Milk as a Complex Multiphase Polydisperse System: Approaches for the Quantitative and Qualitative Analysis
Abstract
1. Introduction
2. Milk as a Complex Multyphase Polydisperse System: Urgent Tasks
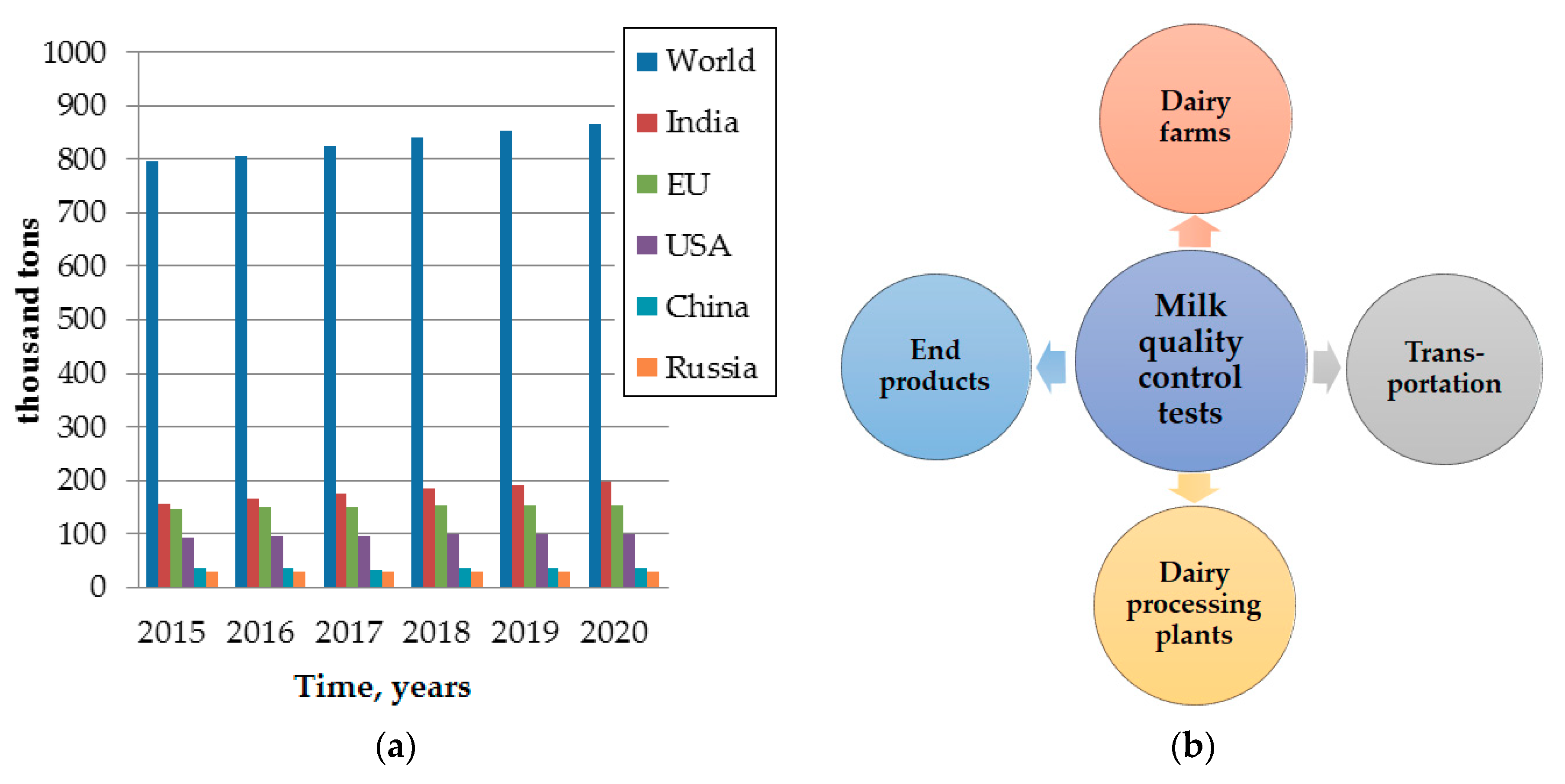
2.1. Milk Industry and Actual Problems
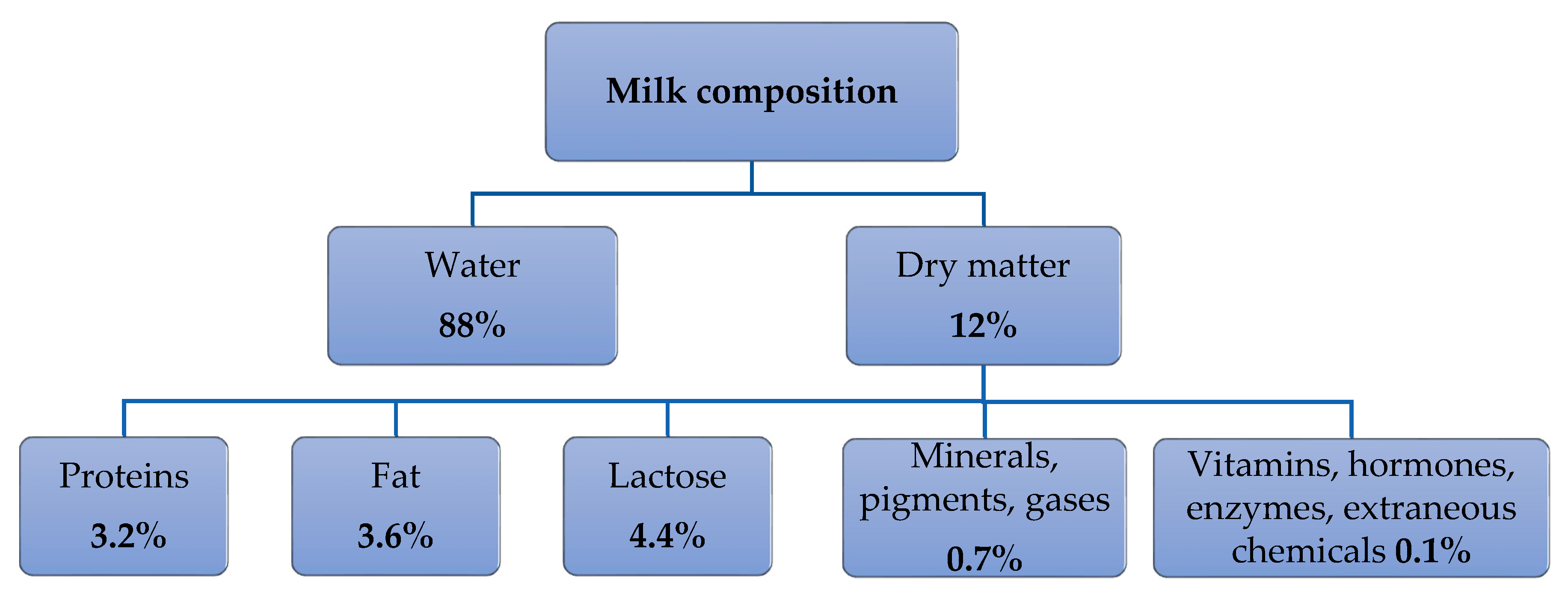
2.2. Milk Composition and Main Parameters
2.2.1. Milk Fat
2.2.2. Milk Proteins
2.2.3. Lactose
2.2.4. Minerals, Vitamins, Urea, Enzymes, Hormones, and Other Ingredients
2.2.5. Falsification
2.3. Commercial Milk Analyzers
3. Optical Spectroscopic Approaches and Techniques for Milk Analysis
3.1. Optical Properties of Milk in a Wide Spectral Range
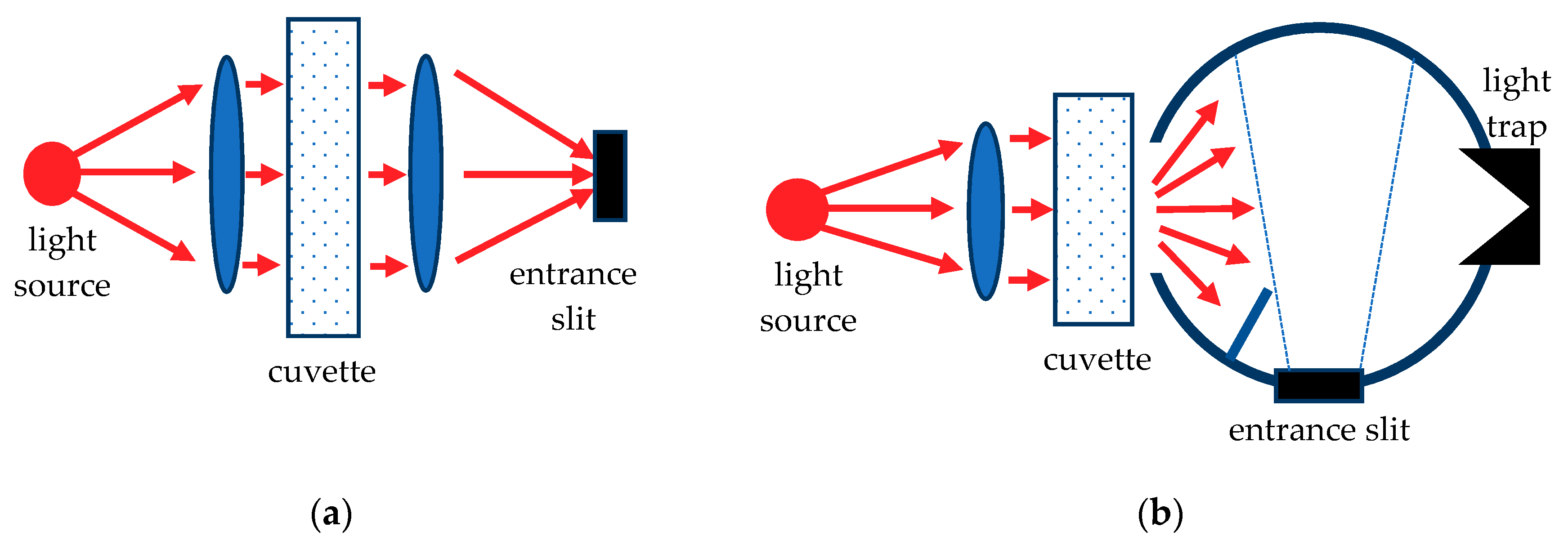
3.2. UV-Visible Spectroscopy
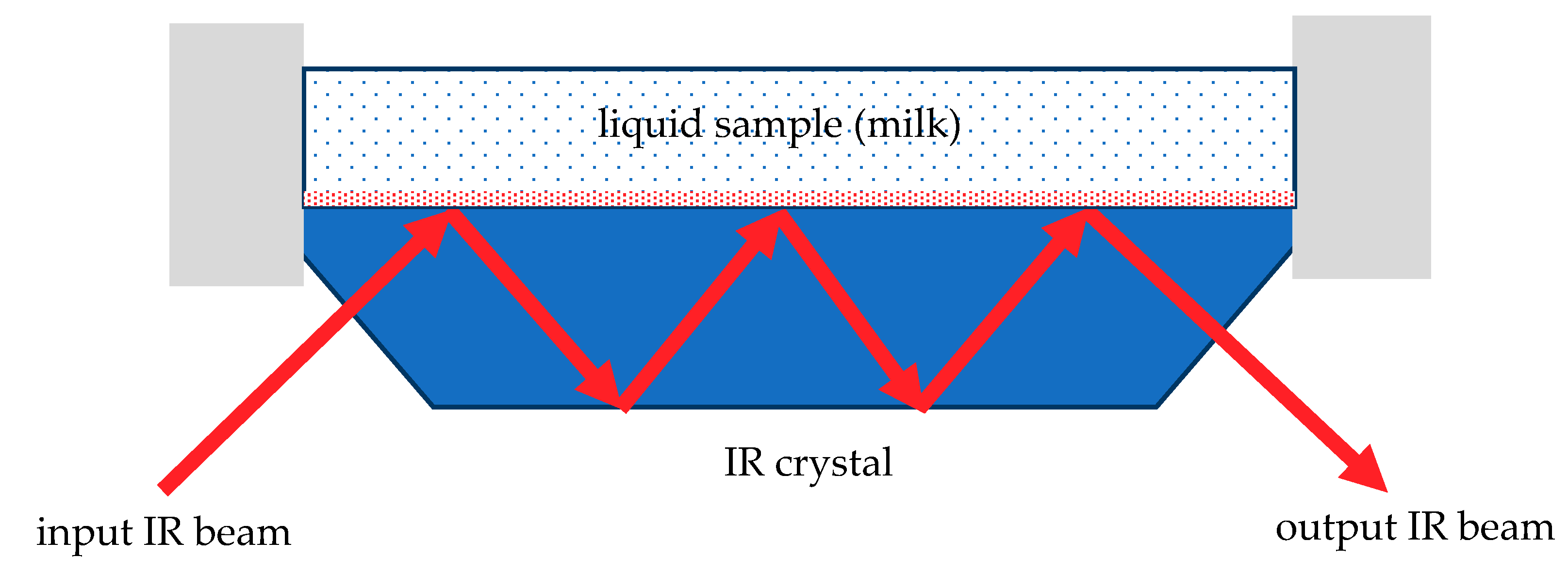
3.3. MIR Spectroscopy
3.4. Near-Infrared (NIR) and Visible Spectroscopy
3.5. Atomic Spectroscopy, Raman Spectrosocopy, Fluorescent Spectrosocopy
3.6. Prospects for the Further Development of Optical Methods and New Devices for Milk Analysis
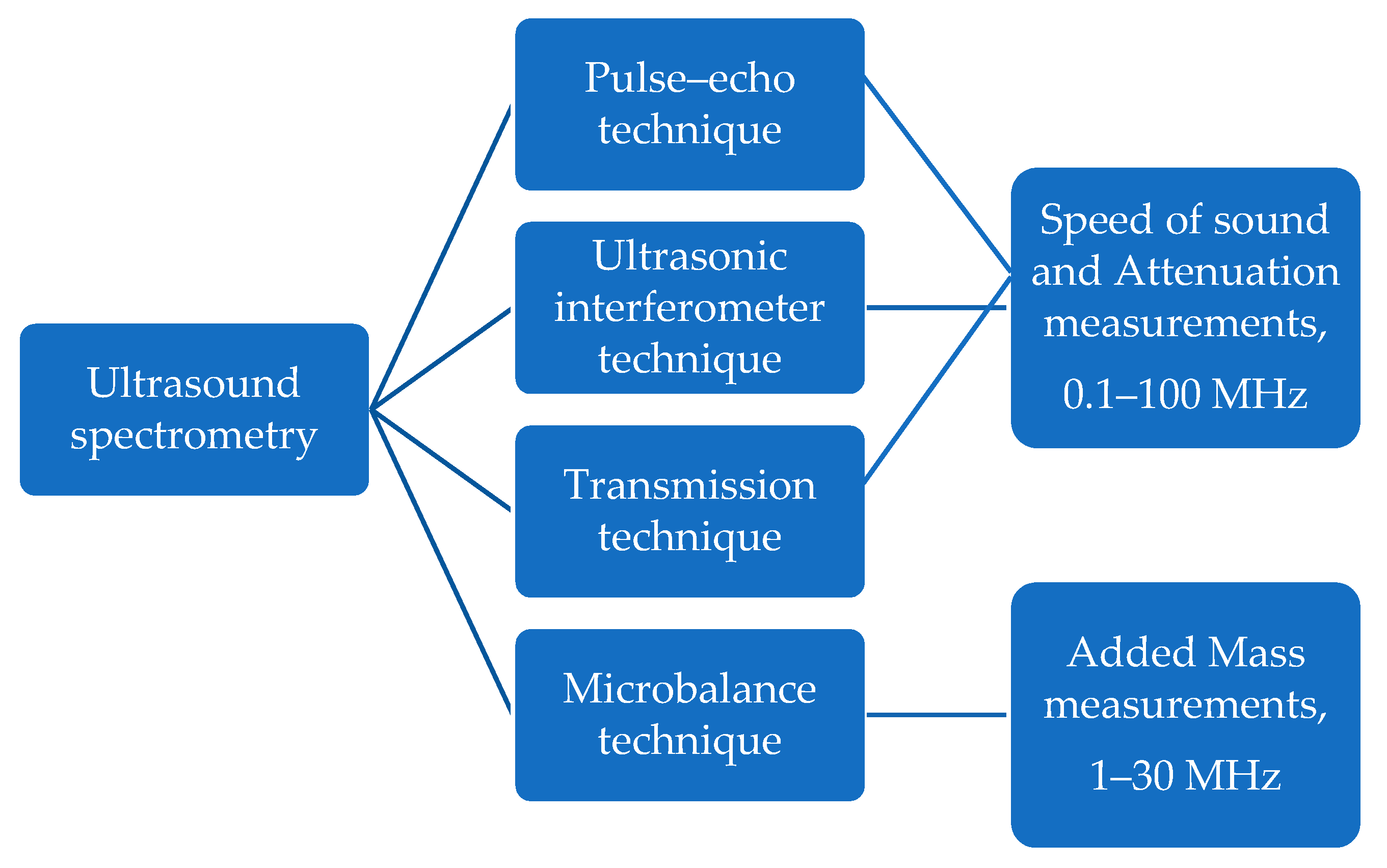
4. Ultrasound Approaches and Techniques for Milk Analysis
4.1. Acoustic Properties of Milk
4.2. Transmission Technique
4.3. Pulse–Echo Technique
4.4. Ultrasonic Interferometer Technique
4.5. Microbalance Technique
4.6. Prospects for the Further Development of Ultrasound Methods and New Devices for Milk Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Caroli, A.; Poli, A.; Ricotta, D.; Banfi, G.; Cocchi, D. Invited review: Dairy intake and bone health: A viewpoint from the state of the art. J. Dairy Sci. 2011, 94, 5249–5262. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, S.; Body, J.-J.; Bruyère, O.; Bergmann, P.; Brandi, M.L.; Cooper, C.; Devogelaer, J.-P.; Gielen, E.; Goemaere, S.; Kaufman, J.-M.; et al. Effects of dairy products consumption on health: Benefits and beliefs—A commentary from the belgian bone club and the european society for clinical and economic aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases. Calcif. Tissue Int. 2016, 98, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Van Lieshout, G.A.A.; Lambers, T.T.; Bragt, M.C.E.; Hettinga, K.A. How processing may affect milk protein digestion and overall physiological outcomes: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2422–2445. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Ludwig, D.S. Milk and Health. N. Engl. J. Med. 2020, 382, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Neumann, C.; Harris, D.M.; Rogers, L.M. Contribution of animal source foods in improving diet quality and function in children in the developing world. Nutr. Res. 2002, 22, 193–220. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th Edition. December 2015. Available online: https://health.gov/our-work/food-and-nutrition/2015-2020-dietary-guidelines/ (accessed on 30 January 2020).
- Order of the Ministry of Health of the Russian Federation of August 19, 2016 No. 614 “On Approval of Recommendations on Rational Norms of Food Consumption that Meet Modern Requirements for Healthy Nutrition”. Available online: https://static-2.rosminzdrav.ru (accessed on 7 May 2020).
- Jenkins, T.C.; McGuire, M.A. Major Advances in Nutrition: Impact on Milk Composition. J. Dairy Sci. 2006, 89, 1302–1310. [Google Scholar] [CrossRef]
- OECD-FAO Agricultural Development Forecast 2020–2029, for Commodities OECD-FAO Agricultural Outlook 2020–2029. Available online: https://stats.oecd.org/viewhtml.aspx?QueryId=98960&vh=0000&vf=0&l&il=&lang=en# (accessed on 2 September 2020).
- Dumuta, A.; Giurgiulescu, L.; Mihaly-Cozmuta, L.; Vosgan, Z. Physical and Chemical Charateristics of Milk. Variation due to Microwave Radiation. Croat. Chem. Acta 2011, 84, 429–433. [Google Scholar] [CrossRef]
- Vaskova, H.; Buckova, M. Spectroscopic Measurement and Analysis of Fat in Mi. In Proceedings of the 26th International DAAAM Symposium 2016; Katalinic, B., Ed.; DAAAM International Vienna: Vienna, Austria, 2016; pp. 365–370. ISBN 9783902734075. [Google Scholar]
- Bansal, B.K.; Hamann, J.; Grabowskit, N.T.; Singh, K.B. Variation in the composition of selected milk fraction samples from healthy and mastitic quarters, and its significance for mastitis diagnosis. J. Dairy Res. 2005, 72, 144–152. [Google Scholar] [CrossRef]
- Mansbridge, R.J.; Blake, J.S. Nutritional factors affecting the fatty acid composition of bovine milk. Br. J. Nutr. 1997, 78 (Suppl. 1), S37–S47. [Google Scholar] [CrossRef]
- Santiago, B.M.; da Silva, F.F.; Silva, R.R.; Costa, E.G.L.; Porto Junior, A.F.; Costa, E.N.; de Souza, D.D. Effect of different roughages sources on performance, milk composition, fatty acid profile, and milk cholesterol content of feedlot feed crossbred cows (Holstein × Zebu). Trop. Anim. Health Prod. 2019, 51, 599–604. [Google Scholar] [CrossRef]
- Pereira, R.; Vicente, A. Novel Technologies for Milk Processing. In Engineering Aspects of Milk and Dairy Products; Coimbra, J., Teixeira, J., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 155–174. ISBN 978-1-4200-9022-2. [Google Scholar]
- Jensen, R.G. Handbook of Milk Composition; Academic Press: San Diego, CA, USA, 1995; ISBN 9780080533117. [Google Scholar]
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef]
- Poonia, A.; Jha, A.; Sharma, R.; Singh, H.B.; Rai, A.K.; Sharma, N. Detection of adulteration in milk: A review. Int. J. Dairy Technol. 2017, 70, 23–42. [Google Scholar] [CrossRef]
- Bauman, D.E.; Griinari, J.M. Nutritional regulation of milk fat synthesis. Annu. Rev. Nutr. 2003, 23, 203–227. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, D.L. Milk Fat: Origin of Fatty Acids and Influence of Nutritional Factors Thereon. In Advanced Dairy Chemistry Volume 2 Lipids; Fox, P.F., McSweeney, P.L.H., Eds.; Springer: Boston, MA, USA, 2006; pp. 43–92. ISBN 978-0-387-26364-9. [Google Scholar]
- Keenan, T.W.; Mather, I.H. Intracellular origin of milk fat globules and the nature of the milk fat globule membrane. In Advanced Dairy Chemistry Volume 2 Lipids; Fox, P.F., McSweeney, P.L.H., Eds.; Springer: Boston, MA, USA, 2006; pp. 137–171. ISBN 978-0-387-26364-9. [Google Scholar]
- Azad, T.; Ahmed, S. Common milk adulteration and their detection techniques. Food Contam. 2016, 3. [Google Scholar] [CrossRef]
- Kala, R.; Samková, E.; Hanuš, O.; Pecová, L.; Sekmokas, K.; Riaukienė, D. Milk Protein Analysis: An Overview of the Methods—Development and Application. Acta Univ. Agric. Silv. Mendel. Brun. 2019, 67, 345–359. [Google Scholar] [CrossRef]
- Bhat, M.Y.; Dar, T.A.; Singh, L.R. Casein Proteins: Structural and Functional Aspects. In Milk Proteins—From Structure to Biological Properties and Health Aspects; Gigli, I., Ed.; InTech: London, UK, 2016; ISBN 978-953-51-2536-5. [Google Scholar]
- Walstra, P.; Walstra, P.; Wouters, J.T.M.; Geurts, T.J. Dairy Science and Technology; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9780429116148. [Google Scholar]
- Rizzoli, R. Dairy products, yogurts, and bone health. Am. J. Clin. Nutr. 2014, 99, 1256S–1262S. [Google Scholar] [CrossRef]
- Evershed, R.; Temple, N. Sorting the Beef from the Bull. The Science of Food Fraud Forensics/Richard Evershed & Nicola Temple; Bloomsbury Sigma: London, UK, 2016; ISBN 9781472911339. [Google Scholar]
- Dave, A.; Banwari, D.; Srivastava, S.; Sadistap, S. Optical sensing system for detecting water adulteration in milk. In Proceedings of the Global Humanitarian Technology Conference (GHTC), Seattle, WA, USA, 13–16 October 2016; pp. 634–639, ISBN 978-1-5090-2432-2. [Google Scholar]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Water in Milk and Dairy Products. In Dairy Chemistry and Biochemistry; Fox, P.F., Uniacke-Lowe, T., McSweeney, P.L.H., O’Mahony, J.A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 299–320. ISBN 978-3-319-14891-5. [Google Scholar]
- Inácio, M.R.C.; de Moura, M.F.V.; de Lima, K.M.G. Classification and determination of total protein in milk powder using near infrared reflectance spectrometry and the successive projections algorithm for variable selection. Vib. Spectrosc. 2011, 57, 342–345. [Google Scholar] [CrossRef]
- Olson, B.J.S.C.; Markwell, J. Assays for determination of protein concentration. Curr. Protoc. Protein Sci. 2007, 38, A.3A.1–A.3A.29. [Google Scholar] [CrossRef]
- ISO. IDF 105:2008 Milk—Determination of Fat Content—Gerber Butyrometers. Available online: https://www.iso.org/standard/51018.html (accessed on 2 September 2020).
- Xin, Q.; Ling, H.Z.; Long, T.J.; Zhu, Y. The rapid determination of fat and protein content in fresh raw milk using the laser light scattering technology. Opt. Lasers Eng. 2006, 44, 858–869. [Google Scholar] [CrossRef]
- Agranovich, D.; Ben Ishai, P.; Katz, G.; Bezman, D.; Feldman, Y. Dielectric spectroscopy study of water dynamics in frozen bovine milk. Colloids Surf. B Biointerfaces 2016, 141, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yu, H.; Xu, H.; Ying, Y. Near infrared spectroscopy for on/in-line monitoring of quality in foods and beverages: A review. J. Food Eng. 2008, 87, 303–313. [Google Scholar] [CrossRef]
- ISO (International Organization for Standardization). Whole Milk—Determination of Milk Fat, Protein and Lactose Content—Guidance on the Operation of Mid-Infrared Instruments; International Standard ISO 9622:2000/IDF 141C:2000; International Dairy Federation: Brussels, Belgium, 2000. [Google Scholar]
- Priev, D.; Ponomarev, V.; Priev, A. Acoustic radiation forces in monitoring of milk composition. J. Acoust. Soc. Am. 2008, 123, 3789. [Google Scholar] [CrossRef]
- Manske, G.A.; Rigo, E.; Gomes, F.J.; Schogor, A.L.B. Infrared or ultrasonic milk analysis can affect its results? Cienc. Rural 2019, 49. [Google Scholar] [CrossRef]
- Operation Manual for Klever-2. Available online: https://www.kleverltd.ru (accessed on 8 August 2020).
- Manual for the Operation of Lactan. Available online: https://sibagropribor.ru/ (accessed on 8 August 2020).
- Ekomilk Operation Manual. Available online: http://ekomilk.ru/ (accessed on 8 August 2020).
- Lactoscan Operation Manual. Available online: https://www.milkotronic.com/downloads.html (accessed on 8 August 2020).
- Operation Manual for Bentley. Available online: http://www.bentleyplemtech.ru/ (accessed on 8 August 2020).
- Zhu, Z.; Guo, W. Recent developments on rapid detection of main constituents in milk: A review. Crit. Rev. Food Sci. Nutr. 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mäntele, W.; Deniz, E. UV-VIS absorption spectroscopy: Lambert-Beer reloaded. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 965–968. [Google Scholar] [CrossRef]
- Gupta, K.; Shenoy, M.R. Method to determine the anisotropy parameter g of a turbid medium. Appl. Opt. 2018, 57, 7559–7563. [Google Scholar] [CrossRef]
- Aernouts, B.; Van Beers, R.; Watté, R.; Huybrechts, T.; Lammertyn, J.; Saeys, W. Visible and near-infrared bulk optical properties of raw milk. J. Dairy Sci. 2015, 98, 6727–6738. [Google Scholar] [CrossRef]
- Steponavičius, R.; Thennadil, S.N. Full correction of scattering effects by using the radiative transfer theory for improved quantitative analysis of absorbing species in suspensions. Appl. Spectrosc. 2013, 67, 526–535. [Google Scholar] [CrossRef]
- Regnima, G.O.; Koffi, T.; Bagui, O.; Kouacou, A.; Kristensson, E.; Zoueu, J.; Berrocal, E. Quantitative measurements of turbid liquids via structured laser illumination planar imaging where absorption spectrophotometry fails. Appl. Opt. 2017, 56, 3929–3938. [Google Scholar] [CrossRef]
- Stocker, S.; Foschum, F.; Krauter, P.; Bergmann, F.; Hohmann, A.; Happ, C.S.; Kienle, A. Broadband Optical Properties of Milk. Appl. Spectrosc. 2017, 71, 951–962. [Google Scholar] [CrossRef]
- Michalski, M.-C.; Briard, V.; Michel, F. Optical parameters of milk fat globules for laser light scattering measurements. Lait 2001, 81, 787–796. [Google Scholar] [CrossRef]
- Aernouts, B.; van Beers, R.; Watté, R.; Huybrechts, T.; Jordens, J.; Vermeulen, D.; Gerven, T.V.; Lammertyn, J.; Saeys, W. Effect of ultrasonic homogenization on the Vis/NIR bulk optical properties of milk. Colloids Surf. B Biointerfaces 2015, 126, 510–519. [Google Scholar] [CrossRef]
- Tsenkova, R.; Atanassova, S.; Kawano, S.; Toyoda, K. Somatic cell count determination in cow’s milk by near-infrared spectroscopy: A new diagnostic tool. J. Anim. Sci. 2001, 79, 2550–2557. [Google Scholar] [CrossRef] [PubMed]
- Stefanescu, R.; Brebu, S.; Matei, M.; Risca, I.M.; Surleva, A.; Drochioiu, G. Contribution to casein determination by UV spectrophotometry. Acta Chem. Iasi. 2017, 25, 112–126. [Google Scholar] [CrossRef]
- McCarthy, O.J. Physical and Physico-Chemical Properties of Milk. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2002; Volume 3, pp. 467–477. ISBN 9780123744029. [Google Scholar]
- Xiong, S.; Adhikari, B.; Chen, X.D.; Che, L. Determination of ultra-low milk fat content using dual-wavelength ultraviolet spectroscopy. J. Dairy Sci. 2016, 99, 9652–9658. [Google Scholar] [CrossRef]
- Miller, E.S.; Mackinney, G.; Zscheile, F.P. Absorption spectra of alpha and beta carotenes and lycopene. Plant Physiol. 1935, 10, 375–381. [Google Scholar] [CrossRef]
- Koziol, J. Studies on flavins in organic solvents-i. Spectral characteristics of riboflavin, riboflavin tetrabutyrate and lumichrome. Photochem. Photobiol. 1966, 5, 41–54. [Google Scholar] [CrossRef]
- Wójcicki, K. Applying NIR spectroscopy to evaluate quality of whey protein supplements available on the polish market. Żywność Naukatechnologia Jakość 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Brandão, M.C.M.P.; Carmo, A.P.; Bell, M.J.V.; Anjos, V.C. Characterization of milk by infrared spectroscopy. Rev. Inst. Latic. Cândid. 2010, 65, 30–33. [Google Scholar]
- Holroyd, S.E. The Use of near Infrared Spectroscopy on Milk and Milk Products. J. Near Infrared Spectrosc. 2013, 21, 311–322. [Google Scholar] [CrossRef]
- Etzion, Y.; Linker, R.; Cogan, U.; Shmulevich, I. Determination of Protein Concentration in Raw Milk by Mid-Infrared Fourier Transform Infrared/Attenuated Total Reflectance Spectroscopy. J. Dairy Sci. 2004, 87, 2779–2788. [Google Scholar] [CrossRef]
- Mohamed, H.; Nagy, P.; Agbaba, J.; Kamal-Eldin, A. Use of near and mid infra-red spectroscopy for analysis of protein, fat, lactose and total solids in raw cow and camel milk. Food Chem. 2020, 334, 127436. [Google Scholar] [CrossRef] [PubMed]
- Bogomolov, A.; Belikova, V.; Galyanin, V.; Melenteva, A.; Meyer, H. Reference-free spectroscopic determination of fat and protein in milk in the visible and near infrared region below 1000 nm using spatially resolved diffuse reflectance fiber probe. Talanta 2017, 167, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Bogomolov, A.; Melenteva, A. Scatter-based quantitative spectroscopic analysis of milk fat and total protein in the region 400–1100 nm in the presence of fat globule size variability. Chemom. Intell. Lab. Syst. 2013, 126, 129–139. [Google Scholar] [CrossRef]
- Bogomolov, A.; Dietrich, S.; Boldrini, B.; Kessler, R.W. Quantitative determination of fat and total protein in milk based on visible light scatter. Food Chem. 2012, 134, 412–418. [Google Scholar] [CrossRef]
- Melenteva, A.; Galyanin, V.; Savenkova, E.; Bogomolov, A. Building global models for fat and total protein content in raw milk based on historical spectroscopic data in the visible and short-wave near infrared range. Food Chem. 2016, 203, 190–198. [Google Scholar] [CrossRef]
- Goulden, J. Analysis of milk by infra-red absorption. J. Dairy Res. 1964, 3, 273–284. [Google Scholar] [CrossRef]
- de Marchi, M.; Penasa, M.; Zidi, A.; Manuelian, C.L. Invited review: Use of infrared technologies for the assessment of dairy products-Applications and perspectives. J. Dairy Sci. 2018, 101, 10589–10604. [Google Scholar] [CrossRef]
- Pereira, C.; Luiz, L.C.; Bell, M.J.V.; Anjos, V. Near and Mid Infrared Spectroscopy to Assess Milk Products Quality: A Review of Recent Applications. J. Dairy Res. Technol. 2020, 3, 100014. [Google Scholar] [CrossRef]
- Balabin, R.M.; Safieva, R.Z.; Lomakina, E.I. Comparison of linear and nonlinear calibration models based on near infrared (NIR) spectroscopy data for gasoline properties prediction. Chemom. Intell. Lab. Syst. 2007, 88, 183–188. [Google Scholar] [CrossRef]
- Balabin, R.M.; Safieva, R.Z.; Lomakina, E.I. Wavelet neural network (WNN) approach for calibration model building based on gasoline near infrared (NIR) spectra. Chemom. Intell. Lab. Syst. 2008, 93, 58–62. [Google Scholar] [CrossRef]
- Andrade, J.; Guimarães Pereira, C.; de Almeida, J.C., Jr.; Viana, C.C.; de Oliveira Neves, L.N.; da Silva, P.H.; Bell, M.J.; dos Anjos, V.D. FTIR-ATR determination of protein content to evaluate whey protein concentrate adulteration. LWT 2019, 99, 166–172. [Google Scholar] [CrossRef]
- Balan, B.; Dhaulaniya, A.S.; Jamwal, R.; Sodhi, K.K.; Kelly, S.D.; Cannavan, A.; Singh, D.K. Application of Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) spectroscopy coupled with chemometrics for detection and quantification of formalin in cow milk. Vib. Spectrosc. 2020, 107, 103033. [Google Scholar] [CrossRef]
- Balan, B.; Dhaulaniya, A.S.; Jamwal, R.; Yadav, A.; Kelly, S.; Cannavan, A.; Singh, D.K. Rapid detection and quantification of sucrose adulteration in cow milk using Attenuated total reflectance-Fourier transform infrared spectroscopy coupled with multivariate analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 240, 118628. [Google Scholar] [CrossRef]
- Franzoi, M.; Niero, G.; Visentin, G.; Penasa, M.; Cassandro, M.; de Marchi, M. Variation of Detailed Protein Composition of Cow Milk Predicted from a Large Database of Mid-Infrared Spectra. Animals 2019, 9, 176. [Google Scholar] [CrossRef]
- Cirak, O.; Icyer, N.; Durak, M. Rapid detection of adulteration of milks from different species using Fourier Transform Infrared Spectroscopy (FTIR). J. Dairy Res. 2018, 85, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Balabin, R.M.; Smirnov, S.V. Melamine detection by mid- and near-infrared (MIR/NIR) spectroscopy: A quick and sensitive method for dairy products analysis including liquid milk, infant formula, and milk powder. Talanta 2011, 85, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Botelho, B.G.; Reis, N.; Oliveira, L.S.; Sena, M.M. Development and analytical validation of a screening method for simultaneous detection of five adulterants in raw milk using mid-infrared spectroscopy and PLS-DA. Food Chem. 2015, 181, 31–37. [Google Scholar] [CrossRef]
- Casarrubias-Torres, L.M.; Meza-Márquez, O.G.; Osorio-Revilla, G.; Gallardo-Velazquez, T. Mid-infrared spectroscopy and multivariate analysis for determination of tetracycline residues in cow’s milk. Acta Vet. Brno 2018, 87, 181–188. [Google Scholar] [CrossRef]
- Rienesl, L.; Khayatzadeh, N.; Köck, A.; Dale, L.; Werner, A.; Grelet, C.; Gengler, N.; Auer, F.J.; Egger-Danner, C.; Massart, X.; et al. Mastitis detection from milk mid-infrared (MIR) spectroscopy in dairy cows. Acta Univ. Agric. Silv. Mendel. Brun. 2019, 67, 1221–1226. [Google Scholar] [CrossRef]
- Libnau, F.O.; Kvalheim, O.M.; Christy, A.A.; Toft, J. Spectra of water in the near- and mid-infrared region. Vib. Spectrosc. 1994, 7, 243–254. [Google Scholar] [CrossRef]
- Linker, R.; Etzion, Y. Potential and limitation of mid-infrared attenuated total reflectance spectroscopy for real time analysis of raw milk in milking lines. J. Dairy Res. 2009, 76, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Aernouts, B.; Polshin, E.; Lammertyn, J.; Saeys, W. Visible and near-infrared spectroscopic analysis of raw milk for cow health monitoring: Reflectance or transmittance? J. Dairy Sci. 2011, 94, 5315–5329. [Google Scholar] [CrossRef] [PubMed]
- Melfsen, A.; Holstermann, M.; Haeussermann, A.; Molkentin, J.; Susenbeth, A.; Hartung, E. Accuracy and application of milk fatty acid estimation with diffuse reflectance near-infrared spectroscopy. J. Dairy Res. 2018, 85, 212–221. [Google Scholar] [CrossRef]
- Katsumata, T.; Aizawa, H.; Komuro, S.; Ito, S.; Matsumoto, T. Quantitative analysis of fat and protein concentrations of milk based on fibre-optic evaluation of back scattering intensity. Int. Dairy J. 2020, 109, 104743. [Google Scholar] [CrossRef]
- Laporte, M.; Paquin, P. Near-infrared analysis of fat, protein, and casein in cow’s milk. J. Agric. Food Chem. 1999, 47, 2600–2605. [Google Scholar] [CrossRef]
- Tsenkova, R.; Atanassova, S.; Toyoda, K.; Ozaki, Y.; Itoh, K.; Fearn, T. Near-Infrared Spectroscopy for Dairy Management: Measurement of Unhomogenized Milk Composition. J. Dairy Sci. 1999, 82, 2344–2351. [Google Scholar] [CrossRef]
- Melfsen, A.; Hartung, E.; Haeussermann, A. Accuracy of milk composition analysis with near infrared spectroscopy in diffuse reflection mode. Biosyst. Eng. 2012, 112, 210–217. [Google Scholar] [CrossRef]
- Surkova, A.; Belikova, V.; Kirsanov, D.; Legin, A.; Bogomolov, A. Towards an optical multisensor system for dairy: Global calibration for fat analysis in homogenized milk. Microchem. J. 2019, 149, 104012. [Google Scholar] [CrossRef]
- Mazivila, S.J.; Páscoa, R.N.M.J.; Castro, R.C.; Ribeiro, D.S.M.; Santos, J.L.M. Detection of melamine and sucrose as adulterants in milk powder using near-infrared spectroscopy with DD-SIMCA as one-class classifier and MCR-ALS as a means to provide pure profiles of milk and of both adulterants with forensic evidence: A short communication. Talanta 2020, 216, 120937. [Google Scholar] [CrossRef]
- Kamboj, U.; Kaushal, N.; Jabeen, S. Near Infrared Spectroscopy as an efficient tool for the Qualitative and Quantitative Determination of Sugar Adulteration in Milk. J. Phys. Conf. Ser. 2020, 1531, 012024. [Google Scholar] [CrossRef]
- Mabood, F.; Ali, L.; Boque, R.; Abbas, G.; Jabeen, F.; Haq, Q.M.; Hussain, J.; Hamaed, A.M.; Naureen, Z.; Al-Nabhani, M.; et al. Robust Fourier transformed infrared spectroscopy coupled with multivariate methods for detection and quantification of urea adulteration in fresh milk samples. Food Sci. Nutr. 2019, 1–10. [Google Scholar] [CrossRef]
- Tsenkova, R.; Meilina, H.; Kuroki, S.; Burns, D.H. Near infrared spectroscopy using short wavelengths and leave-one-cow-out cross-validation for quantification of somatic cells in milk. J. Near Infrared Spectrosc. 2009, 17, 345–351. [Google Scholar] [CrossRef]
- Yang, B.; Zhu, Z.; Gao, M.; Yan, X.; Zhu, X.; Guo, W. A portable detector on main compositions of raw and homogenized milk. Comput. Electron. Agric. 2020, 177, 105668. [Google Scholar] [CrossRef]
- Kawasaki, M.; Kawamura, S.; Tsukahara, M.; Morita, S.; Komiya, M.; Natsuga, M. Near-infrared spectroscopic sensing system for on-line milk quality assessment in a milking robot. Comput. Electron. Agric. 2008, 63, 22–27. [Google Scholar] [CrossRef]
- Diaz-Olivares, J.A.; Adriaens, I.; Stevens, E.; Saeys, W.; Aernouts, B. Online Milk Composition Analysis with an On-farm Near-Infrared Sensor. Available Online: https://www.biorxiv.org/content/10.1101/2020.06.02.129742v1.full.pdf (accessed on 15 October 2020).
- Aleixo, P.C.; Nóbrega, J.A. Direct determination of iron and selenium in bovine milk by graphite furnace atomic absorption spectrometry. Food Chem. 2003, 83, 457–462. [Google Scholar] [CrossRef]
- Birghila, S.; Dobrinas, S.; Stanciu, G.; Soceanu, A. Determination of major and minor elements in milk through ICP-AES. Environ. Eng. Manag. J. 2008, 7, 805–808. [Google Scholar] [CrossRef]
- Asfaw, A.; Wibetoe, G. Simultaneous determination of hydride (Se) and non-hydride-forming (Ca, Mg, K, P, S and Zn) elements in various beverages (beer, coffee, and milk), with minimum sample preparation, by ICP–AES and use of a dual-mode sample-introduction system. Anal. Bioanal. Chem. 2005, 382, 173–179. [Google Scholar] [CrossRef]
- Mazurek, S.; Szostak, R.; Czaja, T.; Zachwieja, A. Analysis of milk by FT-Raman spectroscopy. Talanta 2015, 138, 285–289. [Google Scholar] [CrossRef]
- He, H.; Sun, D.-W.; Pu, H.; Chen, L.; Lin, L. Applications of Raman spectroscopic techniques for quality and safety evaluation of milk: A review of recent developments. Crit. Rev. Food Sci. Nutr. 2019, 595, 770–793. [Google Scholar] [CrossRef]
- Reiner, J.; Protte, K.; Hinrichs, J. Investigation of the Applicability of Raman Spectroscopy as Online Process Control during Consumer Milk Production. Chem. Eng. 2020, 4, 45. [Google Scholar] [CrossRef]
- Soulat, J.; Andueza, D.; Graulet, B.; Girard, C.L.; Labonne, C.; Aït-Kaddour, A.; Martin, B.; Ferlay, A. Comparison of the potential abilities of three spectroscopy methods: Near-infrared, mid-infrared, and molecular fluorescence, to predict carotenoid, vitamin and fatty acid contents in cow milk. Foods 2020, 9, 592. [Google Scholar] [CrossRef]
- Ntakatsane, M.; Chen, P.; Liu, J.; Mosebi, P.; Xu, L.; Matebesi, P.; Wang, Y. Multi-dimensional fluorescence spectroscopy coupled with chemometrics in rapid antibiotic detection and discrimination. J. Food Meas. Charact. 2020, 14, 1892–1900. [Google Scholar] [CrossRef]
- Genis, D.O.; Bilge, G.; Sezer, B.; Durna, S.; Boyaci, I.H. Identification of cow, buffalo, goat and ewe milk species in fermented dairy products using synchronous fluorescence spectroscopy. Food Chem. 2019, 284, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hettinga, K.A.; Erasmus, S.W.; Pustjens, A.M.; van Ruth, S.M. Opportunities for fraudsters: When would profitable milk adulterations go unnoticed by common, standardized FTIR measurements? Food Res. Int. 2020, 136, 109543. [Google Scholar] [CrossRef]
- Villa-Arango, S.; Torres, R.; Kyriacou, P.A.; Lucklum, R. Acoustic spectrometer: Resonant sensing platform for measuring volumetric properties of liquid samples. In Proceedings of the VII Latin American Congress on Biomedical Engineering CLAIB 2016, Bucaramanga, Santander, Colombia, 26–28 October 2016; Torres, I., Bustamante, J., Sierra, D.A., Eds.; Springer: Singapore, 2017; pp. 70–73, ISBN 978-981-10-4085-6. [Google Scholar]
- Szabo, T.L. Diagnostic Ultrasound Imaging. Inside out/Thomas L. Szabo; Elsevier Academic Press: Amsterdam, The Netherlands; Oxford, UK, 2004; ISBN 0-12-680145-2. [Google Scholar]
- Mohammadi, V.; Ghasemi-Varnamkhasti, M.; Ebrahimi, R.; Abbasvali, M. Ultrasonic techniques for the milk production industry. Measurement 2014, 58, 93–102. [Google Scholar] [CrossRef]
- Ali, M.-H.; Ahmad, A. Attenuation of ultrasound in reconstituted milk. Int. J. Sci. Environ. Technol. 2017, 6, 1828–1832. [Google Scholar]
- Adami, A.; Mortari, A.; Morganti, E.; Lorenzelli, L. Microfluidic Sample Preparation Methods for the Analysis of Milk Contaminants. J. Sens. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Dukhin, A.S.; Goetz, P.J.; Travers, B. Use of ultrasound for characterizing dairy products. J. Dairy Sci. 2005, 88, 1320–1334. [Google Scholar] [CrossRef]
- Elvira, L.; Rodríguez, J.; Lynnworth, L.C. Sound speed and density characterization of milk adulterated with melamine. J. Acoust. Soc. Am. 2009, 125, EL177–EL182. [Google Scholar] [CrossRef]
- Halachmi, I.; Guarino, M.; Bewley, J.; Pastell, M. Smart Animal Agriculture: Application of Real-Time Sensors to Improve Animal Well-Being and Production. Annu. Rev. Anim. Biosci. 2019, 7, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, V.; Ghasemi-Varnamkhasti, M.; González, L.A. Analytical measurements of ultrasound propagation in dairy products: A review. Trends Food Sci. Technol. 2017, 61, 38–48. [Google Scholar] [CrossRef]
- McClements, D.J. Ultrasonic characterization of foods and drinks: Principles, methods, and applications. Crit. Rev. Food Sci. Nutr. 1997, 37, 1–46. [Google Scholar] [CrossRef]
- De Luca, M.; Santonico, M.; Pennazza, G.; Iarossi, S. Ultrasound based sensor for fat detection in fresh milk. In Sensors; Baldini, F., D’Amico, A., Di Natale, C., Siciliano, P., Seeber, R., de Stefano, L., Bizzarri, R., Andò, B., Eds.; Springer: New York, NY, USA, 2014; pp. 499–502. ISBN 978-1-4614-3859-5. [Google Scholar]
- Munir, M.; Nadeem, M.; Qureshi, T.M.; Leong, T.S.H.; Gamlath, C.J.; Martin, G.J.O.; Ashokkumar, M. Effects of high pressure, microwave and ultrasound processing on proteins and enzyme activity in dairy systems—A review. Innov. Food Sci. Emerg. Technol. 2019, 57, 102192. [Google Scholar] [CrossRef]
- Paniwnyk, L. Applications of ultrasound in processing of liquid foods: A review. Ultrason. Sonochem. 2017, 38, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.S.; Deshaires, C. Monitoring protein fouling of metal surfaces via a quartz crystal microbalance. J. Colloid Interface Sci. 2000, 227, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.H.; Pallav, P.; Hutchins, D.A. Non-contact ultrasonic quality measurements of food products. J. Food Eng. 2006, 77, 239–247. [Google Scholar] [CrossRef]
- Dukhin, A.S.; Goetz, P.J. Ultrasound for Characterizing Colloids. Particle Sizing, Zeta Potential, Rheology; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- McClements, D.J. Ultrasonic characterisation of emulsions and suspensions. Adv. Colloid Interface Sci. 1991, 37, 33–72. [Google Scholar] [CrossRef]
- Natta, G.; Baccaredda, M. Sulla velocita di propagazione degli ultrasuoni nelle miscele ideali. Atti Accad. Naz. Lincei-Rend. Sc. Fis. Mat. Nat. 1948, 4, 360–366. [Google Scholar]
- Rowlinson, J.S.; Swinton, F. Liquids and Liquid Mixtures, 3rd ed.; Butterworth Scientific: Oxford, UK, 1982. [Google Scholar]
- Flory, P.J. Statistical Thermodynamics of Liquid Mixtures. J. Am. Chem. Soc. 1965, 87, 1833–1838. [Google Scholar] [CrossRef]
- Hahn, P.; Dual, J. A numerically efficient damping model for acoustic resonances in microfluidic cavities. Phys. Fluids 2015, 27, 62005. [Google Scholar] [CrossRef]
- Trujillo, F.J.; Juliano, P.; Barbosa-Cánovas, G.; Knoerzer, K. Separation of suspensions and emulsions via ultrasonic standing waves—A review. Ultrason. Sonochem. 2014, 21, 2151–2164. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Physical Properties of Milk. In Dairy Chemistry and Biochemistry; Fox, P.F., Uniacke-Lowe, T., McSweeney, P.L.H., O’Mahony, J.A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 321–343. ISBN 978-3-319-14891-5. [Google Scholar]
- McCarthy, O.J.; Singh, H. Physico-chemical Properties of Milk. In Advanced Dairy Chemistry; McSweeney, P., Fox, P.F., Eds.; Springer: New York, NY, USA, 2009; pp. 691–758. ISBN 978-0-387-84864-8. [Google Scholar]
- Singh, H.; McCarthy, O.J.; Lucey, J.A. Physico-Chemical Properties of Milk. In Advanced Dairy Chemistry Volume 3; Fox, P.F., Ed.; Springer: Boston, MA, USA, 1997; pp. 469–518. ISBN 978-1-4757-4411-8. [Google Scholar]
- Russel, W.B. Brownian motion of small particles suspended in liquids. Annu. Rev. Fluid Mech. 1981, 13, 425–455. [Google Scholar] [CrossRef]
- Caprita, R.; Caprita, A.; Benscik, I.; Cretescu, I. The Influence of Milk Protein Content on the Surface Tension and Viscosity. Acta Vet. Scand. 2003, 44, P86. [Google Scholar] [CrossRef]
- Vives, A.A. Piezoelectric Transducers and Applications; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-77507-2. [Google Scholar]
- Elvira, L.; Sampedro, L.; de Espinosa, F.M.; Matesanz, J.; Gómez-Ullate, Y.; Resa, P.; Echevarría, F.J.; Iglesias, J.R. Eight-channel ultrasonic device for non-invasive quality evaluation in packed milk. Ultrasonics 2006, 45, 92–99. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in the application of ultrasound in food analysis and processing. Trends Food Sci. Technol. 1995, 6, 293–299. [Google Scholar] [CrossRef]
- Saxena, I.; Pathak, R.N.; Kumar, V.; Devi, R. Introduction of ultrasonic interferometer and experimental techniques for determination of ultrasonic velocity, density, viscosity and various thermodynamic parameters. Int. J. Appl. Res. 2015, 1, 562–569. [Google Scholar] [CrossRef]
- Kucera, D.M.; Ketterson, J.B. Variable path cryogenic acoustic interferometer. Rev. Sci. Instrum. 1998, 69, 4156–4159. [Google Scholar] [CrossRef]
- Sharma, S.; Mishra, U.K.; Yadav, S.; Dubey, P.K. Improved ultrasonic interferometer technique for propagation velocity and attenuation measurement in liquids. Rev. Sci. Instrum. 2019, 90, 45107. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Hirai, M.; Hasegawa, T.; Matsuzawa, K. A new ultrasonic interferometer for velocity measurement in liquids using optical diffraction. J. Phys. D Appl. Phys. 1986, 19, 1439–1447. [Google Scholar] [CrossRef]
- Gülseren, I.; Alexander, M.; Corredig, M. Probing the colloidal properties of skim milk using acoustic and electroacoustic spectroscopy. Effect of concentration, heating and acidification. J. Colloid Interface Sci. 2010, 351, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J.; Wessendorf, K.O.; Gebert, C.T.; Frye, G.C.; Cernosek, R.W.; Casaus, L.; Mitchell, M.A. Measuring liquid properties with smooth- and textured-surface resonators. In Proceedings of the Annual Frequency Control Symposium, Salt Lake City, UT, USA, 2 June 1993; pp. 603–608. [Google Scholar] [CrossRef]
- Ito, T.; Aoki, N.; Tsuchiya, A.; Kaneko, S.; Akiyama, K.; Uetake, K.; Suzuki, K. Detection of Stress Hormone in the Milk for Animal Welfare Using QCM Method. J. Sens. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Sakti, S.P.; Chabibah, N.; Ayu, S.P.; Padaga, M.C.; Aulanni’am, A.A. Development of QCM Biosensor with specific cow milk protein antibody for candidate milk adulteration detection. J. Sens. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Mukhin, N.; Kutia, M.; Oseev, A.; Palis, S.; Steinmann, U.; Lucklum, R. Narrow band solid-liquid composite arrangements: Alternative solutions for phononic crystal-based liquid sensors. Sensors 2019, 19, 3743. [Google Scholar] [CrossRef] [PubMed]
- Mukhin, N.; Lucklum, R. QCM based sensor for detecting volumetric properties of liquids. Curr. Appl. Phys. 2019, 19, 679–682. [Google Scholar] [CrossRef]
- Oseev, A.; Lucklum, R.; Zubtsov, M.; Schmidt, M.-P.; Mukhin, N.V.; Hirsch, S. SAW-Based Phononic Crystal Microfluidic Sensor-Microscale Realization of Velocimetry Approaches for Integrated Analytical Platform Applications. Sensors 2017, 17, 2187. [Google Scholar] [CrossRef] [PubMed]
- Lucklum, R.; Zubtsov, M.; Oseev, A. Phoxonic crystals—A new platform for chemical and biochemical sensors. Anal. Bioanal. Chem. 2013, 405, 6497–6509. [Google Scholar] [CrossRef]



| Components | Average Content, % | Oscillation Limits |
|---|---|---|
| Water | 87.5 | 84–89 |
| Dry matter | 12.5 | 11–16 |
| Including: | ||
| Fat | 3.8 | 2.5–6.8 |
| Protein | 3.3 | 2.7–5.0 |
| Lactose | 4.7 | 3.6–5.6 |
| Ash | 0.7 | 0.6–0.9 |
| Parameters | Diluted with Water | Whole Milk | Skimmed Milk |
|---|---|---|---|
| organoleptic characteristics | weakened taste and odor, decreased viscosity (wateriness), bluish color | specific smell, no foreign impurities, color from white to light cream | taste is significantly reduced, a bluish tint, wateriness is acquired |
| density, kg/m3 | <1027 | 1027–1032 | 1030–1035 |
| viscosity at 20 °C, mPa·s | 1.2 | 2.127 | 1.79 |
| acidity, To | <16 | 16–21 | without changes |
| dry residue, % | <10 | 12.2 | changes slightly |
| fat, % | <3.2 | 3.6 | <2.8 |
| protein, % | <2.5 | 2.8–3.2 | without changes |
| Specifications | Klever-2 [39] | Lactan [40] | Ekomilk [41] | Lactoscan [42] | Bentley [43] | MilkoScan [44] |
|---|---|---|---|---|---|---|
| Manufacturer | Russia | Russia | Bulgaria | Bulgaria | USA | Denmark |
| Price range depending on equipment, € | 464–878 | 340–850 | 645–1530 | from 815 | from 67,000 | from 66,000 |
| Device weight, kg | 1 | 3 | 3.5 | 1.5 | 45.4 | 72 |
| Continuous working time, hours | 12 | no more than 8 | no data | no limits | no limits | no limits |
| Vestigated objects (depending on modification) | Milk and dairy products | Fresh, canned, pasteurized normalized milk | Whole fresh cow, sheep, goat milk | Cow’s, sheep’s, buffalo’s & goat’s milk, cream, serum | Raw & pasteurized milk, whey, fat cream up to 20%, cream up to 50% fat, skim milk, yogurt. | Milk and dairy products |
| Temperature of sample, °C | 5–35 | 15–35 | 5–35 | 5–40 | 38–42 | 5–55 |
| Average measurement time of 1 sample | 22 samples per hour, 3.5 min per sample | No more than 5 min per sample | 65–70 samples per hour, 40 s per sample | 60 s (30 s are optional). | 400–600 samples per hour | 30 s per sample |
| Fat, error, % | 0–20, ±0.06 | 0–10, ±0.05; 5–10, ±0.1 | 0.5–12, ±0.1 | 0.01–25, ±0.06–0.1 | 0–50, ±0.1–0.5 | 0–60, ±0.06–0.5 |
| Protein, error, % | 0.15–6, ±0.15 | 1.5–3.5, ±0.1 | 2–6, ±0.2 | 2–7, ±0.08–0.15 | 0–15, ±0.3 | 0–7, ±0.14–0.3 |
| Density, error, kg/m³ | 1000–1050, ±0.3 | 1000–1040, ±0.3 | 1026–1033, ±0.3 | 1000–1150, ±0.3 | 1000–1100, ±0.3 | 1025–1037, ±0.4 |
| Added water, error, % | 3–70, no data | 0–100, ±1 | 0–60, ± 5 | no data | no data | 0–100, ±1 |
| Spectral Region | Wavelength Interval | Milk Constituents | Absorption Wavelengths/Wavenumbers | Reference |
|---|---|---|---|---|
| Ultraviolet and visible | 0.2–0.7 μm | proteins fat | 280 nm 205, 230, 270 nm | [53,54,55,56] |
| β-carotene | 435 nm, 480 nm | [57] | ||
| riboflavin | 449 nm | [58] | ||
| Near infrared | 0.7–2.5 μm | water | 970, 1200, 1450, 1940, 1953, 1984 nm | [47] |
| whey protein | 1731, 1774, 2056, 2060, 2167, 2180 nm | [59] | ||
| fat | 1220, 1720, 1730, 1740–1770, 1780, 2076, 2270 | [47,60,61] | ||
| 2310, 2340, 2386 nm | ||||
| casein | 1450, 1680, 1720, 1730, 1780, 1820, 1980, 2100, 2310, 2340, 2790 nm | [60,61] | ||
| lactose | 1450, 1820, 2100, 2340 nm | [60] | ||
| Middle infrared | 2.5–25 μm (4000–400 cm−1) | water | 1640, 3300 cm−1 | [62] |
| fat | 1175, 1470, 1754, 2857 cm−1 | [63] | ||
| proteins | 1240, 1600–1700, 1550–1570 cm−1 | [62,63] | ||
| lactose | 1052, 1080, 1157 cm−1 | [63] |
| Measurement Technique | Chemometrics | Spectral Range | Milk Constituents/ Adulterants | Accuracy/ Detection Limit 1 | Reference |
|---|---|---|---|---|---|
| FTIR/ATR | PLS, PCR, ANN | 1600–1500 cm−1 1100–1050 cm−1 | proteins | 0.08–0.20% | [62] |
| FTIR/ATR | PLS, PCR | 3600–2800 cm−1 1800–400 cm−1 | total protein content, whey protein | 4%; 8% (relative) | [73] |
| FTIR/ATR | PLS, PCR | 4000–600 cm−1 | protein, fat, lactose, total solids | – | [63] |
| FTIR/ATR | PCR, PLS, SIMCA | 1025 cm−1 | formalin | 0.5% | [74] |
| FTIR/ATR | PCR, PLS, SIMCA | 996, 1052 cm−1 | sucrose | 0.5% | [75] |
| FTIR/ATR | PLS | 5000–900 cm−1 | protein fractions | – | [76] |
| FTIR/ATR | PCA | 1700–600 cm−1 | milk species discrimination | – | [77] |
| FTIR/ATR | PLS, OPLS, Poly-PLS, ANN | 3850–450 cm−1 | melamine | 1 ppm | [78] |
| FTIR/ATR | PLS | 4000–600 cm−1 | water, starch, sodium citrate, formaldehyde, sucrose | 0.5% | [79] |
| FTIR/ATR | PCR, PLS, SIMCA | 4000–550 cm−1 | tetracycline | 10 μg/L | [80] |
| FTIR | PLS-DA | 5000–925 cm−1 | mastitis detection | – | [81] |
| Measurement Technique | Chemometrics | Spectral Range | Milk Constituents/ Adulterants | Accuracy/ Detection Limit 1 | Reference |
|---|---|---|---|---|---|
| DT | PLS, PCR | 800–2500 nm | protein, fat, lactose, total solids | 8.2%; 9.3%; 5.4%; 2.2% (relative) | [63] |
| DR | PLS | 400–995 nm | fat, protein | 0.10%; 0.008% | [64] |
| DT | PLS | 400–1100 nm | fat, protein | 0.08%; 0.04% | [65] |
| CT | PLS | 400–1000 nm | fat, protein | 0.05%; 0.03% | [66] |
| DT | PLS | 400–1100 nm | fat, protein | 0.09%; 10% | [67] |
| DT | PLS | 400–2500 nm | fat, protein, lactose | 0.04%; 0.13%; 0.13% | [84] |
| DR | PLS | 400–1700 nm | fat, protein, lactose | 0.05%; 0.1%; 0.18% | [84] |
| DT | PLS | 1100–2500 | fat, protein, casein | 0.05–0.07% | [87] |
| DT | PLS | 1100–2500 nm 700–1100 nm | fat; protein; lactose | 0.11%; 0.12%; 0.09% 0.19%; 0.19%; 0.1% | [88] |
| DR | PLS | 851–1649 nm | fat, protein, lactose | 0.03%, 0.07%, 0.09% | [89] |
| DT | PLS | 400–1100 nm | fat | 0.12% | [90] |
| DR | MCR-ALS, SIMCA | 1000–2500 nm | melamine; sucrose | 0.14% (0.8–2%); 0.08% (1–3%) | [91] |
| DR | PCA, PLS | 700–2500 nm | sucrose | 0.04% | [92] |
| CT | PCA, PLS | 1000–2500 nm | urea | 0.48% | [93] |
| FTIR/DT | PLS, OPLS, Poly-PLS, ANN | 1110–2500 nm | melamine | 1 ppm | [78] |
| DT | PLS | 700–1100 nm | somatic cells count | 0.3 LogSCC | [94] |
| Measurement Technique | Measured Parameters | Frequency Range/Centre Frequencies | Milk Constituents/ Adulterants | Accuracy/ Detection Limit 1 | Reference |
|---|---|---|---|---|---|
| Transmission | attenuation | 40–50 MHz | fat | 0.2% | [113] |
| Transmission | attenuation | 1–10 MHz, 1–100 MHz | particle size distribution, particle sizes | single 1-μm particle per 100,000 particles with size 100 nm, from 5 nm | [123] |
| Transmission | attenuation | 0.5, 0.7 MHz | pH | - | [122] |
| Transmission | attenuation | 0.8 MHz | microorganisms | - | [136] |
| Transmission | sound speed | 1–100 MHz | carbohydrates, mineral salts | 10−4 mol/L | [113] |
| Transmission | sound speed | 4 MHz | melamine | 6% | [114] |
| Pulse-echo | sound speed | 1 MHz | carbohydrates | 0.2% | [137] |
| Pulse-echo | sound speed and attenuation | 1–10 MHz | fat globules, casein micelles | - | [137], [124] |
| Interferometer | attenuation | 1 MHz | dry matter | - | [112] |
| Interferometer | attenuation | 3–99.5 MHz | casein micelles size distribution | 0.05 μm | [142] |
| QCM | mass | 5 MHz | β-lactoglobulin | 0.4–0.9 mg/m2 | [121] |
| QCM | mass | 30.8 MHz | cortisol | 0.1 pg/mL | [144] |
| QCM | mass | 10 MHz | PSS 208 protein | 1 ppm | [145] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smirnova, A.; Konoplev, G.; Mukhin, N.; Stepanova, O.; Steinmann, U. Milk as a Complex Multiphase Polydisperse System: Approaches for the Quantitative and Qualitative Analysis. J. Compos. Sci. 2020, 4, 151. https://doi.org/10.3390/jcs4040151
Smirnova A, Konoplev G, Mukhin N, Stepanova O, Steinmann U. Milk as a Complex Multiphase Polydisperse System: Approaches for the Quantitative and Qualitative Analysis. Journal of Composites Science. 2020; 4(4):151. https://doi.org/10.3390/jcs4040151
Chicago/Turabian StyleSmirnova, Alena, Georgii Konoplev, Nikolay Mukhin, Oksana Stepanova, and Ulrike Steinmann. 2020. "Milk as a Complex Multiphase Polydisperse System: Approaches for the Quantitative and Qualitative Analysis" Journal of Composites Science 4, no. 4: 151. https://doi.org/10.3390/jcs4040151
APA StyleSmirnova, A., Konoplev, G., Mukhin, N., Stepanova, O., & Steinmann, U. (2020). Milk as a Complex Multiphase Polydisperse System: Approaches for the Quantitative and Qualitative Analysis. Journal of Composites Science, 4(4), 151. https://doi.org/10.3390/jcs4040151





