Recent Advances in Porous 3D Cellulose Aerogels for Tissue Engineering Applications: A Review
Abstract
:1. Introduction
2. Porous 3D Scaffolds
2.1. Fabrication of Porous 3D Scaffolds
2.2. Parameters Influencing the Freeze-Dried Porous 3D Scaffolds
3. Natural Polymer-Based Scaffolds
3.1. Cellulose Porous 3D Aerogel Scaffolds Preparation by Freeze-Drying
3.2. Cellulose Composite-Based 3D Porous Aerogels
3.3. Cellulose Composite-Based 3D Porous Aerogel Scaffolds with Antibacterial Agents
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Burg, K.J.L.; Porter, S.; Kellam, J.F. Biomaterial developments for bone tissue engineering. Biomaterials 2000, 21, 2347–2359. [Google Scholar] [CrossRef]
- Ko, H.F.; Sfeir, C.; Kumta, P.N. Novel synthesis strategies for natural polymer and composite biomaterials as potential scaffolds for tissue engineering. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 1981–1997. [Google Scholar] [CrossRef]
- Yannas, I.V. Tissue and Organ Regeneration in Adults: Extension of the Paradigm to Several Organs; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Takei, T.; Okonogi, A.; Tateno, K.; Kimura, A.; Kojima, S.; Yazaki, K.; Miura, K.I. The effects of the side chains of hydrophobic aliphatic amino acid residues in an amphipathic polypeptide on the formation of α helix and its association. J. Biochem. 2006, 139, 271–278. [Google Scholar] [CrossRef]
- Supp, D.M.; Boyce, S.T. Engineered skin substitutes: Practices and potentials. Clin. Dermatol. 2005, 23, 403–412. [Google Scholar] [CrossRef]
- Mitrousis, N.; Fokina, A.; Shoichet, M.S. Biomaterials for cell transplantation. Nat. Rev. Mater. 2018, 3, 441–456. [Google Scholar] [CrossRef]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile application of nanocellulose: From industry to skin tissue engineering and wound healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, R.; Sofi, H.S.; Akram, T.; Rather, H.A.; Abdal-hay, A.; Shabir, N.; Vasita, R.; Alrokayan, S.H.; Khan, H.A.; Sheikh, F.A. Fabrication of multifunctional cellulose/TiO2/Ag composite nanofibers scaffold with antibacterial and bioactivity properties for future tissue engineering applications. J. Biomed. Mater. Res. Part A 2020, 108, 947–962. [Google Scholar] [CrossRef]
- Kang, X.; Xie, Y.; Powell, H.M.; Lee, L.J.; Belury, M.A.; Lannutti, J.J.; Kniss, D.A. Adipogenesis of murine embryonic stem cells in a three-dimensional culture system using electrospun polymer scaffolds. Biomaterials 2007, 28, 450–458. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kundu, S.C. Osteogenic and adipogenic differentiation of rat bone marrow cells on non-mulberry and mulberry silk gland fibroin 3D scaffolds. Biomaterials 2009, 30, 5019–5030. [Google Scholar] [CrossRef]
- Wang, J.; Zong, C.; Shi, D.; Wang, W.; Shen, D.; Liu, L.; Tong, X.; Zheng, Q.; Gao, C. Hepatogenic engineering from human bone marrow mesenchymal stem cells in porous polylactic glycolic acid scaffolds under perfusion culture. J. Tissue Eng. Regen. Med. 2012, 6, 29–39. [Google Scholar] [CrossRef]
- Kuboki, Y.; Jin, Q.; Takita, H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J. Bone Jt. Surg. Am. 2001, 83 Pt 2. [Google Scholar] [CrossRef]
- Mygind, T.; Stiehler, M.; Baatrup, A.; Li, H.; Zou, X.; Flyvbjerg, A.; Kassem, M.; Bünger, C. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials 2007, 28, 1036–1047. [Google Scholar] [CrossRef]
- Shor, L.; Güçeri, S.; Chang, R.; Gordon, J.; Kang, Q.; Hartsock, L.; An, Y.; Sun, W. Precision extruding deposition (PED) fabrication of polycaprolactone (PCL) scaffolds for bone tissue engineering. Biofabrication 2009, 1. [Google Scholar] [CrossRef] [Green Version]
- Lien, S.M.; Ko, L.Y.; Huang, T.J. Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater. 2009, 5, 670–679. [Google Scholar] [CrossRef]
- Yannas, I.V.; Lee, E.; Orgill, D.P.; Skrabut, E.M.; Murphy, G.F. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. USA 1989, 86, 933–937. [Google Scholar] [CrossRef] [Green Version]
- Rnjak-Kovacina, J.; Wise, S.G.; Li, Z.; Maitz, P.K.M.; Young, C.J.; Wang, Y.; Weiss, A.S. Tailoring the porosity and pore size of electrospun synthetic human elastin scaffolds for dermal tissue engineering. Biomaterials 2011, 32, 6729–6736. [Google Scholar] [CrossRef]
- Akay, G.; Birch, M.A.; Bokhari, M.A. Microcellular polyHIPE polymer supports osteoblast growth and bone formation in vitro. Biomaterials 2004, 25, 3991–4000. [Google Scholar] [CrossRef]
- Artel, A.; Mehdizadeh, H.; Chiu, Y.C.; Brey, E.M.; Cinar, A. An agent-based model for the investigation of neovascularization within porous scaffolds. Tissue Eng. Part A 2011, 17, 2133–2141. [Google Scholar] [CrossRef]
- Matinfar, M.; Mesgar, A.S.; Mohammadi, Z. Evaluation of physicochemical, mechanical and biological properties of chitosan/carboxymethyl cellulose reinforced with multiphasic calcium phosphate whisker-like fibers for bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 341–353. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Chouhan, D.; Mandal, B.B. 3D functional scaffolds for skin tissue engineering. Funct. 3D Tissue Eng. Scaffolds 2018, 345–365. [Google Scholar] [CrossRef]
- Barrios, E.; Fox, D.; Li Sip, Y.Y.; Catarata, R.; Calderon, J.E.; Azim, N.; Afrin, S.; Zhang, Z.; Zhai, L. Nanomaterials in advanced, high-performance aerogel composites: A review. Polymers 2019, 11, 726. [Google Scholar] [CrossRef] [Green Version]
- Şahin, İ.; Özbakır, Y.; İnönü, Z.; Ulker, Z.; Erkey, C. Kinetics of Supercritical Drying of Gels. Gels 2017, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Baldino, L.; Cardea, S.; Reverchon, E. Natural aerogels production by supercritical gel drying. Chem. Eng. Trans. 2015. [Google Scholar] [CrossRef]
- Nieto-Suárez, M.; López-Quintela, M.A.; Lazzari, M. Preparation and characterization of crosslinked chitosan/gelatin scaffolds by ice segregation induced self-assembly. Carbohydr. Polym. 2016. [Google Scholar] [CrossRef]
- Sola, A.; Bertacchini, J.; D’Avella, D.; Anselmi, L.; Maraldi, T.; Marmiroli, S.; Messori, M. Development of solvent-casting particulate leaching (SCPL) polymer scaffolds as improved three-dimensional supports to mimic the bone marrow niche. Mater. Sci. Eng. C 2019, 96, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the production of polysaccharide aerogel particles. Materials 2018, 11, 2144. [Google Scholar] [CrossRef] [Green Version]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Chuah, C.H. Enhancement of curcumin bioavailability using nanocellulose reinforced chitosan hydrogel. Polymers 2017, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Gauvin, R.; Chen, Y.C.; Lee, J.W.; Soman, P.; Zorlutuna, P.; Nichol, J.W.; Bae, H.; Chen, S.; Khademhosseini, A. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials 2012, 33, 3824–3834. [Google Scholar] [CrossRef] [Green Version]
- Madou, M.J. Fundamentals of Microfabrication: The Science of Miniaturization; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Chen, W.; Xu, Y.; Liu, Y.; Wang, Z.; Li, Y.; Jiang, G.; Mo, X.; Zhou, G. Three-dimensional printed electrospun fiber-based scaffold for cartilage regeneration. Mater. Des. 2019, 179, 107886. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.S.; Edwards, J.R.; Jeon, H. Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef] [Green Version]
- El-Naggar, M.E.; Othman, S.I.; Allam, A.A.; Morsy, O.M. Synthesis, drying process and medical application of polysaccharide-based aerogels. Int. J. Biol. Macromol. 2020, 145, 1115–1128. [Google Scholar] [CrossRef]
- Jovic, T.H.; Kungwengwe, G.; Mills, A.C.; Whitaker, I.S. Plant-Derived Biomaterials: A Review of 3D Bioprinting and Biomedical Applications. Front. Mech. Eng. 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef]
- Lavoine, N.; Bergström, L. Nanocellulose-based foams and aerogels: Processing, properties, and applications. J. Mater. Chem. A 2017, 5, 16105–16117. [Google Scholar] [CrossRef] [Green Version]
- Fereshteh, Z. Freeze-drying technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds: Materials, Technologies, and Applications; Woodhead Publishing: Cambridge, UK, 2018; pp. 151–174. ISBN 9780081009802. [Google Scholar]
- Campodoni, E.; Heggset, E.B.; Rashad, A.; Ramírez-Rodríguez, G.B.; Mustafa, K.; Syverud, K.; Tampieri, A.; Sandri, M. Polymeric 3D scaffolds for tissue regeneration: Evaluation of biopolymer nanocomposite reinforced with cellulose nanofibrils. Mater. Sci. Eng. C 2019, 94, 867–878. [Google Scholar] [CrossRef]
- Morais, A.R.D.V.; Alencar, É.D.N.; Xavier Júnior, F.H.; Oliveira, C.M.D.; Marcelino, H.R.; Barratt, G.; Fessi, H.; Egito, E.S.T.D.; Elaissari, A. Freeze-drying of emulsified systems: A review. Int. J. Pharm. 2016, 503, 102–114. [Google Scholar] [CrossRef]
- Mirab, F.; Eslamian, M.; Bagheri, R. Fabrication and characterization of a starch-based nanocomposite scaffold with highly porous and gradient structure for bone tissue engineering. Biomed. Phys. Eng. Express 2018, 4. [Google Scholar] [CrossRef] [Green Version]
- Nasri-Nasrabadi, B.; Mehrasa, M.; Rafienia, M.; Bonakdar, S.; Behzad, T.; Gavanji, S. Porous starch/cellulose nanofibers composite prepared by salt leaching technique for tissue engineering. Carbohydr. Polym. 2014, 108, 232–238. [Google Scholar] [CrossRef]
- Varshney, D.; Singh, M. History of Lyophilization. In Lyophilized Biologics and Vaccines; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Long, L.Y.; Weng, Y.X.; Wang, Y.Z. Cellulose aerogels: Synthesis, applications, and prospects. Polymers 2018, 8, 623. [Google Scholar] [CrossRef] [Green Version]
- Vasanthan, K.S.; Subramaniam, A.; Krishnan, U.M.; Sethuraman, S. Influence of 3D porous galactose containing PVA/gelatin hydrogel scaffolds on three-dimensional spheroidal morphology of hepatocytes. J. Mater. Sci. Mater. Med. 2015, 26. [Google Scholar] [CrossRef]
- Lim, J.Y.; Lim, D.G.; Kim, K.H.; Park, S.K.; Jeong, S.H. Effects of annealing on the physical properties of therapeutic proteins during freeze drying process. Int. J. Biol. Macromol. 2018, 107, 730–740. [Google Scholar] [CrossRef]
- Yan, J.; Wu, T.; Ding, Z.; Li, X. Preparation and characterization of carbon nanotubes/chitosan composite foam with enhanced elastic property. Carbohydr. Polym. 2016, 136, 1288–1296. [Google Scholar] [CrossRef]
- Jiménez-Saelices, C.; Seantier, B.; Cathala, B.; Grohens, Y. Effect of freeze-drying parameters on the microstructure and thermal insulating properties of nanofibrillated cellulose aerogels. J. Sol-Gel Sci. Technol. 2017, 84, 475–485. [Google Scholar] [CrossRef]
- Asuncion, M.C.T.; Goh, J.C.H.; Toh, S.L. Anisotropic silk fibroin/gelatin scaffolds from unidirectional freezing. Mater. Sci. Eng. C 2016, 67, 646–656. [Google Scholar] [CrossRef]
- Liu, H.; Nakagawa, K.; Chaudhary, D.; Asakuma, Y.; Tadé, M.O. Freeze-dried macroporous foam prepared from chitosan/xanthan gum/montmorillonite nanocomposites. Chem. Eng. Res. Des. 2011, 89, 2356–2364. [Google Scholar] [CrossRef]
- Lederman, M. Stage II Carcinoma of the Cervix. Proc. R. Soc. Med. 1976, 69, 859. [Google Scholar] [CrossRef]
- Ribeiro, D.M.L.; Júnior, A.R.C.; de Macedo, G.H.R.V.; Chagas, V.L.; Silva, L.D.S.; Cutrim, B.D.S.; Santos, D.M.; Soares, B.L.L.; Zagmignan, A.; de Miranda, R.D.C.M.; et al. Polysaccharide-based formulations for healing of skin-related wound infections: Lessons from animal models and clinical trials. Biomolecules 2020, 10, 63. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer Aerogels and Foams: Chemistry, Properties, and Applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef]
- Muthuraj, R.; Grohens, Y.; Seantier, B. Mechanical and thermal insulation properties of elium acrylic resin/cellulose nanofiber based composite aerogels. Nano-Struct. Nano-Objects 2017, 12, 68–76. [Google Scholar] [CrossRef]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef]
- Shen, X.; Shamshina, J.L.; Berton, P.; Gurau, G.; Rogers, R.D. Hydrogels based on cellulose and chitin: Fabrication, properties, and applications. Green Chem. 2015, 18, 53–75. [Google Scholar] [CrossRef] [Green Version]
- Ki, C.S.; Baek, D.H.; Gang, K.D.; Lee, K.H.; Um, I.C.; Park, Y.H. Characterization of gelatin nanofiber prepared from gelatin-formic acid solution. Polymer 2005, 46, 5094–5102. [Google Scholar] [CrossRef]
- Panzavolta, S.; Gioffrè, M.; Focarete, M.L.; Gualandi, C.; Foroni, L.; Bigi, A. Electrospun gelatin nanofibers: Optimization of genipin cross-linking to preserve fiber morphology after exposure to water. Acta Biomater. 2011, 7, 1702–1709. [Google Scholar] [CrossRef]
- Powell, H.M.; Supp, D.M.; Boyce, S.T. Influence of electrospun collagen on wound contraction of engineered skin substitutes. Biomaterials 2008, 29, 834–843. [Google Scholar] [CrossRef]
- Li, X.; Xiao, Z.; Han, J.; Chen, L.; Xiao, H.; Ma, F.; Hou, X.; Li, X.; Sun, J.; Ding, W.; et al. Promotion of neuronal differentiation of neural progenitor cells by using EGFR antibody functionalized collagen scaffolds for spinal cord injury repair. Biomaterials 2013, 34, 5107–5116. [Google Scholar] [CrossRef]
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. Part B Appl. Biomater 2004, 71, 343–354. [Google Scholar] [CrossRef]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioeng. Transl. Med. 2019, 4, 96–115. [Google Scholar] [CrossRef]
- Mirtaghavi, A.; Baldwin, A.; Tanideh, N.; Zarei, M.; Muthuraj, R.; Cao, Y.; Zhao, G.; Geng, J.; Jin, H.; Luo, J. Crosslinked porous three-dimensional cellulose nanofibers-gelatine biocomposite scaffolds for tissue regeneration. Int. J. Biol. Macromol. 2020, 164, 1949–1959. [Google Scholar] [CrossRef]
- Alissandratos, A.; Halling, P.J. Enzymatic acylation of starch. Bioresour. Technol. 2012, 115, 41–47. [Google Scholar] [CrossRef]
- Quraishi, S.; Martins, M.; Barros, A.A.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Novel non-cytotoxic alginate–lignin hybrid aerogels as scaffolds for tissue engineering. J. Supercrit. Fluids 2015, 105, 1–8. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Mathew, A.P.; Oksman, K. Mechanical properties of cellulose nanofiber (CNF) reinforced polylactic acid (PLA) prepared by twin screw extrusion. Compos. Sci. Technol. 2010, 70, 1742–1747. [Google Scholar] [CrossRef]
- Syverud, K. Tissue Engineering Using Plant-Derived Cellulose Nanofibrils (CNF) as Scaffold Material. In Nanocelluloses: Their Preparation, Properties, and Applications; American Chemical Society: Washington, DC, USA, 2017; pp. 171–189. [Google Scholar]
- Jiménez-Saelices, C.; Seantier, B.; Cathala, B.; Grohens, Y. Spray freeze-dried nanofibrillated cellulose aerogels with thermal superinsulating properties. Carbohydr. Polym. 2017, 157, 105–113. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Otoni, C.G.; De France, K.J.; Barud, H.S.; Lona, L.M.F.; Cranston, E.D.; Rojas, O.J. Porous nanocellulose gels and foams: Breakthrough status in the development of scaffolds for tissue engineering. Mater. Today 2020, 37, 126–141. [Google Scholar] [CrossRef]
- Gaihre, B.; Jayasuriya, A.C. Fabrication and characterization of carboxymethyl cellulose novel microparticles for bone tissue engineering. Mater. Sci. Eng. C 2016, 69, 733–743. [Google Scholar] [CrossRef] [Green Version]
- Ninan, N.; Muthiah, M.; Park, I.K.; Elain, A.; Thomas, S.; Grohens, Y. Pectin/carboxymethyl cellulose/microfibrillated cellulose composite scaffolds for tissue engineering. Carbohydr. Polym. 2013, 98, 877–885. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crop. Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Nishino, T.; Matsuda, I.; Hirao, K. All-cellulose composite. Macromolecules 2004, 37, 7683–7687. [Google Scholar] [CrossRef]
- Moreira, S.; Silva, N.B.; Almeida-Lima, J.; Rocha, H.A.O.; Medeiros, S.R.B.; Alves, C.; Gama, F.M. BC nanofibres: In vitro study of genotoxicity and cell proliferation. Toxicol. Lett. 2009, 189, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.; Hirani, A.A.; Colacino, K.R.; Lee, Y.W.; Roman, M. Cytotoxicity and Cellular Uptake of Cellulose Nanocrystals. Nano Life 2012, 2, 1241006. [Google Scholar] [CrossRef]
- Dugan, J.M.; Gough, J.E.; Eichhorn, S.J. Bacterial cellulose scaffolds and cellulose nanowhiskers for tissue engineering. Nanomedicine 2013, 8, 287–298. [Google Scholar] [CrossRef]
- Vandamme, E.J.; De Baets, S.; Vanbaelen, A.; Joris, K.; De Wulf, P. Improved production of bacterial cellulose and its application potential. Polym. Degrad. Stab. 1998, 59, 93–99. [Google Scholar] [CrossRef]
- Kamel, S. Nanotechnology and its applications in lignocellulosic composites, a mini review. Express Polym. Lett. 2007, 1, 546–575. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Frone, A.N.; Panaitescu, D.M.; Nicolae, C.A.; Gabor, A.R.; Trusca, R.; Casarica, A.; Stanescu, P.O.; Baciu, D.D.; Salageanu, A. Bacterial cellulose sponges obtained with green cross-linkers for tissue engineering. Mater. Sci. Eng. C 2020, 110. [Google Scholar] [CrossRef]
- Gutiérrez-Hernández, J.M.; Escobar-García, D.M.; Escalante, A.; Flores, H.; González, F.J.; Gatenholm, P.; Toriz, G. In vitro evaluation of osteoblastic cells on bacterial cellulose modified with multi-walled carbon nanotubes as scaffold for bone regeneration. Mater. Sci. Eng. C 2017, 75, 445–453. [Google Scholar] [CrossRef]
- Pértile, R.; Moreira, S.; Andrade, F.; Domingues, L.; Gama, M. Bacterial cellulose modified using recombinant proteins to improve neuronal and mesenchymal cell adhesion. Biotechnol. Prog. 2012, 28, 526–532. [Google Scholar] [CrossRef] [Green Version]
- Torgbo, S.; Sukyai, P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl. Mater. Today 2018, 11, 34–49. [Google Scholar] [CrossRef]
- Laskowski, J.; Milow, B.; Ratke, L. The effect of embedding highly insulating granular aerogel in cellulosic aerogel. J. Supercrit. Fluids 2015, 106. [Google Scholar] [CrossRef]
- Sirvio, J.; Hyvakko, U.; Liimatainen, H.; Niinimaki, J.; Hormi, O. Periodate oxidation of cellulose at elevated temperatures using metal salts as cellulose activators. Carbohydr. Polym. 2011, 83, 1293–1297. [Google Scholar] [CrossRef]
- Lu, T.; Li, Q.; Chen, W.; Yu, H. Composite aerogels based on dialdehyde nanocellulose and collagen for potential applications as wound dressing and tissue engineering scaffold. Compos. Sci. Technol. 2014, 94, 132–138. [Google Scholar] [CrossRef]
- Saito, T.; Kuramae, R.; Wohlert, J.; Berglund, L.A.; Isogai, A. An ultrastrong nanofibrillar biomaterial: The strength of single cellulose nanofibrils revealed via sonication-induced fragmentation. Biomacromolecules 2013, 14, 248–253. [Google Scholar] [CrossRef]
- Baker, B.M.; Trappmann, B.; Wang, W.Y.; Sakar, M.S.; Kim, I.L.; Shenoy, V.B.; Burdick, J.A.; Chen, C.S. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat. Mater. 2015, 14, 1262–1268. [Google Scholar] [CrossRef] [Green Version]
- Muthuraj, R.; Sachan, A.; Castro, M.; Feller, J.F.; Seantier, B.; Grohens, Y. Vapor and pressure sensors based on cellulose nanofibers and carbon nanotubes aerogel with thermoelectric properties. J. Renew. Mater. 2018, 6, 277–287. [Google Scholar] [CrossRef]
- Rashad, A.; Mustafa, K.; Heggset, E.B.; Syverud, K. Cytocompatibility of Wood-Derived Cellulose Nanofibril Hydrogels with Different Surface Chemistry. Biomacromolecules 2017, 18, 1238–1248. [Google Scholar] [CrossRef]
- Naderi, A.; Lindström, T.; Sundström, J. Carboxymethylated nanofibrillated cellulose: Rheological studies. Cellulose 2014, 21, 1561–1571. [Google Scholar] [CrossRef]
- Hickey, R.J.; Pelling, A.E. Cellulose biomaterials for tissue engineering. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Krontiras, P.; Gatenholm, P.; Hagg, D.A. Adipogenic differentiation of stem cells in three-dimensional porous bacterial nanocellulose scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 195–203. [Google Scholar] [CrossRef]
- Li, V.C.F.; Dunn, C.K.; Zhang, Z.; Deng, Y.; Qi, H.J. Direct Ink Write (DIW) 3D Printed Cellulose Nanocrystal Aerogel Structures. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Håkansson, K.M.O.; Henriksson, I.C.; de la Peña Vázquez, C.; Kuzmenko, V.; Markstedt, K.; Enoksson, P.; Gatenholm, P. Solidification of 3D Printed Nanofibril Hydrogels into Functional 3D Cellulose Structures. Adv. Mater. Technol. 2016, 1. [Google Scholar] [CrossRef]
- Cai, H.; Sharma, S.; Liu, W.; Mu, W.; Liu, W.; Zhang, X.; Deng, Y. Aerogel microspheres from natural cellulose nanofibrils and their application as cell culture scaffold. Biomacromolecules 2014, 15, 2540–2547. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Malinen, M.M.; Lauren, P.; Lou, Y.R.; Kuisma, S.W.; Kanninen, L.; Lille, M.; Corlu, A.; Guguen-Guillouzo, C.; Ikkala, O.; et al. Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture. J. Control. Release 2012, 164, 291–298. [Google Scholar] [CrossRef]
- Vartiainen, J.; Pöhler, T.; Sirola, K.; Pylkkänen, L.; Alenius, H.; Hokkinen, J.; Tapper, U.; Lahtinen, P.; Kapanen, A.; Putkisto, K.; et al. Health and environmental safety aspects of friction grinding and spray drying of microfibrillated cellulose. Cellulose 2011, 18, 775–786. [Google Scholar] [CrossRef]
- Alexandrescu, L.; Syverud, K.; Gatti, A.; Chinga-Carrasco, G. Cytotoxicity tests of cellulose nanofibril-based structures. Cellulose 2013, 20, 1765–1775. [Google Scholar] [CrossRef]
- Liu, Y.; Sui, Y.; Liu, C.; Liu, C.; Wu, M.; Li, B.; Li, Y. A physically crosslinked polydopamine/nanocellulose hydrogel as potential versatile vehicles for drug delivery and wound healing. Carbohydr. Polym. 2018, 188, 27–36. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, F.; Grénman, H.; Spoljaric, S.; Seppälä, J.; Eriksson, J.E.; Willför, S.; Xu, C. Development of nanocellulose scaffolds with tunable structures to support 3D cell culture. Carbohydr. Polym. 2016, 148, 259–271. [Google Scholar] [CrossRef]
- Kilpeläinen, P.; Kitunen, V.; Pranovich, A.; Ilvesniemi, H.; Willför, S. Pressurized hot water flow-through extraction of birch sawdust with acetate pH buffer. BioResources 2013, 8, 5202–5218. [Google Scholar] [CrossRef] [Green Version]
- Shvedova, A.A.; Kisin, E.R.; Yanamala, N.; Farcas, M.T.; Menas, A.L.; Williams, A.; Fournier, P.M.; Reynolds, J.S.; Gutkin, D.W.; Star, A.; et al. Gender differences in murine pulmonary responses elicited by cellulose nanocrystals. Part. Fibre Toxicol. 2016, 13. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, M.M.; Liu, J.C.; Trujillo-de Santiago, G.; Cha, B.H.; Vishwakarma, A.; Ghaemmaghami, A.M.; Khademhosseini, A. Delivery strategies to control inflammatory response: Modulating M1–M2 polarization in tissue engineering applications. J. Control. Release 2016, 240, 349–363. [Google Scholar] [CrossRef] [Green Version]
- Naseri, N.; Poirier, J.M.; Girandon, L.; Fröhlich, M.; Oksman, K.; Mathew, A.P. 3-Dimensional porous nanocomposite scaffolds based on cellulose nanofibers for cartilage tissue engineering: Tailoring of porosity and mechanical performance. RSC Adv. 2016, 6, 5999–6007. [Google Scholar] [CrossRef] [Green Version]
- Pei, Y.; Ye, D.; Zhao, Q.; Wang, X.; Zhang, C.; Huang, W.; Zhang, N.; Liu, S.; Zhang, L. Effectively promoting wound healing with cellulose/gelatin sponges constructed directly from a cellulose solution. J. Mater. Chem. B 2015, 3, 7518–7528. [Google Scholar] [CrossRef]
- Carlström, I.E.; Rashad, A.; Campodoni, E.; Sandri, M.; Syverud, K.; Bolstad, A.I.; Mustafa, K. Cross-linked gelatin-nanocellulose scaffolds for bone tissue engineering. Mater. Lett. 2020, 264, 127326. [Google Scholar] [CrossRef]
- Ghafari, R.; Jonoobi, M.; Amirabad, L.M.; Oksman, K.; Taheri, A.R. Fabrication and characterization of novel bilayer scaffold from nanocellulose based aerogel for skin tissue engineering applications. Int. J. Biol. Macromol. 2019, 136, 796–803. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Y.; Wen, X.; Lin, Q.; Chen, X.; Wu, Z. Silver nanoparticle/bacterial cellulose gel membranes for antibacterial wound dressing: Investigation in vitro and in vivo. Biomed. Mater. 2014, 9. [Google Scholar] [CrossRef]
- Hosseini, H.; Zirakjou, A.; Goodarzi, V.; Mousavi, S.M.; Khonakdar, H.A.; Zamanlui, S. Lightweight aerogels based on bacterial cellulose/silver nanoparticles/polyaniline with tuning morphology of polyaniline and application in soft tissue engineering. Int. J. Biol. Macromol. 2020, 152, 57–67. [Google Scholar] [CrossRef]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of Curcumin: From Mechanism to Biological Implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [Green Version]
- Sahu, A.N. Nanotechnology in herbal medicines and cosmetics. Int. J. Res. Ayurveda Pharm. 2013, 4, 472–474. [Google Scholar] [CrossRef]
- Shefa, A.A.; Sultana, T.; Park, M.K.; Lee, S.Y.; Gwon, J.G.; Lee, B.T. Curcumin incorporation into an oxidized cellulose nanofiber-polyvinyl alcohol hydrogel system promotes wound healing. Mater. Des. 2020, 186, 108313. [Google Scholar] [CrossRef]
- Yin, N.; Du, R.; Zhao, F.; Han, Y.; Zhou, Z. Characterization of antibacterial bacterial cellulose composite membranes modified with chitosan or chitooligosaccharide. Carbohydr. Polym. 2020, 229. [Google Scholar] [CrossRef]
- Ao, H.; Jiang, W.; Nie, Y.; Zhou, C.; Zong, J.; Liu, M.; Liu, X.; Wan, Y. Engineering quaternized chitosan in the 3D bacterial cellulose structure for antibacterial wound dressings. Polym. Test. 2020, 86, 106490. [Google Scholar] [CrossRef]
- Fan, X.; Li, Y.; Li, X.; Wu, Y.; Tang, K.; Liu, J.; Zheng, X.; Wan, G. Injectable antibacterial cellulose nanofiber/chitosan aerogel with rapid shape recovery for noncompressible hemorrhage. Int. J. Biol. Macromol. 2020, 154, 1185–1193. [Google Scholar] [CrossRef]

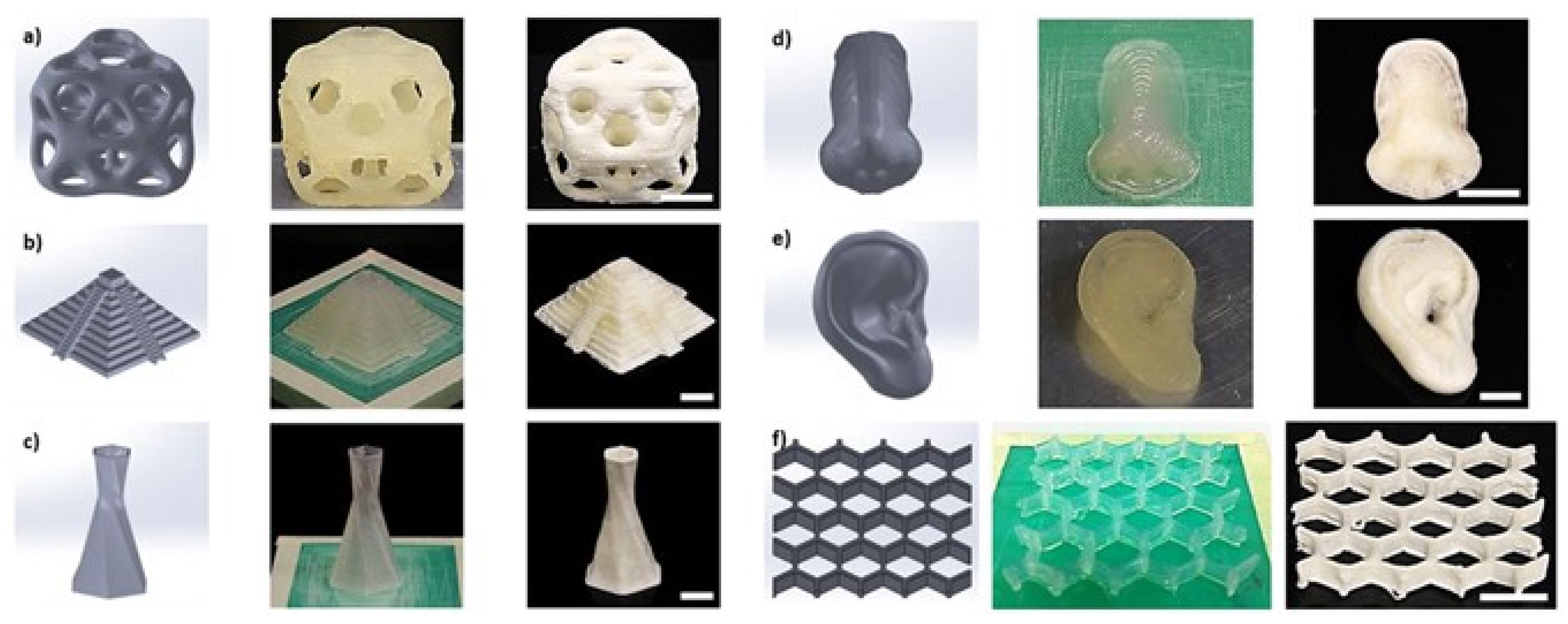
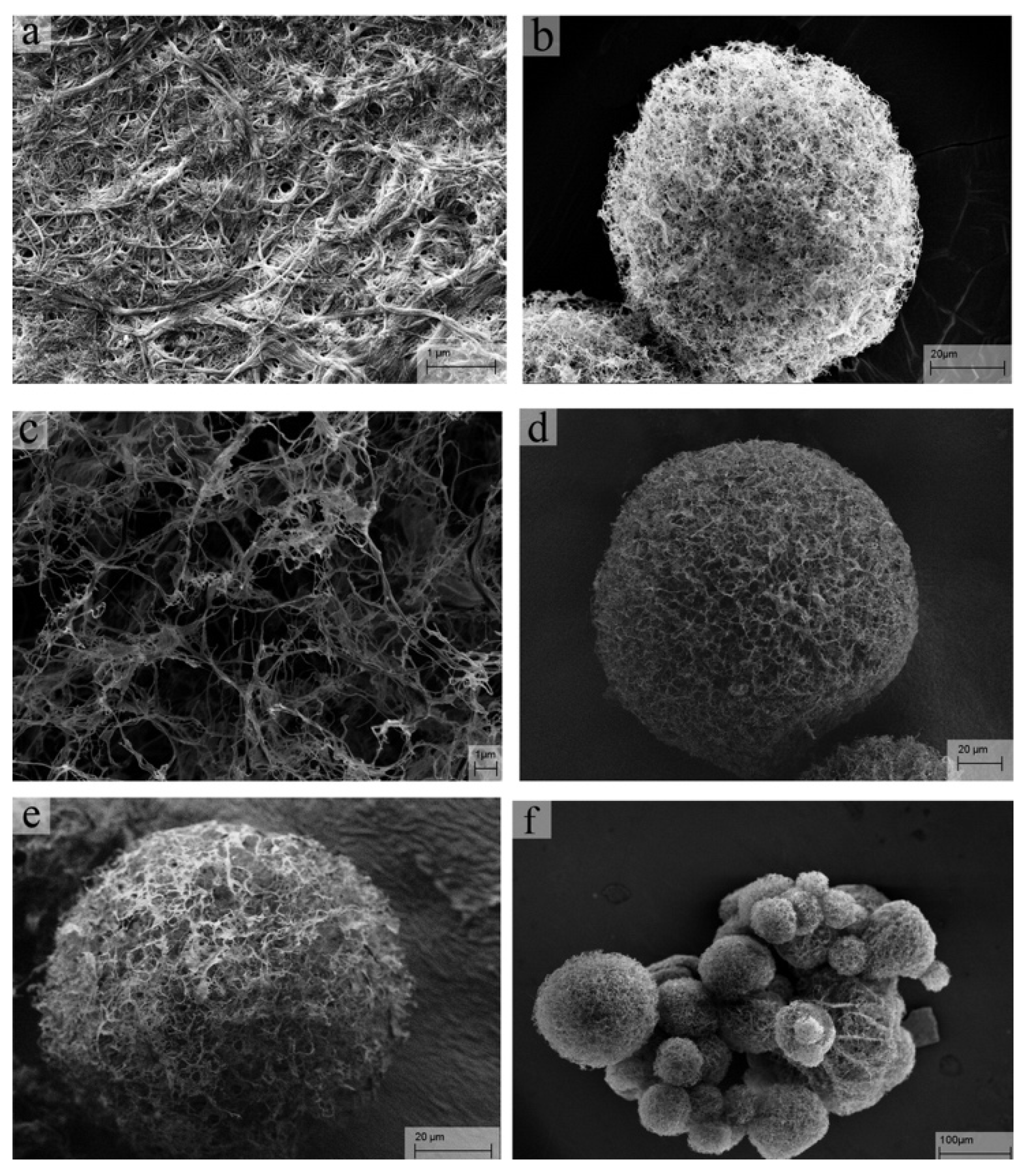
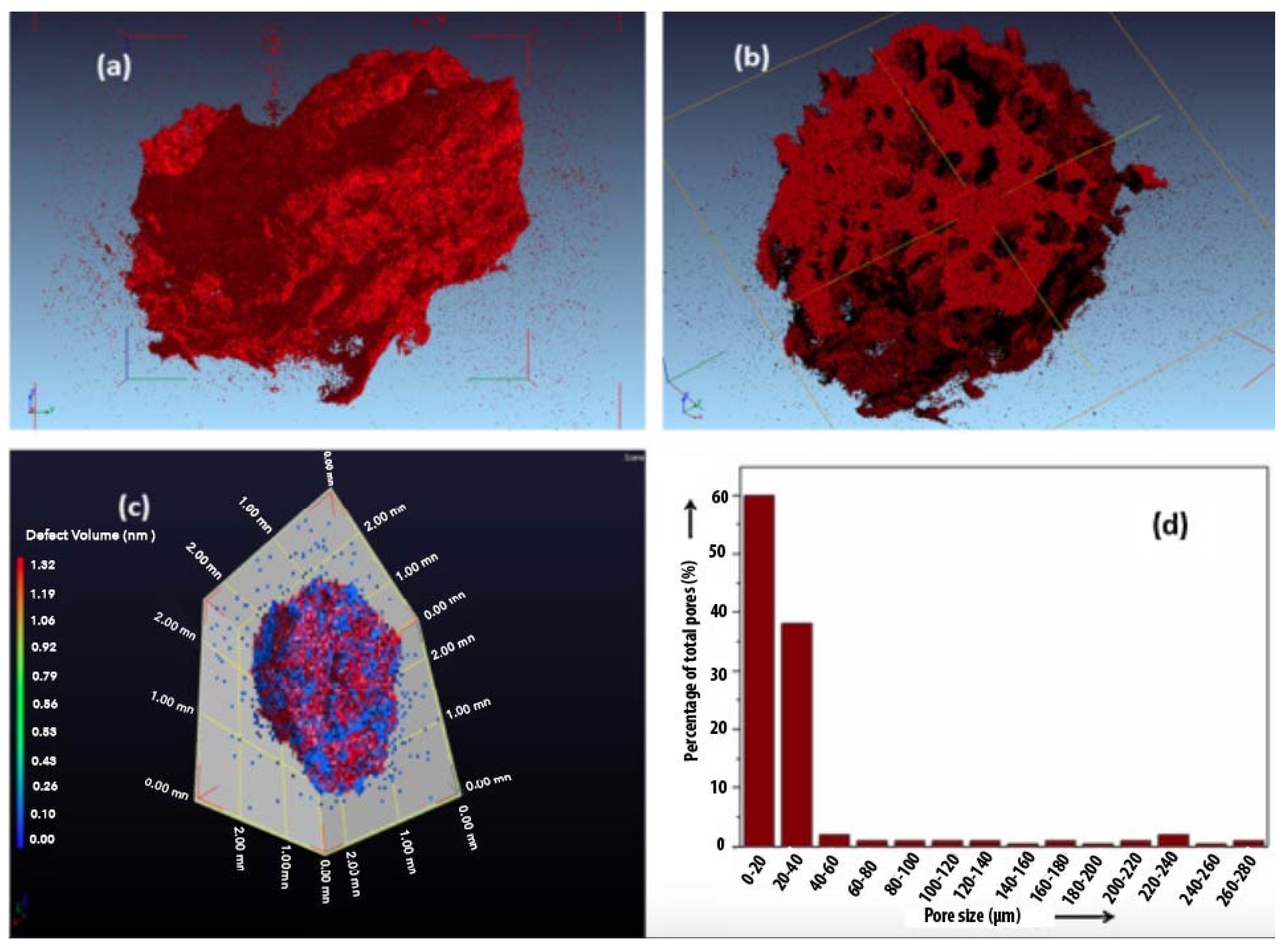


| Scaffold Application | Type of Cell Incorporated In Vivo | Scaffold Type | Fabrication Technique | Pore Size (µm) | Porosity (%) | Reference |
|---|---|---|---|---|---|---|
| Soft tissue regeneration | chicken embryo fibroblast | Cellulose-TiO2-Ag | Freeze-drying | - | 96 | [8] |
| Adipogenesis | Murine embryonic stem cells (rat BMCs) | - PCL - Silk gland fibroin from nonmulberry | Electrospinning Freeze-drying | 6–70 90–110 | 88 97 | [9,10] |
| Chondrogenesis (cartilage regeneration) | - Human ASCs - Porcine chondrocytes - Rabbit MSCs - Porcine BMSCs | - PCL - CNF-gelatin-chitosan - Cellulose/PLA - PEG-chitosan - PCL, PLGA | Centrifugation method Freeze-drying Freeze-gelation Freeze drying and electrospinning | 70–120 190 200 200–500 750 | 95 80 95 30 59 | [11,12,13,14,15] |
| Hepatogenesis (bone marrow regeneration) | Human ASCs Rat bone marrow stem cells | - PLGA - c-PLGA | Low-temperature deposition | 120–200 150–350 | - 85 | [11] |
| Osteogenesis | - In vivo rat implantation - hMSC - In vivo mice implantation -fetal bovine osteoblasts | - HA-BMPs - Coralline HA - β-tricalcium phosphate, natural coral - PCL | Hydrothermal treatment Extrusion deposition | 300–400 200 2–100 350 | - 75 75 65 | [12,13,14] |
| Skin Regeneration | - Primary rat osteoblasts - Guinea pig osteoblasts and epithelial cells | - Type A gelatin - Collagen - CG - Starch | Freeze-gelation Freeze-drying | 20–125 250–500 325 | 85 - - | [15,16] |
| Cell infiltration | - Dermal fibroblasts - Primary rat osteoblasts | - Synthetic human elastin - PHP | Electrospinning Phase separation | 11 100 | 34.4 - | [17,18] |
| Angiogenesis | Multilayer agent-based model simulation | PEG | Freeze-drying | 160–270 | - | [19] |
| Bone tissue regeneration | Human osteosarcoma (MG63) | chitosan/carboxymethyl cellulose | 35–200 | 61–75 | [20] |
| Fabrication Technique | Pros | Cons | References |
|---|---|---|---|
| Solvent casting and particulate leaching | Tunable pore size Control facile process | Limited pore interconnection Not many solvent choices Use of harsh chemicals | [26] |
| Phase separation | Desirable structure control | Limited solvent choice Not user friendly | [27] |
| Gas foaming | High level of pore interconnectivity | Unsustainable processing Low kinetic stability | [28] |
| Hydrogel microfabrication | Fine structure design Tuneable mechanical properties | Limited interconnectivity to obtain 3D structure Limited material choices | [29,30] |
| Electrospinning (ES) | Ultra-Fine fiber size tenability Wide range of material choices | Prone to the ES environment Limited fiber density control Challenging repeated performance Limited interconnectivity of fibers | [31,32] |
| Vacuum drying and microwave drying methods | Interconnected meso- and microporous structure | Large pores and leading to collapse | [33] |
| 3D printing | Facile processing Ultrafine structure control Sustainable Desirable mechanical properties | Limited material options Inadequate printing resolution | [34,35] |
| Supercritical CO2 drying | High surface area High pore volume Prevent shrinkage or collapse of mesopores | Use of organic solvents Safety hazards due to its high-pressure operation Relatively toxic drying process. Energy and time consumption which increases the high overall cost process | [23,36] |
| Freeze-drying | Sustainable Non-toxic solvent used Low temperature process Inexpensive | Limited mechanical properties Large pore size range | [37,38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirtaghavi, A.; Luo, J.; Muthuraj, R. Recent Advances in Porous 3D Cellulose Aerogels for Tissue Engineering Applications: A Review. J. Compos. Sci. 2020, 4, 152. https://doi.org/10.3390/jcs4040152
Mirtaghavi A, Luo J, Muthuraj R. Recent Advances in Porous 3D Cellulose Aerogels for Tissue Engineering Applications: A Review. Journal of Composites Science. 2020; 4(4):152. https://doi.org/10.3390/jcs4040152
Chicago/Turabian StyleMirtaghavi, Ali, Jikui Luo, and Rajendran Muthuraj. 2020. "Recent Advances in Porous 3D Cellulose Aerogels for Tissue Engineering Applications: A Review" Journal of Composites Science 4, no. 4: 152. https://doi.org/10.3390/jcs4040152
APA StyleMirtaghavi, A., Luo, J., & Muthuraj, R. (2020). Recent Advances in Porous 3D Cellulose Aerogels for Tissue Engineering Applications: A Review. Journal of Composites Science, 4(4), 152. https://doi.org/10.3390/jcs4040152






