Highly Hydrophobic, Homogeneous Suspension and Resin by Graft Copolymerization Modification of Cellulose Nanocrystal (CNC)
Abstract
:1. Introduction
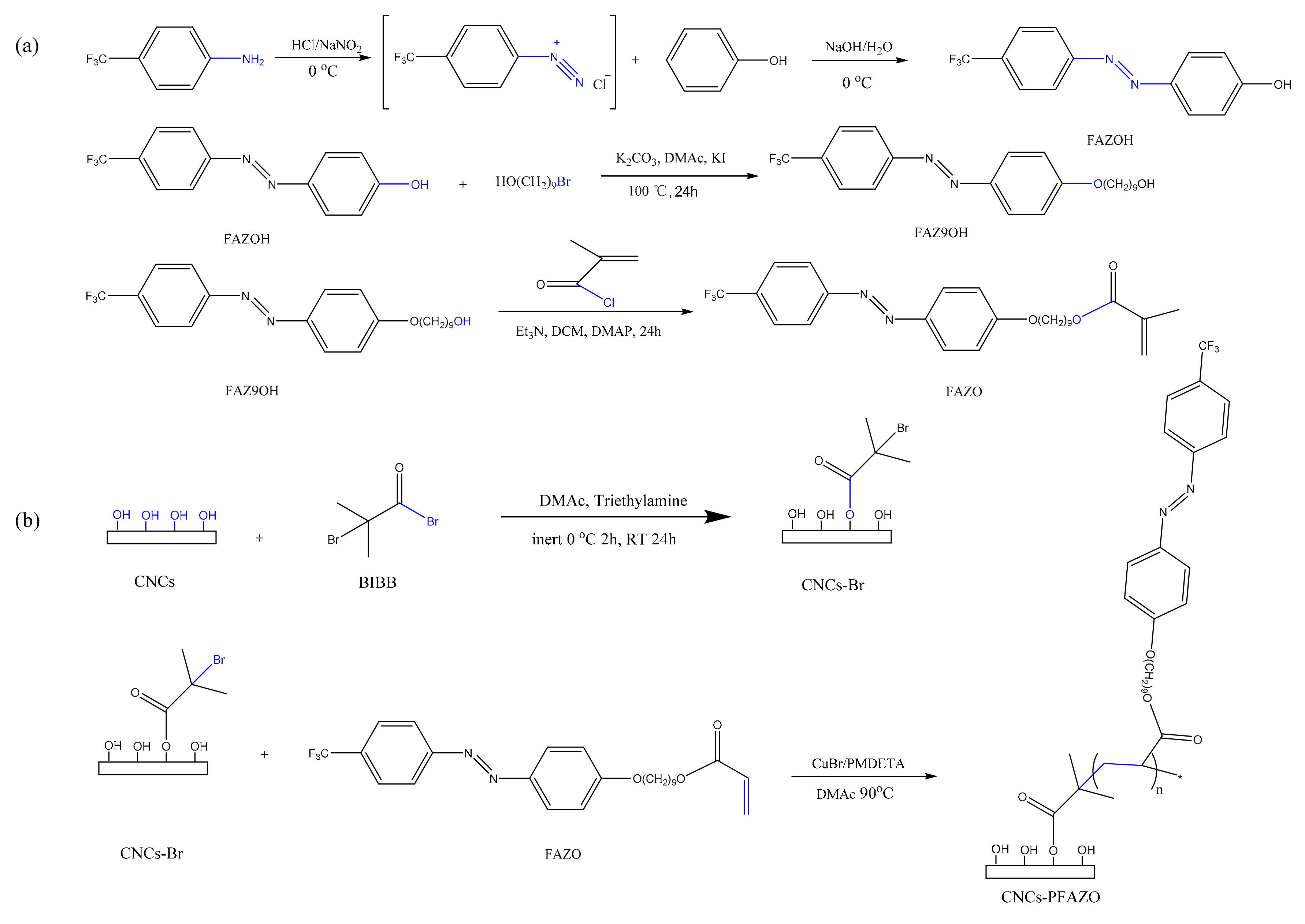
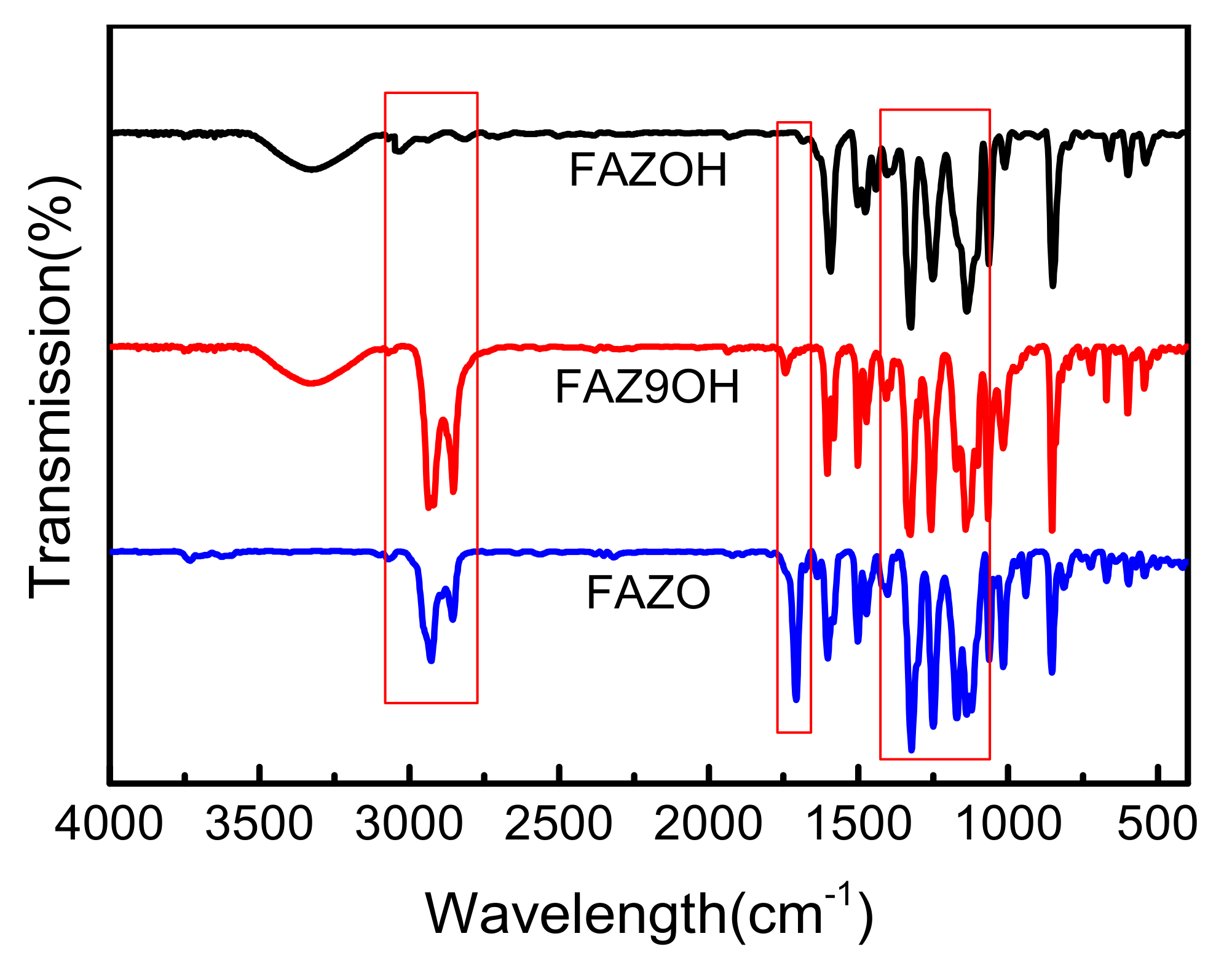
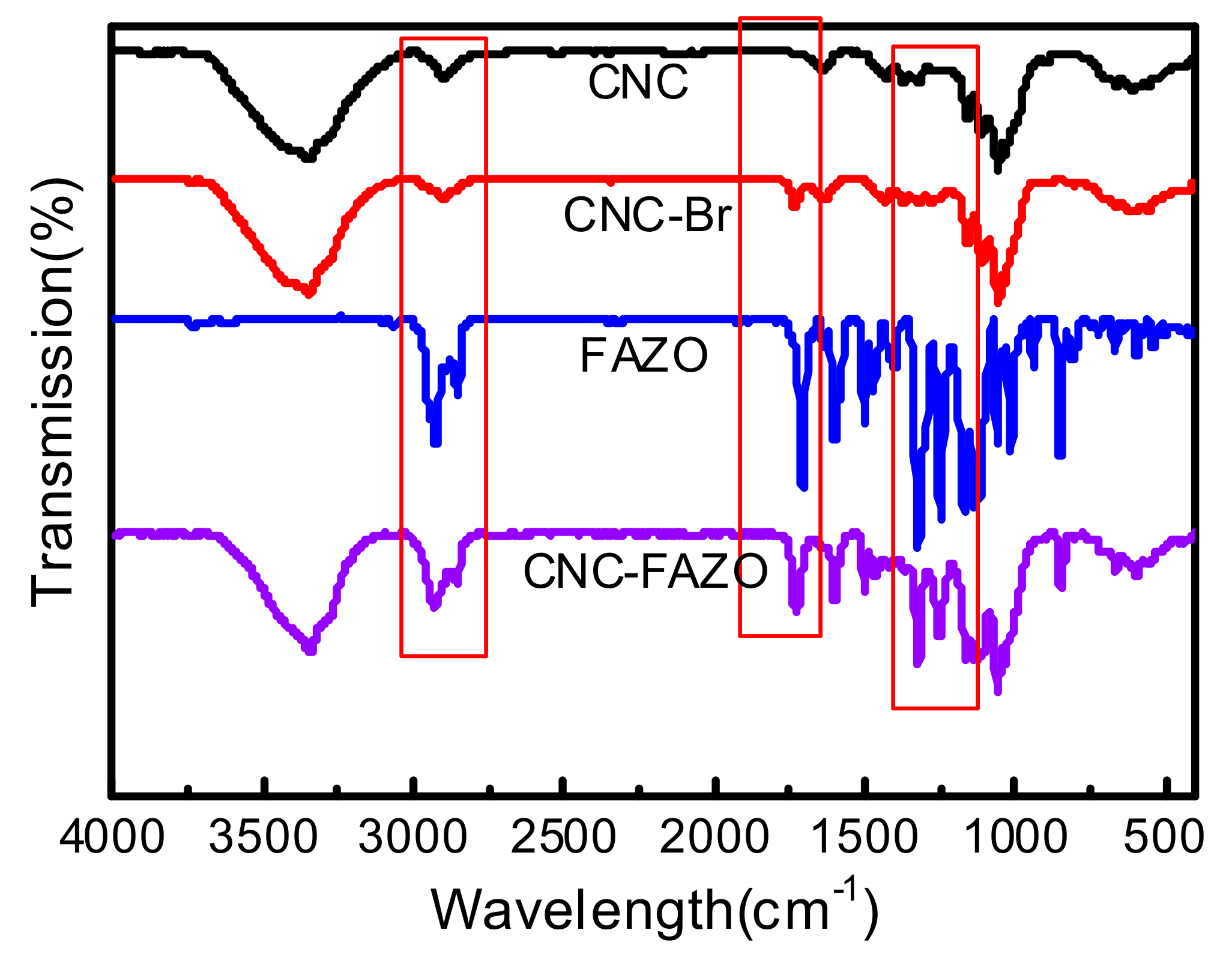
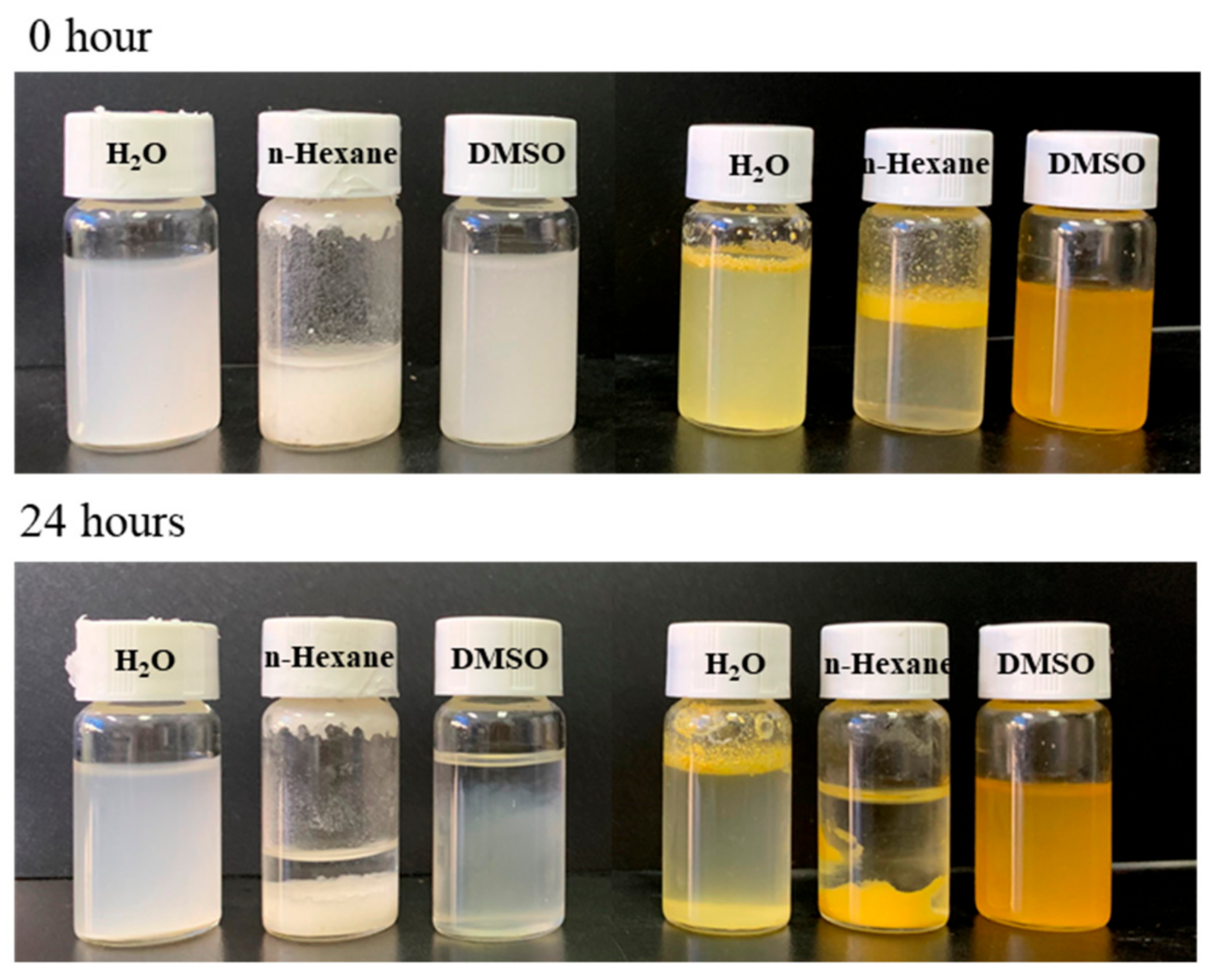
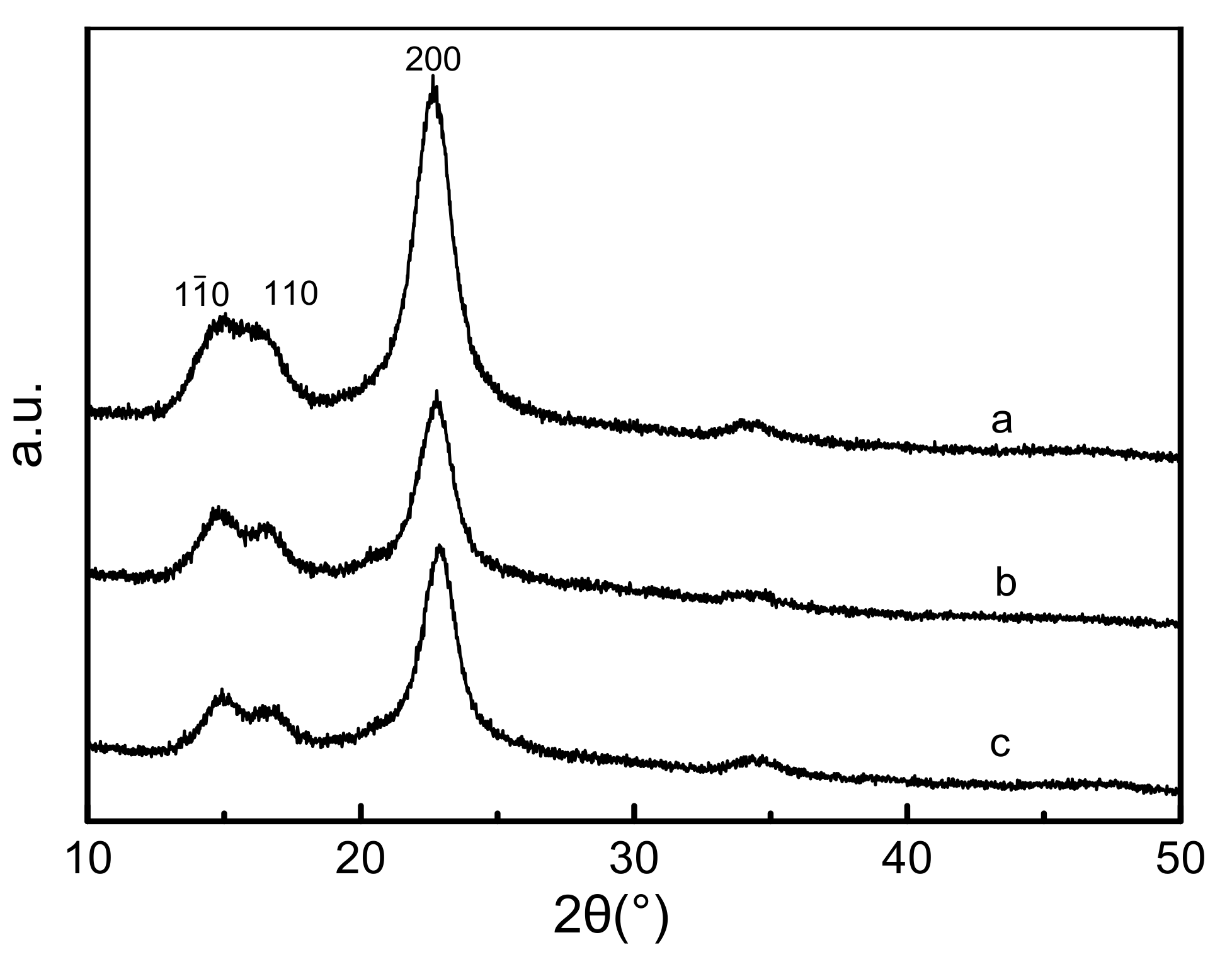
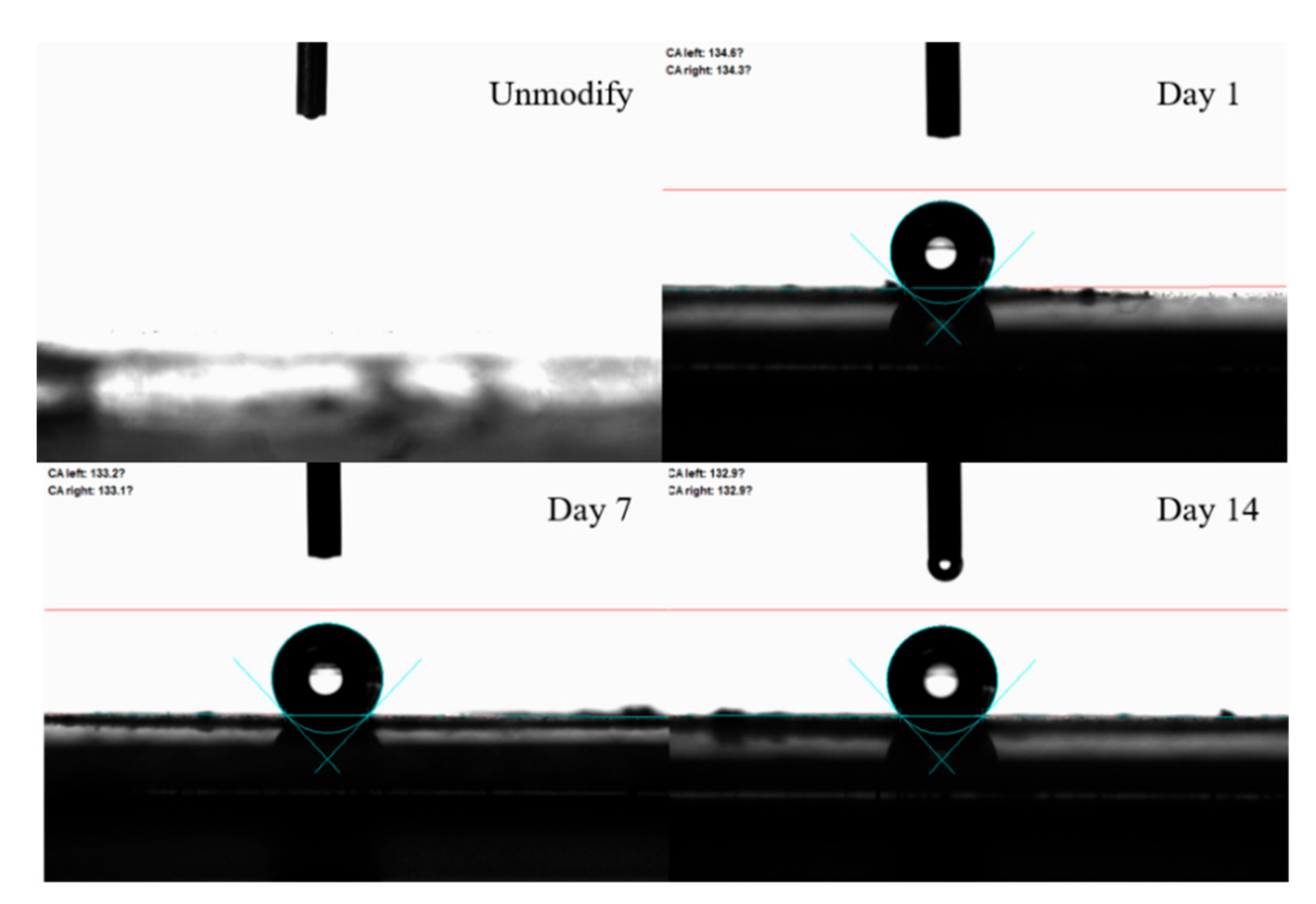
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Šturcová, A.; Davies, G.R.; Eichhorn, S.J. Elastic modulus and stress-transfer properties of tunicate cellulose whiskers. Biomacromolecules 2005, 6, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Cranston, E.D.; Gray, D.G. Morphological and optical characterization of polyelectrolyte multilayers incorporating nanocrystalline cellulose. Biomacromolecules 2006, 7, 2522–2530. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Chowdhury, R.A.; Toomey, M.D.; Betancourt, D.; Montes, F.; Youngblood, J.P. Surface hydrophobization of TEMPO-oxidized cellulose nanofibrils (CNFs) using a facile, aqueous modification process and its effect on properties of epoxy nanocomposites. Cellulose 2019, 26, 9631–9643. [Google Scholar] [CrossRef]
- Chowdhury, R.A.; Clarkson, C.M.; Shrestha, S.; El Awad Azrak, S.M.; Mavlan, M.; Youngblood, J.P. High-Performance Waterborne Polyurethane Coating Based on a Blocked Isocyanate with Cellulose Nanocrystals (CNC) as the Polyol. ACS Appl. Polym. Mater. 2020, 2, 385–393. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Wohlhauser, S.; Delepierre, G.; Labet, M.; Morandi, G.; Thielemans, W.; Weder, C.; Zoppe, J.O. Grafting Polymers from Cellulose Nanocrystals: Synthesis, Properties, and Applications. Macromolecules 2018, 51, 6157–6189. [Google Scholar] [CrossRef] [Green Version]
- Yuan, W.; Wang, C.; Lei, S.; Chen, J.; Lei, S.; Li, Z. Ultraviolet light-, temperature- and pH-responsive fluorescent sensors based on cellulose nanocrystals. Polym. Chem. 2018, 9, 3098–3107. [Google Scholar] [CrossRef]
- Cao, X.; Habibi, Y.; Lucia, L.A. One-pot polymerization, surface grafting, and processing of waterborne polyurethane-cellulose nanocrystal nanocomposites. J. Mater. Chem. 2009, 19, 7137–7145. [Google Scholar] [CrossRef]
- Rosilo, H.; Kontturi, E.; Seitsonen, J.; Kolehmainen, E.; Ikkala, O. Transition to reinforced state by percolating domains of intercalated brush-modified cellulose nanocrystals and poly(butadiene) in cross-linked composites based on thiol-ene click chemistry. Biomacromolecules 2013, 14, 1547–1554. [Google Scholar] [CrossRef]
- Pei, A.; Malho, J.M.; Ruokolainen, J.; Zhou, Q.; Berglund, L.A. Strong nanocomposite reinforcement effects in polyurethane elastomer with low volume fraction of cellulose nanocrystals. Macromolecules 2011, 44, 4422–4427. [Google Scholar] [CrossRef]
- Wang, H.D.; Roeder, R.D.; Whitney, R.A.; Champagne, P.; Cunningham, M.F. Graft modification of crystalline nanocellulose by Cu(0)-mediated SET living radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2800–2808. [Google Scholar] [CrossRef]
- Yin, Y.; Tian, X.; Jiang, X.; Wang, H.; Gao, W. Modification of cellulose nanocrystal via SI-ATRP of styrene and the mechanism of its reinforcement of polymethylmethacrylate. Carbohydr. Polym. 2016, 142, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Carlmark, A.; Larsson, E.; Malmström, E. Grafting of cellulose by ring-opening polymerization—A review. Eur. Polym. J. 2012, 48, 1646–1659. [Google Scholar] [CrossRef] [Green Version]
- Morandi, G.; Heath, L.; Thielemans, W. Cellulose nanocrystals grafted with polystyrene chains through Surface-Initiated Atom Transfer Radical Polymerization (SI-ATRP). Langmuir 2009, 25, 8280–8286. [Google Scholar] [CrossRef] [PubMed]
- Natansohn, A.; Rochon, P. Photoinduced motions in azo-containing polymers. Chem. Rev. 2002, 102, 4139–4176. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Tsutsumi, O. Optical switching and image storage by means of azobenzene liquid-crystal films. Science 1995, 268, 1873–1875. [Google Scholar] [CrossRef]
- Forcén, P.; Oriol, L.; Sánchez, C.; Rodríguez, F.J.; Alcalá, R.; Hvilsted, S.; Jankova, K. Methacrylic azopolymers for holographic storage: A comparison among different polymer types. Eur. Polym. J. 2007, 43, 3292–3300. [Google Scholar] [CrossRef]
- Kumar, G.S.; Neckers, D.C. Photochemistry of Azobenzene-Containing Polymers. Chem. Rev. 1989, 89, 1915–1925. [Google Scholar] [CrossRef]
- Xu, Q.; Yi, J.; Zhang, X.; Zhang, H. A novel amphotropic polymer based on cellulose nanocrystals grafted with azo polymers. Eur. Polym. J. 2008, 44, 2830–2837. [Google Scholar] [CrossRef]
- Geacintov, N.; Stannett, V.; Abrahamson, E.W. The Photo-initiated Grafting of Vinyl Monomers to Cellulose and Other Polymers Using Anthraquinone Vat Dyes. Die Makromol. Chem. Macromol. Chem. Phys. 1960, 36, 52–66. [Google Scholar] [CrossRef]
- Yang, S.; Jacob, M.M.; Li, L.; Cholli, A.L.; Kumar, J. Structural aspects of low-molecular-weight azocellulose polymers: A solid-state 13C NMR study. ACS Symp. Ser. 2002, 834, 58–70. [Google Scholar] [CrossRef]
- Zheng, P.J.; Wang, C.; Hu, X.; Tam, K.C.; Li, L. Supramolecular Complexes of Azocellulose and α-Cyclodextrin: Isothermal Titration Calorimetric and Spectroscopic Studies. Macromolecules 2005, 38, 2859–2864. [Google Scholar] [CrossRef]
- Fink, H.P.; Hofmann, D.; Philipp, B. Some aspects of lateral chain order in cellulosics from X-ray scattering. Cellulose 1995, 2, 51–70. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Peng, S.; Zhou, G.; Xu, X. Highly Hydrophobic, Homogeneous Suspension and Resin by Graft Copolymerization Modification of Cellulose Nanocrystal (CNC). J. Compos. Sci. 2020, 4, 186. https://doi.org/10.3390/jcs4040186
Xu Z, Peng S, Zhou G, Xu X. Highly Hydrophobic, Homogeneous Suspension and Resin by Graft Copolymerization Modification of Cellulose Nanocrystal (CNC). Journal of Composites Science. 2020; 4(4):186. https://doi.org/10.3390/jcs4040186
Chicago/Turabian StyleXu, Zhuofan, Shuting Peng, Guofu Zhou, and Xuezhu Xu. 2020. "Highly Hydrophobic, Homogeneous Suspension and Resin by Graft Copolymerization Modification of Cellulose Nanocrystal (CNC)" Journal of Composites Science 4, no. 4: 186. https://doi.org/10.3390/jcs4040186
APA StyleXu, Z., Peng, S., Zhou, G., & Xu, X. (2020). Highly Hydrophobic, Homogeneous Suspension and Resin by Graft Copolymerization Modification of Cellulose Nanocrystal (CNC). Journal of Composites Science, 4(4), 186. https://doi.org/10.3390/jcs4040186



