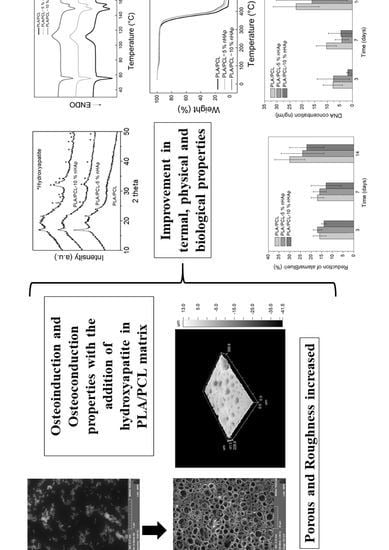
Production and Characterization of Porous Polymeric Membranes of PLA/PCL Blends with the Addition of Hydroxyapatite
Abstract
:1. Introduction
2. Experiment
2.1. Materials
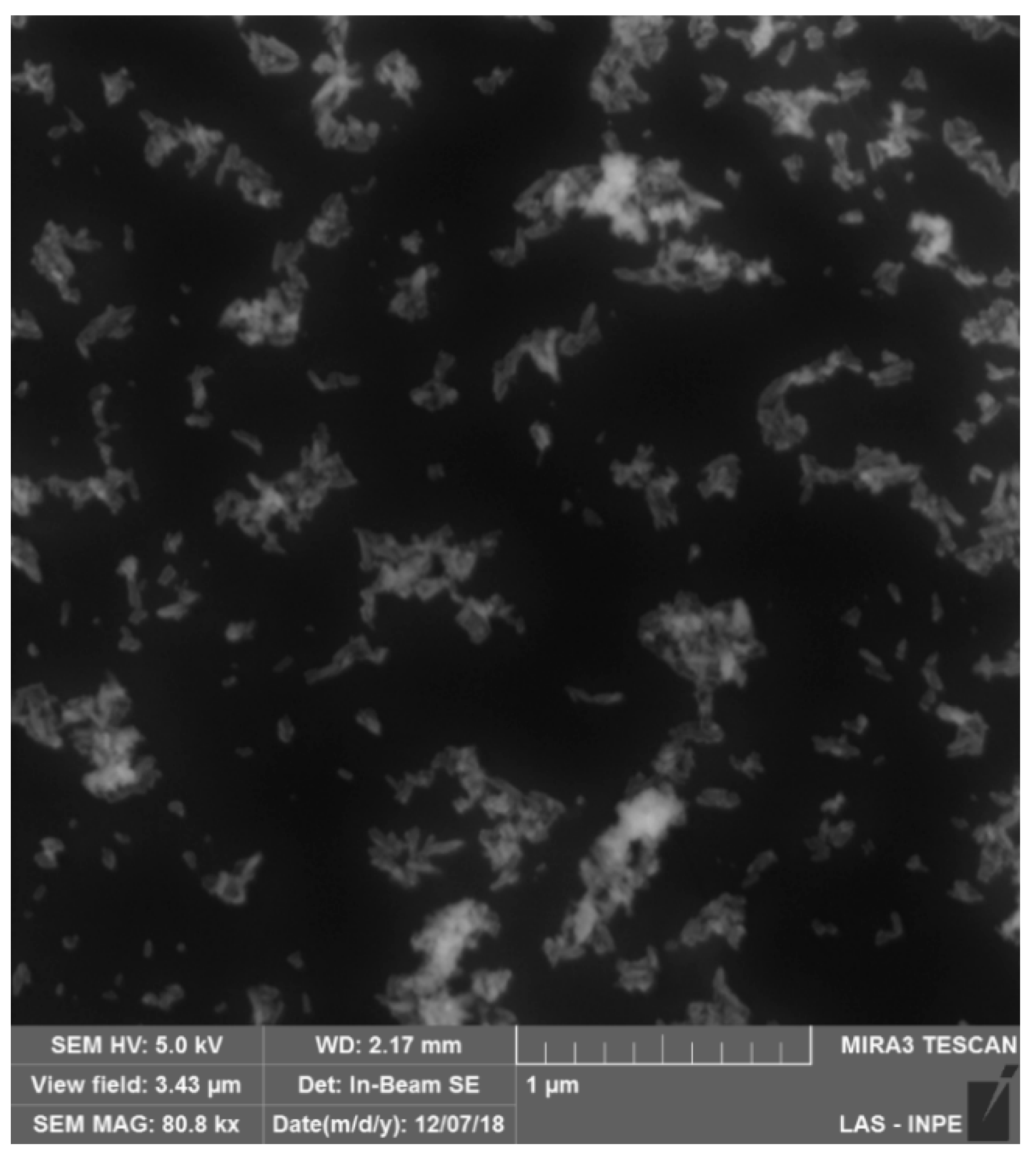
2.2. Synthesis of Hydroxyapatite (HAp)
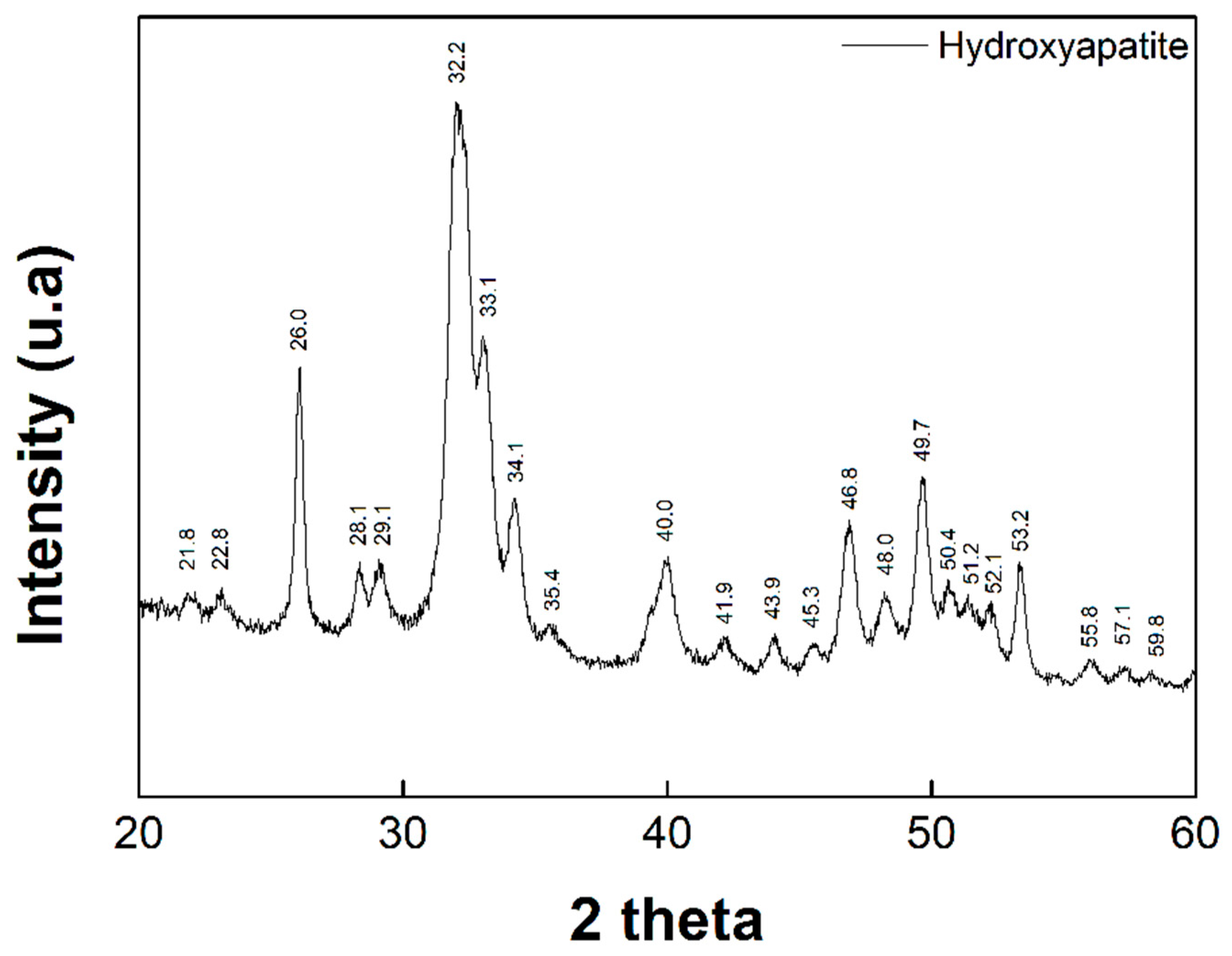
2.3. Characterization of Hydroxyapatite (HAp)
2.4. Preparation of the Porous Membranes of PLA/PCL with Addition of Hydroxyapatite (HAp)
2.5. Structural and Thermal Characterization of the Porous Membranes
2.6. Biological Assays
2.6.1. Cell Culture
2.6.2. Cell Viability
3. Results and Discussion
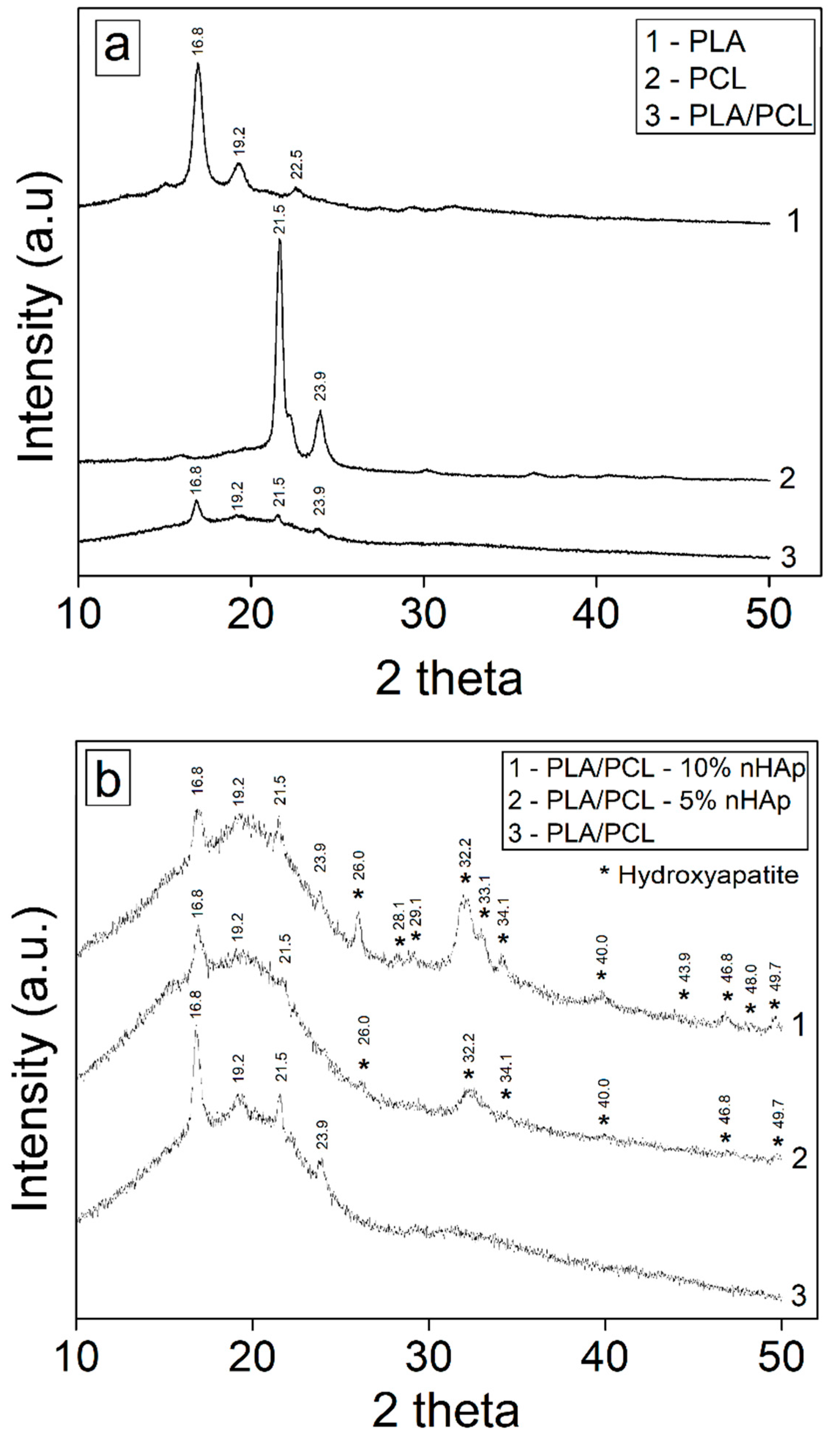
3.1. Synthesis of Hidroxiapatite (HAp)
3.2. Structural and Thermal Characterization of Porous Membranes of PLA/PCL with Hydroxyapatite
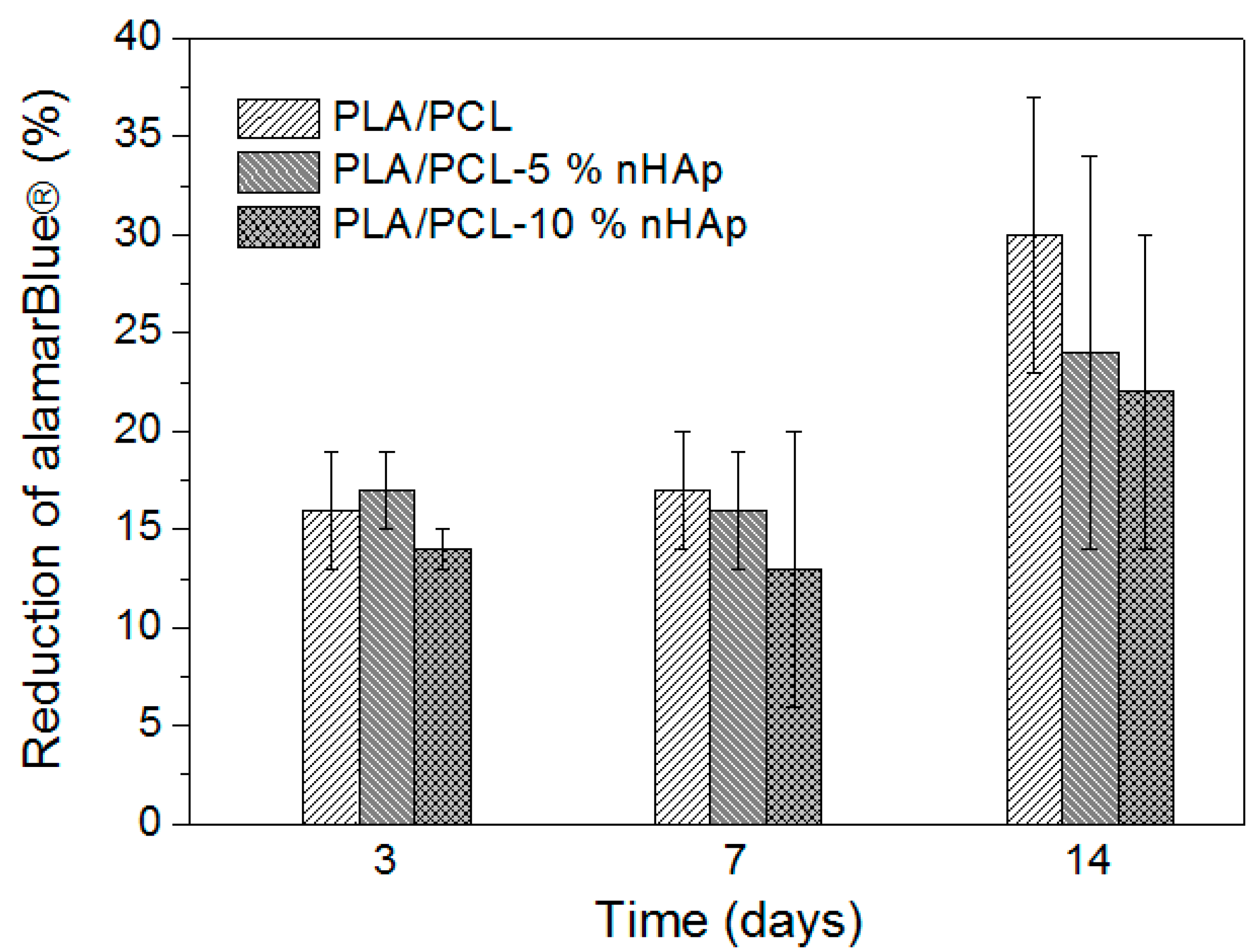
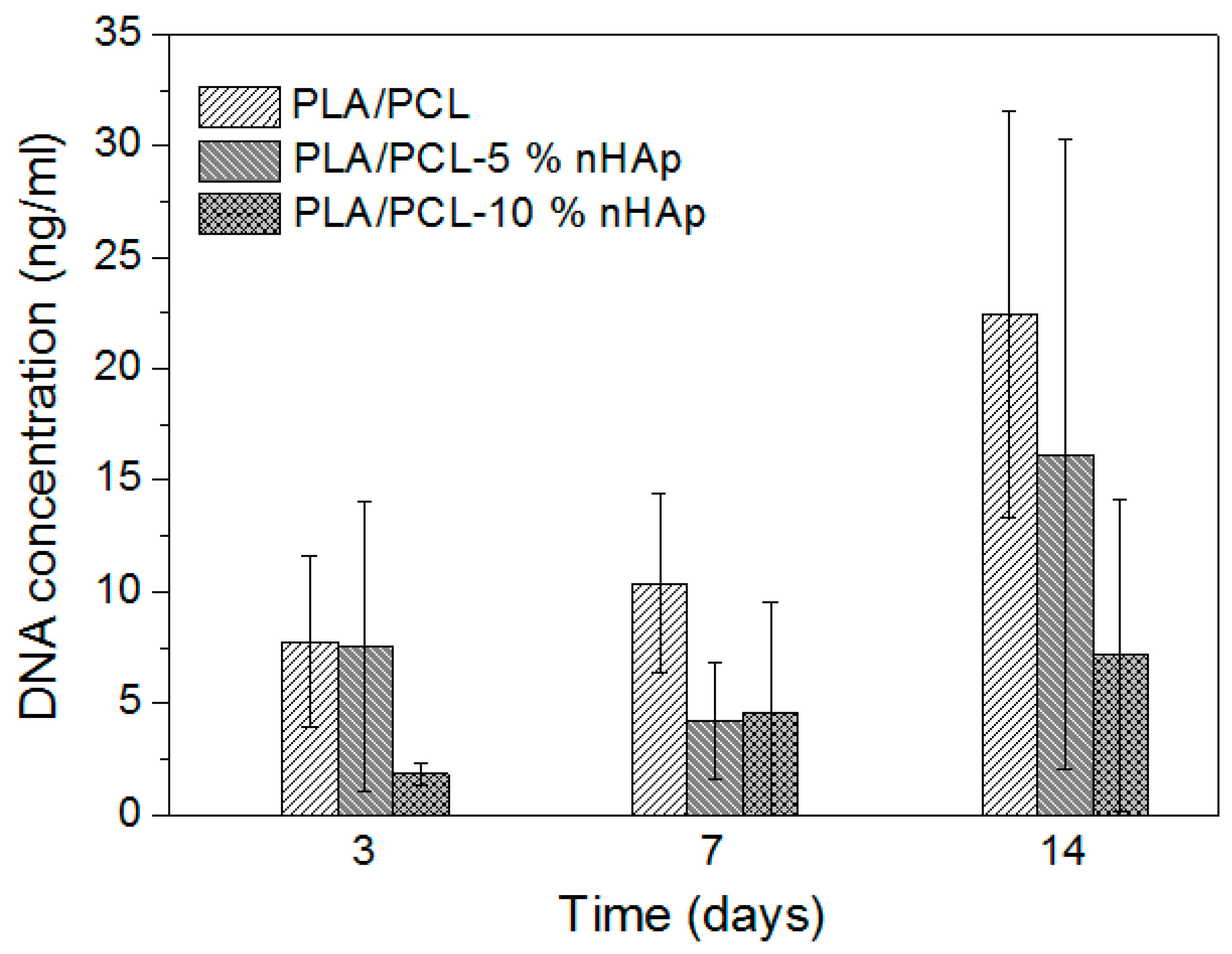
3.3. Cell Viability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable polymer membranes applied in guided bone/tissue regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Maeda, H. Recent developments of functional scaffolds for craniomaxillofacial bone tissue engineering applications. Sci. World J. 2013, 2013, 863157. [Google Scholar] [CrossRef] [PubMed]
- Baolin, G.; Ma, P.X. Synthetic biodegradable functional polymers for tissue engineering: A brief review. Sci. China Chem. 2014, 57, 490–500. [Google Scholar] [CrossRef]
- Gupta, A.P.; Kumar, V. New emerging trends in synthetic biodegradable polymers Polylactide: A critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Auras, R.A.; Harte, B.; Selke, S.; Hernandez, R. Mechanical, physical and barrier properties of poly (lactide) films. J. Plast. Film Sheet. 2003, 19, 123–135. [Google Scholar] [CrossRef]
- Neto, W.A.R.; Pereira, I.H.L.; Ayres, E.; Paula, A.C.C.; Averous, L.; Góes, A.M.; Oréfice, R.L.; Bretas, R.E.S. Influence of the microstructure and mechanical strength of nanofibers of biodegradable polymers with hydroxyapatite in stem cells growth. Electrospinning, characterisation and cell viability. Polym. Degrad. Stab. 2012, 97, 2037–2051. [Google Scholar] [CrossRef]
- Fehri, S.; Cinelli, P.; Coltelli, M.-B.; Anguillesi, I.; Lazzeri, A. Thermal Properties of Plasticized Poly (Lactic Acid) (PLA) Containing Nucleating Agent. Int. J. Chem. Eng. Appl. 2016, 7, 85–88. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Sinha, V.R.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ε-caprolactone microspheres and nanospheres: An overview. Int. J. Pharm. 2004, 278, 1–23. [Google Scholar] [CrossRef]
- Tsuji, H.; Fukui, I.; Daimon, H.; Fujie, K. Poly (l-lactide) XI. Lactide formation by thermal depolymerisation of poly(l-lactide) in a closed system. Polym. Degrad. Stab. 2003, 81, 501–509. [Google Scholar] [CrossRef]
- Shibita, A.; Takase, H.; Shibata, M. Semi-interpenetrating polymer networks composed of poly(l-lactide) and diisocyanate-bridged 4-arm star-shaped 3-caprolactone oligomers. J. Polym. Sci. Part B 2014, 52, 1420–1428. [Google Scholar] [CrossRef]
- Medeiros, K.M.; Lima, D.F.; Lima, C.A.P.; Araujo, E.M.; Lira, H.L.; Medeiros, V. Development of polymer membranes modified with a porogenic Agent. Mater. Sci. Forum 2016, 869, 815–819. [Google Scholar] [CrossRef]
- Arrua, R.D.; Strumia, M.C.; Igarzabal, C.I.A. Macroporous monolithic polymers: Preparation and applications. Materials 2009, 2, 2429–2466. [Google Scholar] [CrossRef]
- Sarmento, B.; Neves, J. Chitosan-Based Systems for Biopharmaceuticals: Delivery, Targeting and Polymer Therapeutics; John Wiley & Sons: Chichester, UK, 2012; ISBN 978-0-470-97832-0. [Google Scholar]
- Mattiasson, B.; Kumar, A.; Galeaey, I.Y. Macroporous Polymers: Production Properties and Biotechnological/Biomedical Applications; CRC Press: New York, NY, USA, 2009; ISBN 978-1-138-11224-7. [Google Scholar]
- Siqueira, I.A.W.B. Desenvolvimento de Membranas Porosas à Base de PDLLA/Nanotubos de Carbono: nHAp para Regeneração Óssea. Ph.D. Thesis, Universidade do Vale do Paraíba, São José dos Campos, Brazil, 2015. Available online: http://biblioteca.univap.br/jspui/bitstream/tede/15/5/00000683.pdf (accessed on 30 January 2019).
- Jamuna-Thevi, K.; Saarani, N.N.; Abdul Kadir, M.R.; Hermawan, H. Triple-layered PLGA/nanoapatite/lauric acid graded composite membrane for periodontal guided bone regeneration. Mater. Sci. Eng. C 2014, 43, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.C.; Jamshidi, P.; Grover, L.M.; Mallick, K.K. Low temperature aqueous precipitation of needle-like nanophase hydroxyapatite. J. Mater. Sci. Mater. Med. 2014, 25, 37–46. [Google Scholar] [CrossRef]
- Cox, S.C.; Jamshidi, P.; Williams, R.L.; Grover, L.M.; Mallick, K.K. The importance of processing conditions on the biological response to apatites. Powder Technol. 2015, 284, 195–203. [Google Scholar] [CrossRef]
- Lobo, A.O.; Zanin, H.; Siqueira, I.A.; Leite, N.C.; Marciano, F.R.; Corat, E.J. Effect of ultrasound irradition on production of nHAp/MWCNT nanocomposites. Mater. Sci. Eng. C 2013, 33, 4305–4312. [Google Scholar] [CrossRef]
- Crescenzi, V.; Manzini, G.; Calzolari, G.; Borri, C. Thermodynamics of fusion of poly-β-propiolactone and poly-ε-caprolactone comparative analysis of the melting of aliphatic polylactone and polyester chains. Eur. Polym. J. 1972, 8, 449–463. [Google Scholar] [CrossRef]
- Yubao, L.; De Groot, K.; De Wijn, J.; Klein, C.P.A.T.; Meer, S.V.D. Morphology and composition of nanograde calcium phosphate needle-like crystals formed by simple hydrothermal treatment. J. Mater. Sci. Mater. Med. 1994, 5, 326–331. [Google Scholar] [CrossRef]
- Zhou, G.; Li, Y.; Xiao, W.; Zhang, L.; Zuo, Y.; Xue, J.; Jansen, J.A. Synthesis, characterization, and antibacterial activities of a novel nanohydroxyapatite/zinc oxide complex. J. Biomed. Mater. Res. A 2008, 85, 929–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, M.C.; Messmer, N.R.; Brazil, T.R.; Marciano, F.R.; Lobo, A.O. The effect of ultrasonic irradiation on the crystallinity of nano-hydroxyapatite produced via the wet chemical method. Mater. Sci. Eng. C 2013, 33, 2620–2625. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Hydroxyapatite, a biomaterial: Its chemical synthesis, characterization and study of biocompatibility prepared from shell of garden snail, Helix aspersa. Bull. Mater. Sci. 2012, 35, 1031–1038. [Google Scholar] [CrossRef]
- Cardoso, G.B.C. Desenvolvimento de Matriz Tridimensional Compósita de poli(ε-caprolactona) e Cerâmica Bioativa para Aplicação em Engenharia Tecidual. Ph.D. Thesis, Universidade Estadual de Campinas, Campinas, Brazil, 2013. Available online: http://repositorio.unicamp.br/bitstream/REPOSIP/263525/1/Cardoso_GuineaBrasilCamargo_D.pdf (accessed on 25 January 2019).
- Fragiadakis, D.; Pissis, P.; Bokobza, L. Glass transition and molecular dynamics in poly(dimethylsiloxane)/silica nanocomposites. Polymer 2005, 46, 6001–6008. [Google Scholar] [CrossRef]
- Can, E.; Udenir, G.; Kanneci, A.I.; Kose, G.; Bucak, S. Investigation of PLLA/PCL blends and paclitaxel release profiles. AAPS PharmSciTech 2011, 12, 1442–1453. [Google Scholar] [CrossRef]
- Szymonowicz, M.; Korczynski, M.; Dobrzynski, M.; Zawisza, K.; Mikulewicz, M.; Karuga-Kuzniewska, E.; Zywickab, B.; Rybak, Z.; Wiglusz, R.J. Cytotoxicity evaluation of high-temperature annealed nanohydroxyapatite in contact with fibroblast cells. Materials 2017, 10, 590. [Google Scholar] [CrossRef] [PubMed]
- Simionescu, B.C.; Drobota, M.; Timpu, D.; Vasiliu, T.; Constantinescu, C.A.; Rebleanu, D.; Calin, M.; David, G. Biopolymers/poly(ε-caprolactone)/polyethylenimine functionalized nano-hydroxyapatite hybrid cryogel: Synthesis, characterization and application in gene delivery. Mater. Sci. Eng. C 2017, 81, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: A review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef] [PubMed]
- Ziv, V.; Weiner, S. Bone crystal size: A comparison of transmission electron microscopic and x-ray diffraction line-width-broadening techniques. Connect. Tissue Res. 1994, 30, 165–175. [Google Scholar] [CrossRef]
- Rho, J.-Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- St. John, K.R. Biocompatibility of dental materials. Dent. Clin. N. Am. 2007, 51, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Gregor, A.; Filová, E.; Novák, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnová, V.; Lukášová, V.; Bartoš, M.; Nečas, A.; et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
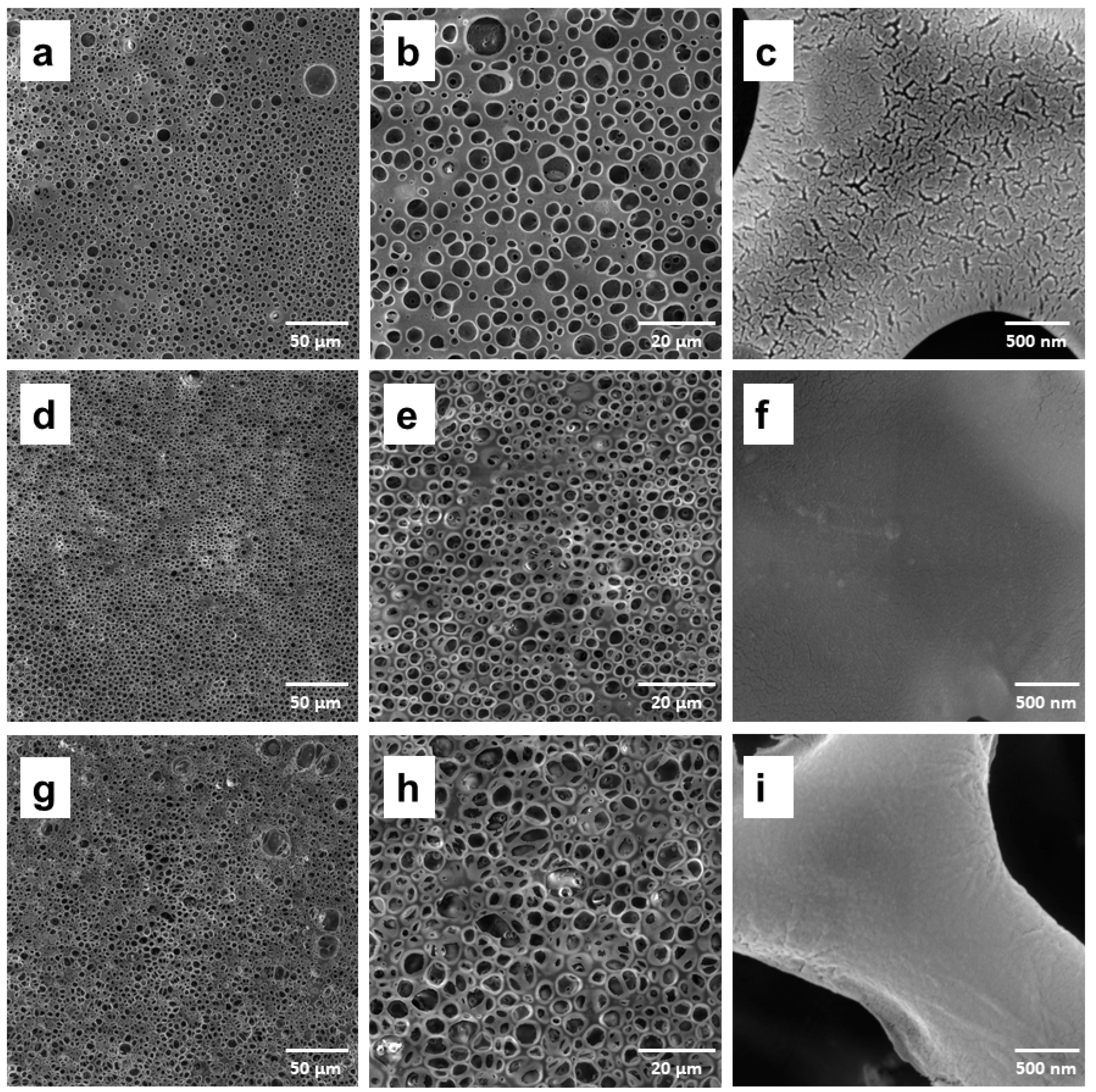
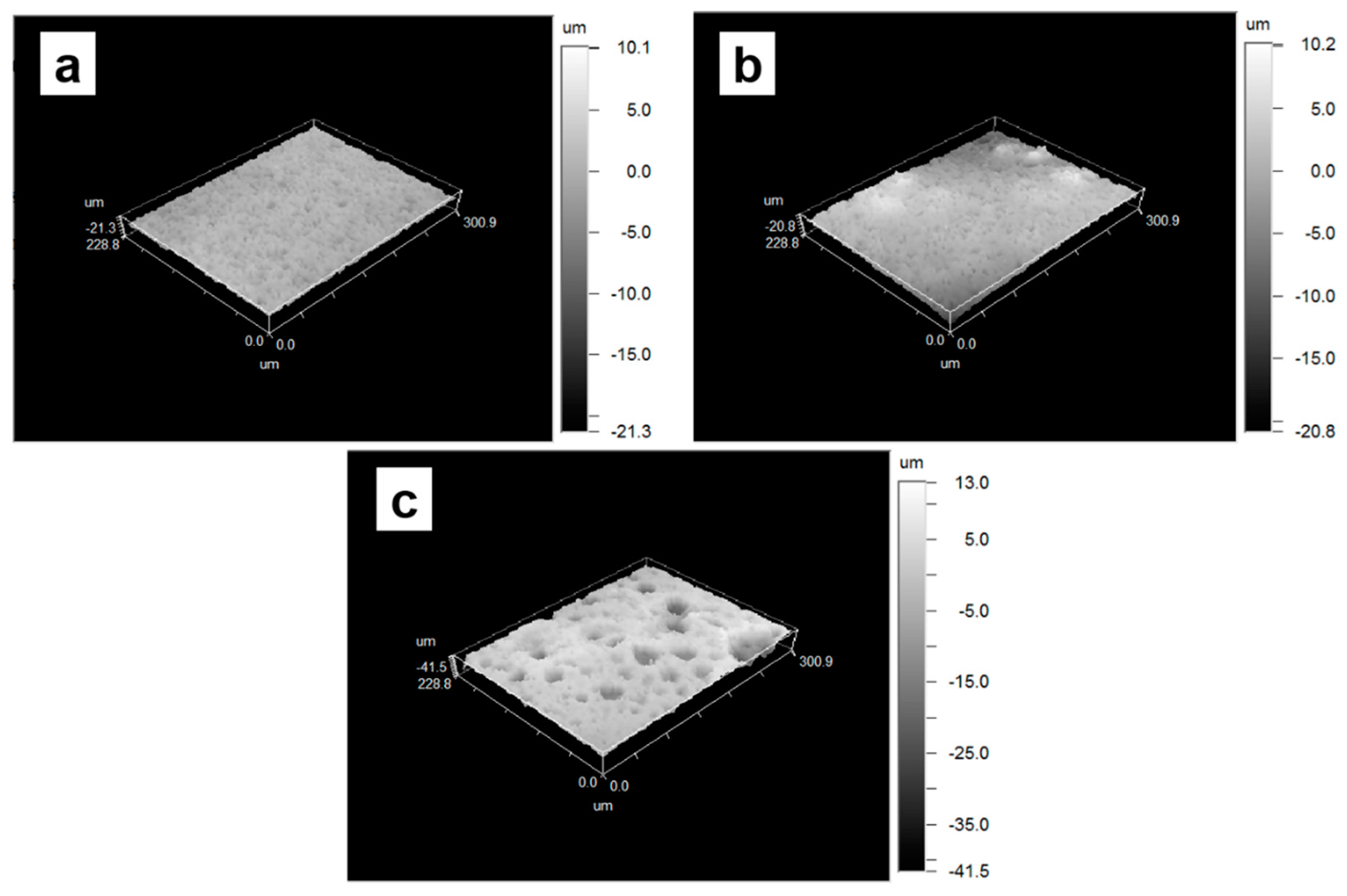


| Samples | Surface Roughness (µm) |
|---|---|
| PLA/PCL | 1.24 ± 0.21 |
| PLA/PCL-5% nHAp | 1.74 ± 0.58 |
| PLA/PCL-10% nHAp | 3.92 ± 0.14 |
| Sample | PLA | PCL | ||||||
|---|---|---|---|---|---|---|---|---|
| Tg (°C) | Tm1 (°C) | ∆Hm1 (J/g) | ∆Hcc1 (J/g) | Xc1 (%) | Tm1 (°C) | ∆Hm1 (J/g) | Xc1 (%) | |
| PCL | __ | __ | __ | __ | __ | 56.0 | 77.4 | 57.0 |
| PLA | 57.8 | 148.4 | 24.7 | 3.0 | 23.4 | __ | __ | __ |
| PLA/PCL | 53.0 | 146.0 | 19.2 | 3.4 | 16.9 | 52.0 | 11.0 | 8.1 |
| PLA/PCL-5% nHAp | 54.3 | 144.0 | 19.2 | 4.7 | 16.4 | 52.0 | 9.9 | 7.7 |
| PLA/PCL-10% nHAp | 54.6 | 146.0 | 17.8 | 1.5 | 19.4 | 52.0 | 6.0 | 4.9 |
| Sample | Tonset of degradation (°C) | Weight (%) | Trreversíble degradation (°C) | Weigh Loss (%) | Residual (%) |
|---|---|---|---|---|---|
| PLA/PCL | 112.7 | 2.39 | 343.2 | 93.59 | 1.25 |
| PLA/PCL-5% nHAp | 79.4 | 1.36 | 357.6 | 92.02 | 5.08 |
| PLA/PCL-10% nHAp | 79.3 | 2.49 | 356.0 | 88.69 | 6.86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moura, N.K.d.; Siqueira, I.A.W.B.; Machado, J.P.d.B.; Kido, H.W.; Avanzi, I.R.; Rennó, A.C.M.; Trichês, E.d.S.; Passador, F.R. Production and Characterization of Porous Polymeric Membranes of PLA/PCL Blends with the Addition of Hydroxyapatite. J. Compos. Sci. 2019, 3, 45. https://doi.org/10.3390/jcs3020045
Moura NKd, Siqueira IAWB, Machado JPdB, Kido HW, Avanzi IR, Rennó ACM, Trichês EdS, Passador FR. Production and Characterization of Porous Polymeric Membranes of PLA/PCL Blends with the Addition of Hydroxyapatite. Journal of Composites Science. 2019; 3(2):45. https://doi.org/10.3390/jcs3020045
Chicago/Turabian StyleMoura, Nayara Koba de, Idália A. W. B. Siqueira, João Paulo de Barros Machado, Hueliton Wilian Kido, Ingrid Regina Avanzi, Ana Claudia Muniz Rennó, Eliandra de Sousa Trichês, and Fabio Roberto Passador. 2019. "Production and Characterization of Porous Polymeric Membranes of PLA/PCL Blends with the Addition of Hydroxyapatite" Journal of Composites Science 3, no. 2: 45. https://doi.org/10.3390/jcs3020045
APA StyleMoura, N. K. d., Siqueira, I. A. W. B., Machado, J. P. d. B., Kido, H. W., Avanzi, I. R., Rennó, A. C. M., Trichês, E. d. S., & Passador, F. R. (2019). Production and Characterization of Porous Polymeric Membranes of PLA/PCL Blends with the Addition of Hydroxyapatite. Journal of Composites Science, 3(2), 45. https://doi.org/10.3390/jcs3020045