1. Introduction
Poly (lactic acid) (PLA) is a polyester and presents good mechanical properties and biocompatibility, and it is bioabsorbable [
1]. Due to these characteristics, the PLA is being widely used in medical applications such as intradermal sutures, fracture recovery devices and tissue regeneration (TR) [
2,
3]. However, PLA does not present osteoinduction behavior when implanted, and one way of conferring this property to it is through the addition of bioactive fillers [
4,
5]. The bioglass developed by Hench (45S5) is the “gold standard” for bone implants; however, other highly bioactive materials have been discovered, as Biosilicate
®. Biosilicate
® has been used in the dental area to treat dentin hypersensitivity, due to its osteoinductive and osteoconductive properties it aids bone healing and growth [
6]. It is interesting to observe that Biosilicate
® also has the potential to be applied in antibacterial coatings due to its antimicrobial properties that inhibit bacterial activity [
7]. It is interesting to point out that this intrinsic property of Biosilicate
® resulted in a considerable reduction of the anaerobic bacteria after 10 min contact tests [
7]. Granito et al. [
8,
9] implanted 45S5 and Biosilicate
® glasses in rat tibial defects and it was observed that Biosilicate
® was more tenacious and more bioactive when compared to the gold standard of the bioglasses (45S5). Biosilicate
® has been widely researched (over 30 scientific papers and 28 theses) and it was demonstrated that this material is highly bioactive and can be used for bone tissue regeneration [
9]. PLA/bioglass composites represent a promising class of materials which can be applied in the tissue regeneration because these composites combine the flexibility, conformability, and bioabsorption capacity of PLA and at the same time combine the stiffness, mechanical resistance, and bioactivity of the bioglasses [
8]. The current techniques used for the preparation of PLA/bioglass composites are based mainly on the use of a solvent such as solvent casting-particulate leaching [
10,
11] and phase inversion [
5,
12]. Blaker et al. [
13] produced PLA scaffolds with different contents of bioglass by the phase inversion method and then evaluated the formation of hydroxyapatite on the surface of the scaffolds after different immersion times in simulated body fluid (SBF). The SBF test evaluated the in vitro bioactivity of biomaterials and it was observed that in the PLA/bioglass composite (95/5 m/m) the formation of hydroxyapatite occurred after only 3 days of immersion. These results are a strong evidence of the bioactivity potential of bioglasses; however further bioactive properties were assessed with cell lines cultures. The authors performed infiltration of osteoblasts into these scaffolds and noted that the cells were able to migrate through the pores of the scaffolds and colonize even deeper regions, indicating that these materials have a biochemical structure and composition capable of supporting the bonds of osteoblasts [
13].
The main disadvantage of phase inversion and solvent casting-particulate leaching methods is the use of solvents which, in most cases, are toxic and can remain in the scaffold produced. In the area of preparation of scaffolds, other methods are being applied, such as 3D printing. However, it is necessary to modify the filament to produce scaffolds for bone tissue regeneration using this technique. Cunha et al. [
14] produced 3D scaffolds of PCL/Biosilicate
® and the observed improvement in the mechanical properties and the addition of Biosilicate
® provided a good environment for cell attachment and proliferation [
14]. It is worth noticing that the printed scaffold used a specific printing setup where the two separated materials were feed in powder form which may lead to some drawbacks as poor filler dispersion. In this work, a different route is proposed to obtain PLA/Biosilicate
® composites. The aim of this study was the development of melt-processed PLA/Biosilicate
® composites using an internal melt mixer. Two different PLA’s were used for composites production and were analyzed through thermogravimetric analysis (TGA), rheological behavior, and molar mass characterization. The effect of Biosilicate
® addition on PLA matrices by this type of processing is promising for the preparation of filaments to produce 3D scaffolds for bone tissue regeneration.
3. Results and Discussion
In this study, PLA and PLA/Biosilicate
® composites were processed using an internal melt mixer. Two different PLA’s were investigated, and
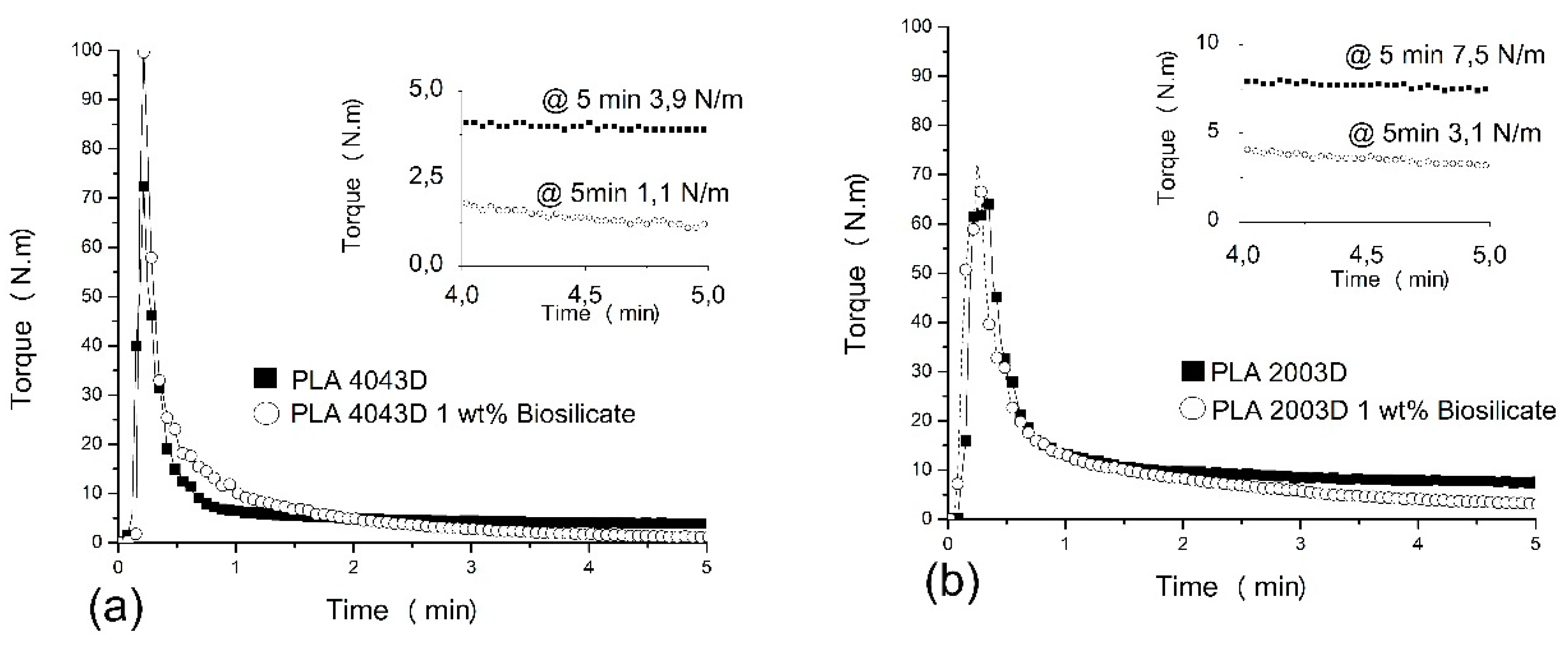
Figure 1 presents the curves of torque versus time obtained by torque rheometry for the both PLAs (PLA 4043D and PLA 2003D) and PLA/Biosilicate
® composites with the addition of 1wt% of Biosilicate
®. The torque peak corresponds to the addition of the compounds. Comparing the results of the different neat PLAs (
Figure 1a,b), it is observed that the maximum torque of PLA 4043D is 73 N m and for the PLA 2003D is 65 N m. The addition of Biosilicate
® changes the maximum torque values for both composites, being more evident for the composite with PLA 4043D. In
Figure 1a it is possible to observe that PLA 4043D presents constant torque (3.9 N m) from 1 to 5 min of mixing and the addition of only 1 wt. % of Biosilicate
® resulted in a continuous decrease in torque obtaining a significantly lower value (1.1 N m) compared to neat PLA 4043D. In
Figure 1b is observed that PLA 2003D shows a similar behavior as PLA 4043D, i.e., the torque is constant from 1 to 5 min. However, for this PLA 2003D, the torque at 5 min is higher than for PLA 4043D (3.1 N m for PLA 2003D and 1.1 N m for PLA 4043D). The addition of Biosilicate
® in PLA 2003D also caused a decrease in the torque during the processing of the composite. The continuous decrease of torque during processing may be associated with the degradation process with the breaking of the polymer chains. The literature shows that the main reaction of the thermo-oxidative degradation mechanism of PLA (neat) is non-radical with intramolecular transesterification. The reactions involved during the thermal degradation of PLA are non-radical scission, backbiting ester interchange, cis-elimination and they are dependent of the hydroxyl groups at the end of PLA chain [
15]. As these reactions proceed, several transitory residues are formed as lactide molecules, oligomeric ring, acetaldehyde with a double bond, volatiles and quite a few of these components have low molecular mass [
16]. These reactions occur only at temperature above 270 °C [
15,
16]. However, thermo-oxidative degradation reactions can occur at temperatures below 270 °C due to the synergistic effect of oxygen and shear [
16]. For both cases, Biosilicate
® might have catalyzed degradation reactions as it can be noticed in
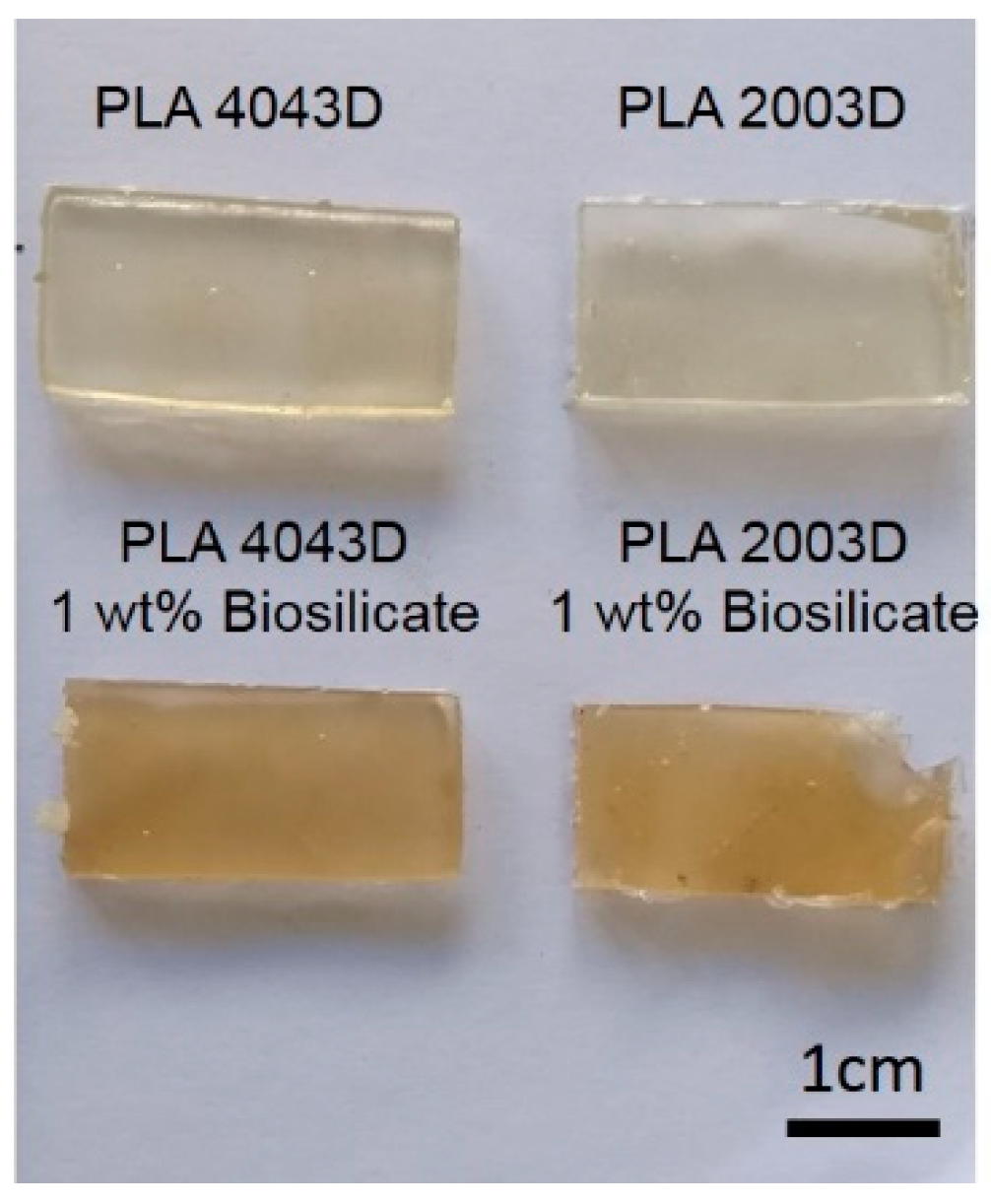
Figure 2 that presents the visual appearance of both PLA after been processed in internal mixer. The neat PLAs did not present color change however, after the processing the sample with addition of Biosilicate
® showed significant changes in the color from transparent yellowish to brown. This fact may be a strong evidence of polymer degradation and corroborates with the results of torque rheometry.
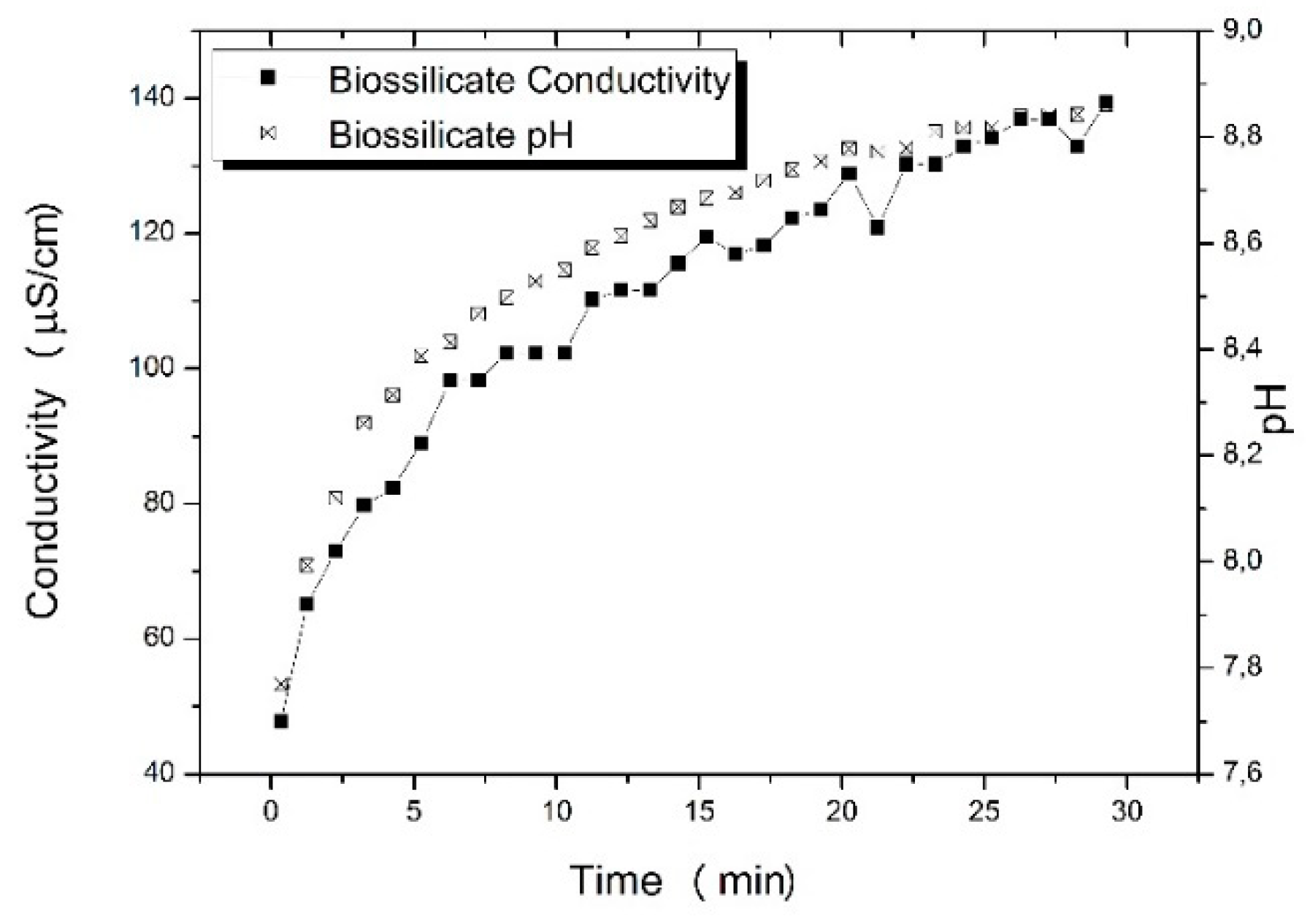
Aiming to understand the effect of the degradation during melt processing of the composites, the zeta potential test was performed for Biosilicate
®. The zeta potential characterization not only provides information about the material’s electrical conductivity but also how the pH of the solution changes with the time. The result is showed in
Figure 3 and it is noticed a rapid increase in the electrical conductivity from 1 to 5 min, from 40 to 100 μs/cm, i.e., the material presents a huge release of ions in contact with aqueous solution. The dissolution of calcium and sodium in Biosilicate
® contributes to the fast increase of the electrical conductivity and pH of the solution, and the rapid dissolution of calcium, which is one of the constituents of bone, might be responsible for Biosilicate
® highly bioactivity [
6,
7,
8,
9]. Blaker et al. [
17] have observed premature degradation of the PLA by addition of the 45S5 during thermal processing and attributed the early degradation of the PLA to the release of counterions of the 45S5 that catalyze thermal degradation reactions, as observed for Biosilicate
®. Although zeta potential test is performed in aqueous solution, it is possible that Biosilicate
® components might react with PLA matrix during mixing in molten state and thus the zeta potential provides useful information about Biosilicate
® high bioactivity which is associated with the capacity of rapid dissolution of some counterions, such as calcium.
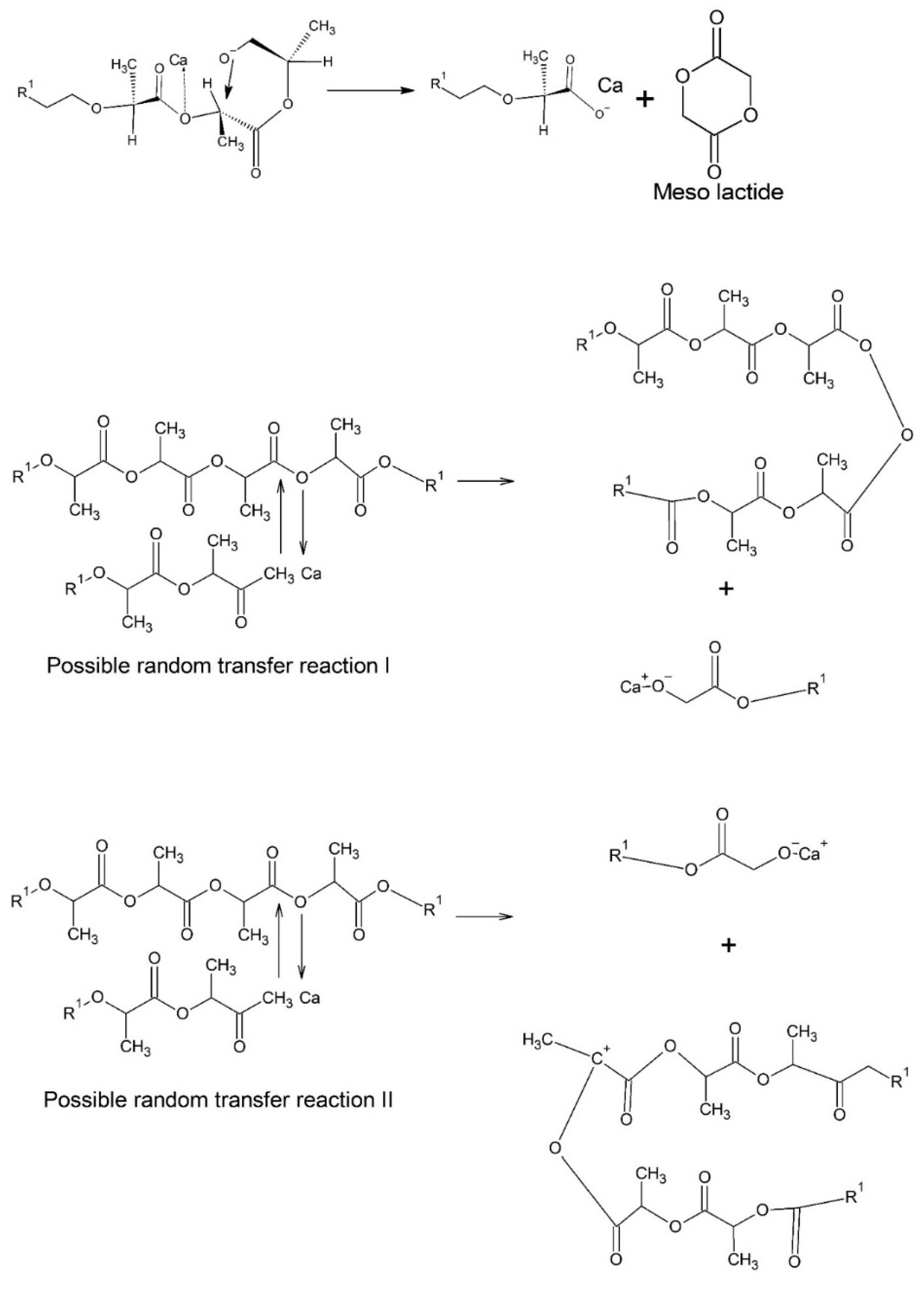
Fan et al. [
18,
19] prepared PLA calcium ion end capped (PLA-Ca) by treating the PLA with CaH
2 and analyzed the thermodegradation of this material under pyrolysis. According to Fan et al., the calcium (Ca) act as a counterion accelerating nucleophilic substitution reactions (SN2) and leading to the racemization of PLA, i.e., the formation of meso-lactide. Another mechanism proposed by Fan et al. [
19] during pyrolysis of PLA-Ca is the random transfer between PLA chains. The reactions proposed are presented in
Figure 4. Thus, it can be concluded that similar effects can occur with the PLA/Biosilicate
® composites produced by torque rheometry.
To understand the effect of the degradation behavior during melt processing, the rheological behavior of the neat PLAs and the composites were evaluated.
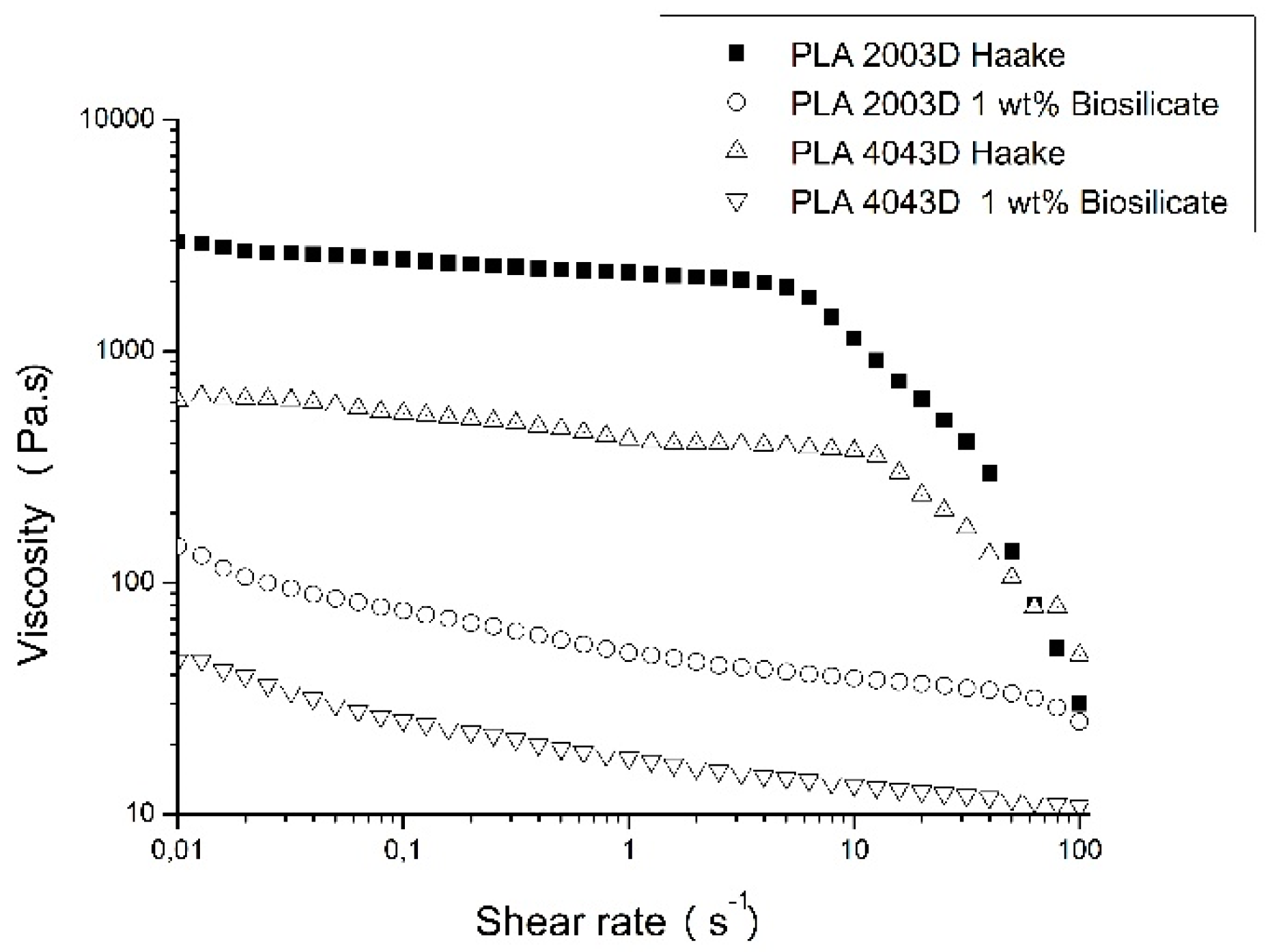
Figure 5 presents the curves of viscosity versus shear strain for the different PLAs and the composites of PLAs with 1 wt. % of Biosilicate
®. The two PLAs used in this work have similar rheological behavior; however, PLA 2003D possess higher viscosity compared to PLA 4043D. The presence of Biosilicate
® reduced the viscosity of both PLAs remarkably, although the viscosity variation presented by both composites remained equivalent when compared to the pure polymers. In addition, a change in the rheological behavior of the composites with the addition of Biosilicate
® is noticed. Both PLAs present a Newtonian behavior (0.01 to 10 s
−1), i.e., the viscosity remained constant with increasing shear rate. The PLA/Biosilicate
® composites exhibited shear thinning (pseudoplastic) behavior even at low shear rates and higher viscosity values than PLA in very low shear rates (up to 0.05 s
−1). These rheological results indicate that the addition of Biosilicate
® could have strongly affected the melt processing of the composites and contribute to the degradation of the PLA matrix.
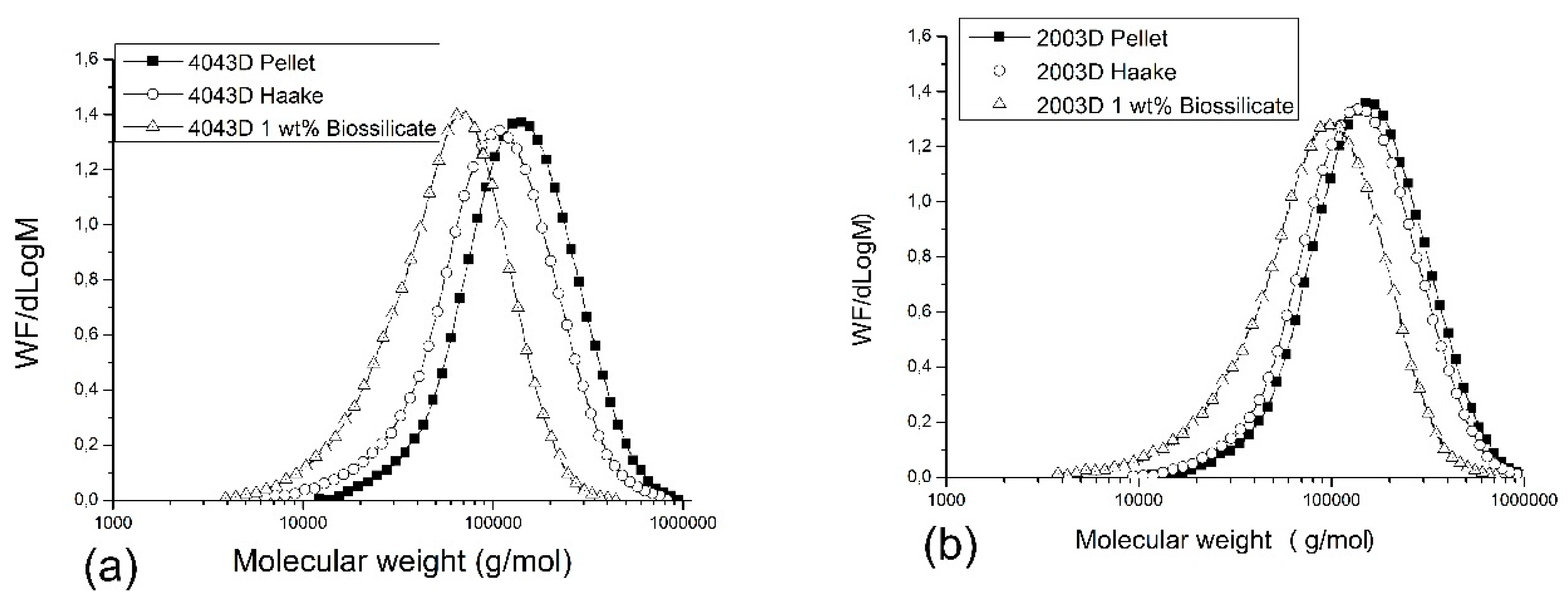
Figure 6 presents the GPC curves of PLA 4043D (a) and PLA 2003D (b), pellets (as received), after torque rheometer (Haake) processing with and without Biosilicate
® (
Table 1) The results show that the PLA 2003D (as received) has a higher number average molar weight (Mn) than PLA 4043D. This result was expected since the PLA 2003D shows higher viscosity baseline when compared to PLA 4043D in the rheological analysis. It is interesting to note, however, that the PLA 2003D presented a reduction of 13% in the Mn and the weight average molecular weight (Mw) was the same after processing by torque rheometer when compared to their pellets (as received). On the other hand, the PLA 4043D presented a reduction of 30% in the Mn and 24% the Mw after processing when compared to its pellets.
To investigate the different behavior of the PLAs their RAC values were determined. The RAC in the PLAs comes from the different functional groups of PLA chain, which might be hydroxyl or carbonyl groups. The RAC in the PLA directly affects its stability and can make the material more susceptible to degradation during shearing process. This is because acidic conditions are known for catalyzing PLA hydrolysis reactions [
15,
16], i.e., the acid group presence in the system during the processing could enhance the degradation processes of the polymer, reducing its molar mass and viscosity. The RAC of the PLAs was determined by titration and a slight difference between them was observed. The PLA 2003D presented acid content of 0.20 ± 0.02% while PLA 4043D 0.30 ± 0.01% [
20,
21]. The difference in RAC for both PLAs corroborates their performance (viscosity and molar mass) after processing. However, PLA 2003D was less sensitive to degradation since it maintained higher M
n and M
w compared to PLA 4043D. On the contrary, the addition of 1 wt. % of Biosilicate
® led to the pronounced shift of the GPC curves to the left side when the Biosilicate
® was added. It was the result of a similar decrease in Mn and Mw for both PLAs, ranging from 50 to 60% of decrease molar mass. As already mentioned, the PLA degradation reactions involved during the processing carried out are thermal degradation under shear, hydrolytic degradation (catalyzed by acid groups—OH and COOH) and thermodegradation in the presence of the calcium ion. Therefore, the results suggest that there may be a predominance of thermodegradation in the presence of the calcium ion during the mixture in the molten state of the PLA with the Biosilicate
®. This can be explained by equivalent reductions of the molar mass independent of the RAC.
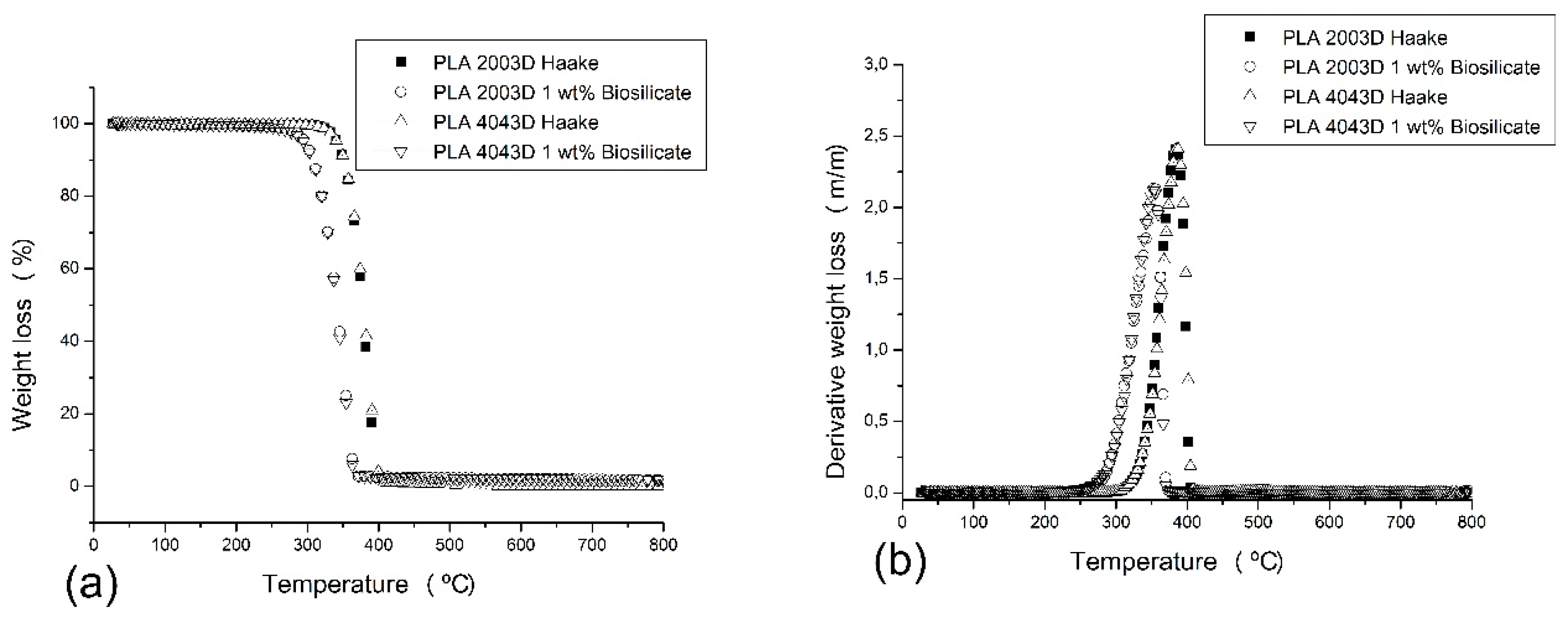
The influence of PLA degradation was analyzed through thermal stability using TGA, while their thermal behavior before decomposition was registered in DSC.
Figure 7 and
Table 2 presents TGA results for the PLAs with and without 1 wt. % of Biosilicate
®. The TGA curve (
Figure 7a) for the two PLAs are similar and the
Tonset presented for PLA 2003D and PLA 4043D is almost equal; however, Biosilicate
® led in both cases to a strong decrease in the thermal stability of the composites, i.e., a reduction of almost 40 °C in the
Tonset and approximately 30 °C in the Derivative weight loss (DWL) at
Figure 7b demonstrating that the PLA underwent different pathways of thermodegradation [
15,
16]. Similar behavior was observed by Fan et al. [
19], where the PLA-Ca, modified with calcium salts, presented a decrease in its thermal stability. The authors quantified the activation energy to initiate thermodegradation reactions and for PLA-Ca it was reduced for the half compared to the same PLA that underwent a process of purification [
19]. It was also noticed that for the samples with the addition of the Biosilicate
® the residue was higher than for the PLAs. It is attributed to the amount of Biosilicate
® that under TGA test conditions are not decomposed. Another possible explanation for this increase in the residue is that Biosilicate
® when present in the composites might lead to the formation of other degradation residues which are not decomposed in the temperature analyzed. Biosilicate
® has in its composition high content of calcium and might underwent some of the reaction as shown in
Figure 4, which directly influences in its thermal stability.
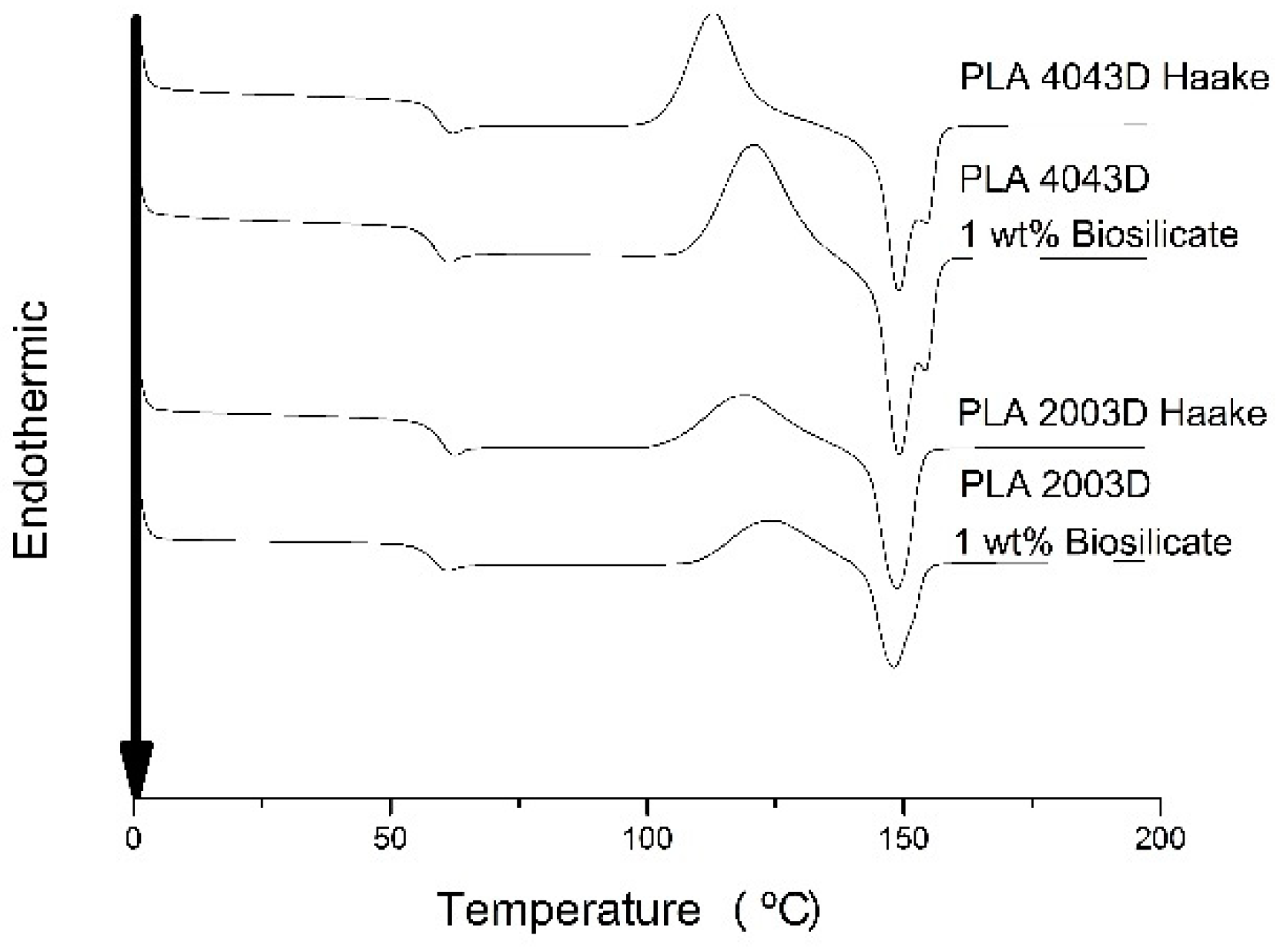
Figure 8 shows DSC curves for the PLAs with and without 1 wt. % of Biosilicate
® and the parameters calculated from those are presents in
Table 3. The results of the second heating of all samples show that PLA was kept almost constant, approximately 60 °C, independent of the polymer molar mass and presence of Biosilicate
®. However, when we analyzed the cold crystallization peak (
Tcc), a shift towards higher temperatures, due to the increase of molecular mass (neat polymers) and the presence of bioglass (for both PLAs), is observed. This means that for the same heating condition, the crystallization of PLA occurs earlier in systems with greater flexibility and mobility of the chains because the molecules are shorter and allow the reorganization of amorphous domains [
15]. PLA can crystallize into three polymorphic forms (α, β and γ) depending on process conditions. The α structure is more stable than the β structure and can be produced from the molten state [
22]. The literature also reports the presence of an α crystalline phase that has a looser and disorderly structure, named α’. The crystallization of PLA in the temperature range from 100 to 120 °C can originate crystals α and α’ at the same time [
23]. In the PLA 4043D and PLA 4043D with addition of Biosilicate
® it was observed one shoulder at the peak fusion of PLA and his evidence can indicate the contribution of both forms α and α’. The α’ form, as mentioned earlier, has a disorderly structure and is fused at lower temperatures (
Tm1) and then recrystallize in the form α, which is subsequently fused in higher temperatures (
Tm2) [
24]. Finally, the molar mass was reduced with the addition of Biosilicate
® and it influenced the crystallinity percentage of the materials studied. During the cooling of the composite could occur crystallization the formation of more crystalline nuclei (due to the presence of bioglass particles), which would act as heterogeneous nuclei. This would facilitate the crystallization of the material [
25]. The presence of bioglass could increase the crystallization rate of the PLA, and this would be demonstrated by a higher percentage of crystallization of the composite PLA, for a given cooling rate. For both PLA it was noticed a slight improvement in the crystallinity of the composites with addition of Biosilicate
®; however, the results showed very low percentages of crystallinity for the studied materials, characterizing almost amorphous materials.