Abstract
Background: En block resection of squamous cell carcinoma (SCC) of the concha represents a reconstruction challenge, due to the complex topography and difficult access. Objective: The objective of the present paper is to describe the chemically assisted dissection (CADISS) of SCC originating in the auricular concha and the following reconstruction of the conchal cavity with a post-auricular island flap (PIF), taking care to minimize injury to the donor site. Methods: Twenty-six patients having a diagnosis of SCC of the auricular concha were included in the study. ‘En bloc’ removal of the tumor was accomplished, leaving the adjacent conchal cartilage attached to the tumor and using the CADISS technique to preserve the deep perichondrium. A PIF was used to repair the auricular conchal defect. Results: Flaps were normal at 10 days and at 1-month follow-up. No tumor recurrence was observed. No complications were observed. According to the SCAR scale, good aesthetic outcomes were achieved in all cases, both at the auricular concha and at the donor site. Conclusion: CADISS facilitates the complete removal of the tumor with the preservation of the surrounding normal tissues. A post-auricular island flap can be easily pulled through a post-auricular tunnel to repair the defect and the donor site can be closed primarily.
1. Introduction
Cutaneous squamous cell carcinoma (SCC) is the second most common non-melanoma skin cancer [1]. The head and neck accounts for the location of more than 75% of skin cancers [2].
The increasing incidence of SCC of the ear is between 1 and 6 cases per million inhabitants [3,4,5]. Primary malignancies of the concha are uncommon, even among otologists caring exclusively for surgical treatment of ear problems. SCCs are less frequent than basal cell carcinomas in the head and neck skin area, with a ratio of 1:4 [6], but the relative percentages are reversed on the auricle [7].
SCCs involving the concha account for 5.5% of tumors of the auricle. They are often observed in Caucasian populations, and there is a clear link between exposure to ultraviolet radiation and white skin cancer [8]. Indeed, the natural pigmentation of darker-skinned people provides natural protection from solar radiation. Sixty percent of cutaneous SCC follows actinic keratoses [9]. SCC is more aggressive in transplant recipients: it grows more rapidly and there is a higher incidence of metastases [10].
Malignancies of the auricle commonly appear as ulcerated or nodular skin lesions. In case of suspicious conchal lesions, there is a high risk of SCC [6]. Therefore, any suspicious lesion over the concha is commonly referred in a short time in order to make an early diagnosis, thus allowing for early treatment and prevention of significant morbidity.
Definitive diagnosis is obtained only with tissue biopsy. Superficial tissue may show patterns of chronic inflammation. In case of high clinical suspicion, deeper tissue should be obtained in order to prevent false-negative biopsies.
SCC of the pinna has a higher rate of metastases than cutaneous SCC that originate in other parts of the body (12–16% vs. 0.5–2%) [11,12]. The best predictor of a poor prognosis is a large tumor with advanced TNM staging, invaded primary excision margins, and multiple SCCs [13].
En bloc resection of the tumor is a well-established principle of ear oncological surgery. It is based on the capability to remove the tumor with clear, normal tissue margins.
Accurate preoperative planning must include consideration of reconstruction that aims to achieve wound healing as well as good cosmetic outcomes. Indeed, reconstruction of auricular conchal defects after removal of SCC is usually necessary to restore the anatomical structure of the concha, mainly for aesthetic reasons [14]. Reconstruction can be difficult because the skin is adherent to the underlying cartilage. Partial-thickness skin grafts and full-thickness skin grafts can lead to delayed wound healing and centripetal contraction, which can lead to deformity of the conchal bowl [15].
The area surrounding the post-auricular sulcus is an excellent donor site for the reconstruction of auricular conchal defects since it is well vascularized via the superficial temporal artery and the posterior auricular artery. Moreover, it is hidden behind the pinna, and its skin is quite similar to the skin of the ear and face. One of the advantages of the post-auricular island flap (PIF) is minimal donor site morbidity. The donor site can be easily closed by primary intention, mobilizing the surrounding tissues [15].
Surgery is the “art of cleavage planes”: during surgical approaches, even normal tissues need to be detached from each other. Up to recent years, such detachment was performed with mechanical instruments and was based on mechanical forces only.
In recent decades, sodium 2-mercaptoethansulfonate (C2H5NaO3S2: mesna) gained attention due to its application in the surgical setting: its intraoperative use facilitates the detachment of pathological tissues from healthy tissues and also the detachment of healthy tissues from other healthy tissues when applied as a topical formulation in the tissues to be dissected [16]. Mesna is included in a class of thiolic drugs that produce tissue separation by breaking the disulfide bridges of the polypeptide chains between different tissue layers.
Various methods for the reconstruction of anterior conchal defects have been reported. The authors describe the surgical technique they used to perform the chemically assisted dissection (CADISS) of the tumor and to repair the tissue defect with a PIF, taking care to minimize injury to the donor site.
2. Materials and Methods
Following approval by the Ethics Committee (Comitato Etico Val Padana—PROT25961), a retrospective medical record review was conducted on all patients diagnosed with SCC of the auricular concha and treated by the first author at our department from August 2010 to May 2022.
A total of 26 patients were identified as meeting the inclusion criteria of a biopsy-proven squamous cell carcinoma of the auricular concha.
Exclusion criteria were patients with previous surgery, with past medical history that included immunosuppressive treatment, and patients with a follow-up <12 months.
Tumors were staged according to the AJCC. Informed consent was obtained.
An operating microscope with a lens of 250 mm and a magnification of 6× was used for the entire duration of the surgical procedure.
The tumor was removed under local anesthesia in all cases. Histopathological examination was required in all cases.
The SCAR scale [17] was used to measure the postoperative scar quality, both at the auricular concha and at the donor site.
3. Surgical Technique
The patient was placed in the supine position and the patient’s head was rotated away from the involved ear. The otologic nurse sat in front of the patient’s face. After premedication with 10 mg diazepam, adequate excision margins were marked all around the lesion (Figure 1). Local anesthesia was performed with lidocaine and epinephrine (final concentration 1:200,000).
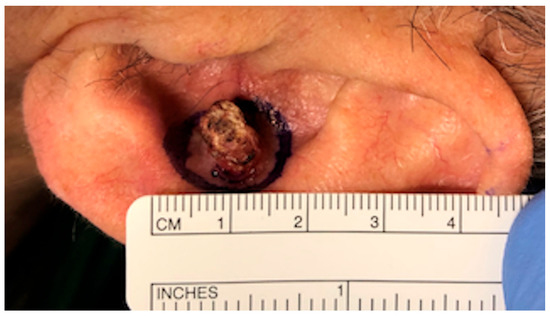
Figure 1.
Squamous cell carcinoma of the auricular concha.
Skin incision was performed with a surgical blade No.15. ‘En bloc’ removal of the tumor (Figure 2) was then accomplished, leaving the adjacent conchal cartilage attached to the tumor and using the CADISS technique to preserve the deep perichondrium, exploiting the ability of mesna to disrupt connections between tissue layers. Mesna was administered as a chemical dissector in the cleavage plane by means of specifically designed dissectors that were used for highlighting and separating healthy tissues from each other. CADISS dissectors (Figure 3) delivered a drop-by-drop 10% mesna solution directly at the tip of the instruments, avoiding the unnecessary spread of the drug. Mesna was delivered by passing through a small channel within the CADISS instrument. Then, the separation of tissues was performed with the same instruments. A PIF (Figure 4) was used to repair the auricular conchal defect.
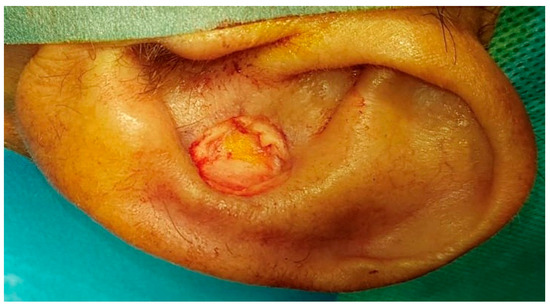
Figure 2.
Conchal defect after removal of the squamous cell carcinoma.
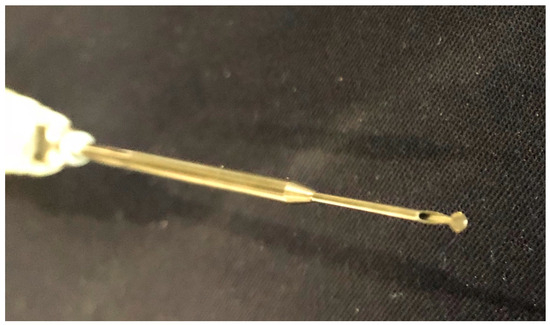
Figure 3.
Dissector delivering a 10% mesna solution directly at the tip of the instrument.
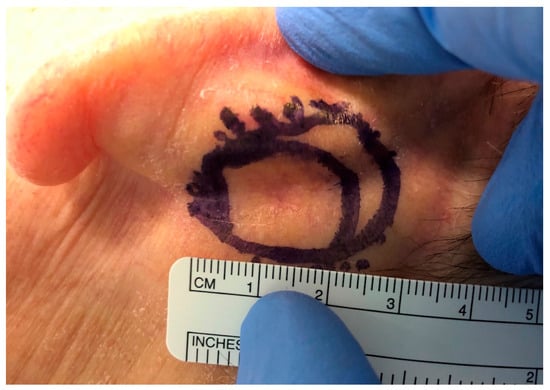
Figure 4.
Post-auricular island flap should be sculptured slightly larger than the conchal defect. Mobilization of the surrounding tissues makes closure of the donor site easy.
The flap was sculptured in the area of the post-auricular sulcus, taking care that the superior limit of the PIF was 0.5–1 cm higher than the horizontal line passing through the superior limit of the tumor. The diameter of the flap was 3 mm larger than the surgical defect.
A subcutaneous pedicle was created at the inferior limit of the flap (length of the subcutaneous pedicle: 0.5–1 cm). The PIF was pulled through a sufficiently large slit created along the post-auricular sulcus. The flap had to pass without resistance in order to reduce the risk of ischemic lesions of the flap.
The skin of the flap was then sutured to the skin of the auricular conchal defect (Figure 5). The donor site was closed primarily with an intradermal suture through mobilization of the surrounding tissues.
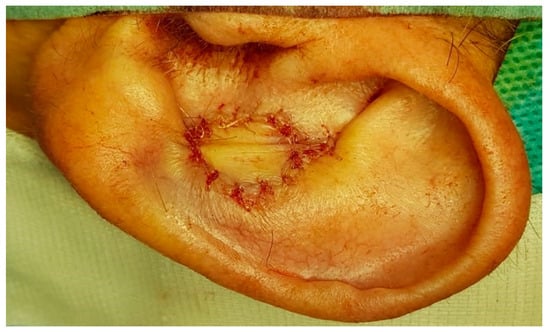
Figure 5.
The postauricular island flap was pulled through a slit created along the post-auricular sulcus.
4. Results
The 26 patients meeting inclusion criteria had a follow-up ranging from 12 to 36 months (mean follow-up: 18 months).
Age ranged from 34 to 72 years (mean age: 56.1 years). A predominance of male patients (15/26) was observed. All patients were Caucasian. A positive history of significant sun exposure was seen in 22/26 patients and 20/26 cases were left sided.
In the present series, none of the patients had more than one lesion. All tumors were classified as superficial (involving the concha only) without deep extension to the external auditory canal, bone, parotid, or neck. Tumors were staged according to the AJCC [18]. In this limited series, all cases were staged as T1N0M0. ‘En bloc’ removal of the tumor was possible in all cases. The auricular conchal defect had a maximum diameter ranging from 1.2 to 1.8 cm (mean diameter: 1.6 cm).
Topical application of mesna facilitated the dissection of the conchal cartilage and attached tumor, perfectly preserving the deep perichondrium intact in all cases.
Reconstruction of auricular conchal defects with a PIF was possible in 26/26 cases, restoring the anatomical structure of the concha.
According to the SCAR scale, good aesthetic outcomes were achieved in 26/26 patients: SCAR scale clinician scores found in this study ranged from 0 to 4 (mean: 2), both at the auricular concha and at the donor site.
Histopathology confirmed the diagnosis of SCC and complete removal of the lesion with negative margins in all cases.
At the last follow-up, all patients were alive and with no evidence of disease. Neither recurrent tumors nor complications were observed.
5. Discussion
SCC of the concha is a malignant tumor that can lead to local destruction and that can metastasize in advanced stages of the disease. Tumor size clearly affects the recurrence rate: a size greater than 3 cm increases recurrence [19]. In the cases included in this series, tumors were much smaller in all cases and no recurrences were observed. Moreover, no metastases were observed by the authors. This may be because the tumors were small and because of the limited number of cases. Moreover, we should consider that transplant recipients in which the tumor tends to be more aggressive, leading to a higher incidence of recurrences and metastases [10], were excluded from this study.
No general consensus exists about when lymph nodes should be treated. The current recommendation is to follow the patient for at least two years [13]. Prophylactic treatment should be given in case the tumor is more than 8 mm deep and/or in case of lympho-vascular invasion [11].
In our cohort, 57.6% of patients were male. This finding is quite discordant with recent studies which show more striking evidence of sex disparity: according to Kovatch, cutaneous SCC of the ears is 10 times more prevalent in men vs. women [20], this is due to different patterns of hair growth in females that shields the ear skin from damaging ultraviolet light which comes either from sun exposure or with exposure to artificial ultraviolet light. This could be explained by the fact that only SCCs limited to the concha were included in our study. Moreover, in our small series, age at diagnosis was lower (mean age: 56.1 years). Interestingly, 20/26 tumors were left sided.
Auricular SCC guidelines recommend surgical excision and histological confirmation of negative margins and close follow-up [21]. Eradication of SCC is the main goal of treatment. Nevertheless, aesthetics should also be considered in the management of SCC of the concha. These concerns make early diagnosis very important.
SCCs limited to the conchal bowl are rarely diagnosed at an early stage, since most of the time they present with advanced disease deserving at least a lateral temporal bone resection [22].
In the case of early diagnosis of conchal bowl SCC, complex reconstructions are described in the case of large full-thickness auricular defects, while reconstruction of small auricular conchal defects is often performed using a skin graft [23]. Potential complications of skin grafting are delayed wound healing, altered color, and contraction of the skin [24]. Some surgeons let the wound epithelize secondarily. This choice can lead to hypertrophic scars [14].
Therefore, flaps for conchal reconstruction should be considered a valid alternative in order to decrease the risk of these local complications. The PIF for reconstruction of auricular defects, including the conchal defects, is a well-known option in the literature. The advantages of the PIF are mainly represented by minimal donor site morbidity and good aesthetic outcomes. The flap is an optimal color match, and it does not need any special dressing technique [14].
The SCAR scale [17] that was used to measure the postoperative scar quality consists of six items scored by the observer and a couple of yes/no questions answered by the patient. Scores were provided via direct observation while patients’ responses for associated symptoms were elicited via verbal responses. The SCAR scale was easily feasible, and raters could complete the entire scale in less than two minutes.
In medicine, mesna has mainly been used as a mucolytic agent and as a protective agent against the toxicity of cyclophosphamide and other chemotherapeutic agents [25,26]. More recently, mesna has been used for CADISS of tissues [27].
CADISS of tissues is based on the combination of the common mechanical surgical instruments with the topical application of mesna, which is a drug capable of loosening the adherences between two different tissues. A series of CADISS dissectors can be used to deliver mesna directly at the tip of the instruments.
The first intraoperative applications of mesna were in the field of ENT surgery [16]. Mesna has proven to be effective in detaching the cholesteatoma matrix from the surrounding tissues [28,29], especially in case of infiltrating cholesteatoma, labyrinthine fistula, and matrices on the exposed facial nerve or dura mater. Later on, mesna was also used to facilitate tissue dissection in other fields of surgery [30].
The fundamental principle of the CADISS technique lies in the richness of disulfide bridges in the adhesions between the different tissue layers. Due to the capability of mesna to break the disulfide bridges of the polypeptide chains along the cleavage plane [31], dissection of tissues is facilitated since CADISS reduces the mechanical forces needed to separate tissues, and it is easier to maintain the proper cleavage plane.
The safety of mesna was demonstrated through an experimental model of guinea pigs in which the toxicity of mesna application into the middle ear on the cochlear anatomy and physiology was evaluated [32]. The toxicity of mesna was assessed by means of transmission electron microscopy, scanning electron microscopy, and auditory brainstem responses: no changes were seen after mesna application. Mesna did not show any other adverse effect when administered topically [32].
The combination of PIF with the CADISS technique, which is not a common technique for ablation of auricular SCC, is a valid alternative that can minimize the risk of complications, making this approach unique.
Due to the chemical properties of mesna [31], dissection of the conchal cartilage and attached tumor with perfect preservation of the deep perichondrium is efficient, safe, and easy.
The donor site can be easily closed primarily, mobilizing the surrounding tissues. The retroauricular skin is highly vascularized and is hidden behind the pinna [33]; moreover, it is very similar to the skin of the concha. Overall, the PIF constitutes an ideal solution for the reconstruction of auricular conchal defects after chemically assisted dissection of squamous cell carcinoma.
6. Conclusions
In our experience, when identified early, SCCs of the concha are highly curable. A limitation of the present study is the small number of participants. Despite the need for a larger series, our surgical study shows that the intraoperative use of mesna can be used as a chemical dissector to remove squamous cell carcinomas originating in the auricular concha. The intraoperative use of mesna facilitates the complete removal of the tumor with atraumatic dissection of tissues. The perfect preservation of the deep perichondrium of the conchal cartilage leads to early tissue repair. A PIF can be easily pulled through a post-auricular tunnel to repair the defect and the donor site can be closed primarily. PIFs have proved effective in the reconstruction of conchal defects and they allow one-stage reconstruction of conchal defects with good aesthetic outcomes [34].
Author Contributions
All authors made substantial contributions to (1) the conception of the study, acquisition, and interpretation of data, (2) drafting the article, (3) the final approval of the version to be submitted, and (4) agreed to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee (Comitato Etico Val Padana-PROT25961).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data supporting reported results can be asked to the corresponding author.
Acknowledgments
We are grateful to Antonella Piazza (Complutense University of Madrid) for her substantial support and contribution to this work, providing language help.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brougham, N.D.; Dennett, E.R.; Cameron, R.; Tan, S.T. The incidence of metastasis from cutaneous squamous cell carcinoma and the impact of its risk factors. J. Surg. Oncol. 2012, 106, 811–815. [Google Scholar] [CrossRef]
- Gaudet, J.E.; Walvekar, R.R.; Arriaga, M.A.; Dileo, M.D.; Nuss, D.W.; Puo, A.M.; Hagan, J.; Lin, J. Applicability of Pittsburgh staging system for advanced cutaneous malignancy of the temporal bone. Skull Base 2010, 20, 409–414. [Google Scholar] [CrossRef]
- Barrs, D.M. Temporal bone carcinoma. Otolaryngol. Clin. N. Am. 2001, 34, 1197–1218. [Google Scholar] [CrossRef]
- Hirsch, B.E.; Chang, C.Y.J. Carcinoma of the temporal bone. In Operative Otolaryngology Head and Neck Surgery; Myers, E.N., Ed.; WB Saunders: Philadelphia, PA, USA, 1997; pp. 1434–1458. [Google Scholar]
- Karia, P.S.; Han, J.; Schmults, C.D. Cutaneous squamous cell carcinoma: Estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J. Am. Acad. Dermatol. 2013, 68, 957–966. [Google Scholar] [CrossRef]
- Ahmad, I.; Gupta, A. Epidemiology of basal cell carcinoma and squamous cell carcinoma of the pinna. J. Laryngol. Otol. 2001, 115, 85–86. [Google Scholar] [CrossRef]
- Blake, G.B.; Wilson, J.S.P. Malignant tumours of the ear and their treatment. Br. J. Plast. Surg. 1974, 27, 67–76. [Google Scholar] [CrossRef]
- Mackie, R.M.; Elwood, J.M.; Hawk, J.L.M. Links between exposure to ultraviolet radiation and skin cancer. J. R. Coll. Physicians Lond. 1987, 21, 91–96. [Google Scholar]
- Marks, R.; Rennie, G.; Selwood, T. Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet 1988, 1, 795–797. [Google Scholar] [CrossRef]
- Redondo, P.; Lloret, P.; Sierra, A.; Gil, P. Aggressive tumors of the concha: Treatment with postauricular island pedicle flap. J. Cutan. Med. Surg. 2003, 7, 339–343. [Google Scholar] [CrossRef]
- Clark, R.R.; Soutar, D.S.; Hunter, K.D. A retrospective analysis of histological prognostic factors for the development of lymph node metastases from auricular squamous cell carcinoma. Histopathology 2010, 57, 138–146. [Google Scholar] [CrossRef]
- Yoon, M.; Chougule, P.; Dufresne, R.; Wanebo, H.J. Localized carcinoma of ther external ear is an unrecognized aggressive disease with a high propensity for local regional recurrences. Am. J. Surg. 1992, 164, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Mayo, E.; Sharma, S.; Horne, J.; Yuen, H.M.; Lee, A.; Gulati, A. Squamous cell carcinoma of the pinna: Which histological features could be used to predict prognosis? Br. J. Oral Maxillofac. Surg. 2017, 55, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, Z.J. Reconstruction of the concha of the ear using a postauricular island flap. Plast. Reconstr. Surg. 1990, 86, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhao, H.; Wu, K.; Lv, C.; Bi, H.-D.; Sun, M.-Y.; Wang, Y.-C.; Xing, X.; Xue, C.-Y. Reconstruction of auricular conchal defects with local flaps. Medicine 2016, 95, e5282. [Google Scholar] [CrossRef] [PubMed]
- Zini, C.; Bacciu, S.; Gandolfi, A.; Piazza, F.; Pasanisi, E. Use of Sodium 2-Mercaptoethanesulfonate in Surgery. U.S. Patent WO016213, 7 November 1998. [Google Scholar]
- Kantor, J. Reliability and photographic equivalency of the scar cosmesis assessment and rating (SCAR) scale, an outcome measure for postoperative scars. JAMA Dermatol. 2017, 153, 55–60. [Google Scholar] [CrossRef]
- Skin Cancer Treatment. National Cancer Institute Website. Available online: http//www.cancer.gov/cancertopics/pdq/treatment//skin/HealthProfessional (accessed on 5 July 2023).
- Rowe, D.E.; Carroll, R.J.; Day, C.L. Prognostic factors for local recurrence metastasis and survival rates in squamous cell carcinoma of the skin, ear, and lip: Implications for treatment modality selection. J. Am. Acad. Dermatol. 1992, 26, 976–990. [Google Scholar] [CrossRef]
- Kotvatch, K.J.; Smith, J.D.; Birkeland, A.C.; Hanks, J.E.; Jawad, R.; McLean, S.A.; Durham, A.B.; Ashok Srinivasan McHugh, J.B.; Basura, G.J. Institutional experience of treatment and outcomes for cutaneous periauricular squamous cell carcinoma. OTO Open 2019, 3, 2473974X19875077. [Google Scholar] [CrossRef]
- Casas, J.; Pachano, O. Postauricular revolving door island flap: Surgical option to concha squamous cell carcinoma. J. Otolaryngol. ENT Res. 2019, 11, 282–286. [Google Scholar] [CrossRef]
- Lovin, B.D.; Gidley, P.W. Squamous cell carcinoma of the temporal bone: A current review. Laryngoscope Investig. Otolaryngol. 2019, 4, 684–692. [Google Scholar] [CrossRef]
- Roche, A.M.; Griffin, M.; Shelton, R.; Urken, M.L. The folded postauricular flap: A novel approach to reconstruction of large full thickness defects of the conchal bowl. Am. J. Otolaryngol. 2017, 38, 706–709. [Google Scholar] [CrossRef]
- Dessy, L.A.; Figus, A.; Fioramonti, P.; Mazzocchi, M.; Scuderi, N. Reconstruction of anterior conchal defect after malignancy excision: Revolving door flap versus full-thickness skin flap. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.W.; Lopez-Vidriero, M.T.; Pavia, D.; Thomson, M.L. The effect of sodium 2 mercapto-ethane sulphonate and hypertonic saline aerosols on bronchial clearance in chronic bronchitis. Br. J. Clin. Pharmacol. 1979, 7, 39–44. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berrigan, M.J.; Marinello, A.J.; Pavelic, Z.; Williams, C.J.; Struck, R.F.; Gurtoo, H.L. Protective role of thiols in cyclophosphamide-induced urotoxicity and depression of hepatic drug metabolism. Cancer Res. 1982, 42, 3688–3695. [Google Scholar] [PubMed]
- Casale, M.; Di Martino, A.; Salvinelli, F.; Trombetta, M.; Denaro, V. Mesna for chemically assisted dissection. Expert Opin. Investig. Drugs 2010, 19, 699–707. [Google Scholar] [CrossRef]
- Zini, C.; Piazza, F.; Vighi, V.; De Franco, A. Chemically-assisted dissection of cholesteatoma. In Proceedings of the Fifth International Conference on Cholesteatoma and Mastoid Surgery, Alghero, Italy, 1–6 September 1996. [Google Scholar]
- Bovi, C.; Luchena, A.; Bivona, R.; Borsetto, D.; Creber, N.; Danesi, G. Recurrence in cholesteatoma surgery: What have we learned and where are we going? A narrative review. Acta Otorhinolaryngol. Ital. 2023, 43 (Suppl. 1), S48–S55. [Google Scholar] [CrossRef]
- Benassi, L.; Lopopolo, G.; Pazzoni, F.; Ricci, L.; Kaihura, C.; Piazza, F.; Vadora, E.; Zini, C. Chemically assisted dissection of tissues: An interesting support in abdominal myomectomy. J. Am. Coll. Surg. 2000, 191, 65–69. [Google Scholar] [CrossRef]
- Trissel, L.A. Mesna. In Handbook on Injectable Drugs; Reynold, J.E.F., Ed.; American Society of Hospital Pharmacists: Bethesda, MD, USA, 1994; pp. 664–666. [Google Scholar]
- Vincenti, V.; Mondain, M.; Pasanisi, E.; Piazza, F.; Puel, J.L.; Bacciu, S.; Quaranta, N.; Uziel, A.; Zini, C. Cochlear effects of Mesna application into the middle ear. Ann. N. Y. Acad. Sci. 1999, 884, 425–432. [Google Scholar] [CrossRef]
- Cordova, A.; D’Arpa, S.; Pirrello, R.; Giambona, C.; Moschella, F. Retroauricular skin: A flaps bank for ear reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2008, 61, S44–S51. [Google Scholar] [CrossRef]
- Iljin, A.; Antoszewski, B.; Durko, M.; Zielinski, T.; Stabryla, P.; Pietruszewska, W. External ear carcinoma: Evaluation of surgical and reconstructive management with postauricular island flap. Postep. Dermatol Alergol 2022, 39, 1134–1140. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).