Advancement of 3D Bioprinting Towards 4D Bioprinting for Sustained Drug Delivery and Tissue Engineering from Biopolymers
Abstract
1. Introduction
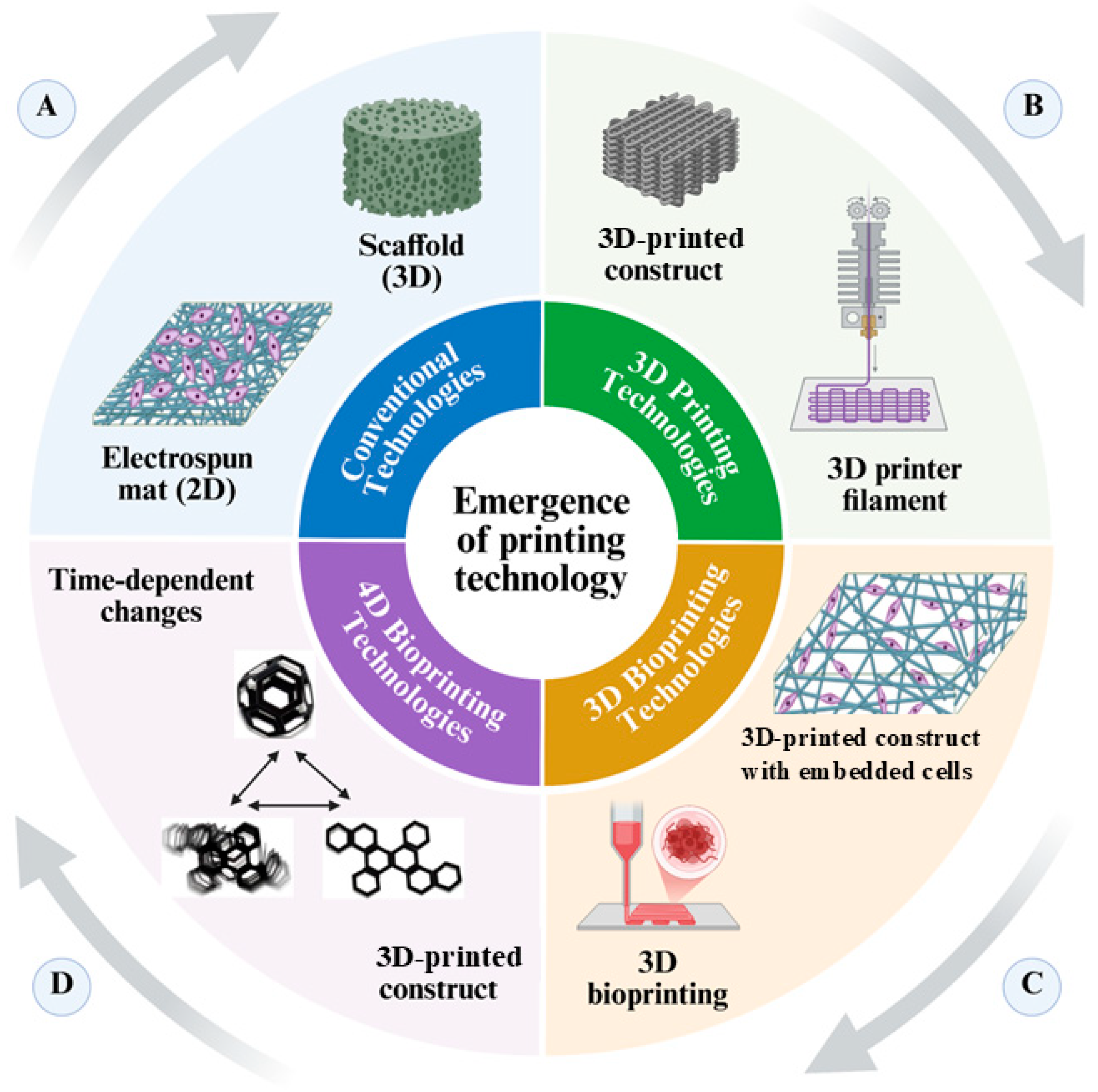
2. Fundamentals of 3D-Bioprinting
Limitations of 3D-Bioprinting
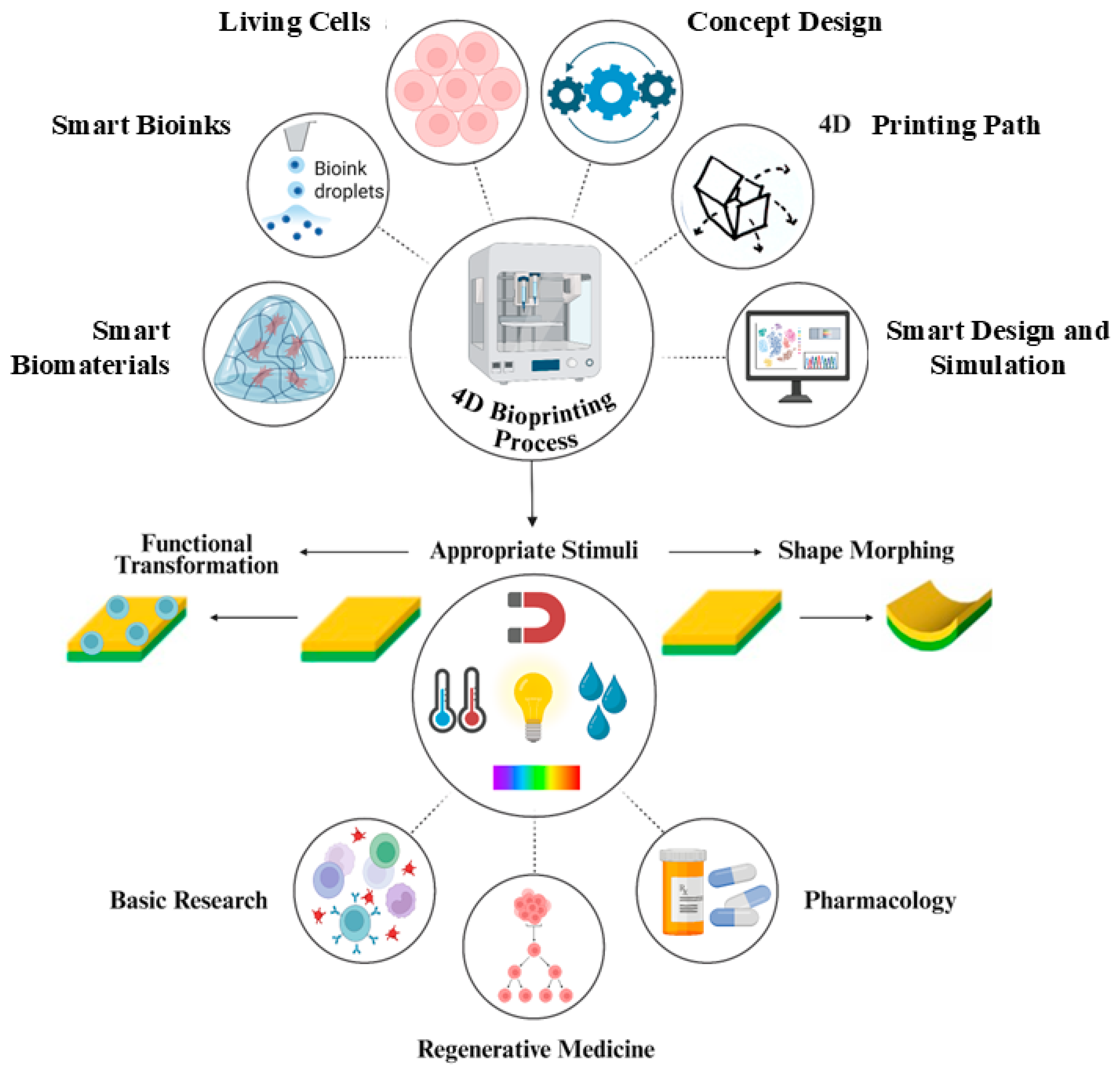
3. Advancements Towards 4D Bioprinting
4. Biopolymers for 4D-Bioprinting
| Polymer | Description | Applications | Stimuli Response Mechanism | Refs. |
|---|---|---|---|---|
| Alginates | Biocompatible, biodegradable gel-forming polymers from brown algae. | Hydrogel scaffolds with shape memory for tissue engineering. | Responsive to temperature and ionic strength (Ca2+ cross-linking). | [96,97] |
| Gelatin | Biocompatible polymer that undergoes thermal gelation. | Dynamic scaffolds mimicking natural tissue healing. | Responds to temperature variations (sol–gel transition). | [98] |
| Chitosan | Antimicrobial polymer derived from chitin. | Scaffolds for controlled drug delivery. | Responds to pH changes (protonation of amino groups). | [99,100] |
| Polydopamine | Enhances polymer responsiveness for dynamic control. | Improves control in hydrogel formulations. | Increases sensitivity to various external stimuli (light, pH, heat, humidity, fields). | [101,102] |
| Carrageenan | Biodegradable polymer with bioactive properties from red algae. | Used in wound healing and skin bioengineering. | Responds to environmental stimuli (moisture, temperature). | [103,104] |
4.1. Mechanisms of Responsive Materials in 4D-Printing
4.1.1. Shape Memory Polymers (SMPs)
4.1.2. Hydrogels
4.1.3. Multi-Responsive Materials
4.1.4. Metal and Ceramic Composites
4.1.5. Biopolymer-Based Smart Materials
| Mechanism | Description | Stimuli | Time Factor | Applications | References |
|---|---|---|---|---|---|
| Shape Memory Polymers (SMPs) | Polymers (e.g., polyurethane) that return to a preset shape when triggered by temperature or electromagnetic fields. | Temperature, electromagnetic fields. | Change occurs within seconds to minutes. | Soft robotics, biomedical devices, dynamic shape changes. | [140,141] |
| Hydrogels | Polymers (e.g., alginate and hyaluronic acid) that swell or shrink in response to temperature, pH, or humidity, enabling shape change. | Temperature, pH, ionic strength, humidity. | Response time varies from minutes to hours. | Bioinspired engineering, drug delivery systems, adaptive structures. | [142,143] |
| Multi-Responsive Materials | Materials (e.g., acrylate-based hydrogels) that react to multiple stimuli for complex behaviors and transformations. | Temperature, light, moisture. | Transformations over minutes to hours. | Advanced bioengineering applications, responsive systems with versatile functions. | [144] |
| Metal and Ceramic Composites | Composites (e.g., alloys) with shape memory properties that respond to thermal or magnetic stimuli. | Thermal, magnetic. | Changes typically occur over seconds to minutes. | Aerospace applications, biomedical engineering where polymers may not suffice. | [145] |
| Biopolymer-based Smart Materials | Biopolymers (e.g., alginate and gelatin) react to pH and biological changes for specific applications. | pH changes, biomolecule presence. | Response time varies from minutes to days. | Drug delivery, tissue engineering applications, targeted therapy strategies. | [146,147] |
4.2. Potential Applications in Sustained Drug Delivery
4.2.1. Stimuli-Responsive Drug Delivery Systems
4.2.2. 4D-Bioprinting for Drug Delivery of Personalized Medicine
4.2.3. Temporal and Spatial Control of Drug Release
4.2.4. Tissue Engineering and Regenerative Medicine
4.2.5. Overcoming Limitations of Conventional Drug Delivery Systems
4.3. Strategies for Enhancing Drug Delivery Efficacy
4.3.1. Stimuli-Responsive Materials
4.3.2. Customization of Drug Release Profiles
4.3.3. Integration of Targeting Mechanisms
4.3.4. Enhancing Biocompatibility and Functional Integration
4.3.5. Developing Personalized Drug Delivery Systems
5. Future Directions and Challenges for Drug Delivery by 4D-Bioprinting
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lazaridou, M.; Bikiaris, D.N.; Lamprou, D.A. 3D bioprinted chitosan-based hydrogel scaffolds in tissue engineering and localised drug delivery. Pharmaceutics 2022, 14, 1978. [Google Scholar] [CrossRef]
- Han, P.; Ivanovski, S. 3D bioprinted extracellular vesicles for tissue engineering-a perspective. Biofabrication 2022, 15, 013001. [Google Scholar] [CrossRef]
- Yazdanpanah, Z.; Johnston, J.D.; Cooper, D.M.; Chen, X. 3D bioprinted scaffolds for bone tissue engineering: State-of-the-art and emerging technologies. Front. Bioeng. Biotechnol. 2022, 10, 824156. [Google Scholar] [CrossRef]
- Cui, H.; Miao, S.; Esworthy, T.; Zhou, X.; Lee, S.J.; Liu, C.; Yu, Z.X.; Fisher, J.P.; Mohiuddin, M.; Zhang, L.G. 3D bioprinting for cardiovascular regeneration and pharmacology. Adv. Drug Deliv. Rev. 2018, 132, 252–269. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Jeong, W.; Atala, A. 3D Bioprinting for Engineered Tissue Constructs and Patient-Specific Models: Current Progress and Prospects in Clinical Applications. Adv. Mater. 2024, 36, 2408032. [Google Scholar] [CrossRef]
- Thomas, D.J.; Jessop, Z.M.; Whitaker, I.S. 3D Bioprinting for Reconstructive Surgery: Techniques and Applications; Woodhead Publishing: Cambridge, UK, 2017. [Google Scholar]
- Gupta, S.; Kumar, G.; Dubey, S.; Sharma, G.; Singh, S.; Minz, S. 3D Bioprinting for Skin and Tissue Engineering. In 3D Printing and Microfluidics in Dermatology; CRC Press: Boca Raton, FL, USA, 2025; pp. 125–156. [Google Scholar]
- Lee, A.; Hudson, A.; Shiwarski, D.; Tashman, J.; Hinton, T.; Yerneni, S.; Bliley, J.; Campbell, P.; Feinberg, A. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Piluso, S.; Skvortsov, G.A.; Altunbek, M.; Afghah, F.; Khani, N.; Koç, B.; Patterson, J. 3D bioprinting of molecularly engineered PEG-based hydrogels utilizing gelatin fragments. Biofabrication 2021, 13, 045008. [Google Scholar] [CrossRef] [PubMed]
- Özenler, A.K.; Distler, T.; Akkineni, A.R.; Tihminlioglu, F.; Gelinsky, M.; Boccaccini, A.R. 3D bioprinting of mouse pre-osteoblasts and human MSCs using bioinks consisting of gelatin and decellularized bone particles. Biofabrication 2024, 16, 025027. [Google Scholar] [CrossRef]
- Khati, V.; Ramachandraiah, H.; Pati, F.; Svahn, H.A.; Gaudenzi, G.; Russom, A. 3D bioprinting of multi-material decellularized liver matrix hydrogel at physiological temperatures. Biosensors 2022, 12, 521. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Luo, Y.; Luo, G.; Gelinsky, M.; Huang, P.; Ruan, C. 3D bioprinting scaffold using alginate/polyvinyl alcohol bioinks. Mater. Lett. 2017, 189, 295–298. [Google Scholar] [CrossRef]
- Hull, S.M.; Lindsay, C.D.; Brunel, L.G.; Shiwarski, D.J.; Tashman, J.W.; Roth, J.G.; Myung, D.; Feinberg, A.W.; Heilshorn, S.C. 3D bioprinting using UNIversal orthogonal network (UNION) bioinks. Adv. Funct. Mater. 2021, 31, 2007983. [Google Scholar] [CrossRef]
- Antezana, P.E.; Municoy, S.; Ostapchuk, G.; Catalano, P.N.; Hardy, J.G.; Evelson, P.A.; Orive, G.; Desimone, M.F. 4D printing: The development of responsive materials using 3D-printing technology. Pharmaceutics 2023, 15, 2743. [Google Scholar] [CrossRef]
- Noroozi, R.; Arif, Z.U.; Taghvaei, H.; Khalid, M.Y.; Sahbafar, H.; Hadi, A.; Sadeghianmaryan, A.; Chen, X. 3D and 4D bioprinting technologies: A game changer for the biomedical sector? Ann. Biomed. Eng. 2023, 51, 1683–1712. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Luo, Y.; Liang, Q.; Liu, Y.; Zhong, G.; Yu, Y.; Chen, F. 3D Contour Printing of Anatomically Mimetic Cartilage Grafts with Microfiber-Reinforced Double-Network Bioink. Macromol. Biosci. 2022, 22, 2200179. [Google Scholar] [CrossRef]
- Ceylan, H.; Dogan, N.O.; Yasa, I.C.; Musaoglu, M.N.; Kulali, Z.U.; Sitti, M. 3D printed personalized magnetic micromachines from patient blood–derived biomaterials. Sci. Adv. 2021, 7, eabh0273. [Google Scholar] [CrossRef]
- Wu, S.-D.; Hsu, S.-h. 4D bioprintable self-healing hydrogel with shape memory and cryopreserving properties. Biofabrication 2021, 13, 045029. [Google Scholar] [CrossRef]
- Chen, Q.; Kalpoe, T.; Jovanova, J. Design of mechanically intelligent structures: Review of modelling stimuli-responsive materials for adaptive structures. Heliyon 2024, 10, e34026. [Google Scholar] [CrossRef] [PubMed]
- Willemen, N.G.; Morsink, M.A.; Veerman, D.; Da Silva, C.F.; Cardoso, J.C.; Souto, E.B.; Severino, P. From oral formulations to drug-eluting implants: Using 3D and 4D printing to develop drug delivery systems and personalized medicine. Bio-Des. Manuf. 2021, 5, 85–106. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Aghda, N.H.; Pillai, A.R.; Thakkar, R.; Nokhodchi, A.; Maniruzzaman, M. Emerging 3D printing technologies for drug delivery devices: Current status and future perspective. Adv. Drug Deliv. Rev. 2021, 174, 294–316. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Feng, X.; Wang, Z.; Zhan, Y.; Wu, X.; Xie, W.; Wang, Z.; Zhang, G. Techniques and applications in 3D bioprinting with chitosan bio-inks for drug delivery: A review. Int. J. Biol. Macromol. 2024, 278, 134752. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Ahmed, W.; Arshad, H. A review on four-dimensional (4D) bioprinting in pursuit of advanced tissue engineering applications. Bioprinting 2022, 27, e00203. [Google Scholar] [CrossRef]
- Rizzo, M.; Turco, S.; Spina, F.; Costantino, A.; Visi, G.; Baronti, A.; Maiese, A.; Di Paolo, M. 3D printing and 3D bioprinting technology in medicine: Ethical and legal issues. La Clin. Ter. 2023, 174, 80–84. [Google Scholar]
- Naghib, S.M.; Zarrineh, M.; Mozafari, M.R. 3D Printing chitosan-based nanobiomaterials for biomedicine and drug delivery: Recent advances on the promising bioactive agents and technologies. Curr. Org. Chem. 2024, 28, 510–525. [Google Scholar] [CrossRef]
- Mahesh Krishna, B.; Francis Luther King, M.; Robert Singh, G.; Gopichand, A. 3D printing in drug delivery and healthcare. In Advanced Materials and Manufacturing Techniques for Biomedical Applications; Wiley: Hoboken, NJ, USA, 2023; pp. 241–274. [Google Scholar]
- Wang, X.; Mu, M.; Yan, J.; Han, B.; Ye, R.; Guo, G. 3D printing materials and 3D printed surgical devices in oral and maxillofacial surgery: Design, workflow and effectiveness. Regen. Biomater. 2024, 11, rbae066. [Google Scholar] [CrossRef]
- Parupelli, S.K.; Desai, S. The 3D printing of nanocomposites for wearable biosensors: Recent advances, challenges, and prospects. Bioengineering 2023, 11, 32. [Google Scholar] [CrossRef]
- Sharma, V.; Roozbahani, H.; Alizadeh, M.; Handroos, H. 3D printing of plant-derived compounds and a proposed nozzle design for the more effective 3D FDM printing. IEEE Access 2021, 9, 57107–57119. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, R.; Zepeda, H.; Zeng, L.; Qiu, J.; Wang, S. 3D Printing Super Strong Hydrogel for Artificial Meniscus. ACS Appl. Polym. Mater. 2019, 1, 2023–2032. [Google Scholar] [CrossRef]
- Khan, M.; Khan, N.; Ullah, M.; Hamayun, S.; Makhmudov, N.; Mbbs, R.; Safdar, M.; Bibi, A.; Wahab, A.; Naeem, M.; et al. 3D Printing Technology and Its Revolutionary Role in Stent Implementation in Cardiovascular Disease. Curr. Probl. Cardiol. 2024, 49, 102568. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Wahab, A.; Khan, S.U.; Naeem, M.; ur Rehman, K.; Ali, H.; Ullah, A.; Khan, A.; Khan, N.R.; Rizg, W.Y. 3D printing technology: A new approach for the fabrication of personalized and customized pharmaceuticals. Eur. Polym. J. 2023, 195, 112240. [Google Scholar] [CrossRef]
- Pavan Kalyan, B.; Kumar, L. 3D printing: Applications in tissue engineering, medical devices, and drug delivery. AAPS PharmSciTech 2022, 23, 92. [Google Scholar] [CrossRef]
- Jeong, Y.M.; Bang, C.; Park, M.; Shin, S.; Yun, S.; Kim, C.M.; Jeong, G.; Chung, Y.J.; Yun, W.S.; Lee, J.H.; et al. 3D-Printed Collagen Scaffolds Promote Maintenance of Cryopreserved Patients-Derived Melanoma Explants. Cells 2021, 10, 589. [Google Scholar] [CrossRef]
- Javkhlan, Z.; Hsu, S.-H.; Chen, R.-S.; Chen, M.-H. 3D-printed polycaprolactone scaffolds coated with beta tricalcium phosphate for bone regeneration. J. Formos. Med. Assoc. 2024, 123, 71–77. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, S.; Ravichandran, D.; Ramanathan, A.; Sobczak, M.T.; Sacco, A.F.; Patil, D.; Thummalapalli, S.V.; Pulido, T.V.; Lancaster, J.N. 3D-Printed Polymeric Biomaterials for Health Applications. Adv. Healthc. Mater. 2025, 14, 2402571. [Google Scholar] [CrossRef]
- Aftab, M.; Ikram, S.; Ullah, M.; Khan, N.; Naeem, M.; Khan, M.A.; Bakhtiyor o’g’li, R.B.; Qizi, K.S.S.; Erkinjon Ugli, O.O.; Abdurasulovna, B.M. Recent Trends and Future Directions in 3D Printing of Biocompatible Polymers. J. Manuf. Mater. Process. 2025, 9, 129. [Google Scholar] [CrossRef]
- Feng, P.; Jia, J.; Yu, L.; Min, A.; Yang, S.; Shuai, C. Accelerated degradation of poly (l-lactide) bone scaffold: Crystallinity and hydrophilicity. Mater. Chem. Phys. 2021, 266, 124545. [Google Scholar] [CrossRef]
- Kozaily, E.; Geagea, M.; Akdogan, E.R.; Atkins, J.; Elshazly, M.B.; Guglin, M.; Tedford, R.J.; Wehbe, R.M. Accuracy and consistency of online large language model-based artificial intelligence chat platforms in answering patients’ questions about heart failure. Int. J. Cardiol. 2024, 408, 132115. [Google Scholar] [CrossRef]
- Kumar, H.; Yadav, S. Additive and Good Manufacturing Practices in Conformity Assessment. In Handbook of Quality System, Accreditation and Conformity Assessment; Springer: Singapore, 2024; pp. 971–988. [Google Scholar]
- Dai, X.j.; Li, W.J.; Xie, D.D.; Liu, B.x.; Gong, L.; Han, H.H. Stimuli-Responsive Nano Drug Delivery Systems for the Treatment of Neurological Diseases. Small 2025, 21, 2410030. [Google Scholar] [CrossRef]
- Narupai, B.; Smith, P.T.; Nelson, A. 4D printing of multi-stimuli responsive protein-based hydrogels for autonomous shape transformations. Adv. Funct. Mater. 2021, 31, 2011012. [Google Scholar] [CrossRef]
- Jain, P.; Kathuria, H.; Dubey, N. Advances in 3D bioprinting of tissues/organs for regenerative medicine and in-vitro models. Biomaterials 2022, 287, 121639. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, X.; Shan, M.; Hao, Z.; Zhang, X.; Meng, L.; Zhai, Z.; Zhang, L.; Liu, X.; Wang, X. Convergence of 3D bioprinting and nanotechnology in tissue engineering scaffolds. Biomimetics 2023, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Saadi, M.; Maguire, A.; Pottackal, N.T.; Thakur, M.S.H.; Ikram, M.M.; Hart, A.J.; Ajayan, P.M.; Rahman, M.M. Direct ink writing: A 3D printing technology for diverse materials. Adv. Mater. 2022, 34, 2108855. [Google Scholar] [CrossRef]
- Capuana, E.; Lopresti, F.; Carfì Pavia, F.; Brucato, V.; La Carrubba, V. Solution-based processing for scaffold fabrication in tissue engineering applications: A brief review. Polymers 2021, 13, 2041. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, Y.; Peng, X.; Lu, C.; Wu, Z.; Xu, H.; Qin, Y.; Xu, Y.; Wang, Q.; Hao, Y. Comprehensive Overview of Interface Strategies in Implant Osseointegration. Adv. Funct. Mater. 2025, 35, 2418849. [Google Scholar] [CrossRef]
- Law, A.C.C.; Wang, R.; Chung, J.; Kucukdeger, E.; Liu, Y.; Barron, T.; Johnson, B.N.; Kong, Z. Process parameter optimization for reproducible fabrication of layer porosity quality of 3D-printed tissue scaffold. J. Intell. Manuf. 2024, 35, 1825–1844. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Zheng, Z.; Wei, X.; Chen, L.; Wu, Y.; Huang, W.; Yang, L. Strategies for improving the 3D printability of decellularized extracellular matrix bioink. Theranostics 2023, 13, 2562. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Guo, B.; Sun, D.; Xiao, Y.; Yang, Z.; Liu, R.; Chen, J.; Wu, B.; Zhao, P. Jammed pickering emulsion gels. Adv. Sci. 2024, 11, 2409678. [Google Scholar] [CrossRef]
- Khopade, A.J.; Shah, M. Challenges for Commercial Translation of Nanomedicines: From Lab Scale to Production Scale. In Commercial Scale Production of Nanomedicines; CRC Press: Boca Raton, FL, USA, 2025; pp. 1–40. [Google Scholar]
- Vanaei, H.R.; Khelladi, S.; Tcharkhtchi, A. 3D Printing as a Multidisciplinary Field. In Industrial Strategies and Solutions for 3D Printing: Applications and Optimization; Vanaei, H.R., Khelladi, S., Tcharkhtchi, A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2024; pp. 1–24. [Google Scholar]
- Wang, J.; Huo, Y.; Mahe, J.; Ge, Z.; Liu, Z.; Wang, W.; Zhang, L. Developing an ethical regulatory framework for artificial intelligence: Integrating systematic review, thematic analysis, and multidisciplinary theories. IEEE Access 2024, 12, 179383–179395. [Google Scholar] [CrossRef]
- Thompson, M.J.; Frampton, J.P. Bioengineered Models of Nerve Regeneration. Adv. Mater. Technol. 2025. early view. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Jia, Z.; Jiao, K.; Liu, C.; Deng, Z.; Bai, Y.; Wei, X.; Zhou, X. Bioprinted hydrogels in bone regeneration: A bibliometric analysis. Front. Pharmacol. 2025, 16, 1532629. [Google Scholar] [CrossRef]
- Amukarimi, S.; Ramakrishna, S.; Mozafari, M. Smart biomaterials—A proposed definition and overview of the field. Curr. Opin. Biomed. Eng. 2021, 19, 100311. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, X.; Yao, B.; Zhu, S.; Zhu, P.; Huang, S. Tailoring bioinks of extrusion-based bioprinting for cutaneous wound healing. Bioact. Mater. 2022, 17, 178–194. [Google Scholar] [CrossRef]
- Deptuła, M.; Zawrzykraj, M.; Sawicka, J.; Banach-Kopeć, A.; Tylingo, R.; Pikuła, M. Application of 3D-printed hydrogels in wound healing and regenerative medicine. Biomed. Pharmacother. 2023, 167, 115416. [Google Scholar] [CrossRef]
- Jamieson, C.; Keenan, P.; Kirkwood, D.A.; Oji, S.; Webster, C.; Russell, K.A.; Koch, T.G. A review of recent advances in 3D bioprinting with an eye on future regenerative therapies in veterinary medicine. Front. Vet. Sci. 2021, 7, 584193. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Ullah, M.; Saeed, S.; Saleh, E.A.M.; Kassem, A.F.; Arbi, F.M.; Wahab, A.; Rehman, M.; ur Rehman, K.; Khan, D. Nanotherapeutic approaches for transdermal drug delivery systems and their biomedical applications. Eur. Polym. J. 2024, 207, 112819. [Google Scholar] [CrossRef]
- Suhag, D. Regulatory and Ethical Considerations. In Handbook of Biomaterials for Medical Applications, Volume 2: Applications; Springer: Berlin/Heidelberg, Germany, 2024; pp. 355–372. [Google Scholar]
- Sekar, M.P.; Budharaju, H.; Zennifer, A.; Sethuraman, S.; Vermeulen, N.; Sundaramurthi, D.; Kalaskar, D.M. Current standards and ethical landscape of engineered tissues—3D bioprinting perspective. J. Tissue Eng. 2021, 12, 20417314211027677. [Google Scholar] [CrossRef]
- Rawal, P.; Tripathi, D.M.; Ramakrishna, S.; Kaur, S. Prospects for 3D bioprinting of organoids. Bio-Des. Manuf. 2021, 4, 627–640. [Google Scholar] [CrossRef]
- Gao, J.; Feng, L.; Chen, B.; Fu, B.; Zhu, M. The role of rare earth elements in bone tissue engineering scaffolds—A review. Compos. Part B Eng. 2022, 235, 109758. [Google Scholar] [CrossRef]
- Salg, G.A.; Poisel, E.; Neulinger-Munoz, M.; Gerhardus, J.; Cebulla, D.; Bludszuweit-Philipp, C.; Vieira, V.; Nickel, F.; Herr, I.; Blaeser, A. Toward 3D-bioprinting of an endocrine pancreas: A building-block concept for bioartificial insulin-secreting tissue. J. Tissue Eng. 2022, 13, 20417314221091033. [Google Scholar] [CrossRef]
- Souri, M.; Soltani, M.; Kashkooli, F.M.; Shahvandi, M.K.; Chiani, M.; Shariati, F.S.; Mehrabi, M.R.; Munn, L.L. Towards principled design of cancer nanomedicine to accelerate clinical translation. Mater. Today Bio 2022, 13, 100208. [Google Scholar] [CrossRef]
- Buj-Corral, I.; Tejo-Otero, A.; Fenollosa-Artés, F. Use of FDM technology in healthcare applications: Recent advances. In Fused Deposition Modeling Based 3D Printing; Dave, H.K., Davim, J.P., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 277–297. [Google Scholar] [CrossRef]
- Szychlinska, M.A.; Bucchieri, F.; Fucarino, A.; Ronca, A.; D’Amora, U. Three-dimensional bioprinting for cartilage tissue engineering: Insights into naturally-derived bioinks from land and marine sources. J. Funct. Biomater. 2022, 13, 118. [Google Scholar] [CrossRef]
- Mahendiran, B.; Muthusamy, S.; Sampath, S.; Jaisankar, S.; Popat, K.C.; Selvakumar, R.; Krishnakumar, G.S. Recent trends in natural polysaccharide based bioinks for multiscale 3D printing in tissue regeneration: A review. Int. J. Biol. Macromol. 2021, 183, 564–588. [Google Scholar] [CrossRef]
- Altıparmak, S.C.; Yardley, V.A.; Shi, Z.; Lin, J. Extrusion-based additive manufacturing technologies: State of the art and future perspectives. J. Manuf. Process. 2022, 83, 607–636. [Google Scholar] [CrossRef]
- Zennifer, A.; Subramanian, A.; Sethuraman, S. Design considerations of bioinks for laser bioprinting technique towards tissue regenerative applications. Bioprinting 2022, 27, e00205. [Google Scholar] [CrossRef]
- Dou, C.; Perez, V.; Qu, J.; Tsin, A.; Xu, B.; Li, J. A state-of-the-art review of laser-assisted bioprinting and its future research trends. ChemBioEng Rev. 2021, 8, 517–534. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Jahangiri, S.; Zirak Hassan Kiadeh, S.; Rezvaninejad, S.; Ahmadi, Z.; Ahmadi, S.; Safarkhani, M.; Rabiee, N. Stimuli-responsive biomaterials: Smart avenue toward 4D bioprinting. Crit. Rev. Biotechnol. 2024, 44, 860–891. [Google Scholar] [CrossRef]
- Tamir, T.S.; Teferi, F.B.; Hua, X.; Leng, J.; Xiong, G.; Shen, Z.; Liu, Q. A review of advances in 3D and 4D bioprinting: Toward mass individualization paradigm. J. Intell. Manuf. 2024, 35, 1–30. [Google Scholar] [CrossRef]
- Zheng, F.; Tian, R.; Lu, H.; Liang, X.; Shafiq, M.; Uchida, S.; Chen, H.; Ma, M. Droplet Microfluidics Powered Hydrogel Microparticles for Stem Cell-Mediated Biomedical Applications. Small 2024, 20, 2401400. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, J.; Kumar, R.; Najser, J.; Frantik, J.; Manuja, A.; Sunnam, N.; Praveenkumar, S. Advances in bioink-based 3D printed scaffolds: Optimizing biocompatibility and mechanical properties for bone regeneration. Biomater. Sci. 2025, 13, 2556–2579. [Google Scholar] [CrossRef] [PubMed]
- Pourmasoumi, P.; Moghaddam, A.; Nemati Mahand, S.; Heidari, F.; Salehi Moghaddam, Z.; Arjmand, M.; Kühnert, I.; Kruppke, B.; Wiesmann, H.-P.; Khonakdar, H.A. A review on the recent progress, opportunities, and challenges of 4D printing and bioprinting in regenerative medicine. J. Biomater. Sci. Polym. Ed. 2023, 34, 108–146. [Google Scholar] [CrossRef]
- Bănică, C.-F.; Sover, A.; Anghel, D.-C. Printing the future layer by layer: A comprehensive exploration of additive manufacturing in the era of industry 4.0. Appl. Sci. 2024, 14, 9919. [Google Scholar] [CrossRef]
- Sadraei, A.; Naghib, S.M. 4D printing of physical stimuli-responsive hydrogels for localized drug delivery and tissue engineering. Polym. Rev. 2025, 65, 104–168. [Google Scholar] [CrossRef]
- Park, T.; Leem, J.W.; Kim, Y.L.; Lee, C.H. Photonic Nanomaterials for Wearable Health Solutions. Adv. Mater. 2025. early view. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Zolfagharian, A.; Bodaghi, M. 4D bioprinting of smart polymers for biomedical applications: Recent progress, challenges, and future perspectives. React. Funct. Polym. 2022, 179, 105374. [Google Scholar] [CrossRef]
- Amirthalingam, S.; Rajendran, A.K.; Moon, Y.G.; Hwang, N.S. Stimuli-responsive dynamic hydrogels: Design, properties and tissue engineering applications. Mater. Horiz. 2023, 10, 3325–3350. [Google Scholar] [CrossRef]
- Maan, Z.; Masri, N.Z.; Willerth, S.M. Smart bioinks for the printing of human tissue models. Biomolecules 2022, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Almaz, A.F.; El-Agouz, E.; Abdelfatah, M.T.; Mohamed, I.R. The future role of Artificial Intelligence (AI) design’s integration into architectural and interior design education is to improve efficiency, sustainability, and creativity. Civ. Eng. Archit. 2024, 12, 1749–1772. [Google Scholar] [CrossRef]
- Liu, Q.; Leng, J.; Yan, D.; Zhang, D.; Wei, L.; Yu, A.; Zhao, R.; Zhang, H.; Chen, X. Digital twin-based designing of the configuration, motion, control, and optimization model of a flow-type smart manufacturing system. J. Manuf. Syst. 2021, 58, 52–64. [Google Scholar] [CrossRef]
- Biswal, T. Biopolymers for tissue engineering applications: A review. Mater. Today Proc. 2021, 41, 397–402. [Google Scholar] [CrossRef]
- Marques, D.M.; Silva, J.C.; Serro, A.P.; Cabral, J.M.; Sanjuan-Alberte, P.; Ferreira, F.C. 3D bioprinting of novel κ-carrageenan bioinks: An algae-derived polysaccharide. Bioengineering 2022, 9, 109. [Google Scholar] [CrossRef]
- Berg, J.; Kurreck, J. Clean bioprinting-fabrication of 3D organ models devoid of animal components. ALTEX-Altern. Anim. Exp. 2021, 38, 269–288. [Google Scholar] [CrossRef]
- Hasan, N.; Aftab, M.; Ullah, M.; Nguyen, P.T.; Agustina, R.; Djabir, Y.Y.; Tockary, T.A.; Uchida, S. Nanoparticle-based drug delivery system for Oral Cancer: Mechanism, challenges, and therapeutic potential. Results Chem. 2025, 14, 102068. [Google Scholar] [CrossRef]
- Mahmood, A.; Maher, N.; Amin, F.; Alqutaibi, A.Y.; Kumar, N.; Zafar, M.S. Chitosan-based materials for dental implantology: A comprehensive review. Int. J. Biol. Macromol. 2024, 268, 131823. [Google Scholar] [CrossRef] [PubMed]
- Samyn, P. Polydopamine and cellulose: Two biomaterials with excellent compatibility and applicability. Polym. Rev. 2021, 61, 814–865. [Google Scholar] [CrossRef]
- Ding, A.; Lee, S.J.; Ayyagari, S.; Tang, R.; Huynh, C.T.; Alsberg, E. 4D biofabrication via instantly generated graded hydrogel scaffolds. Bioact. Mater. 2022, 7, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Udayakumar, S.; Girigoswami, A.; Girigoswami, K. Biological activities of carrageenan from red algae: A mini review. Curr. Pharmacol. Rep. 2024, 10, 12–26. [Google Scholar] [CrossRef]
- Park, S.-J.; Sharma, A.; Lee, H.-J. An update on the chemical constituents and biological properties of selected species of an underpinned genus of red algae: Chondrus. Mar. Drugs 2024, 22, 47. [Google Scholar] [CrossRef]
- Tiwari, J. Trends In therapeutic and Prevention Strategies for Management of Bovine Mastitis: An Overview. J. Vaccines Vaccin. 2013, 4, 1000176. [Google Scholar] [CrossRef]
- Ullah, N.; Hasnain, S.Z.U.; Baloch, R.; Amin, A.; Nasibova, A.; Selakovic, D.; Rosic, G.L.; Islamov, S.; Naraliyeva, N.; Jaradat, N. Exploring essential oil-based bio-composites: Molecular docking and in vitro analysis for oral bacterial biofilm inhibition. Front. Chem. 2024, 12, 1383620. [Google Scholar] [CrossRef]
- Andreazza, R.; Morales, A.; Pieniz, S.; Labidi, J. Gelatin-based hydrogels: Potential biomaterials for remediation. Polymers 2023, 15, 1026. [Google Scholar] [CrossRef]
- Nuc, Z.; Dobrzycka-Krahel, A. From chitin to chitosan–a potential natural antimicrobial agent. Prog. Chem. Appl. Chitin Its Deriv. 2021, 26, 23–40. [Google Scholar] [CrossRef]
- Chakravarty, J.; Edwards, T.A. Innovation from waste with biomass-derived chitin and chitosan as green and sustainable polymer: A review. Energy Nexus 2022, 8, 100149. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, F.; Zhang, Z.; Cheng, Y.; Wang, Z.; Li, Y. Stimuli-responsive polydopamine-based smart materials. Chem. Soc. Rev. 2021, 50, 8319–8343. [Google Scholar] [CrossRef]
- Lv, H.; Chi, H.; Yang, X.; Peng, J.; Wang, W.; Tang, D. Polydopamine-assisted shape memory of polyurethane nanofibers with light-induced tunable responsiveness and improved cell adhesiveness. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127100. [Google Scholar] [CrossRef]
- Gomaa, M. Biodegradable plastics based on algal polymers: Recent advances and applications. In Handbook of Biodegradable Materials; Ali, G.A.M., Makhlouf, A.S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 501–531. [Google Scholar]
- James, J.; Verma, M.; Sharma, N. Nanotechnology-driven improvisation of red algae-derived carrageenan for industrial and bio-medical applications. World J. Microbiol. Biotechnol. 2024, 40, 4. [Google Scholar] [CrossRef]
- Bonetti, L.; Scalet, G. 4D fabrication of shape-changing systems for tissue engineering: State of the art and perspectives. Prog. Addit. Manuf. 2025, 10, 1913–1943. [Google Scholar] [CrossRef]
- Hasan, N.; Palungan, J.; Ullah, M. Gene Editing Techniques in Cancer Research; In Methods in Cell Biology (in press). Elsevier: Amsterdam, The Netherlands, 2025. [CrossRef]
- Naghib, S.M.; Hosseini, S.N.; Beigi, A. 3D 4D printing of chitosan-based scaffolds for wound dressing applications. Carbohydr. Polym. Technol. Appl. 2024, 8, 100594. [Google Scholar] [CrossRef]
- Veeman, D.; Sai, M.S.; Sureshkumar, P.; Jagadeesha, T.; Natrayan, L.; Ravichandran, M.; Mammo, W.D. Additive manufacturing of biopolymers for tissue engineering and regenerative medicine: An overview, potential applications, advancements, and trends. Int. J. Polym. Sci. 2021, 2021, 4907027. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A potential biopolymer in drug delivery and biomedical applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef]
- Singh, R.; Ahn, Y.H. Progress of Biopolymers in 3D and 4D Printing for Biomedical Applications. In Biopolymers for Biomedical Applications; Wiley: Hoboken, NJ, USA, 2024; pp. 479–507. [Google Scholar]
- Kashyap, S.; Kirtania, S.; Banerjee, S. Smart Biomaterials for Thermal Regulation in Biomedical Applications. In Engineering Materials for Efficient Energy Storage and Conversion; IGI Global: Hershey, PA, USA, 2024; pp. 377–418. [Google Scholar]
- Al-Jarwany, Q.A.; Al-Kubaisy, T.; Hasan, A.; Kotb, Y.E. Does nano-drug delivery systems based on biopolymers offer to transform medication dosage form and enhance patient care. Arch. Eng. Knowl. 2024, 10. [Google Scholar]
- Alharbi, H.M. Exploring the Frontier of Biopolymer-Assisted Drug Delivery: Advancements, Clinical Applications, and Future Perspectives in Cancer Nanomedicine. Drug Des. Dev. Ther. 2024, 18, 2063–2087. [Google Scholar] [CrossRef] [PubMed]
- Bodaghi, M.; Wang, L.; Zhang, F.; Liu, Y.; Leng, J.; Xing, R.; Dickey, M.D.; Vanaei, S.; Elahinia, M.; Hoa, S.V. 4D printing roadmap. Smart Mater. Struct. 2024, 33, 113501. [Google Scholar] [CrossRef]
- Safdar, M.; Ullah, M.; Bibi, A.; Khan, M.; Rehman, M.; Fatima, Z.; Hussain, M.; Awan, U.; Naeem, M. The Evolving Landscape of Biosafety and Biosecurity: A Review of International Guidelines and Best Practices. J. Women Med. Dent. Coll. 2023, 2. [Google Scholar] [CrossRef]
- Yarali, E. 3D and 4D Printed Meta-Biomaterials for Bone Tissue Engineering. Ph.D. Thesis, Delft University of Technology (TU Delft), Delft, The Netherlands, 2025. Print ISBN: 978-94-6496-389-2. [Google Scholar]
- Omar, A.M. Artificial Stimuli-Responsive Constructs Through 4D Fabrication. Ph.D. Thesis, University of Manchester, Manchester, UK, 2024. [Google Scholar]
- Dayyoub, T.; Maksimkin, A.V.; Filippova, O.V.; Tcherdyntsev, V.V.; Telyshev, D.V. Shape memory polymers as smart materials: A review. Polymers 2022, 14, 3511. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Wahab, A.; Khan, S.U.; Zaman, U.; ur Rehman, K.; Hamayun, S.; Naeem, M.; Ali, H.; Riaz, T.; Saeed, S. Stent as a novel technology for coronary artery disease and their clinical manifestation. Curr. Probl. Cardiol. 2023, 48, 101415. [Google Scholar] [CrossRef]
- Agarwal, T.; Hann, S.Y.; Chiesa, I.; Cui, H.; Celikkin, N.; Micalizzi, S.; Barbetta, A.; Costantini, M.; Esworthy, T.; Zhang, L.G. 4D printing in biomedical applications: Emerging trends and technologies. J. Mater. Chem. B 2021, 9, 7608–7632. [Google Scholar] [CrossRef]
- Vatanparast, S.; Boschetto, A.; Bottini, L.; Gaudenzi, P. New trends in 4D printing: A critical review. Appl. Sci. 2023, 13, 7744. [Google Scholar] [CrossRef]
- Akbar, I.; El Hadrouz, M.; El Mansori, M.; Lagoudas, D. Toward enabling manufacturing paradigm of 4D printing of shape memory materials: Open literature review. Eur. Polym. J. 2022, 168, 111106. [Google Scholar] [CrossRef]
- Li, Y.; Hu, J.; Liu, Z. A constitutive model of shape memory polymers based on glass transition and the concept of frozen strain release rate. Int. J. Solids Struct. 2017, 124, 252–263. [Google Scholar] [CrossRef]
- Idumah, C.I. Multifunctional properties optimization and stimuli-responsivity of shape memory polymeric nanoarchitectures and applications. Polym. Eng. Sci. 2023, 63, 1857–1873. [Google Scholar] [CrossRef]
- Waidi, Y.O. Recent Advances in 4D-Printed Shape Memory Actuators. Macromol. Rapid Commun. 2025, 46, 2401141. [Google Scholar] [CrossRef]
- Shehzad, A.; Mukasheva, F.; Moazzam, M.; Sultanova, D.; Abdikhan, B.; Trifonov, A.; Akilbekova, D. Dual-crosslinking of gelatin-based hydrogels: Promising compositions for a 3D printed organotypic bone model. Bioengineering 2023, 10, 704. [Google Scholar] [CrossRef]
- Aftab, M.; Javed, F.; Haider, S.; Khan, R.; Khan, S.U.; Alam, K.; Amir, A.; Ullah, F.; Shah, N.A. Design and characterization of chitosan-based smart injectable hydrogel for improved sustained release of antinarcotics. Pharmaceuticals 2024, 17, 749. [Google Scholar] [CrossRef]
- Kalogeropoulou, M.; Díaz-Payno, P.J.; Mirzaali, M.J.; van Osch, G.J.; Fratila-Apachitei, L.E.; Zadpoor, A.A. 4D printed shape-shifting biomaterials for tissue engineering and regenerative medicine applications. Biofabrication 2024, 16, 022002. [Google Scholar] [CrossRef]
- Guo, S.; Agarwal, T.; Song, S.; Sarkar, K.; Zhang, L.G. Development of novel multi-responsive 4D printed smart nanocomposites with polypyrrole coated iron oxides for remote and adaptive transformation. Mater. Horiz. 2025, 12, 3907–3917. [Google Scholar] [CrossRef]
- Lee, J.; Kwak, D.; Kim, H.; Ullah, M.; Kim, J.; Naeem, M.; Hwang, S.; Im, E.; Yoon, I.S.; Jung, Y. Elucidating a Tumor-Selective Nanoparticle Delivery Mechanism at the Colorectal Lumen–Tumor Interface for Precise Local Cancer Therapy. Small 2025, 21, e2409994. [Google Scholar] [CrossRef]
- Fink, J.K. 3D Industrial Printing with Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2018; ISBN 978-1-119-49696-1. [Google Scholar]
- Ullah, M.; Wahab, A.; Khan, D.; Saeed, S.; Khan, S.U.; Ullah, N.; Saleh, T.A. Modified gold and polymeric gold nanostructures: Toxicology and biomedical applications. Colloid Interface Sci. Commun. 2021, 42, 100412. [Google Scholar] [CrossRef]
- Mahmood, A.; Perveen, F.; Chen, S.; Akram, T.; Irfan, A. Polymer composites in 3D/4D printing: Materials, advances, and prospects. Molecules 2024, 29, 319. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U.; Noroozi, R.; Zolfagharian, A.; Bodaghi, M. 4D printing of shape memory polymer composites: A review on fabrication techniques, applications, and future perspectives. J. Manuf. Process. 2022, 81, 759–797. [Google Scholar] [CrossRef]
- Osman, A.; Song, Y.H.; Ullah, M.; Kim, Y.; Lee, H.; Yoo, J.-W.; Hwang, D.S.; Seo, J.H. Sugar Nanocluster Adhesive Boosts Wound Healing in Diabetic Mice. Carbohydr. Polym. Technol. Appl. 2025, 11, 100933. [Google Scholar] [CrossRef]
- Faheed, N.K. Advantages of natural fiber composites for biomedical applications: A review of recent advances. Emergent Mater. 2024, 7, 63–75. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, A. An overview of recent advancements in 4D printing of alginate hydrogels for tissue regeneration. J. Biomater. Sci. Polym. Ed. 2025, 36, 1001–1034. [Google Scholar] [CrossRef]
- Swain, S.; Sahoo, S.; Rautray, T.R.; You, C. Current Approaches to Smart Drug Delivery. In Smart Micro-and Nanomaterials for Drug Delivery; CRC Press: Boca Raton, FL, USA, 2024; pp. 18–46. [Google Scholar]
- Singh, J.; Nayak, P. pH-responsive polymers for drug delivery: Trends and opportunities. J. Polym. Sci. 2023, 61, 2828–2850. [Google Scholar] [CrossRef]
- Xia, Y.; He, Y.; Zhang, F.; Liu, Y.; Leng, J. A review of shape memory polymers and composites: Mechanisms, materials, and applications. Adv. Mater. 2021, 33, 2000713. [Google Scholar] [CrossRef]
- Pisani, S.; Genta, I.; Modena, T.; Dorati, R.; Benazzo, M.; Conti, B. Shape-memory polymers hallmarks and their biomedical applications in the form of nanofibers. Int. J. Mol. Sci. 2022, 23, 1290. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, P.; Liu, S.; Chen, J.; Chen, R.; He, X.; Ma, G.; Lei, Z. Factors affecting the properties of superabsorbent polymer hydrogels and methods to improve their performance: A review. J. Mater. Sci. 2021, 56, 16223–16242. [Google Scholar] [CrossRef]
- Sudheer, S.; Bandyopadhyay, S.; Bhat, R. Sustainable polysaccharide and protein hydrogel-based packaging materials for food products: A review. Int. J. Biol. Macromol. 2023, 248, 125845. [Google Scholar] [CrossRef]
- Bisht, N.; Jaiswal, S.; Vishwakarma, J.; Gupta, S.K.; Yeo, R.J.; Sankaranarayanan, S.; Dhand, C.; Dwivedi, N. Mxene enhanced shape memory polymer composites: The rise of MXenes as fillers for stimuli-responsive materials. Chem. Eng. J. 2024, 498, 155154. [Google Scholar] [CrossRef]
- Namathoti, S.; PS, R.S. A review on progress in magnetic, microwave, ultrasonic responsive Shape-memory polymer composites. Mater. Today Proc. 2022, 56, 1182–1191. [Google Scholar] [CrossRef]
- Zidarič, T.; Maver, U. Biopolymer Thin Films as “Smart” Materials in Biomedical Applications. Funct. Biomater. Des. Dev. Biotechnol. Pharmacol. Biomed. 2023, 1, 239–268. [Google Scholar]
- Jaiswal, R.; Sherje, A.P. Recent advances in biopolymer-based smart hydrogel for wound healing. J. Drug Deliv. Sci. Technol. 2024, 99, 105990. [Google Scholar] [CrossRef]
- Ullah, M.; Waleed, A.; Hamayun, S.; Shaukat, A.; Safdar, M.; Hussain, M.; Waleed, T.; Akber, K.; Ali, H.; Amjad, M.; et al. Nanotechnology and Biomedical Devices Used as a Novel Tool in Biosensing and Bioimaging of Disease. J. Women Med. Dent. Coll. 2023, 1, 4. [Google Scholar] [CrossRef]
- Marques, L.; Costa, B.; Pereira, M.; Silva, A.; Santos, J.; Saldanha, L.; Silva, I.; Magalhães, P.; Schmidt, S.; Vale, N. Advancing precision medicine: A review of innovative in silico approaches for drug development, clinical pharmacology and personalized healthcare. Pharmaceutics 2024, 16, 332. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Song, Y. Printable smart materials and devices: Strategies and applications. Chem. Rev. 2021, 122, 5144–5164. [Google Scholar] [CrossRef]
- Caceres-Alban, J.; Sanchez, M.; Casado, F.L. Bioprinting: A strategy to build informative models of exposure and disease. IEEE Rev. Biomed. Eng. 2022, 16, 594–610. [Google Scholar] [CrossRef]
- Mirzababaei, S.; Towery, L.A.K.; Kozminsky, M. 3D and 4D assembly of functional structures using shape-morphing materials for biological applications. Front. Bioeng. Biotechnol. 2024, 12, 1347666. [Google Scholar] [CrossRef]
- Cecen, B.; Hassan, S.; Li, X.; Zhang, Y.S. Smart biomaterials in biomedical applications: Current advances and possible future directions. Macromol. Biosci. 2024, 24, 2200550. [Google Scholar] [CrossRef]
- Khan, S.; Zaman, U.; Rehman, K.; Faryal, U.; Khan, D.; Saeed, S.; Jadoon, M.; Khan, M.; Khan, U.; Ullah, M.; et al. Antibacterial Assessment of Biofabricated Magnesium Oxide Nanoparticles (MgO NPs) using Conocarpus erectus Leaf Extract. J. Women Med. Dent. Coll. 2022, 1, 2. [Google Scholar] [CrossRef]
- Alifah, N.; Palungan, J.; Ardayanti, K.; Ullah, M.; Nurkhasanah, A.; Mustopa, A.; Lallo, S.; Agustina, R.; Yoo, J.-W.; Hasan, N. Development of Clindamycin-Releasing Polyvinyl Alcohol Hydrogel with Self-Healing Property for the Effective Treatment of Biofilm-Infected Wounds. Gels 2024, 10, 482. [Google Scholar] [CrossRef]
- Zu, S.; Wang, Z.; Zhang, S.; Guo, Y.; Chen, C.; Zhang, Q.; Liu, T.; Liu, Q.; Zhang, Z. A bioinspired 4D printed hydrogel capsule for smart controlled drug release. Mater. Today Chem. 2022, 24, 100789. [Google Scholar] [CrossRef]
- Ullah, M.; Awan, U.A.; Muhaymin, A.; Naeem, M.; Yoo, J.-W.; Mehreen, A.; Safdar, A.; Hasan, N.; Haider, A. Cancer nanomedicine: Smart arsenal in the war against cancer. Inorg. Chem. Commun. 2025, 174, 114030. [Google Scholar] [CrossRef]
- Ahmed, Z.; Ullah, M.; Zeshan, D.; Khan, S.U.; Ali, F.; Wahab, A. Exploring the tumor microenvironment in solid cancer: From biology to therapy. Methods Cell Biol. 2025, in press. [Google Scholar]
- Won, C.; Kwon, C.; Park, K.; Seo, J.; Lee, T. Electronic drugs: Spatial and temporal medical treatment of human diseases. Adv. Mater. 2021, 33, 2005930. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Osouli-Bostanabad, K.; Masalehdan, T.; Kapsa, R.M.; Quigley, A.; Lalatsa, A.; Bruggeman, K.F.; Franks, S.J.; Williams, R.J.; Nisbet, D.R. Traction of 3D and 4D printing in the healthcare industry: From drug delivery and analysis to regenerative medicine. ACS Biomater. Sci. Eng. 2022, 8, 2764–2797. [Google Scholar] [CrossRef]
- Raikar, A.S.; Kalaskar, D.M.; Bhilegaonkar, S.; Somnache, S.N.; Bodaghi, M. Revolutionizing drug delivery by bioinspired 4D transdermal microneedles: Advances and future horizons. Eur. Polym. J. 2024, 210, 112952. [Google Scholar] [CrossRef]
- Safdar, M.; Amin, Z.; Ullah, M.; Wahab, A.; Hasan, N.; Naeem, M. Cancer stem cell analysis and targeting. In Methods in Cell Biology; Academic Press: Cambridge, MA, USA, 2025. [Google Scholar] [CrossRef]
- Nawaz, K.; Ullah, M.; Yoo, J.-W.; Wahab, A.; Hasan, N.; Naeem, M. Tissue Engineering for Wound Healing: Recent Advancements and Opportunities. In Nanotechnology in Wound Healing; CRC Press: Boca Raton, FL, USA, 2025; pp. 149–167. [Google Scholar]
- Malekmohammadi, S.; Sedghi Aminabad, N.; Sabzi, A.; Zarebkohan, A.; Razavi, M.; Vosough, M.; Bodaghi, M.; Maleki, H. Smart and biomimetic 3D and 4D printed composite hydrogels: Opportunities for different biomedical applications. Biomedicines 2021, 9, 1537. [Google Scholar] [CrossRef]
- Mahmoud, D.B.; Schulz-Siegmund, M. Utilizing 4D printing to design smart gastroretentive, esophageal, and intravesical drug delivery systems. Adv. Healthc. Mater. 2023, 12, 2202631. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, H.; Esworthy, T.; Mei, D.; Wang, Y.; Zhang, L.G. Emerging 4D printing strategies for next-generation tissue regeneration and medical devices. Adv. Mater. 2022, 34, 2109198. [Google Scholar] [CrossRef]
- Dowaidar, M. Guidelines for biomimetic 3D/4D printing in drug delivery. Mater. Chem. Phys. 2024, 325, 129692. [Google Scholar] [CrossRef]
- Ullah, M.; Awan, U.A.; Ali, H.; Wahab, A.; Khan, S.U.; Naeem, M.; Ruslin, M.; Mustopa, A.Z.; Hasan, N. Carbon Dots: New Rising Stars in the Carbon Family for Diagnosis and Biomedical Applications. J. Nanotheranostics 2024, 6, 1. [Google Scholar] [CrossRef]
- Moroni, S.; Bingham, R.; Buckley, N.; Casettari, L.; Lamprou, D.A. 4D printed multipurpose smart implants for breast cancer management. Int. J. Pharm. 2023, 642, 123154. [Google Scholar] [CrossRef]
- Yuan, C.; Lu, T.; Wang, T. Mechanics-based design strategies for 4D printing: A review. Forces Mech. 2022, 7, 100081. [Google Scholar] [CrossRef]
- Park, D.; Lee, S.J.; Park, J.-W. Aptamer-based smart targeting and spatial trigger–response drug-delivery systems for anticancer therapy. Biomedicines 2024, 12, 187. [Google Scholar] [CrossRef]
- Senthil, M.K.; Sekar, M.; Vasanthanathan, A.; Amudhan, K. 4D bioprinting for personalized medicine, innovations in implant fabrication and regenerative therapies. Polym.-Plast. Technol. Mater. 2025, 64, 1839–1864. [Google Scholar]
- Ramezani, M.; Mohd Ripin, Z. 4D printing in biomedical engineering: Advancements, challenges, and future directions. J. Funct. Biomater. 2023, 14, 347. [Google Scholar] [CrossRef]
- Wan, X.; He, Y.; Liu, Y.; Leng, J. 4D printing of multiple shape memory polymer and nanocomposites with biocompatible, programmable and selectively actuated properties. Addit. Manuf. 2022, 53, 102689. [Google Scholar] [CrossRef]
- Li, S.; Huan, Y.; Zhu, B.; Chen, H.; Tang, M.; Yan, Y.; Wang, C.; Ouyang, Z.; Li, X.; Xue, J. Research progress on the biological modifications of implant materials in 3D printed intervertebral fusion cages. J. Mater. Sci. Mater. Med. 2022, 33, 2. [Google Scholar] [CrossRef]
- Tran, T.S.; Balu, R.; Mettu, S.; Roy Choudhury, N.; Dutta, N.K. 4D printing of hydrogels: Innovation in material design and emerging smart systems for drug delivery. Pharmaceuticals 2022, 15, 1282. [Google Scholar] [CrossRef]
- Ullah, M.; Lee, J.; Hasan, N.; Hakim, M.; Kwak, D.; Kim, H.; Lee, E.; Ahn, J.; Mun, B.; Lee, E.; et al. Clindamycin-Loaded Polyhydroxyalkanoate Nanoparticles for the Treatment of Methicillin-Resistant Staphylococcus aureus-Infected Wounds. Pharmaceutics 2024, 16, 1315. [Google Scholar] [CrossRef]
- Uboldi, M.; Perrotta, C.; Moscheni, C.; Zecchini, S.; Napoli, A.; Castiglioni, C.; Gazzaniga, A.; Melocchi, A.; Zema, L. Insights into the safety and versatility of 4D printed intravesical drug delivery systems. Pharmaceutics 2023, 15, 757. [Google Scholar] [CrossRef]
- Ullah, M.; Hamayun, S.; Wahab, A.; Khan, S.U.; Rehman, M.U.; Haq, Z.U.; Rehman, K.U.; Ullah, A.; Mehreen, A.; Awan, U.A. Smart technologies used as smart tools in the management of cardiovascular disease and their future perspective. Curr. Probl. Cardiol. 2023, 48, 101922. [Google Scholar] [CrossRef]
- López Ruiz, A.; Ramirez, A.; McEnnis, K. Single and multiple stimuli-responsive polymer particles for controlled drug delivery. Pharmaceutics 2022, 14, 421. [Google Scholar] [CrossRef]
- Serrano, D.R.; Kara, A.; Yuste, I.; Luciano, F.C.; Ongoren, B.; Anaya, B.J.; Molina, G.; Diez, L.; Ramirez, B.I.; Ramirez, I.O. 3D printing technologies in personalized medicine, nanomedicines, and biopharmaceuticals. Pharmaceutics 2023, 15, 313. [Google Scholar] [CrossRef]
- Jurak, M.; Wiącek, A.E.; Ładniak, A.; Przykaza, K.; Szafran, K. What affects the biocompatibility of polymers? Adv. Colloid Interface Sci. 2021, 294, 102451. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Seidi, F.; Azarfam, M.Y.; Yazdi, M.K.; Erfani, A.; Barani, M.; Chauhan, N.P.S.; Rabiee, N.; Kuang, T.; Kucinska-Lipka, J. Biopolymer-based composites for tissue engineering applications: A basis for future opportunities. Compos. Part B Eng. 2023, 258, 110701. [Google Scholar] [CrossRef]
- Biswas, M.C.; Jony, B.; Nandy, P.K.; Chowdhury, R.A.; Halder, S.; Kumar, D.; Ramakrishna, S.; Hassan, M.; Ahsan, M.A.; Hoque, M.E. Recent advancement of biopolymers and their potential biomedical applications. J. Polym. Environ. 2021, 30, 51–74. [Google Scholar] [CrossRef]
- Ariga, K. Responsive materials nanoarchitectonics at interfaces. Responsive Mater. 2024, 2, e20240011. [Google Scholar] [CrossRef]
- Maridevaru, M.C.; Lu, H.; Roy, S.; Yan, Y.; Wang, F.; Soe, S.K.; Ullah, Z.; Sang, H.; Shang, J.; Guo, B. Development of Polymer-Based Piezoelectric Materials for the Bone Tissue Regeneration. Macromol. Biosci. 2025, 25, e2500031. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial intelligence in pharmaceutical technology and drug delivery design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef]
- Hamayun, S.; Ullah, M.; Rehman, M.U.; Hussain, M.; Khan, A.I.; Shah, S.N.A.; Waleed, A.; Muneeb, T.; Khan, T. Rational Therapeutic Approaches for the Management of Congestive Cardiac Failure. J. Bashir Inst. Health Sci. 2023, 4, 62–68. [Google Scholar] [CrossRef]
- Bider, F.; Gunnella, C.; Reh, J.T.; Clejanu, C.-E.; Kuth, S.; Beltrán, A.M.; Boccaccini, A.R. Enhancing alginate dialdehyde-gelatin (ADA-GEL) based hydrogels for biofabrication by addition of phytotherapeutics and mesoporous bioactive glass nanoparticles (MBGNs). J. Biomater. Appl. 2025, 39, 524–556. [Google Scholar] [CrossRef]
- Gilad, Y.; Gellerman, G.; Lonard, D.M.; O’malley, B.W. Drug combination in cancer treatment—From cocktails to conjugated combinations. Cancers 2021, 13, 669. [Google Scholar] [CrossRef]
- Yu, J.; Mu, Q.; Fung, M.; Xu, X.; Zhu, L.; Ho, R.J. Challenges and opportunities in metastatic breast cancer treatments: Nano-drug combinations delivered preferentially to metastatic cells may enhance therapeutic response. Pharmacol. Ther. 2022, 236, 108108. [Google Scholar] [CrossRef] [PubMed]
- Ajalik, R.E.; Alenchery, R.G.; Cognetti, J.S.; Zhang, V.Z.; McGrath, J.L.; Miller, B.L.; Awad, H.A. Human organ-on-a-chip microphysiological systems to model musculoskeletal pathologies and accelerate therapeutic discovery. Front. Bioeng. Biotechnol. 2022, 10, 846230. [Google Scholar] [CrossRef]
- Sharma, G.; Rawat, K.S.; Sood, S.K. Adoption of controls and saga of development in 3D printed bioimplants: A business perspective. In IEEE Engineering Management Review; IEEE: New York, NY, USA, 2024. [Google Scholar]
- Fidan, I.; Huseynov, O.; Ali, M.; Alkunte, S.; Rajeshirke, M.; Gupta, A.; Hasanov, S.; Tantawi, K.; Yasa, E.; Yilmaz, O. Recent Inventions in Additive Manufacturing: Holistic Review. Inventions 2023, 8, 103. [Google Scholar] [CrossRef]
- Abbas, A.; Raza, A.; Ullah, M.; Akbar, F.; Khan, S.; Zaman, U.; Saeed, S.; Rehman, K.; Sultan, S.; Alissa, M. A Comprehensive Review: Epidemiological Strategies, Catheterization and Biomarkers used as a Bioweapon in Diagnosis and Management of Cardio Vascular Diseases. Curr. Probl. Cardiol. 2023, 48, 101661. [Google Scholar] [CrossRef]
- Tian, Y.; Xia, L.; Song, X.; Chen, Y. Dissolving Microneedles as In Situ Chemical Reaction Chambers: From Design Strategies to Versatile Biomedical Applications. Adv. Funct. Mater. 2025, 35, 2422274. [Google Scholar] [CrossRef]
- Tian, Y.; Cheng, T.; Sun, F.; Zhou, Y.; Yuan, C.; Guo, Z.; Wang, Z. Effect of biophysical properties of tumor extracellular matrix on intratumoral fate of nanoparticles: Implications on the design of nanomedicine. Adv. Colloid Interface Sci. 2024, 326, 103124. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R. Overview of 3D and 4D printing techniques and their emerging applications in medical sectors. Curr. Mater. Sci. Former. Recent Pat. Mater. Sci. 2023, 16, 143–170. [Google Scholar] [CrossRef]
- Pesqueira, A.; Almeida, D. Rethinking the Future of Mental Health: AI, Bioprinting, and the Ethical Evolution of Diagnostics for ADHD and OCB. In Smart Healthcare, Clinical Diagnostics, and Bioprinting Solutions for Modern Medicine; IGI Global Scientific Publishing: Hershey, PA, USA, 2025; pp. 233–258. [Google Scholar]
- Ding, A.; Tang, F.; Alsberg, E. 4D printing: A comprehensive review of technologies, materials, stimuli, design, and emerging applications. Chem. Rev. 2025, 125, 3663–3771. [Google Scholar] [CrossRef] [PubMed]
- Chikwendu, O.C.; Emeka, U.C. Artificial Intelligence Applications for Customized Products Design in Manufacturing. Int. J. Multidiscip. Res. Growth Eval. 2025, 6, 1796–1806. [Google Scholar]
- Kumari, V.; Majumder, M.K. AI-Enabled 3D Integration. In AI-Enabled Electronic Circuit and System Design: From Ideation to Utilization; Springer Nature: Cham, Switzerland, 2025; pp. 257–308. [Google Scholar]



| Limitation | Description | Consequences | Potential Solutions | Refs. |
|---|---|---|---|---|
| Microarchitecture Control | Difficult to achieve precise control over tissue structure and cell distribution during bioprinting. | Impaired tissue functionality and poor integration with host tissue. | Improved calibration and advanced printing techniques, with improved parameters (e.g., nozzle geometry, pressure, speed). | [56,57] |
| Bioink Selection | Existing bioinks often lack ideal mechanical strength and biocompatibility. | Limits the range of functional tissues; raises the risk of implant failure. | New bio-inks with improved mechanical properties and biocompatibility, e.g., peptide self-assembled or temperature-responsive hydrogels. | [58,59] |
| Scalability | Hard to scale up from lab-scale to clinically or commercially viable production. | Limited commercial applications and reduced patient access. | Standardize and automate processes; adopt modular, multi-nozzle, or parallel printing platforms. | [60,61] |
| Ethical Considerations | Questions around consent, material sourcing, and the implications of bioprinted tissues. | Potential legal hurdles and erosion of public trust. | Establish multidisciplinary ethical guidelines and oversight frameworks. | [62,63] |
| Integration with Living Organisms | Lack of vascularization restricts nutrient/waste transport in printed tissues. | Reduced tissue survival and compromised function. | Incorporate vascular cues (e.g., VEGF), sacrificial bioinks, coaxial techniques, and 4D-bioprinting approaches. | [64,65] |
| Strategy | Mechanism | Materials | Significance | Limitations | Refs. |
|---|---|---|---|---|---|
| Stimuli-Responsive Materials | React to stimuli (pH, temperature) for controlled release. | Shape memory polymers (SMPs), hydrogels. | Enables precise drug release timing and dosage. | Variable response times can affect predictability. | [102,181] |
| Customization of Drug Release | Tailors’ profiles based on local conditions. | Bioprinted components with porosity. | Aligns with patient-specific needs, reducing side effects. | Designing multi-layered structures can be complex. | [16,182] |
| Integration of Targeting Mechanisms | Uses ligands and magnetic nanoparticles for localization. | Ligand-enhanced carriers, magnetic nanoparticles. | Increases efficacy by minimizing systemic exposure. | Design complexity due to need for suitable ligands; potential off-target effects. | [16,182] |
| Enhancing Biocompatibility | Uses biopolymers and living cells for active therapy. | Alginate, chitosan, gelatin. | Promotes better acceptance and integration in biological systems. | Biocompatibility does not ensure full efficacy; risks in cellular viability. | [183,184] |
| Developing Personalized Systems | Customizes systems to fit individual patient needs. | Patient-derived materials, customizable polymers. | Enhance treatment accuracy for better outcomes. | High costs and design time may limit adoption. | [185] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aftab, M.; Ikram, S.; Ullah, M.; Khan, S.U.; Wahab, A.; Naeem, M. Advancement of 3D Bioprinting Towards 4D Bioprinting for Sustained Drug Delivery and Tissue Engineering from Biopolymers. J. Manuf. Mater. Process. 2025, 9, 285. https://doi.org/10.3390/jmmp9080285
Aftab M, Ikram S, Ullah M, Khan SU, Wahab A, Naeem M. Advancement of 3D Bioprinting Towards 4D Bioprinting for Sustained Drug Delivery and Tissue Engineering from Biopolymers. Journal of Manufacturing and Materials Processing. 2025; 9(8):285. https://doi.org/10.3390/jmmp9080285
Chicago/Turabian StyleAftab, Maryam, Sania Ikram, Muneeb Ullah, Shahid Ullah Khan, Abdul Wahab, and Muhammad Naeem. 2025. "Advancement of 3D Bioprinting Towards 4D Bioprinting for Sustained Drug Delivery and Tissue Engineering from Biopolymers" Journal of Manufacturing and Materials Processing 9, no. 8: 285. https://doi.org/10.3390/jmmp9080285
APA StyleAftab, M., Ikram, S., Ullah, M., Khan, S. U., Wahab, A., & Naeem, M. (2025). Advancement of 3D Bioprinting Towards 4D Bioprinting for Sustained Drug Delivery and Tissue Engineering from Biopolymers. Journal of Manufacturing and Materials Processing, 9(8), 285. https://doi.org/10.3390/jmmp9080285





