, M.; Qu, T.; Li, J.; Man, Y. Three-dimensional electrospun nanofibrous scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1311–1321. [Google Scholar] [CrossRef]
- Bhushan, B.; Caspers, M. An overview of additive manufacturing (3D printing) for microfabrication. Microsyst. Technol. 2017, 23, 1117–1124. [Google Scholar] [CrossRef]
- Bendtsen, S.T.; Quinnell, S.P.; Wei, M. Development of a novel alginate-polyvinyl alcohol-hydroxyapatite hydrogel for 3D bioprinting bone tissue engineered scaffolds. J. Biomed. Mater. Res. Part A 2017, 105, 1457–1468. [Google Scholar] [CrossRef]
- Sachlos, E.; Czernuszka, J. Making tissue engineering scaffolds work. Review on the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur. Cells Mater. 2003, 5, 29–39. [Google Scholar]
- Bose, S.; Ke, D.; Sahasrabudhe, H.; Bandyopadhyay, A. Additive manufacturing of biomaterials. Prog. Mater. Sci. 2018, 93, 45–111. [Google Scholar] [CrossRef]
- Rychter, P.; Pamula, E.; Orchel, A.; Posadowska, U.; Krok-Borkowicz, M.; Kaps, A.; Smigiel-Gac, N.; Smola, A.; Kasperczyk, J.; Prochwicz, W.; et al. Scaffolds with shape memory behavior for the treatment of large bone defects. J. Biomed. Mater. Res. Part A 2015, 103, 3503–3515. [Google Scholar] [CrossRef]
- Schmidleithner, C.; Malferrari, S.; Palgrave, R.G.; Bomze, D.; Schwentenwein, M.; Kalaskar, D.M. Application of high resolution DLP stereolithography for fabrication of tricalcium phosphate scaffolds for bone regeneration. Biomed. Mater. 2019, 14, 045018. [Google Scholar] [CrossRef]
- Madrid, A.P.M.; Vrech, S.M.; Sanchez, M.A.; Rodriguez, A.P. Advances in additive manufacturing for bone tissue engineering scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 631–644. [Google Scholar] [CrossRef]
- Distler, T.; Fournier, N.; Grünewald, A.; Polley, C.; Seitz, H.; Detsch, R.; Boccaccini, A.R. Polymer-Bioactive Glass Composite Filaments for 3D Scaffold Manufacturing by Fused Deposition Modeling: Fabrication and Characterization. Front. Bioeng. Biotechnol. 2020, 8, 552. [Google Scholar] [CrossRef]
- Mazzoli, A. Selective laser sintering in biomedical engineering. Med. Biol. Eng. Comput. 2013, 51, 245–256. [Google Scholar] [CrossRef]
- Qin, T.; Li, X.; Long, H.; Bin, S.; Xu, Y. Bioactive Tetracalcium Phosphate Scaffolds Fabricated by Selective Laser Sintering for Bone Regeneration Applications. Materials 2020, 13, 2268. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Hasan, A.; Kaarela, O.; Byambaa, B.; Sheikhi, A.; Gaharwar, A.K.; Khademhosseini, A. Advancing Frontiers in Bone Bioprinting. Adv. Healthc. Mater. 2019, 8, e1801048. [Google Scholar] [CrossRef]



| Materials | Advantages | Disadvantages | |
|---|---|---|---|
| Poly-lactic acid (PLA) [46,51] | 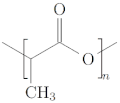 | - Water-soluble - Crystallinity tunable by changing hydroxylation degree | - Non-hydrophobic - Shortage of cell adhesion |
| Poly-glycolic acid (PGA) [46] |  | - Water-soluble - Crystallinity tunable by changing hydroxylation degree | - Non-hydrophobic - Shortage of cell adhesion |
| Polylactic-co-glycolic acid (PLGA) [52,53] | 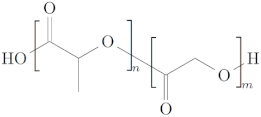 | - Water-soluble - Crystallinity tunable by changing hydroxylation degree - Easily synthesized - Biodegradable in non-toxic by-products - Controlled degradation time | - Non-hydrophobic - Shortage of cell adhesion |
| Polycaprolactone (PCL) [54] |  | - Crosslink in situ and print by injection - Elastic behavior | - Degradation rate in years |
| Polyvynil alcohol (PVA) [55,56] | 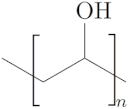 | - Ability to manufacture scaffolds with various characteristics such as shape, porosity, and degradation rate - Flexibility - Mechanically strong - Water-soluble - Compatible with several polymers | - Non-soluble in organic solvents - Cross-linking of polymers to maintain integrity |
| Polypropylene fumarate (PPF) [57] | 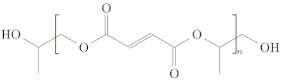 | - Adjustable mechanically strong - Adjustable rates of degradation | - Cross-linking of polymers to maintain integrity |
| Hydroxyapatite (HAp) [44,46,58] | 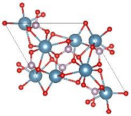 | - Highly biocompatibility - Nontoxic - Hydrophilicity - Provides calcium and phosphorus for new tissue | - Poor mechanical strength - Lack of organic phase |
| Tricalcium phosphates (TCP) [59,60] |  | - Provides calcium and phosphorus for new tissue | - Poor mechanical strength - Lack of organic phase |
| Bioactive glass (BG) [46,61] |  | - Bioactive - Bond-bonding affinity | - High solubility - Limitation of shaping |
| Zirconia (ZrO2) [62,63] |  | - Mechanically strong - High fracture toughness - Osseointegration potential - Radiopacity | - No biological activity |
| Technique | Description | Scaffold Materials |
|---|---|---|
| Solvent casting and particle leaching [154] | A polymer solution is dissolved in a solvent rich in crystals of soluble salts or organic particles. After removing the solvent by evaporation, these particles come together to form a matrix. The system is immersed in water, allowing the dissolution of the salt matrix and the removal of the produced polymeric structure, which is highly porous. The structures produced are simple but may contain some solvent residue. The centrifugation and layer technique can be combined to minimize these limitations. | - PLGA |
| Freeze drying [155,156] | The polymeric material is dissolved in a solvent and the solution obtained is cooled below its freezing point taking the solvent to solidification. This system is taken to a freeze dryer, previously adjusted with a temperature below the freezing point of the solvent and a pressure below atmospheric pressure to promote the sublimation of the solvent. The result is the formation of a porous structure, with multiple empty spaces and channels connected. | - Gelatine - HAp - PLA - PCL - Chitosan |
| Thermally-induced phase separation [148,157] | A polymer is dissolved in a solvent at high temperature, followed by rapid cooling. The solvent is separated from the polymeric structure due to the change in the solubility coefficient caused by the temperature reduction, forming one phase rich in polymer and another poor. The polymeric phase solidifies, while the other phase is removed, resulting in a highly porous polymeric structure. This technique can be used in association with other techniques to manufacture 3D structures with controlled pore morphology, such as leaching. | - PPLA - Chitosan |
| Gas foaming [158] | Blowing agents are used to pressurize molded polymers. These agents generate gas bubbles that act as porosity builders, causing expansion in volume and reduction in the density of polymers. When associated with the replica technique, the polymeric foam is impregnated with a ceramic suspension. The structure sintered at high temperature, degrades the polymer, resulting in a porous ceramic structure. | - HAp - β-TCP |
| Powder-forming [116] | A suspension of ceramic particles is prepared in an appropriate liquid to form a paste. From this paste, green bodies are produced in different ways. Subsequent sintering results in porous scaffolds. | - PLGA - HAp |
| Electrospinning [159,160] | An electric field is used to form fibers with diameters ranging from micrometer to nanometer scale. A typical apparatus consists of an infusion pump, syringe set, and metallic needle for the formation of the spinning droplet, a collector, and the electrical system. The potential difference applied by the electrical system generates high electric fields and its strength exceeds the surface tension of the droplet, elongating it. After evaporation of the solvent, the fibers are collected. | - PLA - β-TCP |
| Techniques | Description | Scaffold Materials |
|---|---|---|
| Stereolithography (SL) [166,167] | Solid objects are manufactured, layer by layer, by curing a photoreticulable liquid resin of ultraviolet or visible light beams, directed by a dynamic mirror system. A mobile platform moves the cured part. Therefore, another layer can solidify producing a three-dimensional structure. | - PEG - PPF |
| Fused Deposition Modeling (FDM) [168] | Thermoplastic filaments, consisting of an extruded material or composite, are melted and deposited layerwise on a build platform until the object is formed. | - ABS - PLA - nylon |
| Selective Laser Sintering (SLS) [169,170] | In this technique, also known as selective laser melting (SLM), the poorly compacted powder is sintered with a high-power laser (e.g., CO2), particle by particle, uniting them in a controlled manner, forming thin layers. The layers are joined to each other according to predefined computer-aided data (CAD) parameters. The interaction of the laser beam with the powder increases the temperature of the powder above the glass transition temperature and below the melting temperature, causing the melting and bonding of the particles to form a solid mass. The process results in solid or porous structures with superior mechanical properties, custom density, and elastic modulus, and a post-processing phase is required to remove the remaining power. | - PPLA - HPa |
| Bioprinting [171] | Cells and biomaterials are printed using inkjet, extrusion, or laser-assisted bioprinting techniques with micrometric precision. Jet-based bioprinting produces 2D and 3D structures by applying layers of bio-ink on a substrate. In extrusion-based bioprinting, a mixture of hydrogels is injected by pressure. Afterward, the hydrogels are solidified physically or chemically, and the 3D structures are manufactured by stacking. In laser bioprinting, a receptor material made of glass covered with a layer of gold absorbs the laser, and in this way, a drop is created at high pressure, which in turn transfers materials to the substrate. | - alginate - chitosan - collagen - fibrin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özcan, M.; Hotza, D.; Fredel, M.C.; Cruz, A.; Volpato, C.A.M. Materials and Manufacturing Techniques for Polymeric and Ceramic Scaffolds Used in Implant Dentistry. J. Compos. Sci. 2021, 5, 78. https://doi.org/10.3390/jcs5030078
Özcan M, Hotza D, Fredel MC, Cruz A, Volpato CAM. Materials and Manufacturing Techniques for Polymeric and Ceramic Scaffolds Used in Implant Dentistry. Journal of Composites Science. 2021; 5(3):78. https://doi.org/10.3390/jcs5030078
Chicago/Turabian StyleÖzcan, Mutlu, Dachamir Hotza, Márcio Celso Fredel, Ariadne Cruz, and Claudia Angela Maziero Volpato. 2021. "Materials and Manufacturing Techniques for Polymeric and Ceramic Scaffolds Used in Implant Dentistry" Journal of Composites Science 5, no. 3: 78. https://doi.org/10.3390/jcs5030078
APA StyleÖzcan, M., Hotza, D., Fredel, M. C., Cruz, A., & Volpato, C. A. M. (2021). Materials and Manufacturing Techniques for Polymeric and Ceramic Scaffolds Used in Implant Dentistry. Journal of Composites Science, 5(3), 78. https://doi.org/10.3390/jcs5030078









