1. Introduction
Intracranial aneurysms are localized pathological dilations of cerebral arteries that occur in approximately 4% of the population [
1]. Approximately 90% of intracranial aneurysms are saccular aneurysms, which are commonly formed at arterial bifurcations of the circle of Willis [
2]. In the United States, approximately 30,000 people suffer from rupture of aneurysm annually, which leads to subarachnoid hemorrhage (SAH). SAH has a high case-fatality rate of 25–50% caused by an initial bleed or immediate complications, and half of the survivors have permanent neurological damage [
3,
4]. Since the development of detachable coiling using a microcatheter in 1990, endovascular treatment has been the standard treatment method for intracranial aneurysms, substituting invasive craniotomies necessary for surgical clipping [
5]. However, recanalization is a major problem that occurs in up to 25% of aneurysms treated with platinum coils, especially in wide-neck or large-to-giant aneurysms [
6,
7]. One of the most significant contributing factors of aneurysm recurrence is coil compaction, which is caused by the water-hammer effect of pulsatile blood flow [
8]. To prevent coil compaction, increasing the packing density of an embolization device is critical. However, it is not usually achievable in wide-neck aneurysms or large aneurysms with volumes larger than 600 mm
3 [
9].
Polyurethane shape memory polymer (SMP) foams have shown great promise in endovascular embolization devices due to long-term biocompatibility, rapid thrombosis, and unique shape recovery capabilities [
10,
11,
12,
13]. SMP foams are soft and open-celled porous materials that can be crimped into a secondary shape and maintain the shape until actuated by a thermal stimulus to recover their original shape. This allows a low-volumetric SMP foam to be delivered to the desired spot (e.g., aneurysm sac) using a microcatheter where the foam can expand to its porous primary shape when actuated by body temperature or an external heat stimulus. SMP foams have interconnected pores that allow blood to flow inside the scaffold and form interconnected thrombus, promoting subsequent cell infiltration that triggers the tissue healing process in the matrix [
13,
14]. SMP foam-coated coils showed a bulk packing density of 93% in an in vitro wide-neck aneurysm, which was significantly higher than previously reported packing densities of bare platinum coils (BPC; 30–31%) or hydrogel coated coils (76–85%) [
12]. In vivo studies in porcine sidewall aneurysm models demonstrated connective tissue formation in the SMP foam-treated aneurysms and neointima layer formation over the neck of the treated aneurysms [
14,
15]. SMP foam-treated aneurysms have shown more mature connective tissue, thicker fibrous cap across the aneurysm neck, and more rapid healing compared to coil-treated porcine vein pouch aneurysms [
15]. SMP foam material was also used in a clinical study to embolize a false lumen of a post-dissection aneurysm in a 69-year-old patient, which resulted in complete and stable thrombosis of the false lumen at 15 months [
16]. However, SMP foams do not have inherent radiopacity, which limits their clinical utility as endovascular devices since X-ray visualization is necessary for safely delivering the device to the target site, properly packing devices into an aneurysm, and tracking the implant over time.
One approach to circumventing the lack of SMP X-ray visibility is to incorporate high-Z markers into the device design. Kashyap et al. three-dimensionally (3D) printed and salt leached thermoplastic SMP containing tungsten particulates to fabricate radiopaque and porous endovascular embolization material [
17]. However, the fabricated SMP had too high density (~1 g/cm
3) and too stiff modulus (~1 GPa) to be used as a neurovascular device. In the case of low-density SMP foams, one of the methods is to use BPCs as a backbone for SMP foams to visualize the foam devices being delivered to the aneurysm sites [
11,
12]. However, the use of BPC restricts the aneurysm lesion shrinkage when the collagenous connective tissue formed around the device is contracted in the late healing stage, which would delay the overall healing [
15]. Hasan et al. physically incorporated tungsten nanoparticles into SMP foams to increase the X-ray visibility. Sufficient radiopacity was achieved at 6 vol% tungsten foams, but the mechanical toughness was diminished significantly [
18]. Nash et al. reported chemically modified, triiodobenzene monomer (5-amino-2,4,6-triiodoisophthalic acid; ATIPA)-containing SMP foams with increased X-ray visibility and mechanical toughness. However, the degree of radiopacity was still not sufficient for the neurovascular device scale (2 mm diameter) [
19], motivating the need for further opacification.
Tantalum is a good candidate material as an X-ray-visible additive due to its excellent biocompatibility, ductility, corrosion resistance, and radiopacity [
20,
21]. Tantalum has been in clinical use since 1940 and has shown great in vivo bioactivity, exhibiting abundant cellular adherence, growth, and extracellular matrix formation on tantalum coated surface [
20,
22]. In this study, tantalum microparticles were loaded in the ATIPA foaming mixtures to further enhance X-ray visibility of the ATIPA SMP foam. In addition, cell opening additives were added during the foaming process to make a more open cell structure, which will prevent the foam from shrinking during curing. Thermal properties, mechanical properties, chemical properties, radiopacity, cytocompatibility, and extractables were characterized. The effect of E-beam sterilization on chemical, thermal, and mechanical properties of tantalum-loaded ATIPA foams (AT_T) was also analyzed, in addition to the in vitro degradation behavior (oxidative and hydrolytic) after E-beam sterilization.
2. Materials and Methods
2.1. Materials
The 5-amino-2,4,6-triiodoisophthalic acid (ATIPA; Sigma Aldrich, St. Louis, MO, USA), 3-methyl-1,5-pentanediol (MPD; Sigma Aldrich), 1,2,6-hexanetriol (HT; Sigma Aldrich), 2-butyl-2-ethyl propanediol (BEP; VWR Scientific, Radnor, PA, USA), hemxamethylene diisocyanate (HDI; TCI America, Portland, OR, USA), Enovate 245fa Blowing Agent (Honeywell International, Houston, TX, USA), tantalum powder (APS~2 μm, 99.9%; Alfa Aesar, Tewksbury, MA), Tegostab B8523 (Evonik, Essen, Germany), 2-propanol 99% (IPA; VWR Scientific), tetrahydrofuran (THF; Millipore, Billerica, MA, USA), Dulbecco’s Modified Eagle Medium (DMEM; Sigma Aldrich), and deionized (DI) water (>17 MΩcm purity; Millipore water purifier system; Millipore) were used as received.
2.2. Synthesis of Tantalum-Loaded ATIPA SMP Foam
ATIPA SMP foams with tantalum microparticles (AT_T) were synthesized based on the protocol described by Nash et al. [
19]. Hydroxyl (OH) premix was prepared one day before foaming by adding 0.6 equivalent of ATIPA, MPD, BEP, and HT. The OH premix was mixed for 30 s at 3500 rpm using a Flacktek high-speed shear mixer, heated at 50 °C for one hour, mixed for 30 s at 3500 rpm, heated overnight at 50 °C, and mixed for 30 s at 3500 rpm before foaming. Isocyanate (NCO) premix was prepared two days before foaming in a desiccated glove box by adding 0.4 equivalent of ATIPA, MPD, BEP, and HT. The NCO premix was mixed at 3500 rpm for 30 s, heated at 50 °C for an hour, mixed at 3500 rpm for 30 s, and heated at 50 °C overnight. On the next day, 1 equivalent of HDI was added to the NCO premix and it was mixed for 10 min at 3500 rpm and placed on a room temperature shaker overnight at 60 rpm. On the foaming day, the viscosity of NCO premix was checked to be like honey and if it was less viscous, it was shaken for an additional day. An amount of 0, 2, 4, and 8 vol% tantalum powder was added to the NCO premix and mixed for 30 s at 3500 rpm. The mixture of 4 wt% of surfactant DC1990 and 0.025 wt% of Tegostab B8523 was added to the NCO premix and mixed for 30 s at 3500 rpm. The OH premix was added to the NCO premix and mixed for 30 s. After adding 1.5 mL Enovate and mixing for 15 s, the mixture was cured for 20 min at 90 °C. The foam skin was removed by a razor blade and then post-cured at 50 °C overnight. The detailed foam composition is shown in
Table 1. All the foam samples were thoroughly cleaned with reverse osmosis (RO) water (30 min, sonicated), IPA (30 min × 4 times, sonicated) and RO water again (15 min × 4 times, sonicated). Foam samples were dried at 50 °C overnight and stored at room temperature under vacuum before use.
2.3. Physical and Morphological Characterization
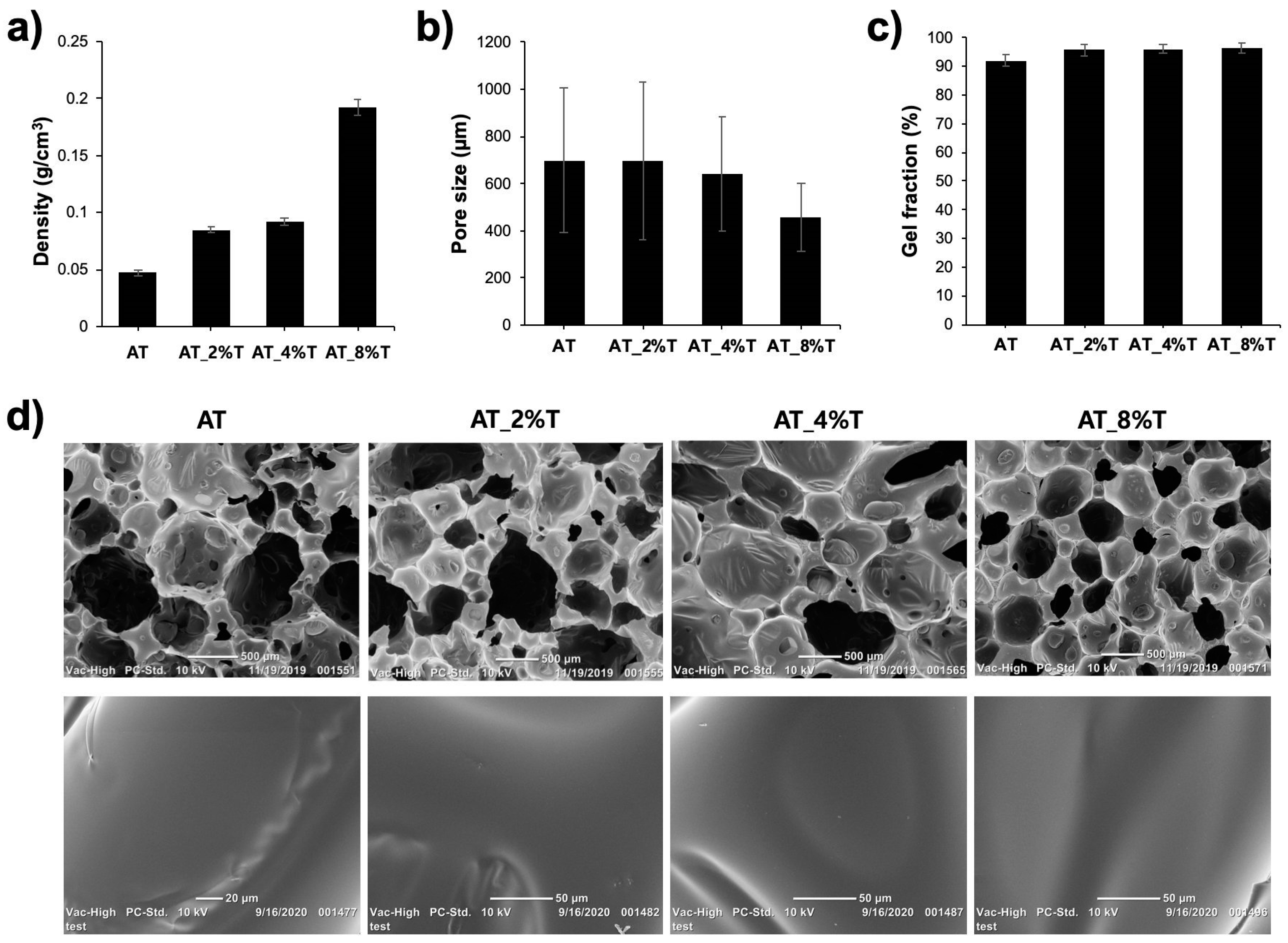
Foam density was calculated by foam mass divided by the foam’s bulk volume. Foams were cut into 1 cm3 cubes and the mass was measured (n = 5).
Pore size was measured using images taken from scanning electron microscopy (SEM; JCM-5000 Neoscope, JEOL, Peabody, MA, USA). For SEM sample preparation, foams were cut into slices with a thickness of 3 mm and sputter coated. Five images were taken for each foam composition. Random 5–6 pores were selected per image and their diameters were measured using ImageJ software (n = 20).
Gel fraction was calculated by the final sample mass divided by the original sample mass. Foam samples were cut into 1 cm3 cubes. The samples were cleaned three times with IPA (1:20 volume ratio) for 30 min each using sonication to remove any residual surfactants. Then the samples were dried at 100 °C overnight and the mass was recorded. THF (1:20 volume ratio) was added to the samples, heated at 50 °C for 48 h, and dried at 70 °C after removing THF for 36 h. The final mass of the samples was recorded (n = 5).
2.4. ATR-FTIR Spectroscopy
The absorbance of foam samples was measured using attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy (Bruker ALPHA, diamond ATR crystal, Bruker, Billerica, MA, USA). Bruker OPUS Spectroscopy software was used to analyze the spectra.
2.5. Differential Scanning Calorimetry (DSC)
Glass transition temperatures (Tg) of dry samples (dry Tg) and water-plasticized samples (wet Tg) were analyzed using differential scanning calorimetry (DSC Q200, TA Instruments, New Castle, DE, USA,). For the dry Tg measurement, dry foam samples (2–4 mg, n = 3) were loaded into Tzero pans and two heating cycles were run, of which the second cycle was used to obtain the dry Tg (heating range: −40~120 °C, ramp rate: 10 °C/min, 1 min equilibration at each endpoint). For wet Tg measurement, the dry mass of the samples were measured (2–4 mg, n = 3) and the samples were submerged in 50 °C water for 30 min. Excess water was squeezed out using a laboratory wipe until the water uptake was smaller than 10% of the original sample mass. Samples were loaded into Tzero pans and only one cycle was run to determine the wet Tg (heating range: −40~120 °C, ramp rate: 10 °C/min). Pyris software was used to determine the Tgs of the samples.
2.6. Dynamic Mechanical Analysis (DMA)
Foam samples were punched into cylinders with a diameter of 6 mm and a thickness of 5 mm. Thermomechanical properties of the foams were analyzed using a TA Q800 Dynamic Mechanical Analyzer (TA Instruments, New Castle, DE, USA) with a compression mode. Foams were equilibrated at 20 °C for 5 min and heated to 100 °C at a rate of 3 °C/min, with an amplitude of 40 μm at 1 Hz and a preload of 0.01 N.
2.7. Expansion Study
Foams were punched into cylindrical shapes with a diameter of 2 mm and a length of 1.5 cm (n = 3). The samples were axially threaded over 0.006″ wires. The foam crimper (Machine Solutions SC250) was pre-heated to 100 °C for 15 min. Foams were radially compressed at 100 °C, constrained while cooling to room temperature, and released from the crimper. Crimped foams were placed under vacuum for 24 h before the expansion test. Foams over wires were submerged in 37 °C water and the expanding foams were imaged every one minute until five minutes, and every five minutes until 40 min. Diameters of the expanding foams at five sites along the length were measured using ImageJ software.
2.8. Mechanical Testing
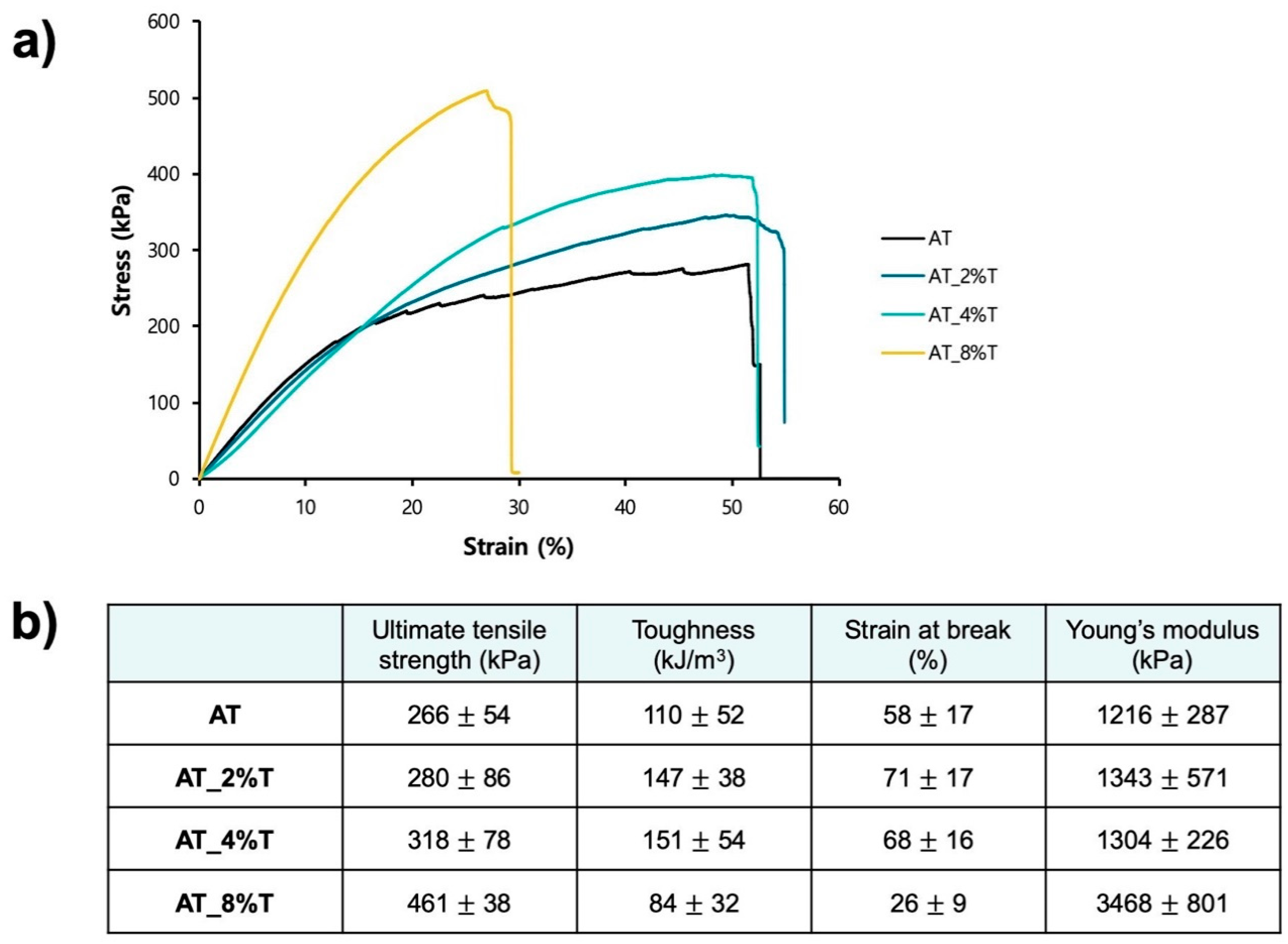
Samples were prepared by cutting them into an ASTM D-638-V dog bone shape. The shorter ends of the dog bone samples were epoxy glued to wooden blocks and they were rested for 24 h for complete cure. The attached wooden blocks were fixed to clamps on MTS Insight 30 Universal Tensile Tester. Strain-to-failure experiments were performed on the foam samples (n = 5), with a rate of 5 mm/min at room temperature.
2.9. X-ray Spectroscopy
Samples were cut into 1.5 cm × 1.5 cm squares with thicknesses of 8, 4, 2, and 1 mm. Neurovascular-scale foams which were expanded and crimped cylindrical samples with a diameter of 2 mm were prepared. All the foam samples were taped on a plastic tray as well as two bare platinum coils as positive controls (0.008″ OD 90/10 Pt/Ir coil and 0.008″ OD 92/8 Pt/W coil). X-ray images of the plastic tray containing all the samples were taken using OrthoScan C-arm system (Mobile DI Model 1000-0005). X-ray images were obtained with and without an 0.5″ aluminum plate over the sample tray which was used as a skull analog that attenuates X-rays and imitates the clinical setting of endovascular treatment [
23]. Grayscale values were measured on the background of an 8 bit X-ray image and on the samples using ImageJ software. The background value was subtracted from the sample’s mean grey scale value.
2.10. Cytocompatibility Test
Extraction media tests were performed on the ATIPA foams with a 3 cm2/mL extraction ratio according to ISO10993-5. Cell culture media consisted of DMEM, 10% newborn calf serum, 1% penicillin/streptomycin, and 0.1% fungizone. The extracts of each specimen were obtained by submerging discs, with a diameter of 5 mm and a thickness of 2 mm, cut by biopsy punch into 2 mL of media solution. The size of the discs was chosen through consideration of the pore sizes and volume to represent the accessible surface area. The samples were then placed on a shaking incubator for 72 h at 37 °C. Three replicate extraction media samples were made for each foam.
NIH/3T3 mouse fibroblast cells were seeded at an initial 7500 cells per well onto 96-well polystyrene plates and cultured for 24 h at 37 °C and 5% CO2 in an incubator before treatment with extract media samples. At 24 h, 200 µL of extraction media was used for each well. Cell viability was evaluated using a resazurin assay to quantify metabolic activity 48 h after the addition of extraction media. Resazurin solution was added to cell culture media for a 5% final concentration. Cells were then incubated in the media and resazurin solution for 3 h, where the increase in absorbance at 590 nm relative to a blank (media and 5% resazurin without cells) was measured using a plate reader (Tecan Infinite M200 Pro, Männedorf, Switzerland). The absorbance values for the extract media samples were compared to a negative control (media and 5% resazurin with cells and without extract media) as a percentage, with the negative control representing 100% cell viability.
2.11. E-Beam Sterilization
Samples were packaged in foil film bags (Beacon Converters, Saddle Brook, NJ, USA) with humidity indicators. The packages were vacuum sealed with heat after purging with nitrogen gas using an AVN packaging system (AmeriVacS, San Diego, CA, USA). The sealed packages were E-beam-sterilized at 43.6 kGy E-beam dose (10 MeV, 18 kW) at the National Center for Electron Beam Research (College Station, TX, USA). Alanine films (Kodak, Rochester, NY, USA) were used to measure the actual dose of E-beam using a Bruker E-scan spectrometer (Bruker, Billerica, MA, USA).
2.12. Degradation Analysis
In vitro degradability of foam samples was analyzed in an accelerated oxidative condition (20% H2O2) and an accelerated hydrolytic condition (0.1 N NaOH). Samples were prepared by cutting foams into 1 cm3 cubes (n = 3) and the initial sample mass was recorded. Samples were submerged in 20% H2O2 or 0.1 N NaOH with a 1:20 volume ratio (foam:solution) and stored at 37 °C. Degradation solutions were exchanged with fresh solutions every three days to ensure stable pH or ion concentration. The mass change of the samples was recorded every six days. Samples were washed with RO water and ethanol (1:20 volume ratio) and dried at 50 °C under vacuum overnight. The mass of the samples were recorded and the samples were put into fresh degradation solutions. To analyze the chemical and morphological changes of the sample foams over degradation time, ATR-FTIR and SEM analysis were performed every 18 days of degradation.
2.13. Tantalum Extraction Analysis
The AT_2%T was chosen for the extractables study due to its optimal radiopacity and the oxidative stability. The extraction was performed under exaggerated extraction conditions. Foam samples were prepared in neurovascular device scale (2 mm diameter, 1 cm length), and submerged in 5 mL of water (polar extraction) and hexane (non-polar extraction) at 50 °C for 72 h.
To study the effect of the oxidative and hydrolytic degradation solutions on the extraction of tantalum particles from the foam samples, AT_2%T was submerged in 5 mL of 20% H2O2 and 0.1 N NaOH at 37 °C for 72 h.
Foam samples were removed from the four extraction solutions. After gently mixing the solutions, 1 mL aliquot was taken and 0.1 mL of concentrated hydrofluoric acid (HF) was added for complete dissolution of tantalum. The aliquot equilibrated for a minimum of one hour in a polypropylene acid digestion tube. For the hexane extraction solution, hexane was evaporated using nitrogen evaporation. Then deionized water was added to the samples to a final volume of 10 mL. A method blank and lab control spike solutions were prepared and got the same treatment as the samples. The samples were analyzed by inductively coupled plasma-optical emission spectrometry (ICP-OES) to determine the amount of tantalum in the extraction solutions. The ICP-OES analysis including the HF treatment was performed at Legend Technical Services Inc. The total amount of tantalum extracted from the neurovascular device was calculated by multiplying the result by the volume of the remaining solutions accounting for the evaporated solvents in the extraction solutions.
4. Discussion
The goal of this study was to develop SMP foam compositions with appropriate X-ray visibility on the neurovascular scale. Previously developed ATIPA foams showed higher X-ray visibility with increasing density but showed limited visibility both in the crimped and expanded forms at the neurovascular scale [
19]. Tantalum microparticles (~2 um) were added to ATIPA foam premix to combine the chemical approach of incorporating iodine motifs in the foam system and the physical approach of loading radio-dense tantalum microparticles to achieve sufficient X-ray visualization. In a clinical setting, a NED foam should be visible in its crimped shape through fluoroscopy to enable device visualization during delivery into an intracranial aneurysm. Once the delivered foam expands to fill the aneurysm sac, clinicians check whether the aneurysm is properly embolized or not by imaging a contrast agent introduced through the bloodstream (angiography). The expanded device should not be too highly radio-dense since it can block the visibility of the contrast agent flowing into the aneurysm that is not properly embolized, in which case more NED foams are delivered to pack the aneurysm. All of the fabricated AT_T foams (AT_2%T, AT_4%T, and AT_8%T) showed sufficient X-ray visibility in their crimped shape. However, AT_2%T and AT_4%T showed more desirable X-ray visibility in the expanded form, being less radiopaque than AT_8%T. Therefore, the optimal X-ray visibility could be achieved in the AT_2%T and AT_4%T foam compositions.
The tensile strength was increased with the increasing tantalum % in the foam, and the strain at break and toughness were decreased at a higher tantalum loading % (AT_8%T). This trend is consistent with Hasan et al.’s work, where the tungsten-loaded SMP foam’s material stiffness and strength were increased up to 4 vol% tungsten and higher loading of the additives resulted in decreasing toughness and strain at break [
18]. The tungsten-loaded SMP foams could achieve sufficient X-ray visibility at tungsten content greater than 6 vol%, of which the mechanical properties were diminished [
18]. It is noteworthy that AT_2%T and AT_4%T foams showed sufficient X-ray visibility at the neurovascular scale with slightly improved mechanical properties. Further, ATIPA foams including AT_8%T have much higher toughness compared to previously developed low-density SMP foams due to the aromatic structures from the ATIPA monomer, which demonstrates that ATIPA foams can be mechanically stable under blood flow, lowering the risk of forming emboli due to foam fragmentation.
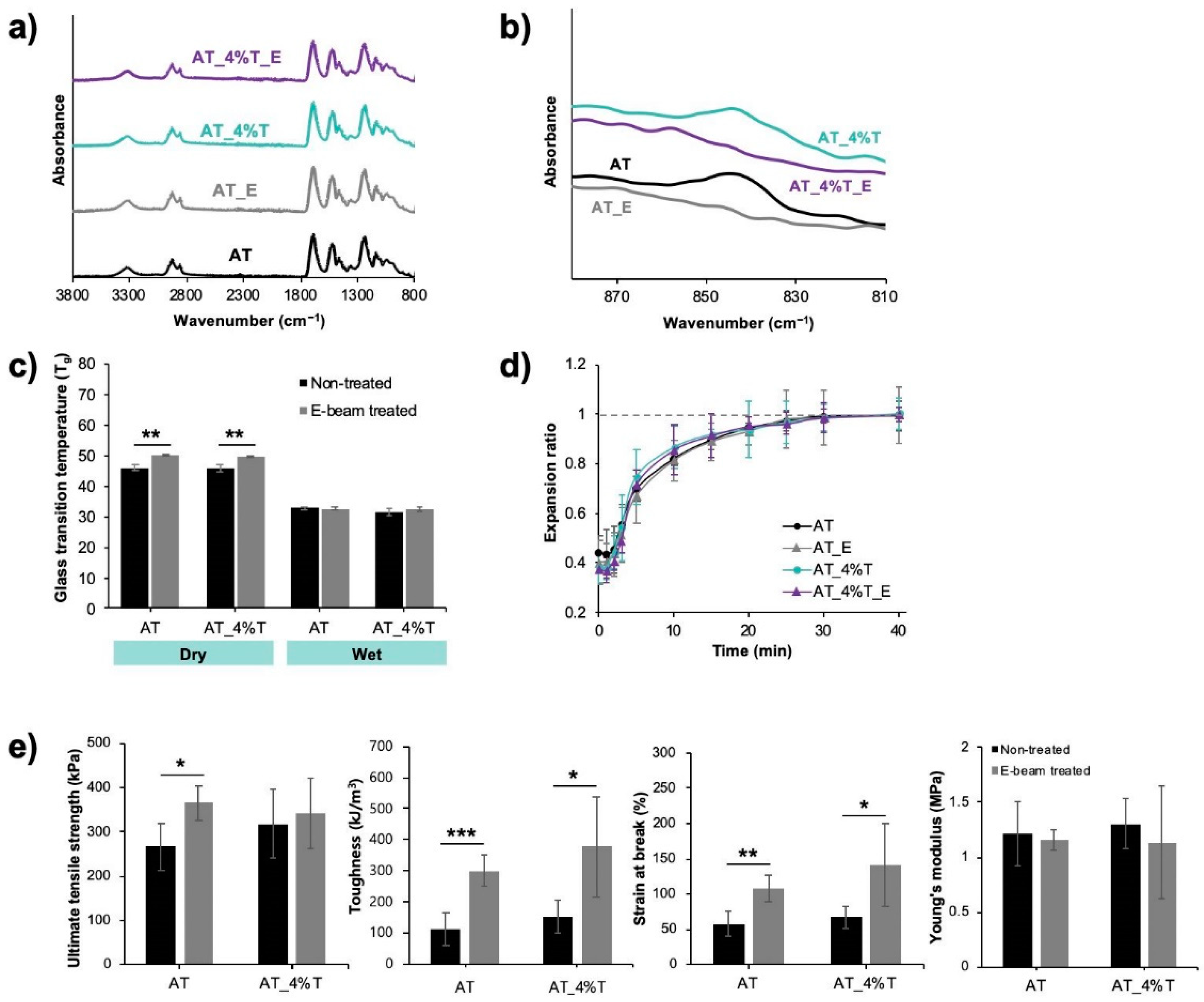
Biomedical devices can be sterilized in various ways, including ethylene oxide gas sterilization, E-beam radiation, and gamma radiation. E-beam radiation was chosen for a sterilization method in this study since E-beam radiation has more favorable sterilization conditions than the traditional ethylene oxide sterilization method (e.g., high heat, high humidity) for polyurethanes and also has shown to not prematurely actuate or alter any critical properties of SMP foam device in a previous study [
32]. After E-beam sterilization, ultimate tensile strength (for AT foam only), toughness, and strain at break of ATIPA foams were increased but Young’s modulus was not significantly impacted. Polymer crosslinking usually increases the modulus, ultimate tensile strength, and decreases the strain at break due to the crosslinking of intra- or intermolecular chains restricting the mobility of the polymer chains [
28]. However, in highly porous material such as ATIPA foams, the modulus is not affected by the crosslinking of polymer chains [
33]. The strain at break was increased after the E-beam radiation due to the elongation of pores to a higher extent resulting from the formation of tougher material after chain crosslinking.
In addition to the polymer chain crosslinking, E-beam sterilization changed the color of ATIPA foams to a more yellowish color (
Figure S1a). The discoloration is commonly observed in various types of polymers after being irradiated by X-ray, gamma, or E-beam. The radiation can cause either permanent color change by rearranging bonds and forming covalent double bonds, or annealable color change by forming free radicals that are trapped in the polymeric matrix [
28]. The discoloration observed in ATIPA foams was the latter, where the original color could be recovered after washing the material in water. The trapped free radicals can react with water molecules and anneal out the color center. After the recovery of the original color, radiopacity, T
g, and chemical properties of the ATIPA foams were analyzed. There was no significant difference in those properties after the washing step (
Figure S1b–d), which indicates that the increase in T
g and change in the IR after E-beam radiation (
Figure 6) are not relevant to the color center formation but are due to the chain crosslinking.
In the in vitro degradation studies, it should be noted that AT foams showed significantly improved biostability in the oxidative degradation condition compared to the previously developed low-density SMP foams (H40 in this study) due to the absence of the tertiary amine-containing monomers which are susceptible to oxidative degradation [
29]. Therefore, the mass loss observed for AT_T foams over 90 days is due to the tantalum dissolution. However, the mass loss over 90 days (24, 55, and 72 wt% for AT_2%T, AT_4%T, and AT_8%T, respectively) was larger than the tantalum wt% in the foams (22, 36, and 52 wt%) and the mass consistently decreased during 90 days. This indicates that the mass loss is also associated with the loss of foam particulates, as the foam structure becomes weaker during the tantalum dissolution. Since fragments from neurovascular foam possess the possibility of generating emboli in the bloodstream, the more oxidatively biostable AT_2%T is more appropriate composition for neurovascular devices than AT_4%T. However, it is very unlikely for the degrading material to enter the circulation since the developing clot isolates the foam material a few hours after the device implantation, and the foam is trapped in thrombus and developing scar tissue.
Extraction studies showed that tantalum was extracted out of the AT_2%T device only in 20% H
2O
2, but not in water, hexane, or 0.1 N NaOH extraction solutions. This is attributed to the higher solubility of tantalum in the H
2O
2 solution compared to other extraction solutions, which supports our degradation study results [
30]. However, the extracted amounts were still very small, which did not exceed 20 μg per neurovascular device over three days in the accelerated condition. For a large aneurysm with a diameter of 25 mm, 234 cm of foam devices is needed to achieve a packing density of 90%, which is a high-end estimation for the amount of implanted foams. In this case, 0.031 mg/kg tantalum will be extracted per day for a 50 kg adult, which is five orders of magnitude lower than the LD50 (lethal dose) level of tantalum (8000 mg/kg) [
34]. The extraction study results along with the cell viability results ensure the biocompatibility of the AT_2%T device even with a much longer length of the foam device.
One limitation of AT_T foams is the short working time (~2 min), as shown in the expansion profile (
Figure 2d). Clinicians require a working time of approximately 5–10 min, which is a time to deliver the crimped foam through the blood vessel into the aneurysm sac using a microcatheter. Several methods can increase the working time of AT_T foams. First, more hydrophobic monomers could be used in the foam synthesis to decrease the water penetration rate into the crimped foam. Second, the foams can be crimped to a smaller diameter, which would decrease the water penetration rate as well. In order to crimp foams more tightly, foams with a lower density could be fabricated by increasing the amount of blowing agent or using different surfactants. The foams can also be mechanically crimped into a smaller diameter since the foams could only be crimped down to a diameter of 0.6 mm in this study due to the crimper used, which had a minimum crimping diameter of 0.6 mm. Therefore, a crimper that has a smaller minimum crimping diameter can decrease the crimped diameter of the AT_T foams. For feasible delivery of the foam device into a tortuous human neurovasculature, the use of 0.017″ (0.43 mm) or smaller inner diameter (ID) catheter is required. Thus, the foams would need to be crimped to a smaller diameter than 0.43 mm to be used for a neurovascular embolization device. In addition to increasing the working time and decreasing the crimped diameter, the biocompatibility of the degraded products needs to be analyzed in vitro and in vivo for future work to ensure the safety of using AT_T foams in neurovascular embolization applications.