From Magneto-Dielectric Biocomposite Films to Microstrip Antenna Devices
Abstract
:1. Introduction
2. Materials and Methods
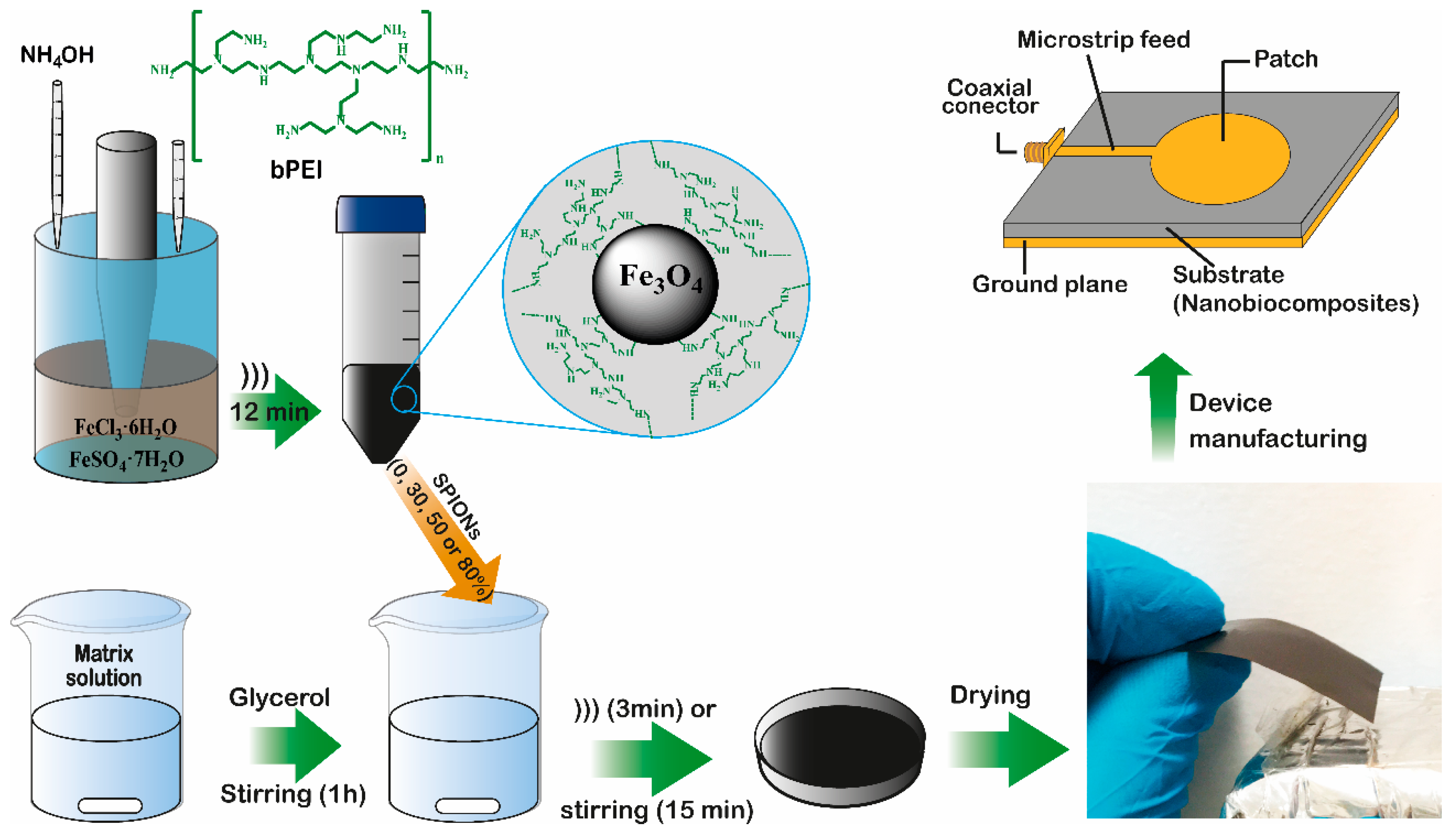
2.1. Biopolymers Obtention
2.2. Synthesis of SPIONs
2.3. Composite Films Preparation
2.4. Characterization
3. Results
3.1. Microstructural Analysis
3.1.1. XRD
3.1.2. FTIR
3.1.3. SEM
3.2. Thermogravimetric Analysis
3.3. Dielectric and Magnetic Properties
3.3.1. Microwave Dielectric Spectroscopy
3.3.2. Vibrating Sample Magnetometer
3.4. Design and Characterization of the Microstrip Patch Antennas
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahman, A.; Islam, M.T.; Singh, M.J.; Misran, N. Sol-gel synthesis of transition-metal doped ferrite compounds with potential flexible, dielectric and electromagnetic properties. RSC Adv. 2016, 6, 84562–84572. [Google Scholar] [CrossRef]
- Vural, M.; Crowgey, B.; Kempel, L.C.; Kofinas, P. Nanostructured flexible magneto-dielectrics for radio frequency applications. J. Mater. Chem. C 2014, 2, 756–763. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Fang, L.; Jiang, P.; Huang, X. Role of reduced graphene oxide in dielectric enhancement of ferroelectric polymers composites. Appl. Surf. Sci. 2019, 470, 348–359. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, H.; Jin, L.; Li, Y.; Li, J.; Gan, G.; Wei, M.; Li, M.; Liao, Y. Controllably degradable transient electronic antennas based on water-soluble PVA/TiO2 films. J. Mater. Sci. 2018, 53, 2638–2647. [Google Scholar] [CrossRef]
- Varghese, J.; Dinesh, A.; Nair, R.; Mohanan, P.; Sebastian, M.T. Dielectric, thermal and mechanical properties of zirconium silicate reinforced high density polyethylene composites for antenna applications. Phys. Chem. Chem. Phys. 2015, 17, 14943–14950. [Google Scholar] [CrossRef]
- Morales, C.; Dewdney, J.; Pal, S.; Skidmore, S.; Stojak, K.; Srikanth, H.; Weller, T.; Wang, J. Tunable Magneto-Dielectric Polymer Nanocomposites for Microwave Applications. IEEE Trans. Microw. Theory Tech. 2011, 59, 302–310. [Google Scholar] [CrossRef]
- Sun, D.; Chen, F.; Gao, Y.; Huang, S.; Wang, Y. Polymer-derived SiCN ceramics as fillers for polymer composites with high dielectric constants. J. Mater. Sci. 2019, 54, 6982–6990. [Google Scholar] [CrossRef]
- Sun, H.-S.; Chiu, Y.-C.; Chen, W.-C. Renewable polymeric materials for electronic applications. Polym. J. 2017, 49, 61–73. [Google Scholar] [CrossRef]
- Ambikeswari, N.; Manivannan, S. Superior magnetodielectric properties of room temperature synthesized superparamagnetic cobalt ferrite—Graphene oxide composite. J. Alloys Compd. 2018, 763, 711–718. [Google Scholar] [CrossRef]
- Syed Nasser, S.S.; Liu, W.; Chen, Z.N. Wide Bandwidth and Enhanced Gain of a Low-Profile Dipole Antenna Achieved by Integrated Suspended Metasurface. IEEE Trans. Antennas Propag. 2018, 66, 1540–1544. [Google Scholar] [CrossRef]
- Alqadami, A.S.M.; Jamlos, M.F.; Lago, H.; Babarinde, O.J. Bandwidth enhancement of a microstrip antenna array using magneto-dielectric polymer substrate (PDMS-Fe3O4). In Proceedings of the 2014 IEEE Symposium on Wireless Technology and Applications (ISWTA), Kota Kinabalu, Malaysia, 28 September–1 October 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 152–155. [Google Scholar]
- Ali-zade, R.A. Influence of Magnetic Field on Dielectric Permittivity of Nanocomposites on the Base of Polymeric Matrix: Collagen, Polystyrole, and Magnetite Nanoparticles. IEEE Trans. Magn. 2015, 51, 1–8. [Google Scholar] [CrossRef]
- Stojak, K.; Pal, S.; Srikanth, H.; Morales, C.; Dewdney, J.; Weller, T.; Wang, J. Polymer nanocomposites exhibiting magnetically tunable microwave properties. Nanotechnology 2011, 22, 135602. [Google Scholar] [CrossRef]
- Mallmann, E.J.J.; Goes, J.C.; Figueiro, S.D.; Ricardo, N.M.P.S.; Denardin, J.C.; Sombra, A.S.B.; Maia, F.J.N.; Mazzeto, S.E.; Fechine, P.B.A. Microstructure and magneto-dielectric properties of the chitosan/gelatin-YIG biocomposites. Express Polym. Lett. 2011, 5, 1041–1049. [Google Scholar] [CrossRef]
- Souza, N.D.G.; Freire, R.M.; Cunha, A.P.; Da Silva, M.A.S.; Mazzetto, S.E.; Sombra, A.S.B.; Denardin, J.C.; Ricardo, N.M.P.S.; Fechine, P.B.A. New magnetic nanobiocomposite based in galactomannan/glycerol and superparamagnetic nanoparticles. Mater. Chem. Phys. 2015, 156, 113–120. [Google Scholar] [CrossRef]
- Figueiro, S.D.; Mallmann, E.J.J.; Góes, J.C.; Ricardo, N.M.P.S.; Denardin, J.C.; Sombra, A.S.B.; Fechine, P.B.A. New ferrimagnetic biocomposite film based in collagen and yttrium iron garnet. Express Polym. Lett. 2010, 4, 790–797. [Google Scholar] [CrossRef]
- Gheorghiu, F.; Stanculescu, R.; Curecheriu, L.; Brunengo, E.; Stagnaro, P.; Tiron, V.; Postolache, P.; Buscaglia, M.T.; Mitoseriu, L. PVDF–ferrite composites with dual magneto-piezoelectric response for flexible electronics applications: Synthesis and functional properties. J. Mater. Sci. 2020, 55, 3926–3939. [Google Scholar] [CrossRef]
- Castro, J.; Morales, C.; Weller, T.; Wang, J.; Srikanth, H. Synthesis and characterization of low-loss Fe3O4-PDMS magneto-dielectric polymer nanocomposites for RF applications. In Proceedings of the WAMICON 2014, Tampa, FL, USA, 6 June 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 1–5. [Google Scholar]
- Muhamad, W.A.W.; Ngah, R.; Jamlos, M.F.; Soh, P.J.; Jamlos, M.A.; Lago, H. Antenna array bandwidth enhancement using polymeric nanocomposite substrate. Appl. Phys. A 2016, 122, 426. [Google Scholar] [CrossRef]
- Jafari-Soghieh, F.; Maleki, B.; Behniafar, H. Effect of dendrimer-functionalized magnetic iron oxide nanoparticles on improving thermal and mechanical properties of DGEBA/IPD epoxy networks. High Perform. Polym. 2019, 31, 24–31. [Google Scholar] [CrossRef]
- Zhu, P.; Weng, L.; Zhang, X.; Wang, X.; Guan, L.; Liu, L. Graphene@poly(dopamine)-Ag core–shell nanoplatelets as fillers to enhance the dielectric performance of polymer composites. J. Mater. Sci. 2020, 1–15. [Google Scholar] [CrossRef]
- Sun, L.; Hou, H.; Li, B.; Zhang, Y. Characterization of acid- and pepsin-soluble collagen extracted from the skin of Nile tilapia (Oreochromis niloticus). Int. J. Biol. Macromol. 2017, 99, 8–14. [Google Scholar] [CrossRef]
- Lima, H.L.S.; Gonçalves, C.; Cerqueira, M.Â.; Do Nascimento, E.S.; Gama, M.F.; Rosa, M.F.; Borges, M.d.F.; Pastrana, L.M.; Brígida, A.I.S. Bacterial cellulose nanofiber-based films incorporating gelatin hydrolysate from tilapia skin: Production, characterization and cytotoxicity assessment. Cellulose 2018, 25, 6011–6029. [Google Scholar] [CrossRef] [Green Version]
- Hestrin, S.; Schramm, M. Synthesis of cellulose by Acetobacter xylinum. II. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Andrade Neto, D.M.; Freire, R.M.; Gallo, J.; Freire, T.M.; Queiroz, D.C.; Ricardo, N.M.P.S.; Vasconcelos, I.F.; Mele, G.; Carbone, L.; Mazzetto, S.E.; et al. Rapid Sonochemical Approach Produces Functionalized Fe3O4Nanoparticles with Excellent Magnetic, Colloidal, and Relaxivity Properties for MRI Application. J. Phys. Chem. C 2017, 121, 24206–24222. [Google Scholar] [CrossRef]
- Kent, G. An Evanescent-Mode Tester for Ceramic Dielectric Substrates. IEEE Trans. Microw. Theory Tech. 1988, 36, 1451–1454. [Google Scholar] [CrossRef]
- Sebastian, M.T.; Silva, M.A.S.; Sombra, A.S.B. Measurement of Microwave Dielectric Properties and Factors Affecting Them. In Microwave Materials and Applications; Sebastian, M.T., Jantunen, H., Ubic, R., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 1–51. ISBN 9781119208549. [Google Scholar]
- Cobos, M.; González, B.; Fernández, M.J.; Fernández, M.D. Chitosan-graphene oxide nanocomposites: Effect of graphene oxide nanosheets and glycerol plasticizer on thermal and mechanical properties. J. Appl. Polym. Sci. 2017, 134, 45092. [Google Scholar] [CrossRef]
- Guerrero, P.; Muxika, A.; Zarandona, I.; De la Caba, K. Crosslinking of chitosan films processed by compression molding. Carbohydr. Polym. 2019, 206, 820–826. [Google Scholar] [CrossRef]
- Giannakas, A.; Grigoriadi, K.; Leontiou, A.; Barkoula, N.-M.; Ladavos, A. Preparation, characterization, mechanical and barrier properties investigation of chitosan–clay nanocomposites. Carbohydr. Polym. 2014, 108, 103–111. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Wang, L.; Schütz, C.; Salazar-Alvarez, G.; Titirici, M.-M. Carbon aerogels from bacterial nanocellulose as anodes for lithium ion batteries. RSC Adv. 2014, 4, 17549–17554. [Google Scholar] [CrossRef] [Green Version]
- Tabarsa, T.; Sheykhnazari, S.; Ashori, A.; Mashkour, M.; Khazaeian, A. Preparation and characterization of reinforced papers using nano bacterial cellulose. Int. J. Biol. Macromol. 2017, 101, 334–340. [Google Scholar] [CrossRef]
- Wada, M.; Okano, T. Localization of Iα and Iβ phases in algal cellulose revealed by acid treatments. Cellulose 2001, 8, 183–188. [Google Scholar] [CrossRef]
- Jin, E.; Guo, J.; Yang, F.; Zhu, Y.; Song, J.; Jin, Y.; Rojas, O.J. On the polymorphic and morphological changes of cellulose nanocrystals (CNC-I) upon mercerization and conversion to CNC-II. Carbohydr. Polym. 2016, 143, 327–335. [Google Scholar] [CrossRef]
- Kloster, G.A.; Marcovich, N.E.; Mosiewicki, M.A. Composite films based on chitosan and nanomagnetite. Eur. Polym. J. 2015, 66, 386–396. [Google Scholar] [CrossRef]
- Matet, M.; Heuzey, M.-C.; Pollet, E.; Ajji, A.; Avérous, L. Innovative thermoplastic chitosan obtained by thermo-mechanical mixing with polyol plasticizers. Carbohydr. Polym. 2013, 95, 241–251. [Google Scholar] [CrossRef] [PubMed]
- De Morais Lima, M.; Bianchini, D.; Guerra Dias, A.; Da Rosa Zavareze, E.; Prentice, C.; Da Silveira Moreira, A. Biodegradable films based on chitosan, xanthan gum, and fish protein hydrolysate. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Khoury, C.; Gazit, O.M. Self-Organized Porous Titanium-Chitosan Hybrid Materials with Tunable Functions. ChemNanoMat 2018, 4, 353–360. [Google Scholar] [CrossRef]
- Freire, T.M.; Dutra, L.M.U.; Queiroz, D.C.; Ricardo, N.M.P.S.; Barreto, K.; Denardin, J.C.; Wurm, F.R.; Sousa, C.P.; Correia, A.N.; De Lima-Neto, P.; et al. Fast ultrasound assisted synthesis of chitosan-based magnetite nanocomposites as a modified electrode sensor. Carbohydr. Polym. 2016, 151, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L.; Yi, R.; Xu, N.; Gao, R.; Hong, B. Extraction and characterization of acid-soluble collagen from scales and skin of tilapia (Oreochromis niloticus). LWT Food Sci. Technol. 2016, 66, 453–459. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Gad, A.; Abu-Hussein, A.E.-H.G.; Habib, S.I.; Badr, N.A.; Hashem, A.A. Chemical and biological evaluation of Egyptian Nile Tilapia (Oreochromis niloticas) fish scale collagen. Int. J. Biol. Macromol. 2015, 79, 618–626. [Google Scholar] [CrossRef]
- Chen, J.-H.; Liu, J.-G.; Su, Y.-Q.; Xu, Z.-H.; Li, M.-C.; Ying, R.-F.; Wu, J.-Q. Preparation and properties of microfibrillated cellulose with different carboxyethyl content. Carbohydr. Polym. 2019, 206, 616–624. [Google Scholar] [CrossRef]
- Gea, S.; Reynolds, C.T.; Roohpour, N.; Wirjosentono, B.; Soykeabkaew, N.; Bilotti, E.; Peijs, T. Investigation into the structural, morphological, mechanical and thermal behaviour of bacterial cellulose after a two-step purification process. Bioresour. Technol. 2011, 102, 9105–9110. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, M.; Dong, L.; Xie, H.; Xiong, C. Particle Size Dependence of the Dielectric Properties of Polyvinyledene Fluoride/Silver Composites. J. Macromol. Sci. Part B 2013, 52, 1073–1081. [Google Scholar] [CrossRef]
- Zhao, C.; Wei, X.; Huang, Y.; Ma, J.; Cao, K.; Chang, G.; Yang, J. Preparation and unique dielectric properties of nanoporous materials with well-controlled closed-nanopores. Phys. Chem. Chem. Phys. 2016, 18, 19183–19193. [Google Scholar] [CrossRef] [PubMed]
- Iswariya, S.; Bhanukeerthi, A.V.; Velswamy, P.; Uma, T.S.; Perumal, P.T. Design and development of a piscine collagen blended pullulan hydrogel for skin tissue engineering. RSC Adv. 2016, 6, 57863–57871. [Google Scholar] [CrossRef]
- Maria, V.D.; Bernal, C.; Francois, N.J.; Francois, N.J. Development of Biodegradable Films Based on Chitosan/Glycerol Blends Suitable for Biomedical Applications. J. Tissue Sci. Eng. 2016, 7. [Google Scholar] [CrossRef]
- Li, J.; Li, G. The thermal behavior of collagen in solution: Effect of glycerol and 2-propanol. Int. J. Biol. Macromol. 2011, 48, 364–368. [Google Scholar] [CrossRef]
- Augustine, R.; Kalappura, U.G.; Laheurte, J.-M.; Mathew, K.T. Biocompatibility study of beta tricalcium phosphate bioceramics and chitosan biopolymer and their use as phantoms for medical imaging applications. Microw. Opt. Technol. Lett. 2009, 51, 2923–2927. [Google Scholar] [CrossRef]
- Da Silva, A.L.; Da Silva, L.R.R.; Camargo, I.d.A.; Agostini, D.L.d.S.; Denardin, J.C.; Rosa, D.d.S.; Mele, G.; De Oliveira, D.L.V.; Fechine, P.B.A.; Mazzetto, S.E. Superparamagnetic nano-biocomposites for application as dielectric resonator antennas. Mater. Chem. Phys. 2017, 185, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Vikram Babu, B.; Vijaya Babu, K.; Tewodros Aregai, G.; Seeta Devi, L.; Madhavi Latha, B.; Sushma Reddi, M.; Samatha, K.; Veeraiah, V. Structural and electrical properties of Li4Ti5O12 anode material for lithium-ion batteries. Results Phys. 2018, 9, 284–289. [Google Scholar] [CrossRef]
- Bibikov, S.B.; Kulikovskij, E.I.; Sharafiev, R.S.; Bychkova, A.V.; Ol’khov, A.A. Nanocomposites for antenna-feeder systems. In Proceedings of the 2013 IX Internatioal Conference on Antenna Theory and Techniques, Odesa, Ukraine, 16–20 September 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 500–502. [Google Scholar]
- Balanis, C.A. Antenna Theory: Analysis and Design, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; ISBN 0-471-66782-X. [Google Scholar]
- Melone, L.; Rossi, B.; Pastori, N.; Panzeri, W.; Mele, A.; Punta, C. TEMPO-Oxidized Cellulose Cross-Linked with Branched Polyethyleneimine: Nanostructured Adsorbent Sponges for Water Remediation. Chempluschem 2015, 80, 1408–1415. [Google Scholar] [CrossRef]
- Nunes, W.C.; Cebollada, F.; Knobel, M.; Zanchet, D. Effects of dipolar interactions on the magnetic properties of γ-Fe2O3 nanoparticles in the blocked state. J. Appl. Phys. 2006, 99, 08N705. [Google Scholar] [CrossRef] [Green Version]
- Tung, T.T.; Chen, S.J.; Fumeaux, C.; Losic, D. Scalable realization of conductive graphene films for high-efficiency microwave antennas. J. Mater. Chem. C 2016, 4, 10620–10624. [Google Scholar] [CrossRef]
- Yousefi, L.; Mohajer-Iravani, B.; Ramahi, O.M. Enhanced bandwidth artificial magnetic ground plane for low-profile antennas. IEEE Antennas Wirel. Propag. Lett. 2007, 6, 289–292. [Google Scholar] [CrossRef]
- Sharifi, M.; Rezaei, P. Designing and modeling of compact microstrip antennas using new nanocomposite materials. ARPN J. Eng. Appl. Sci. 2017, 12, 6825–6833. [Google Scholar]
- Jose, S.K.; Suganthi, S. Circular-Rectangular Microstrip Antenna for Wireless Applications. Int. J. Microw. Appl. 2015, 4, 6–10. [Google Scholar]
- De Morais, J.E.V.; De Castro, A.J.N.; Oliveira, R.G.M.; Do Carmo, F.F.; Sales, A.J.M.; Sales, J.C.; Silva, M.A.S.; Gouveia, D.X.; Costa, M.M.; Rodrigues, A.R.; et al. Magneto Tuning of a Ferrite Dielectric Resonator Antenna Based on LiFe5O8 Matrix. J. Electron. Mater. 2018, 47, 3829–3835. [Google Scholar] [CrossRef]
- Fechine, P.B.A.; Moretzsohn, R.S.T.; Costa, R.C.S.; Derov, J.; Stewart, J.W.; Drehman, A.J.; Junqueira, C.; Sombra, A.S.B. Magneto-dielectric properties of the Y3Fe5O12 and Gd3Fe5O12 dielectric ferrite resonator antennas. Microw. Opt. Technol. Lett. 2008, 50, 2852–2857. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Saraswat, S.; Gulati, G.; Shekhar, S.; Joshi, K.; Sharma, K. Dual band multi frequency rectangular patch microstrip antenna with flyswatter shaped slot for wireless systems. In Proceedings of the AIP Conference Proceedings, Rajasthan, India, 18–20 October 2015; AIP Publishing: Melville, NY, USA, 2016; Volume 1715, p. 020033. [Google Scholar]








| Samples | GL (%) | SPION (%) | Thickness (μm) |
|---|---|---|---|
| Ch0 | 20 | 0 | 88 |
| Ch30 | 20 | 30 | 67 |
| Ch50 | 20 | 50 | 61 |
| Ch80 | 20 | 80 | 66 |
| Col0 | 20 | 0 | 43 |
| Col30 | 20 | 30 | 127 |
| Col50 | 20 | 50 | 148 |
| Col80 | 20 | 80 | 155 |
| BC0 | 25 | 0 | 29 |
| BC30 | 25 | 30 | 47 |
| BC50 | 25 | 50 | 54 |
| BC80 | 25 | 80 | 65 |
| Samples | 1st Event | 2nd Event | 3rd Event | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TR | TM | WL | TR | TM | WL | TR | TM | WL | RW | |
| Ch0 | 30–119 | 62 | 14.76 | 119–212 | 177 | 13.77 | 212–402 | 284 | 36.85 | 3.59 |
| Ch80 | 30–106 | 60 | 8.05 | 106–218 | 174 | 10.61 | 218–390 | 288 | 21.26 | 38.18 |
| Col0 | 30–119 | 62 | 8.64 | 119–233 | 191 | 13.66 | 233–500 | 315 | 51.16 | 0.16 |
| Col80 | 30–124 | 52 | 6.68 | 124–239 | 217 | 8.72 | 239–423 | 300 | 18.68 | 37.86 |
| BC0 | 30–108 | 60 | 7.57 | 108–224 | 203 | 17.16 | 224–375 | 329 | 44.71 | 2.61 |
| BC80 | 30–107 | 55 | 5.80 | 107–215 | 189 | 7.06 | 215–371 | 333 | 33.12 | 38.18 |
| Fe3O4@bPEI | 30–151 | 54 | 3.02 | 151–280 | 244 | 3.35 | 280–434 | 342 | 5.52 | 86.97 |
| Samples | ε′ (4.33 GHz) | fo (GHz) Calc. | fo (GHz) Exp. | Error (%) | BW (%) |
|---|---|---|---|---|---|
| Ch0 | 5.2 | 5.89 | 5.55 | 6.12 | 5.47 |
| Ch30 | 6.1 | 5.44 | 5.41 | 0.55 | 3.40 |
| Ch50 | 6.8 | 5.18 | 5.23 | 0.96 | 5.35 |
| Ch80 | 8.3 | 4.67 | 4.69 | 0.43 | 5.58 |
| Col0 | 5.9 | 5.55 | 5.44 | 2.02 | 6.36 |
| Col30 | 7.2 | 5.02 | 5.33 | 5.82 | 3.27 |
| Col50 | 7.5 | 4.90 | 5.03 | 2.58 | 4.81 |
| Col80 | 9.1 | 4.46 | 4.93 | 9.53 | 5.61 |
| BC0 | 6.7 | 5.20 | 5.18 | 0.39 | 5.57 |
| BC30 | 7.3 | 4.98 | 4.97 | 0.20 | 6.34 |
| BC50 | 7.5 | 4.92 | 4.72 | 4.24 | 5.65 |
| BC80 | 8.4 | 4.66 | 4.63 | 0.65 | 6.10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Menezes, F.L.; Andrade Neto, D.M.; Rodrigues, M.d.L.L.; Lima, H.L.S.; Paiva, D.V.M.; da Silva, M.A.S.; Fechine, L.M.U.D.; Sombra, A.S.B.; Freire, R.M.; Denardin, J.C.; et al. From Magneto-Dielectric Biocomposite Films to Microstrip Antenna Devices. J. Compos. Sci. 2020, 4, 144. https://doi.org/10.3390/jcs4040144
de Menezes FL, Andrade Neto DM, Rodrigues MdLL, Lima HLS, Paiva DVM, da Silva MAS, Fechine LMUD, Sombra ASB, Freire RM, Denardin JC, et al. From Magneto-Dielectric Biocomposite Films to Microstrip Antenna Devices. Journal of Composites Science. 2020; 4(4):144. https://doi.org/10.3390/jcs4040144
Chicago/Turabian Stylede Menezes, Fernando Lima, Davino Machado Andrade Neto, Maria do Livramento Linhares Rodrigues, Helder Levi Silva Lima, Denis Valony Martins Paiva, Marcelo Antônio Santos da Silva, Lillian Maria Uchôa Dutra Fechine, Antônio Sérgio Bezerra Sombra, Rafael Melo Freire, Juliano Casagrande Denardin, and et al. 2020. "From Magneto-Dielectric Biocomposite Films to Microstrip Antenna Devices" Journal of Composites Science 4, no. 4: 144. https://doi.org/10.3390/jcs4040144








