The Use of Conductive Polymers Embedded Macro Porous Pei and Ionic Liquid Form of Pei Cryogels for Potential Conductometric Sensor Application to CO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Macro Porous PEI and PIL PEI Cryogels
2.3. In situ Preparation of Conductive Polymers within PEI Cryogels
2.4. Instruments
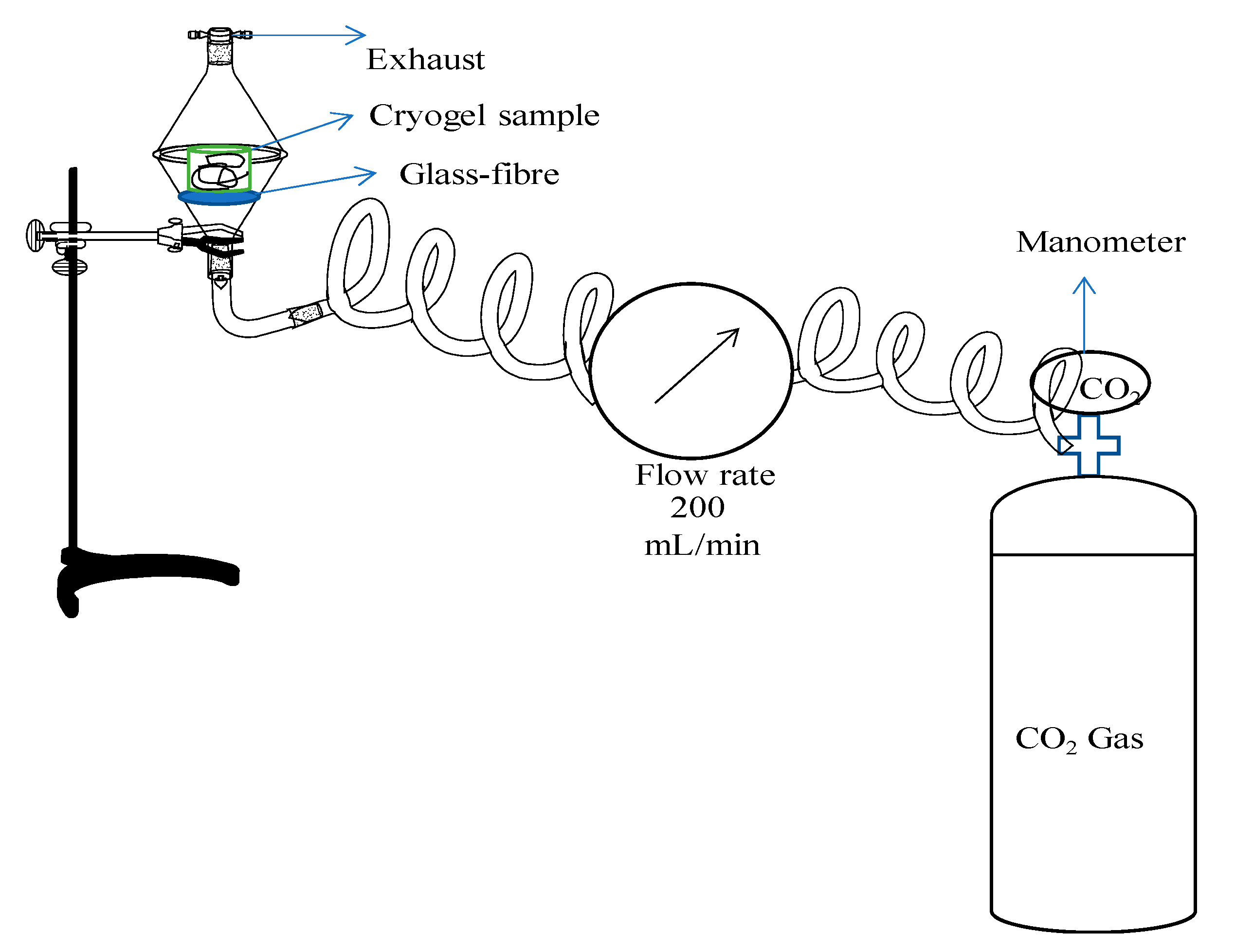
2.5. Conductometric Sensor Application of PEI-Based Composites against CO2 Gas
3. Results
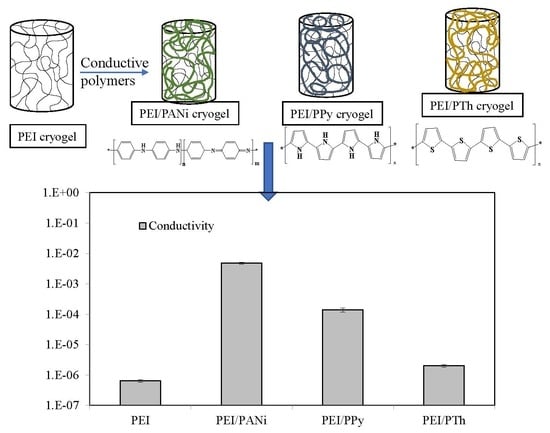
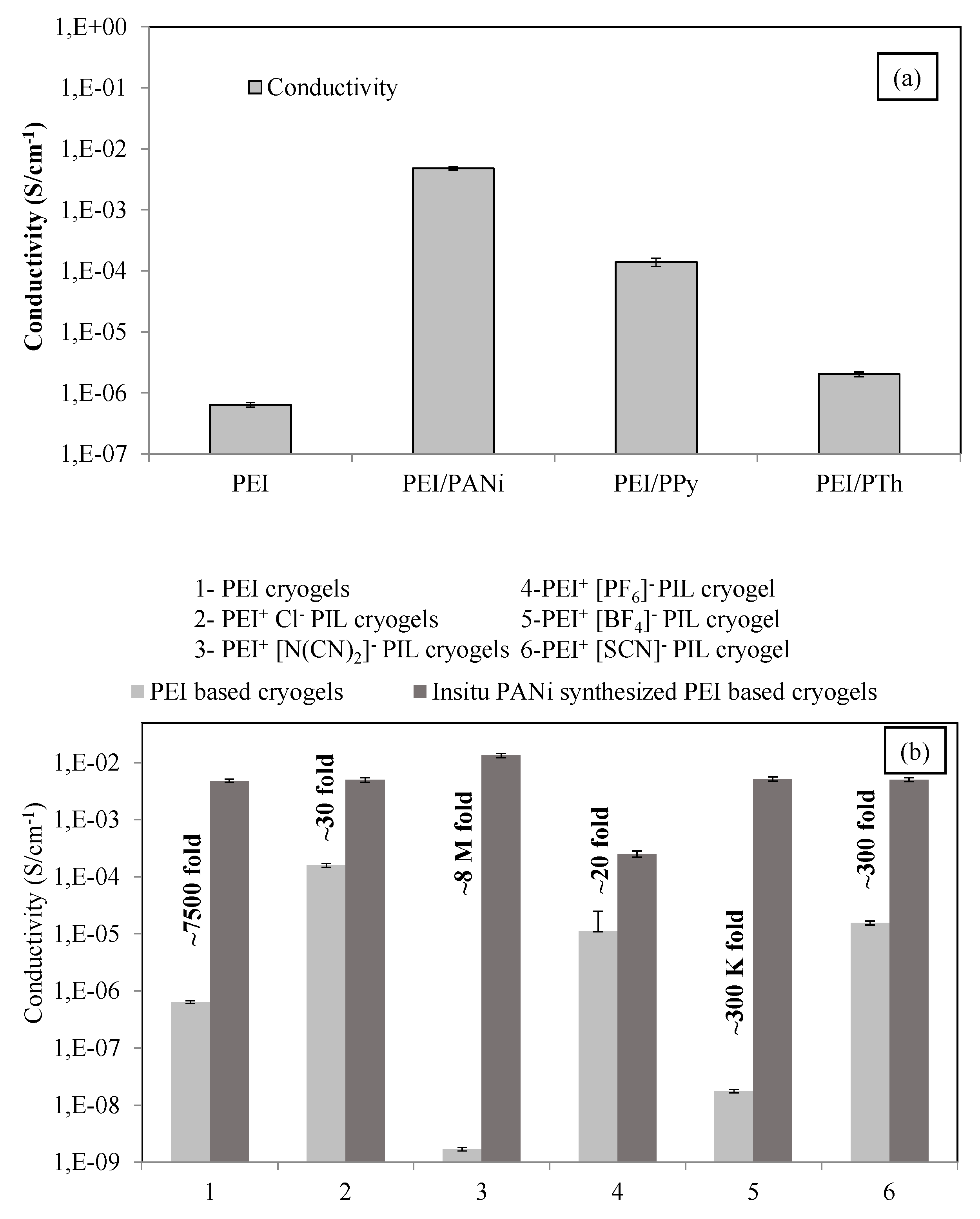
3.1. Synthesis and Characterization of PEI Cryogel/Conductive Polymer Composites
Conductivity Measurements
3.2. Sensor Application
3.2.1. PEI/Conductive Polymer Cryogel Composites as Sensing Material
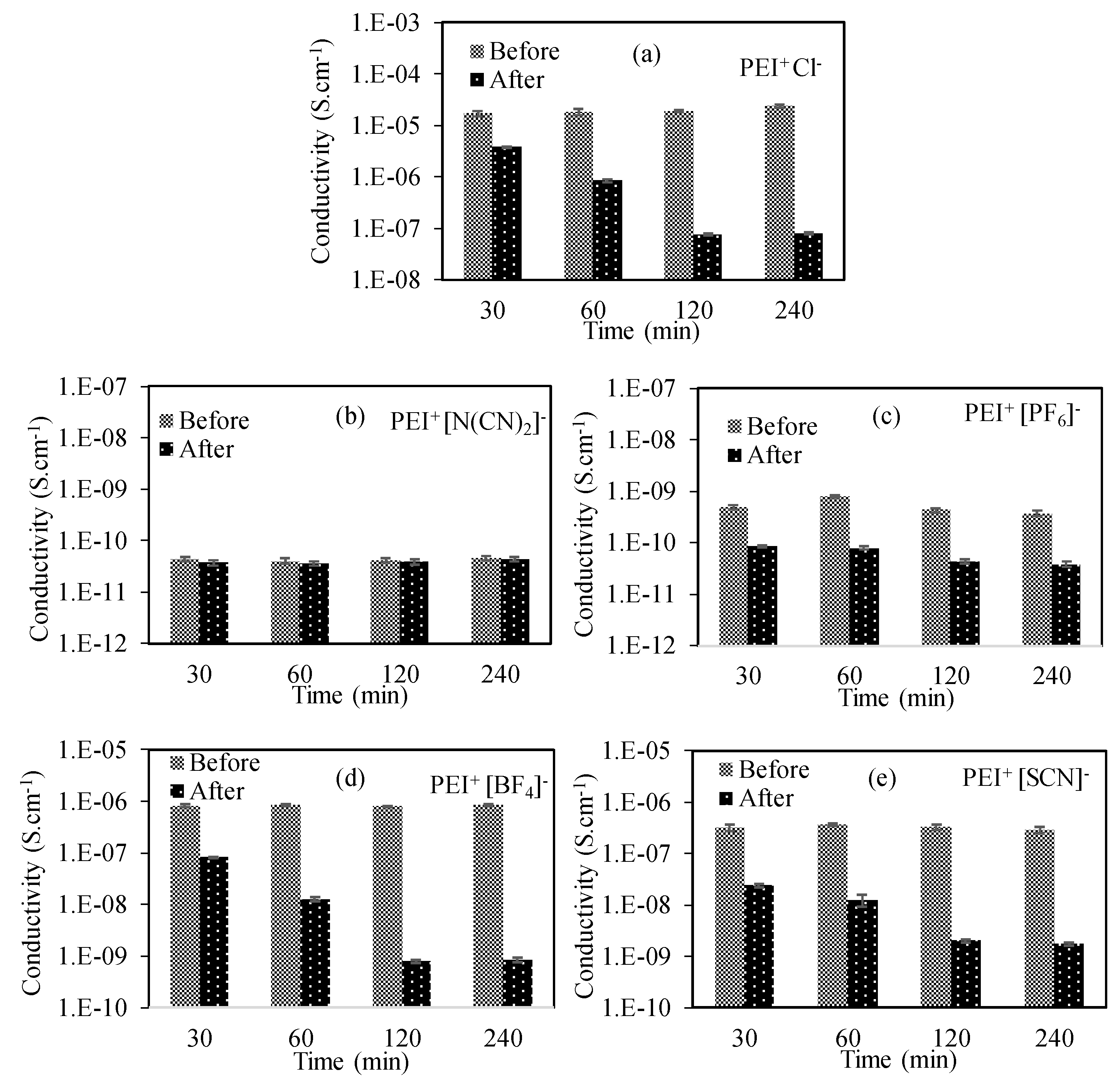
3.2.2. The Use of IL Forms of Pei Cryogels
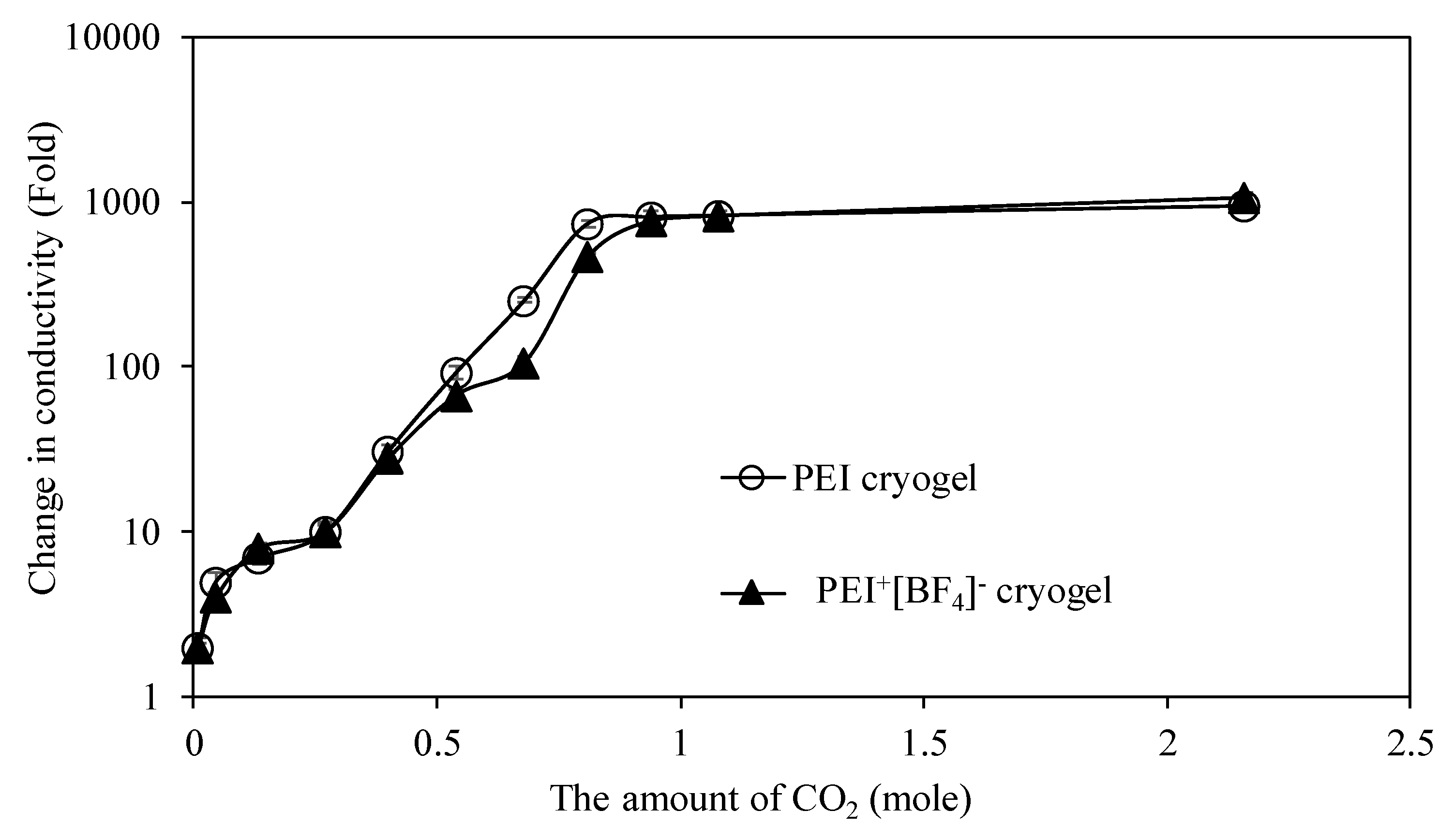
3.2.3. Sensitivity of PEI-Based Cryogels as a CO2 Gas Sensor
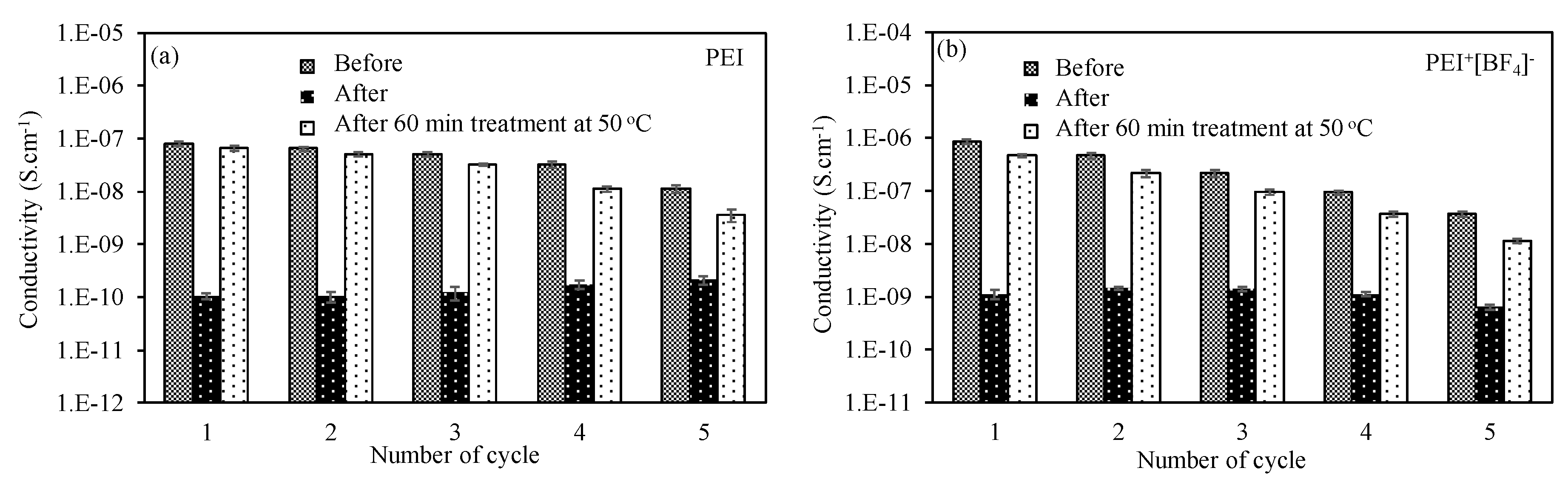
3.2.4. Reusability of PEI-Based Cryogel as a CO2 Gas Sensor
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Metz, B.; Davidson, O.; de Coninck, H.; Loos, M.; Meyer, L. IPCC Special Report on Carbon Dioxide Capture And Storage, Prepared by Working Group Iii of The Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2005. [Google Scholar]
- Halmann, M.M.; Stenberg, M. Greenhouse Gas Carbon Dioxide Mitigation; CRC Press LLC: Boca Raton, FL, USA, 1999. [Google Scholar]
- Stewart, C.; Hessami, M.A. A study of methods of carbon dioxide capture and sequestration-the sustainability of a photosynthetic bioreactor approach. Energy Convers. Manage. 2005, 46, 403–420. [Google Scholar] [CrossRef]
- The Scientific Basis, Impacts, Adaptation and Vulnerability Mitigation; International Panel on Climate Change: Geneve, Switzerland, 2001.
- Zosel, J.; Oelssner, W.; Decker, M.; Gerlach, G.; Guth, U. The measurement of dissolved and gaseous carbon dioxide concentration. Meas. Sci. Technol. 2011, 22, 72001. [Google Scholar] [CrossRef]
- Perez de Vargas-Sansalvador, I.M.; Fay, C.; Phelan, T.; Fernandez-Ramos, M.D.; Capitan-Vallvey, L.F.; Diamond, D.; Benito-Lopez, F. A new light emitting diode light emitting diode portable carbon dioxide gas sensor based on an interchangeable membrane system for industrial applications. Anal. Chim. Acta 2011, 699, 216–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herber, S.; Bomer, J.; Olthuis, W.; Bergveld, P.; van den Berg, A. A miniaturized carbon dioxide gas sensor based on sensing of pH-sensitive hydrogel swelling with a pressure sensor. Biomed. Microdevices 2005, 7, 197–204. [Google Scholar] [CrossRef]
- Trung, D.D.; Toan, L.D.; Hong, H.S.; Lam, T.D.; Trung, T.; Van Hieu, N. Selective detection of carbon dioxide using LaOCl-functionalized SnO2 nanowires for air-quality monitoring. Talanta 2012, 88, 152–159. [Google Scholar] [CrossRef]
- Dell’Amico, D.B.; Calderazzo, F.; Labella, L.; Marchetti, F.; Pampaloni, G. Converting carbon dioxide into carbamato derivatives. Chem. Rev. 2003, 103, 3857–3898. [Google Scholar] [CrossRef]
- Rackley, S.A. Carbon Capture and Storage; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar]
- Sahiner, N.; Demirci, S. Poly ionic liquid cryogel of polyethyleneimine: Synthesis, characterization, and testing in absorption studies. J. Appl. Polym. Sci. 2016, 133, 43478. [Google Scholar] [CrossRef]
- Sahiner, N.; Demirci, S. The use of p(4-VP) cryogel as template for in situ preparation of p(An), p(Py), and p(Th) conductive polymer and their potential sensor applications. Synth. Metal. 2017, 227, 11–20. [Google Scholar] [CrossRef]
- Sahiner, N.; Demirci, S. Conducting semi-interpenetrating polymeric composites via the preparation of poly(aniline), poly(thiophene), and poly(pyrrole) polymers within superporous poly(acrylic acid) cryogels. React. Funct. Polym. 2016, 105, 60–65. [Google Scholar] [CrossRef]
- Vera, R.A.; Romero, H.B.; Ahumada, E. Synthesis and characterization of polyaniline and poly-ortho-methoxyaniline. Behavior against carbon steel corrosion. J. Chil. Chem. Soc. 2003, 48, n.1. [Google Scholar] [CrossRef]
- Xu, C.; Sun, J.; Gao, L. Synthesis of novel hierarchical graphene/polypyrrole nanosheet composites and their superior electrochemical performance. J. Mater. Chem. 2011, 21, 11253–11258. [Google Scholar] [CrossRef]
- Liao, J.; Wang, Y.; Chang, L.; Bao, W. A process for de sulfurization of coking benzene by a two-step method with reuse of sorbent/thiophene and its key procedures. Green Chem. 2015, 17, 3164–3175. [Google Scholar] [CrossRef]
- Fatoni, A.; Numnuam, A.; Kanatharana, P.; Limbut, W.; Thavarungkul, P. A novel molecularly imprinted chitosan-acrylamide, graphene, ferrocene composite cryogel biosensor used to detect microalbumin. Analyst 2014, 139, 6160–6167. [Google Scholar] [CrossRef]
- Kangkamano, T.; Mumnuam, A.; Limbut, W.; Kanatharana, P.; Thavarungkul, P. Chitosan cryogel with embedded gold nanoparticles decorated multıwalled carbon nanotubes modified electrode for highly sensitive flow based non-enzymatic glucose sensor. Sens. Actuat. B Chem. 2017, 246, 85–863. [Google Scholar] [CrossRef]
- Choi, W.; Min, K.; Kim, C.; Koo, Y.S.; Jeon, J.W.; Seo, H.; Park, Y.K.; Choi, M. Epozide-functionalization of polyethyleneimne for synthesis of stable carbon dioxide adsorbent in temperature swing adsorption. Nat. Commun. 2016, 7, 12640. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.S.; Lee, S.Y.; Park, S.J. Preparation and characterization of multiwalled carbon nanotubes imprenated with polyethyleneimine for carbondioxide capture. Int. J. Hydrogen Energy 2015, 40, 3415–3421. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, Q.; Li, M.; Zheng, W.; Christianson, D.; Economy, J. Adsorptive removal of carbon dioxide using polyethyleneimne loaded glass fiber in a fixed bed. Colloid. Surf. A Physicochem. Eng. Aspect. 2015, 481, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Star, A.; Han, T.R.; Joshi, V.; Gabriel, J.C.P.; Grüner, G. Nanoelectronic carbon dioxide sensors. Adv. Mater. 2004, 16, 22. [Google Scholar] [CrossRef]
- Srinives, S.; Sarkar, T.; Hernandez, R.; Mulchandani, A. Aminiature chemiresistor sensor for carbon dioxide. Anal. Chim. Acta 2015, 874, 54–58. [Google Scholar] [CrossRef]
- Selampinar, F.; Toppare, L.; Akbulut, U.; Yalcin, T.; Suzer, S. A conducting composite of polypyrrole II. As a gas sensor. Synth. Metal. 1995, 68, 109–116. [Google Scholar] [CrossRef]
- Willa, C.; Yuan, J.; Niedeberger, M.; Koziej, D. When nanoparticles meet poly(ionic liquid)s: Chemoreistive CO2 sensing at room temperature. Adv. Funct. Mater. 2015, 25, 2537–2542. [Google Scholar] [CrossRef] [Green Version]
- Behera, K.; Pandey, S.; Kadyan, A.; Pandey, S. Ionic liquid-based optical and electrochemical carbon dioxide sensors. Sensors 2015, 15, 30487–30503. [Google Scholar] [CrossRef] [Green Version]
- Brennecke, J.F.; Gurkan, B.E. Ionic liquids for CO2 capture and emission reduction. J. Phys. Chem. Lett. 2010, 1, 3459–3464. [Google Scholar] [CrossRef]
- Lu, B.; Wang, X.; Xia, Y.; Liu, N.; Li, S.; Li, W. Kinetics of carbon dioxide absorption into mixed aqueous solutions of MEA+ [Bmim]BF4 using a double stirred cell. Energy Fuels 2013, 27, 6002–6009. [Google Scholar] [CrossRef]
- Dutcher, B.; Fan, M.; Russell, A.G. Amine-based CO2 capture technology development from the beginning of 2013-A review. ACS Appl. Mater. Interfaces 2015, 7, 2137–2148. [Google Scholar] [CrossRef]
- Carvalho, P.J.; Alvarez, V.H.; Marrucho, I.M.; Aznar, M.; Couthinho, J.A.P. High pressure phase behavior or carbon dioxide in 1-buthyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide and 1-butyl-3-methylimidazolium dicyanamide ionic liquids. J. Supercrit. Flu. 2009, 50, 105–111. [Google Scholar] [CrossRef]
- Erdmann, C.A.; Apte, M.G. Mucous membrane and lower respiratory building related symptoms in relation to indoor carbon dioxide concentrations in the 100-building BASE dataset. Indoor Air 2004, 14, 127–134. [Google Scholar] [CrossRef]
- Lin, Y.; Fan, Z. Compositing strategies to enhance the performance of chemiresistive CO2 gas sensors. Mater. Sci. Semicond. Process 2020, 107, 104820. [Google Scholar] [CrossRef]

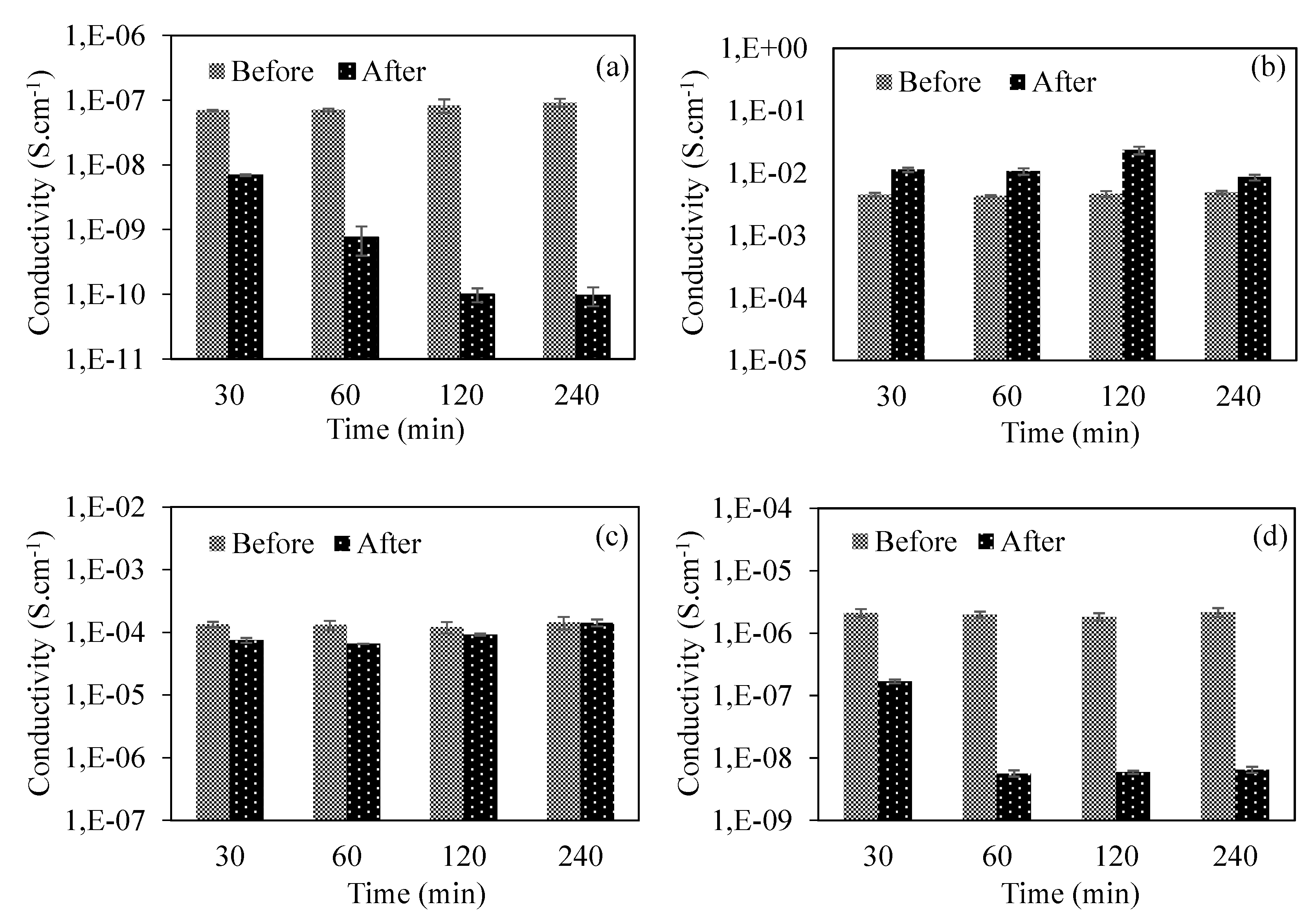
| Cryogel Composite | Conductivity (S.cm−1) | ||||
|---|---|---|---|---|---|
| 30 min | 60 min | 120 min | 240 min | ||
| PEI | Before | 7.0 × 10−8 | 7.1 × 10−8 | 8.3 × 10−8 | 9.2 × 10−8 |
| After | 6.9 × 10−9 | 7.6 × 10−10 | 1.0 × 10−10 | 9.7 × 10−11 | |
| PEI/PANi | Before | 4.5 × 10−3 | 4.3 × 10−3 | 4.6 × 10−3 | 4.9 × 10−3 |
| After | 1.1 × 10−2 | 1.1 × 10−2 | 2.3 × 10−2 | 8.5 × 10−3 | |
| PEI/PPy | Before | 1.3 × 10−4 | 1.3 × 10−4 | 1.2 × 10−4 | 1.4 × 10−4 |
| After | 7.5 × 10−5 | 6.5 × 10−5 | 9.2 × 10−5 | 1.4 × 10−4 | |
| PEI/PTh | Before | 2.1 × 10−6 | 2.0 × 10−6 | 1.8 × 10−6 | 2.2 × 10−6 |
| After | 1.7 × 10−7 | 5.7 × 10−9 | 5.9 × 10−9 | 6.5 × 10−9 | |
| The Decrease in Conductivity (Fold) | ||||||
|---|---|---|---|---|---|---|
| PEI | PEI+ Cl− | PEI+ [N(CN)2]− | PEI+ [PF6]− | PEI+ [BF4]− | PEI+ [SCN]− | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 30 | 10 | 4.4 | 1 | 5 | 10 | 13.3 |
| 60 | 100 | 20 | 1 | 10 | 66.7 | 30 |
| 120 | 1000 | 250 | 1 | 10 | 1000 | 160 |
| 240 | 1000 | 300 | 1 | 10 | 1000 | 300 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirci, S.; Sahiner, N. The Use of Conductive Polymers Embedded Macro Porous Pei and Ionic Liquid Form of Pei Cryogels for Potential Conductometric Sensor Application to CO2. J. Compos. Sci. 2020, 4, 27. https://doi.org/10.3390/jcs4010027
Demirci S, Sahiner N. The Use of Conductive Polymers Embedded Macro Porous Pei and Ionic Liquid Form of Pei Cryogels for Potential Conductometric Sensor Application to CO2. Journal of Composites Science. 2020; 4(1):27. https://doi.org/10.3390/jcs4010027
Chicago/Turabian StyleDemirci, Sahin, and Nurettin Sahiner. 2020. "The Use of Conductive Polymers Embedded Macro Porous Pei and Ionic Liquid Form of Pei Cryogels for Potential Conductometric Sensor Application to CO2" Journal of Composites Science 4, no. 1: 27. https://doi.org/10.3390/jcs4010027
APA StyleDemirci, S., & Sahiner, N. (2020). The Use of Conductive Polymers Embedded Macro Porous Pei and Ionic Liquid Form of Pei Cryogels for Potential Conductometric Sensor Application to CO2. Journal of Composites Science, 4(1), 27. https://doi.org/10.3390/jcs4010027