1. Introduction
Short-term nitriding of cutting and rolling tools (drills, taps, knurling tools, etc.) made of high-speed steel increases their wear resistance by 1.5–2 times. Strength, especially impact strength after nitriding, decreases with increasing depth of the diffusion layer. The best combination of mechanical properties, wear resistance, and heat resistance for high-speed steel tools is achieved with a nitrided layer thickness of 0.01–0.025 mm. For these purposes, it is recommended to nitride high-speed steel tools at 510–520 °C. To increase impact strength and more uniformly release carbides throughout the grain volume, it is recommended to temper three times at 550 °C for one hour at each stage after chemical–thermal treatment. After the specified nitriding modes, a nitrided layer with a high hardness of 1340–1460 HV and heat resistance (hardness of 700 HV is maintained up to 700 °C) is formed. The hardness of the nitrided layer increases as the α solid solution is alloyed [
1]. In nitrided products, favorable compressive residual stresses arise, which are maintained throughout the entire depth of the diffusion layer and increase the resistance of the material to alternating loads [
2].
Good results are obtained by the nitrohardening method in combination with surface induction heating or glow discharge heating [
3,
4]. The process of nitriding followed by induction hardening also contributes to an increase in corrosion resistance since, with a short austenitization time, nitrogen and carbon do not have time to be removed from the layer, and the anticorrosive carbonitride ε-phase is preserved on the surface of the steel being processed.
The method of strengthening crankshafts is based on the combination of induction hardening and nitriding processes. After nitriding at 510–565 °C, induction hardening of the main and connecting rod journals is carried out, and then induction hardening of the fillets. During the hardening process, the white layer of iron nitrides disappears. The surface hardness reaches 700 HV. The prospects for using nitriding on carburized products have been noted [
5].
In some cases, such as for dies and molds that are subject to high loads but not subject to grinding, nitriding is used before hardening [
6]. A significant effect has been noted when combining nitriding with hardening on high-carbon steels [
7]. This process, called the “marstressing process”, ensures high hardness, increased fatigue limit, and wear resistance, as well as high corrosion resistance of high-carbon steels. It is noted that the nitrided layer has high wear resistance and impact strength, which leads to an increase in the service life of cutting dies by three to four times compared to dies that have undergone conventional heat treatment [
8].
Thus, nitriding allows for improving the performance characteristics of tool steels significantly.
Progressive technologies of chemical–thermal treatment include vacuum nitriding (VN) and plasma electrolytic treatment. VN combines the advantages of vacuum heat treatment and gas nitriding. It is characterized by a stronger connection between the nitrided layer and the substrate than gas nitriding. VN for 12 h at a temperature of 520 °C significantly increases the hardness and wear resistance of steel with a high manganese content due to the appearance of iron nitrides Fe
3N and Fe
4N in the structure, as well as the ε-phase. The resulting nitride layer exhibits high adhesion to the substrate. A positive effect of nitriding on corrosion resistance has been noted [
9]. VN of punches made of high-speed tool steel for 1 h after quenching in oil from a temperature of 1220 °C and triple tempering at 560 °C leads to the formation of nitrides of alloying elements V, W, Mo, and Cr in the diffusion layer, which ensures an increase in the service life of punches up to 8 times [
10]. VN allows for obtaining precision nitrided parts with a controlled thickness of the ε-phase on the surface, depending on the operating conditions of a specific part [
11].
Plasma electrolytic treatment is a high-speed surface treatment method. High heating rates in electrolytic plasma, reaching 100 °C/s, promote an increase in the diffusion susceptibility of blocks inside austenite grains. This leads to a reduction in the formation time of diffusion layers and the total duration of treatment to 5–10 min with anodic polarity of the workpiece [
12]. This method can be used for carburizing [
13], nitriding [
14,
15,
16], carbonitriding [
17,
18], nitrocarburizing [
19], and boriding [
20]. This technology has found application in improving the performance characteristics of products made of various materials, such as titanium alloys [
21,
22], carbon steels [
23,
24], and alloy steels, including 42CrMo [
25], 40Cr [
26], 30CrMnSiA [
27], AISI H13 [
28], T8 [
29], 38CrMoAl [
30], 20CrMnTi [
31], 30CrMnSi [
32], and others.
Under cathodic plasma electrolytic nitriding (PEN) conditions, a similar high-speed tool steel was previously treated at a temperature of 550–750 °C for 5 min with the formation of a fine-grained martensitic structure with dispersed inclusions of insoluble carbides and nitrides [
14]. The same results were obtained with PEN of P9 and P18 steels [
15]. According to the available results of elemental analysis, the highest concentration of nitrogen is observed in martensite grains (5.87%), and carbonitride grains contain 1.37% nitrogen [
16]. Light grains of carbides are enriched with tungsten and molybdenum, and dark grains are enriched with vanadium.
Thus, VN and cathodic PEN enable the formation of structures with altered phase composition in the surface layers of high-alloy steels. This paper presents the results of studying the possibility of nitriding M2 tool high-speed steel using plasma electrolytic treatment methods with anodic polarity of the processed products (anodic PEN) and VN. The use of anodic polarity opens up prospects for combining diffusion saturation and polishing, which, in the future, will improve the manufacturability of production [
33]. The results of structural and phase changes in nitrided samples, as well as tribological tests, are presented. In addition to considering each technology separately, a combination of VN with subsequent hardening under plasma electrolytic heating conditions is proposed. This combination is proposed to solve the problem of nitrogen diffusion from the surface layers into the volume of the part during traditional hardening of nitrided tool steels, when the parts are slowly heated in a furnace to a hardening temperature of 1230 °C. As a result, the nitrided surface layer is completely destroyed. At the heating rates for hardening in the plasma electrolytic treatment (about 100 °C per second), the diffusion of nitrogen from the saturated surface does not have time to occur, and the modified nitrided layer is preserved. VN ensures a greater depth of the nitrided layer than PEN and the absence of surface oxidation in a vacuum. The combination of high-speed plasma hardening performed after VN, in addition to preserving the nitrided layer, prevents grain coarsening due to rapid heating and the associated deterioration in the volume properties of tool steel. All this determines the scientific novelty and originality of this study.
2. Materials and Methods
2.1. Materials
Cylindrical samples of M2 high-speed tool steel with a height of 15 mm and a diameter of 10 mm were subjected to nitriding (
Table 1). Before and after treatment, the samples were washed in an ultrasonic bath with acetone and then dried.
2.2. Processing
2.2.1. Plasma Electrolytic Nitriding
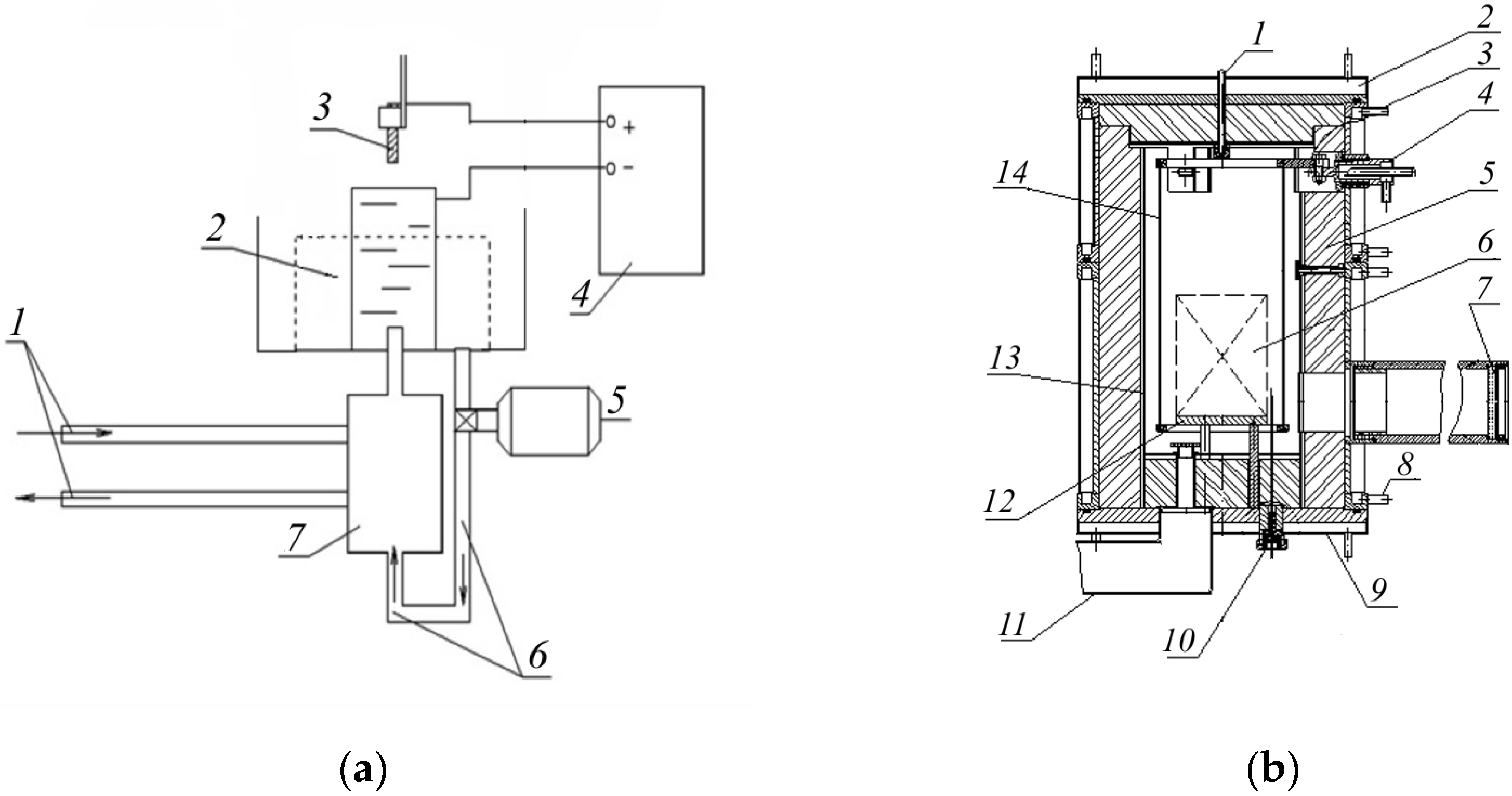
PEN was carried out on a pilot setup with a vertical flow of electrolyte around the workpiece (
Figure 1a). This design allowed for uniform heating of the workpiece along the vertical coordinate and obtaining uniform properties. During processing, the sample was connected to a DC source. An electrolyzer was connected to the opposite pole of the current source. The electrolyte was fed into the electrolyzer using a pump at a constant rate of 2.5 L/min through a heat exchanger, which ensured a constant electrolyte temperature of 22 ± 2 °C. The heating temperature was controlled by regulating the voltage.
PEN was carried out in an aqueous solution of ammonia (5%) and ammonium chloride (10%) at a temperature of 550 °C with a holding time of 10 min (voltage is 120 V, current is 12 A). To maintain a stable temperature during low-temperature nitriding, diffusion saturation was carried out with the anodic polarity of the processed samples. After diffusion saturation, hardening was carried out in the same setup by changing the electrophysical parameters of the processing. To avoid cracking due to the low thermal conductivity of steel, the samples were slowly heated to a temperature of 850 °C (voltage of 180 V; current of 8 A) with a subsequent holding time of 1.5 min. Then, the polarity was switched on the current source, and heating was carried out to a temperature of 1230 °C (voltage of 105 V; current of 15 A) with cathodic polarity of the sample. The change in polarity from anodic to cathodic was due to the impossibility of reaching a temperature above 1100 °C with anodic polarity. The selected quenching temperature ensured the production of high-alloy martensite due to the transition of the maximum amount of special carbides into solution. After holding for 1.5 min at this temperature, the sample was removed from the electrolyte and quenched in air.
In order to transform the residual austenite remaining in the structure after quenching (Q) into martensite, three tempering (T) cycles were performed at a temperature of 555 °C with a 60 min hold and air cooling between heating cycles. In this process, dispersed carbides are released from martensite and residual austenite. Austenite loses carbon and alloying elements, becomes less stable, and undergoes martensitic transformation. A single tempering creates additional stresses. The second and third tempering cycles allow these stresses to be relieved and higher mechanical properties to be obtained compared to a single tempering cycle. Removal of internal stresses is especially important for complex-shaped tools.
2.2.2. Vacuum Nitriding
Vacuum nitriding was carried out on a pilot setup. The setup consists of a reaction chamber, vacuum–gas and electrical systems, and systems for regulating the main process parameters. The reaction chamber of the setup is a sealed water-cooled vessel (
Figure 1b). The cage is located on a special table installed at the bottom of the chamber. In one cycle, the cage can accommodate up to 15 samples of 15 mm in height and 10 mm in diameter.
The power supply system of the setup provides a method of heating the parts by radiation from resistance heaters installed in the chamber. Carbon–graphite fabric placed on specially designed current leads is used as the heating element. Thermal insulation made of carbon–graphite felt is installed between the heaters and the chamber walls to reduce heat loss.
The gas preparation system ensures a metered supply of the working environment into the chamber. It consists of a cylinder station, a pipeline system with reducers, and automatic regulators of the gas environment component flow rate.
The gas mixture of the required concentration, prepared with the help of regulators, is fed from the mixer into the chamber’s gas manifold, which ensures a uniform flow of gas atmosphere throughout its volume.
The vacuum system, including a forevacuum pump, provides preliminary vacuum, pumping of the gas medium, and pressure regulation.
Two sensors are used to control and regulate the temperature. One of them controls the temperature of the internal space of the working chamber, and the other controls the temperature of the reference sample.
The sensors are a thermocouple cable, hermetically inserted into the working space of the chamber and protected from soot emission by a ceramic cap.
The setup is fully automated. It provides for the regulation of the saturation process temperature, gas medium pressure, and its composition, as well as measurement and recording of all technological and auxiliary process parameters.
Ammonia was used as a saturating gas.
VN was carried out according to the following scheme. After placing the samples on the table, the chamber was evacuated and then heated to the nitriding temperature of 540 °C in an ammonia atmosphere (ammonia flow rate of 5 dm3/h; pressure of 1.1 kPa). Upon reaching the specified temperature, the holding time was counted down (8 h). Cooling of the samples to the delivery temperature was carried out in ammonia.
In order to improve performance properties after VN, some of the samples were subjected to plasma electrolytic quenching, as described in
Section 2.2.1. The formation of nitrogenous austenite in the diffusion layer when nitriding is combined with quenching leads to a decrease in the temperatures of the beginning and end of the martensitic transformation in steel and causes a delay in the martensitic transformation in the surface volumes. The formation of martensite on the surface occurs under stress conditions caused by the martensitic transformation in deeper layers of the sample. Such a sequence of γ → α transition leads to an increase in compressive stresses in the surface zone and, as a consequence, to an improvement in the performance of parts under alternating loads.
After VN and quenching, some of the samples were subjected to three-fold tempering, as described in
Section 2.2.1.
2.3. Sample Testing
Samples for electron microscopic studies and microhardness measurements were subjected to preliminary grinding, polishing, and etching in a 4% solution of nitric acid in ethyl alcohol (Kurpatov reagent) for 5–7 s. After etching, the samples were washed in an ultrasonic bath.
To determine the phase composition of the treated surface, a PANalytical Empyrean X-ray diffractometer (Malvern Panalytical, Malvern, UK) with PANalytical High Score Plus software (v.2.0.0) [
34] and ICCD PDF-2 and COD databases [
35] was used. A cobalt X-ray tube was used for the analysis. An iron filter was used to transmit only Kα radiation and absorb Kβ radiation. The wavelength of cobalt Kα radiation was 0.1791 nm. The Soller slits were set to 0.08° to reduce the X-ray beam divergence and minimize the geometric broadening of the diffraction peak. The automatic slit had a spot size of 5 by 5 mm. A tracking anti-scatter slit with the same angle on the diffracted beam was used. A 128-channel Pixel detector with a scanning step of 0.026° was used.
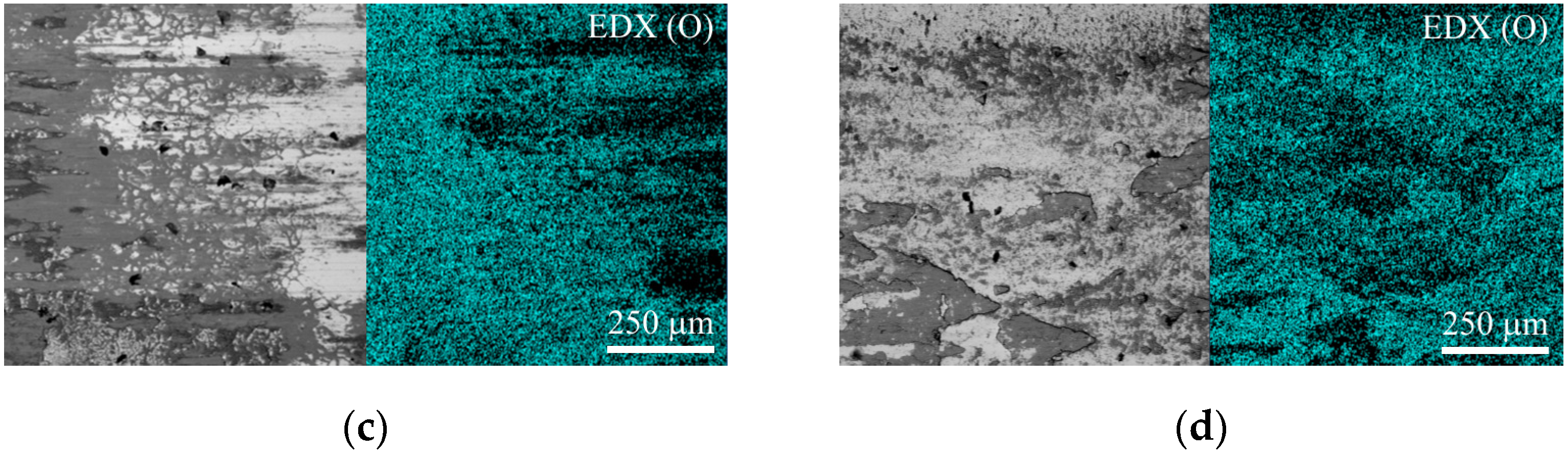
The cross-sectional structure of the treated samples and their elemental composition were analyzed using a Tescan Vega 3 scanning electron microscope (SEM) (Tescan, Brno, Czech Republic) and X-Act energy dispersive analysis (EDX) detector (Oxford Instruments, Abingdon, UK).
The distribution of microhardness in the surface layer of the treated samples was determined using a Vickers microhardness tester (Falcon 503, Innovatest Europe BV, Maastricht, The Netherlands) under a 10 g load.
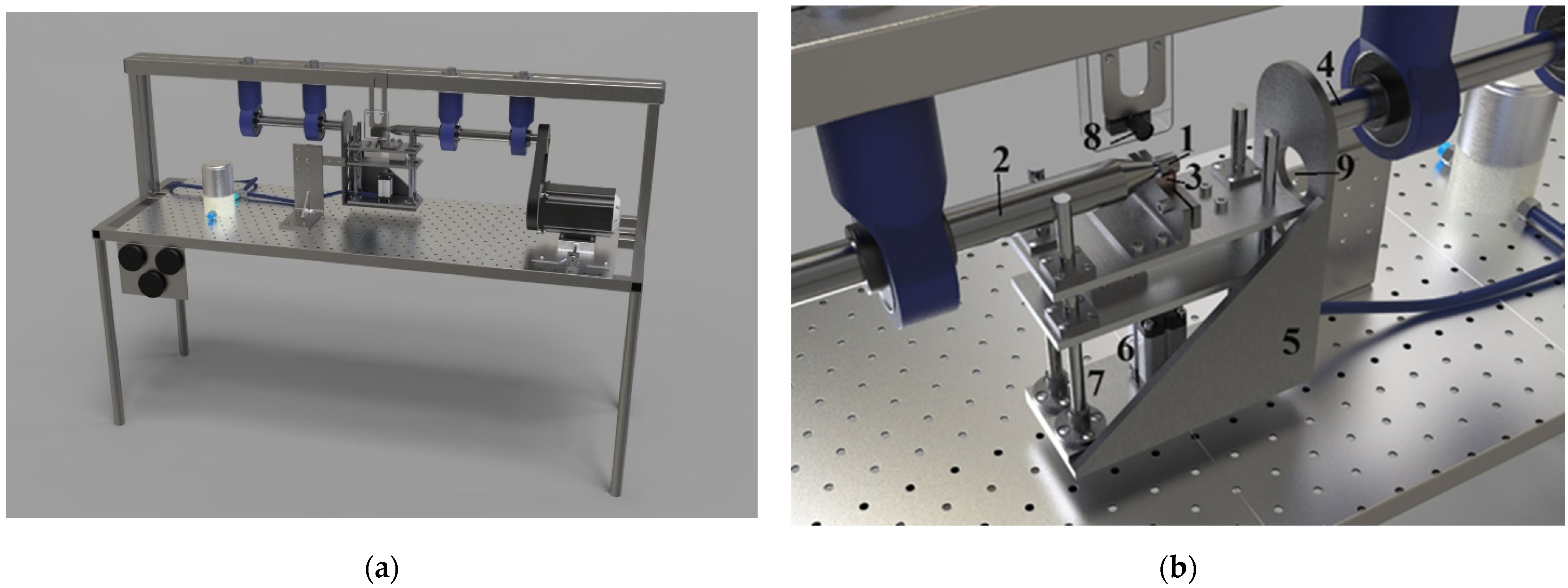
Tribological tests were carried out under dry friction of the lateral surface of the samples using the shaft-bushing scheme with an overlap coefficient of 0.5 on a pilot setup (
Figure 2). The tests were carried out under a normal load of 10 N and sliding speed variation from 0.46 to 3.25 m/s. The friction distance was 1000 m. Tool alloy steel (wt.%: 0.9–1.2 Cr, 1.2–1.6 W, 0.8–1.1 Mn, and 0.9–1.05 C) was used as a counter body.
This friction pattern allows for layer-by-layer wear of multilayer thin coatings, unlike the commonly used ball-on-disc patterns. The ball-on-disc friction unit is designed such that as the friction pair wears, a depression forms in the test surface. As the duration of tribological testing increases, this depression increases in volume. If the counter body surface contains several functional layers with different structures, friction will occur across all layers simultaneously, and the actual contact area of the ball with each layer will vary. Thus, this design of tribological coupling fundamentally does not allow one to determine the influence of each of the successively abraded layers on the wear resistance of the entire coating. The friction machine used in this research was specially designed for studying the tribological parameters of thin multilayer coatings.
2.4. Methodology for Calculating Contact Deformations During Friction
In assessing the results of tribological tests, the roughness of the surfaces of both the nitrided samples and the counter body was taken into account [
36]. The distribution of the material in the rough layer is described by the curve of the support surface, which is approximated by the Demkin power function [
37,
38,
39,
40]:
where the approximating coefficients
b and
ν are determined by the height parameters of the microgeometry of rough surfaces, taken directly from the profilometer;
y is the level of the profile section, measured from the line of protrusions;
Rmax is the maximum height of the irregularities;
ε =
y/
Rmax is the relative height of the profile;
Ar is the actual contact area;
A is the nominal contact area;
Pr is the average actual pressure on the friction contact; and
N is the load in the friction unit.
The parameters
b and
ν are related to the roughness parameters by the following relationships [
41,
42,
43,
44]:
where index 1 refers to the surface parameters of the sample and index 2 to the counter body;
Rp is the smoothing height or the distance from the protrusion line to the center line within the base length;
Ra is the arithmetic mean deviation of the profile; and
tm is the relative support length of the profile at the level of the center line [
36,
45,
46,
47]:
where
l is the base length and Δ
li are the lengths of the segments cut off by the center line in the profile. The relative support length of the rough surface profile
tm was measured directly on the profilometer. At least thirty measurements were taken on each friction track, which were averaged to find the average experimental value of
tm. The measurements were taken in the friction pair, i.e., both on the friction tracks on the sample and on the counter body.
Each unevenness on the mating rough surfaces was replaced by a body of double curvature with the radius of curvature of its vertex [
38,
42,
48]:
where
Sm is the average pitch of profile irregularities.
To assess the nature of the deformations of irregularities, the plasticity criterion was used [
38,
42,
48]:
where
Ei and
μi are the elastic moduli and Poisson’s ratios of the modified sample and the counter body;
d is the diameter of the imprint; and
D is the diameter of the indenter. In the case of elastic deformations, the actual pressure was determined by solving the classical Hertz problem, and in the case of plastic flow, it was taken to be numerically equal to the hardness HV.
Substituting into Equation (1) the value of absolute penetration of the sample into the surface of the counter body
h instead of the section level
y allowed us to obtain an expression for the absolute penetration of surfaces in friction tests [
38,
42,
48]:
The ratio of the absolute penetration (7) to the average radius of curvature of the protrusion (5) h/r was used to analyze the results of tribological tests. In the case of dry friction of iron- and titanium-based alloys, h/r < 0.01 is necessary for the destruction of frictional bonds because of elastic displacement. In this case, the surface layer will be destroyed mainly due to fatigue, and a large number of repeated test cycles will be required to destroy the material. If traces of residual deformation are visible on the friction tracks after the tests and h/r < 0.1, then the frictional bonds are destroyed because of plastic displacement of the softer material by roughness microprotrusions of the more rigid body. In the case of h/r > 0.1, the surfaces in the tribological contact are destroyed because of microcutting, and microchips are formed.
The calculation results of parameters (5)–(7) are presented in
Table 2.
4. Discussion
A comparative assessment of the results of structural and phase changes during various types of processing based on plasma electrolyte and vacuum nitriding made it possible to identify both distinctive features and certain similarities.
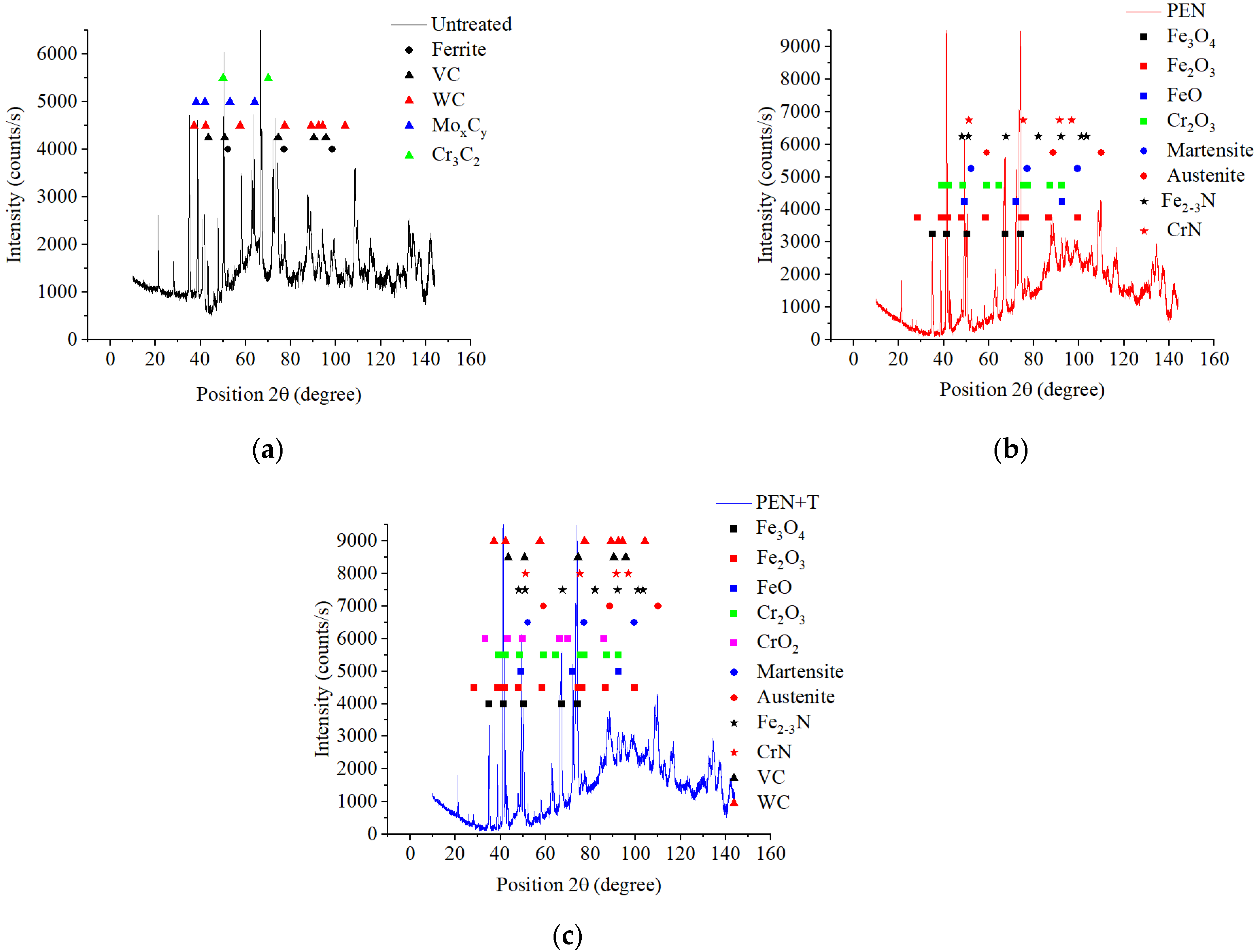
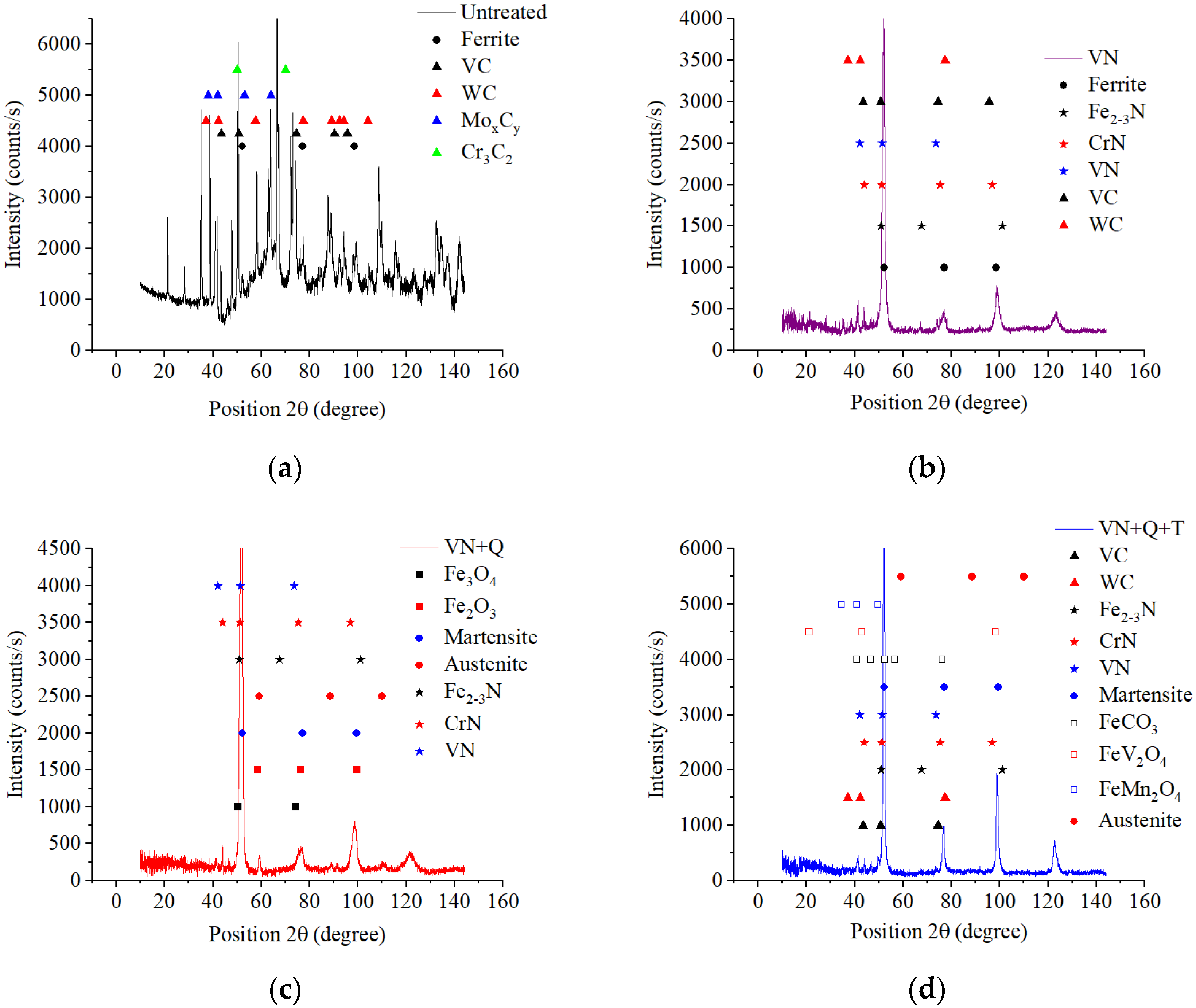
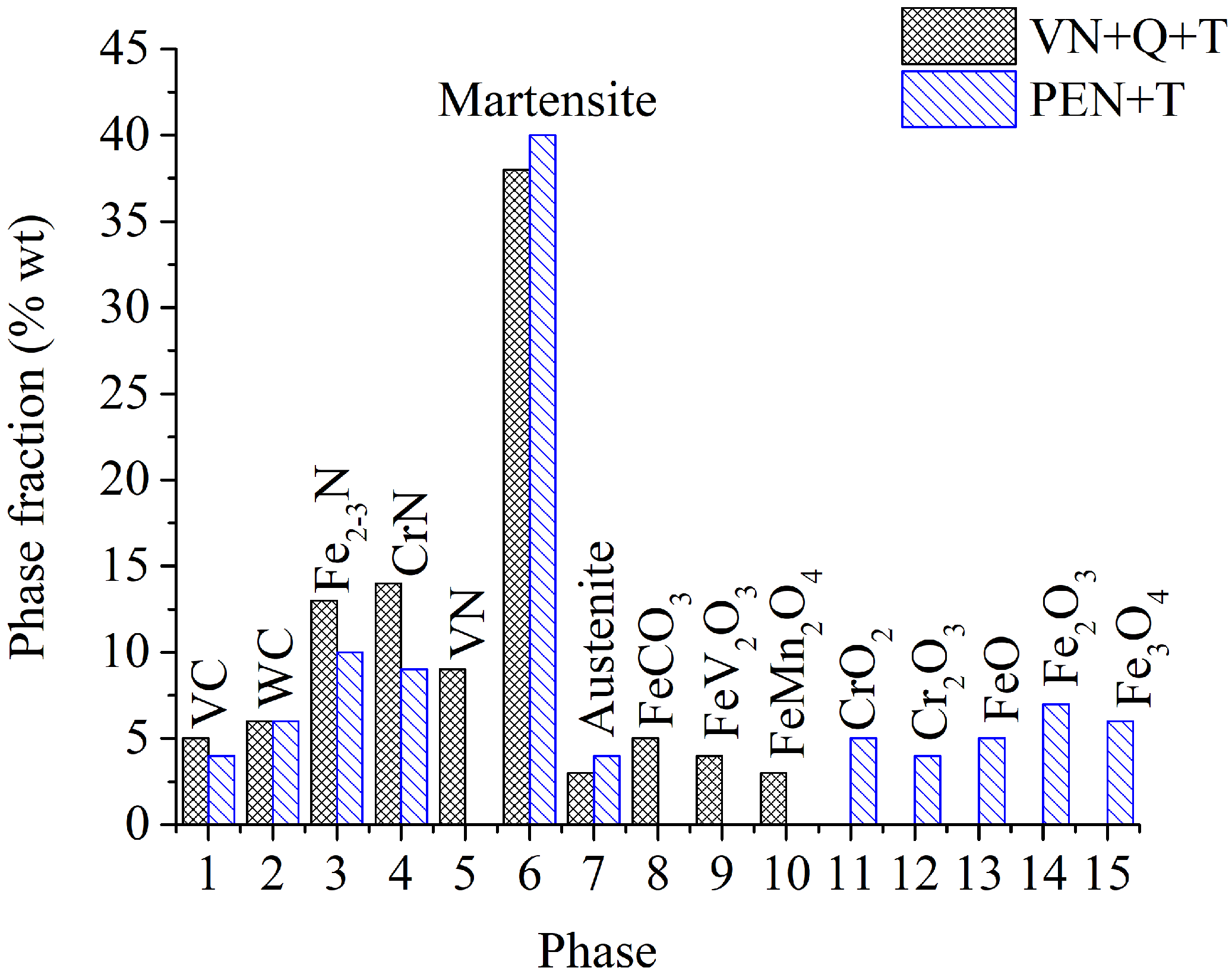
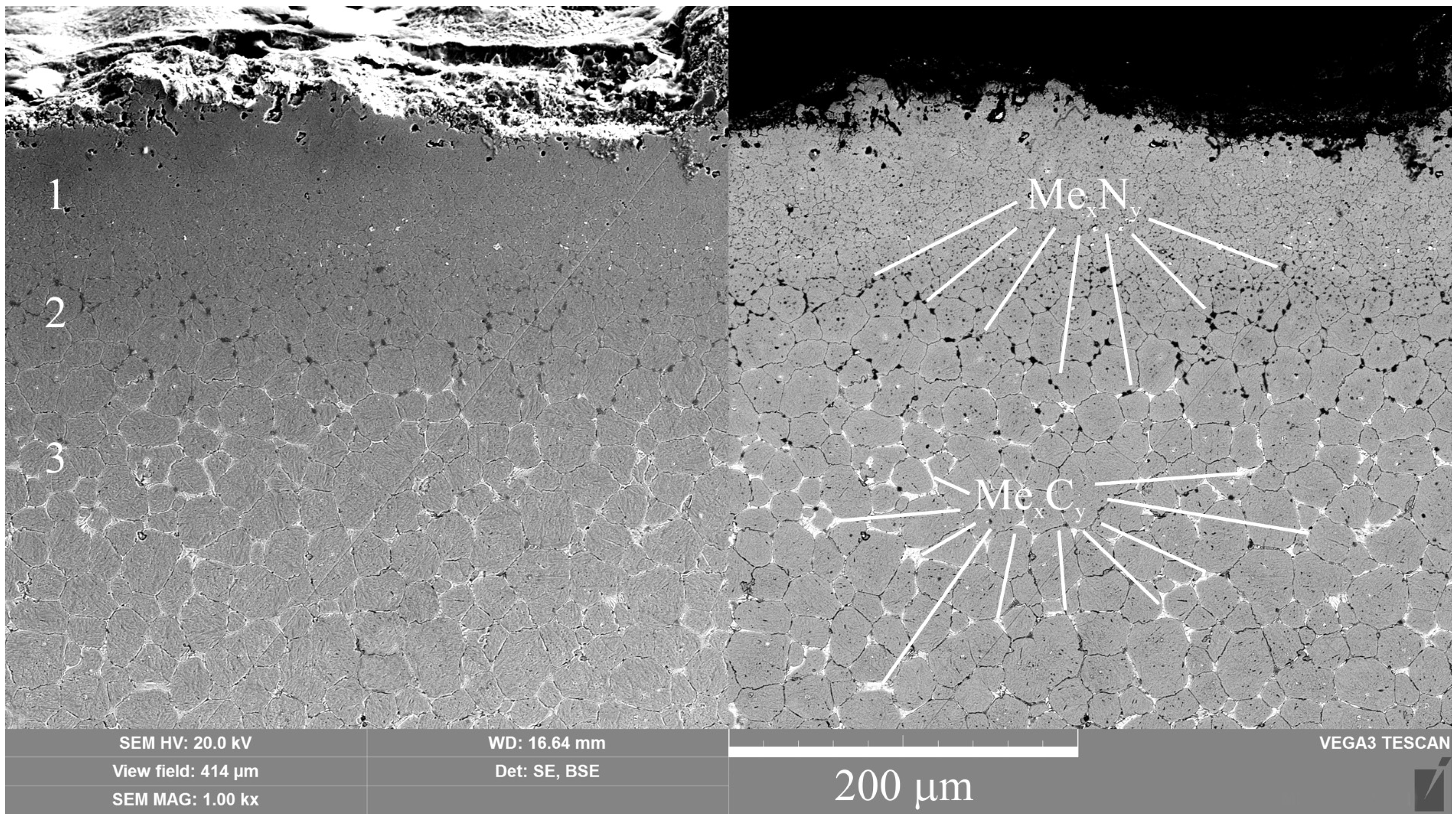
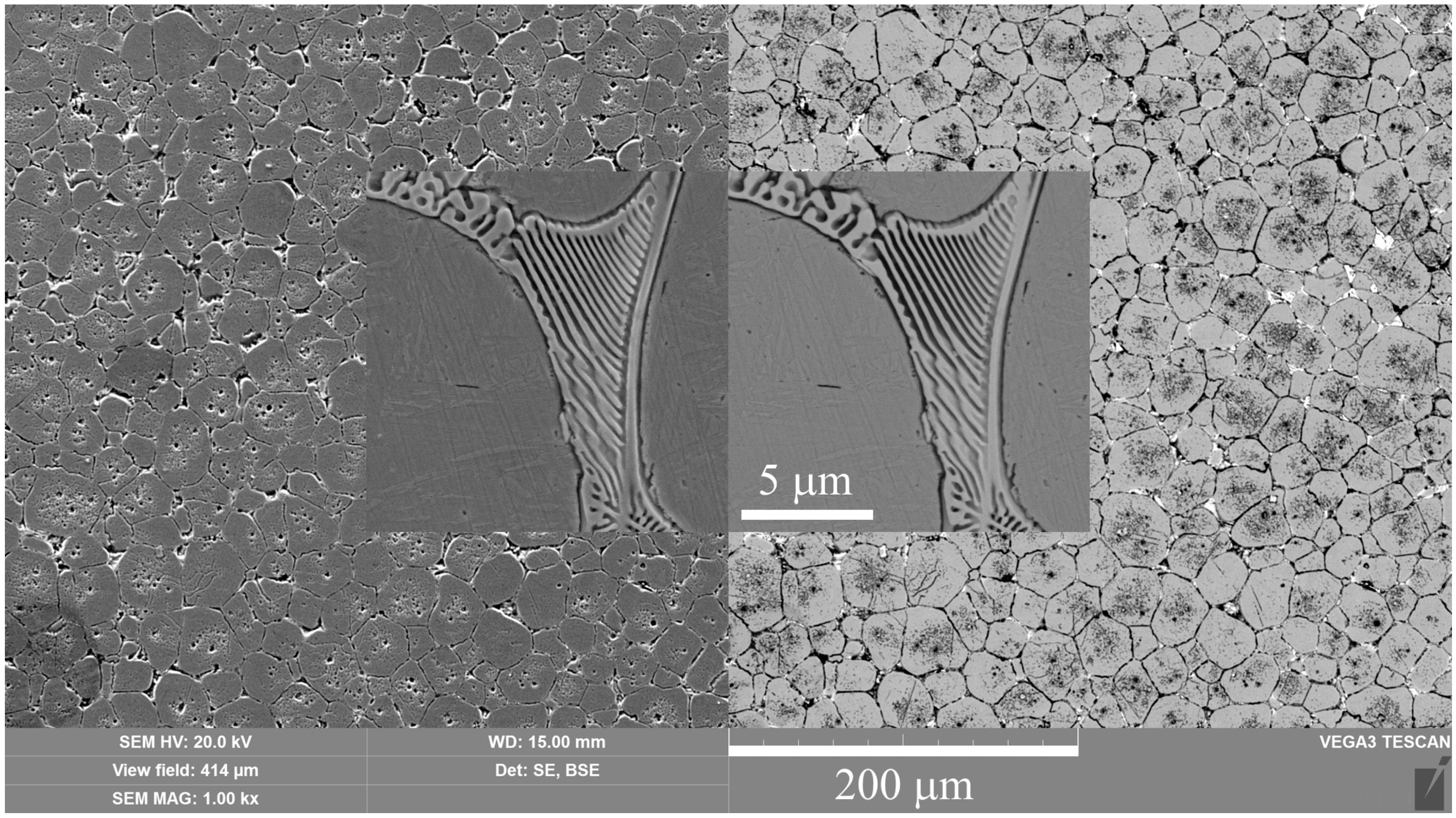
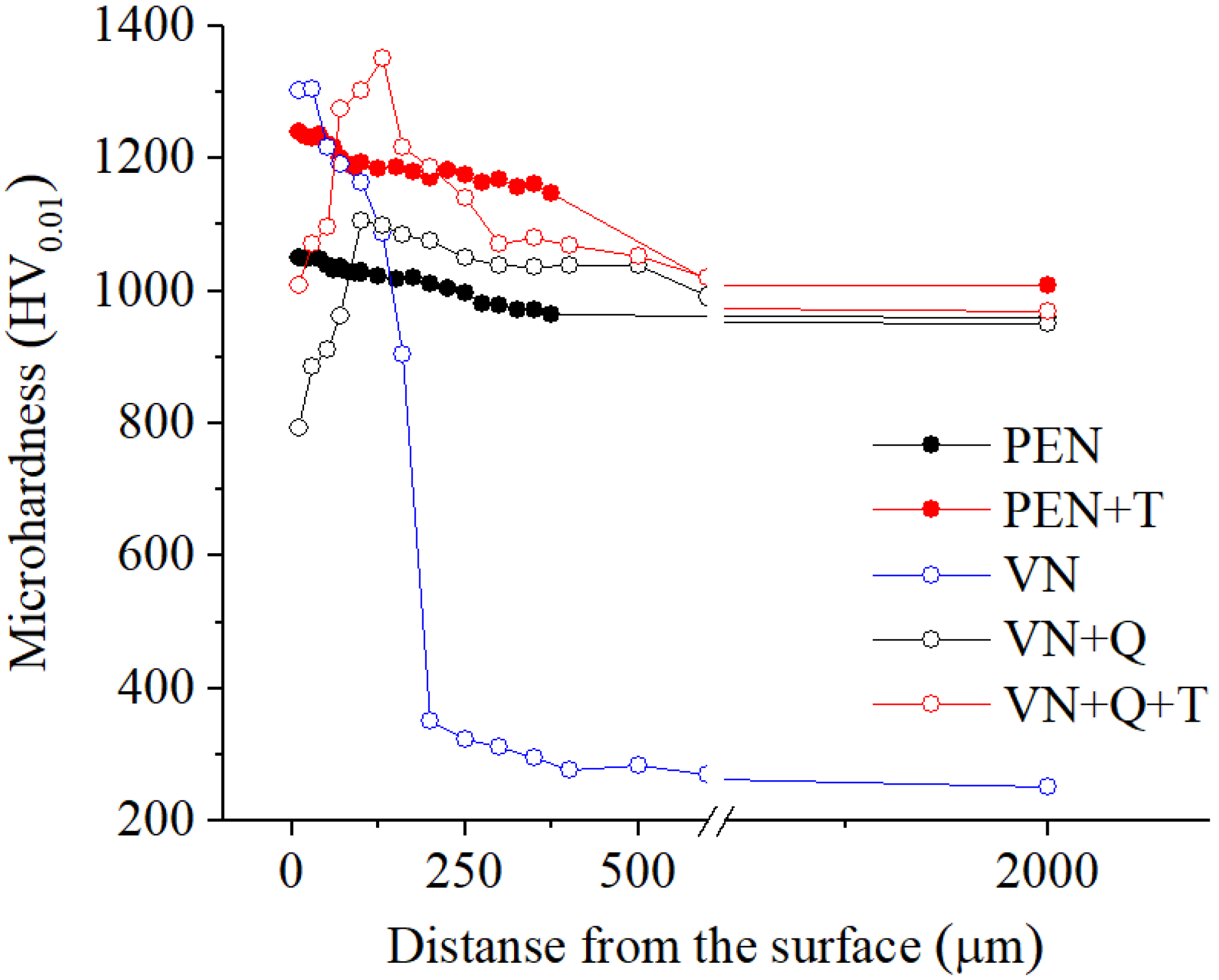
High-speed PEN, which consists of diffusion saturation of the steel surface with nitrogen and subsequent quenching, ensures the formation of nitrogen martensite, which forms a continuous martensite layer with increased microhardness up to 1050 HV. Below this layer, a hardened sublayer with dispersedly distributed nitrides Fe2-3N and chromium CrN is revealed. The formation of such a structure is due to a higher cooling rate on the surface from the quenching temperature (1230 °C), which leads to martensitic transformation of almost all austenite with diffuse nitrogen dissolved in it, as well as carbides VC, WC, MoxCy, and Cr3C2, which were part of the initial matrix. At a certain depth from the surface, the cooling rate decreases, as does the concentration of diffused nitrogen. This leads to incomplete martensitic transformation, the formation of residual austenite after quenching, and the coagulation of nitrides. The hardness of such a structure is slightly reduced. Below the nitrogen diffusion zone, at an even lower cooling rate, the precipitation of carbides along the grain boundaries is observed while maintaining a sufficiently high hardness of 960 HV.
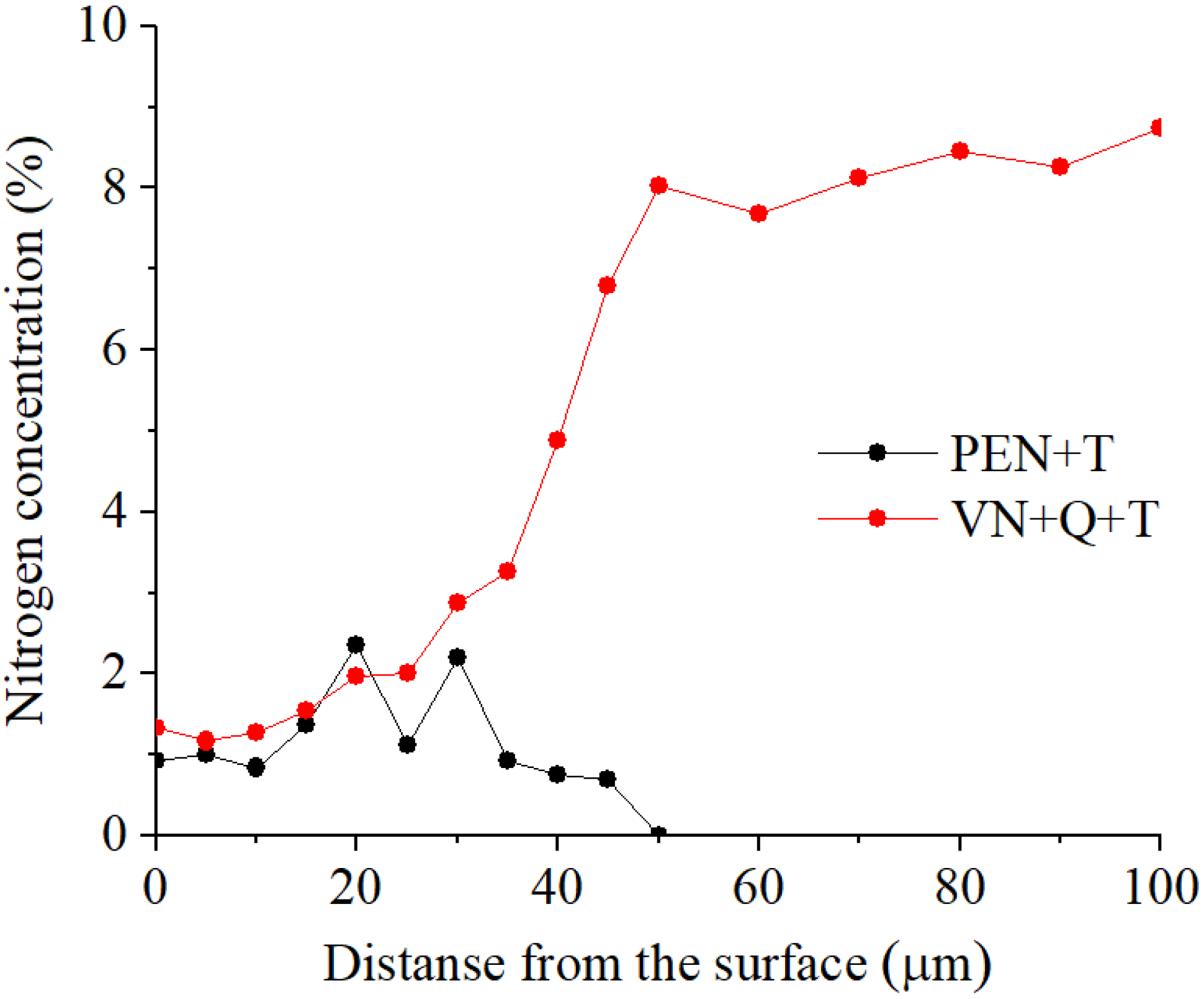
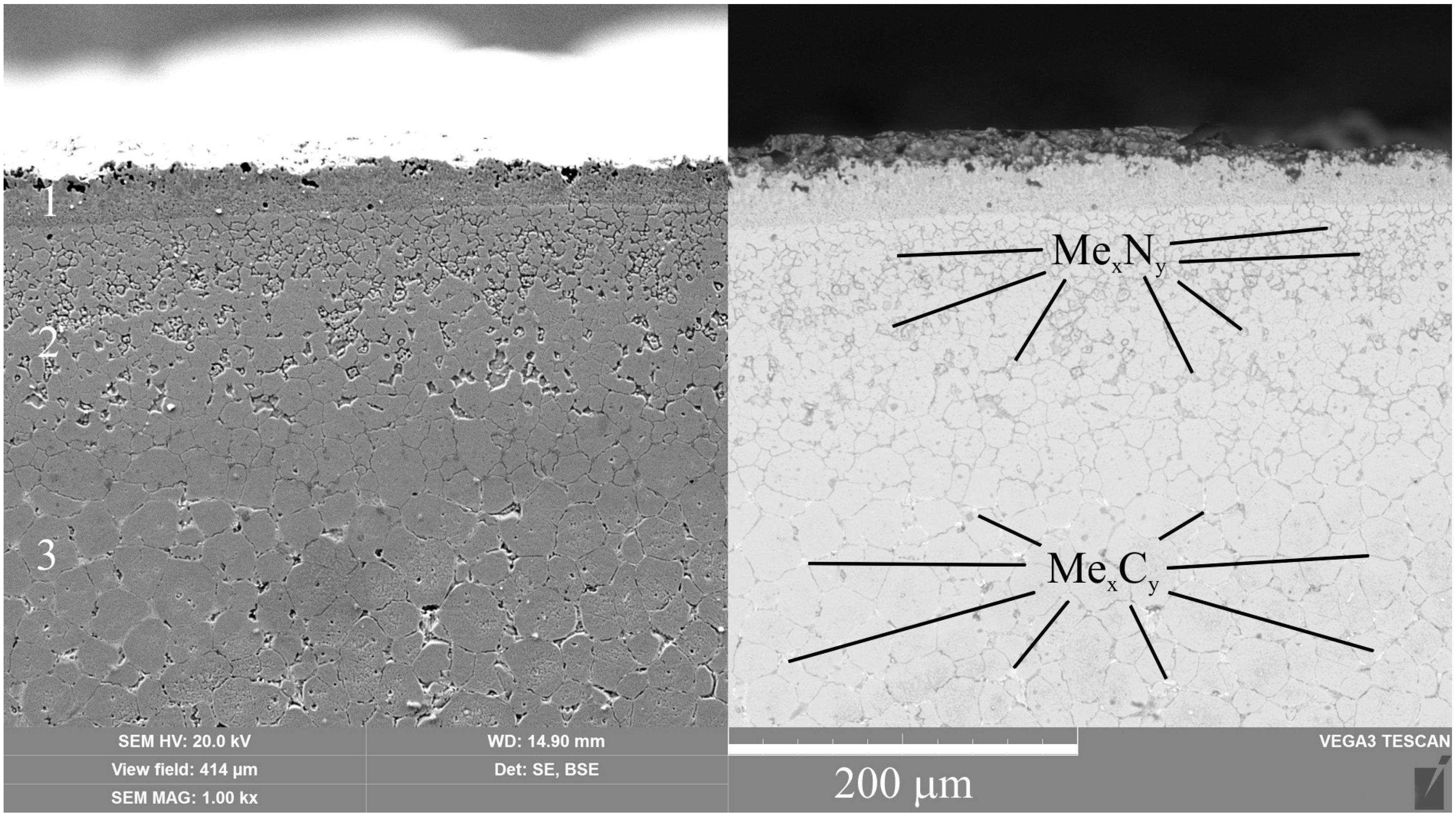
A significantly longer holding time in a saturated ammonia environment at VN promotes greater nitrogen diffusion and formation of iron, chromium, and vanadium nitrides, unlike PEN. Low-temperature nitriding without heating to the austenitization temperature does not result in the dissolution of the initial carbides. The high concentration of nitrides in the diffusion zone promotes a significant increase in hardness, reaching 1300 HV. At the same time, the hardness of the core is significantly lower than the hardness of steel before treatment (about 1000 HV) because of tempering, which does not allow the use of VN technology without subsequent heat treatment.
The use of plasma electrolytic heating to a temperature of 1230 °C ensures the austenitization of steel and the dissolution of carbides. Subsequent air-cooling leads to hardening with martensitic transformation. In this case, similar processes are observed as in PEN with hardening. At the same time, a distinctive feature is a decrease in hardness at the edge of the surface after hardening. This could be due to the displacement of carbon by a large amount of nitrogen during diffusion and the partial dissolution of nitrides in austenite during subsequent heating for hardening. Thus, after heating to a temperature of 1230 °C, carbon depletion is observed in the surface layer, and almost all the nitrogen from solid nitrides dissolves in austenite and, during hardening, is formed into nitrogen martensite. At a certain depth, with a decrease in the nitrogen concentration in steel and an increase in the carbon concentration, the hardness begins to increase. Additionally, a decrease in the cooling rate promotes the release and coagulation of nitrides of increased hardness.
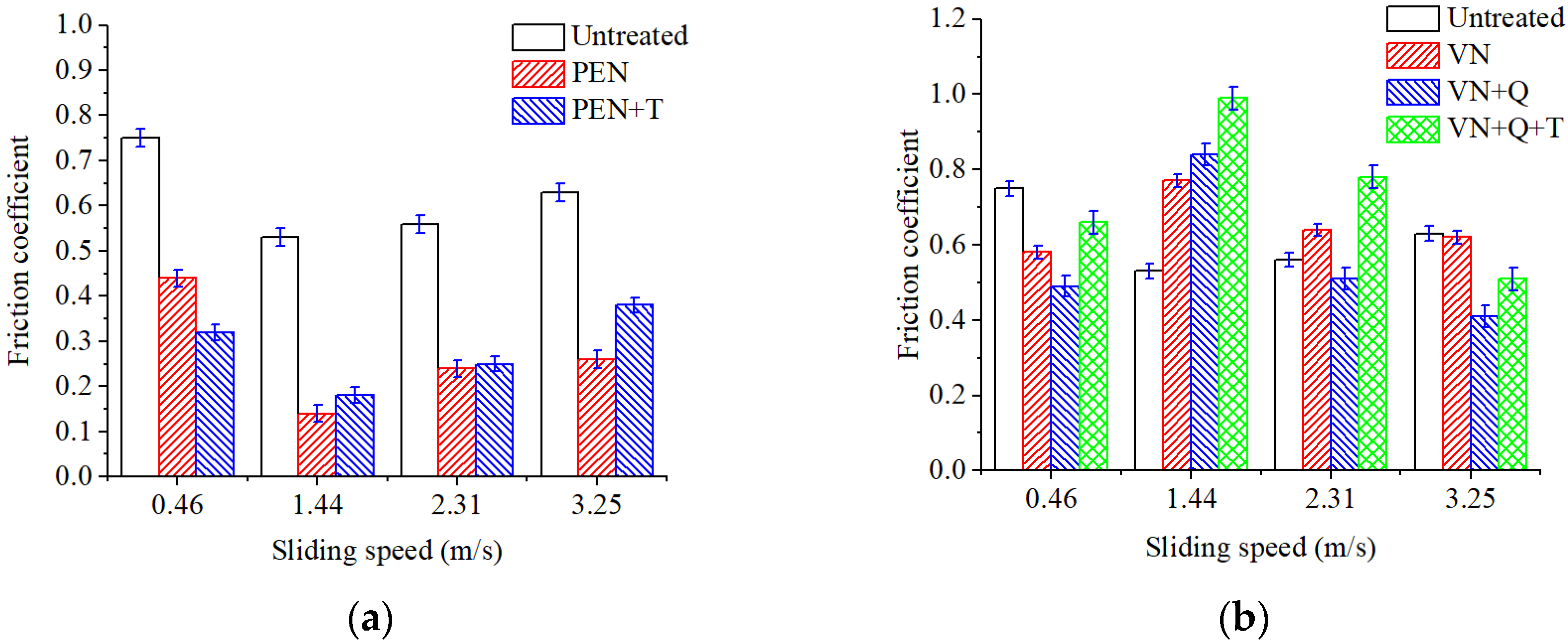
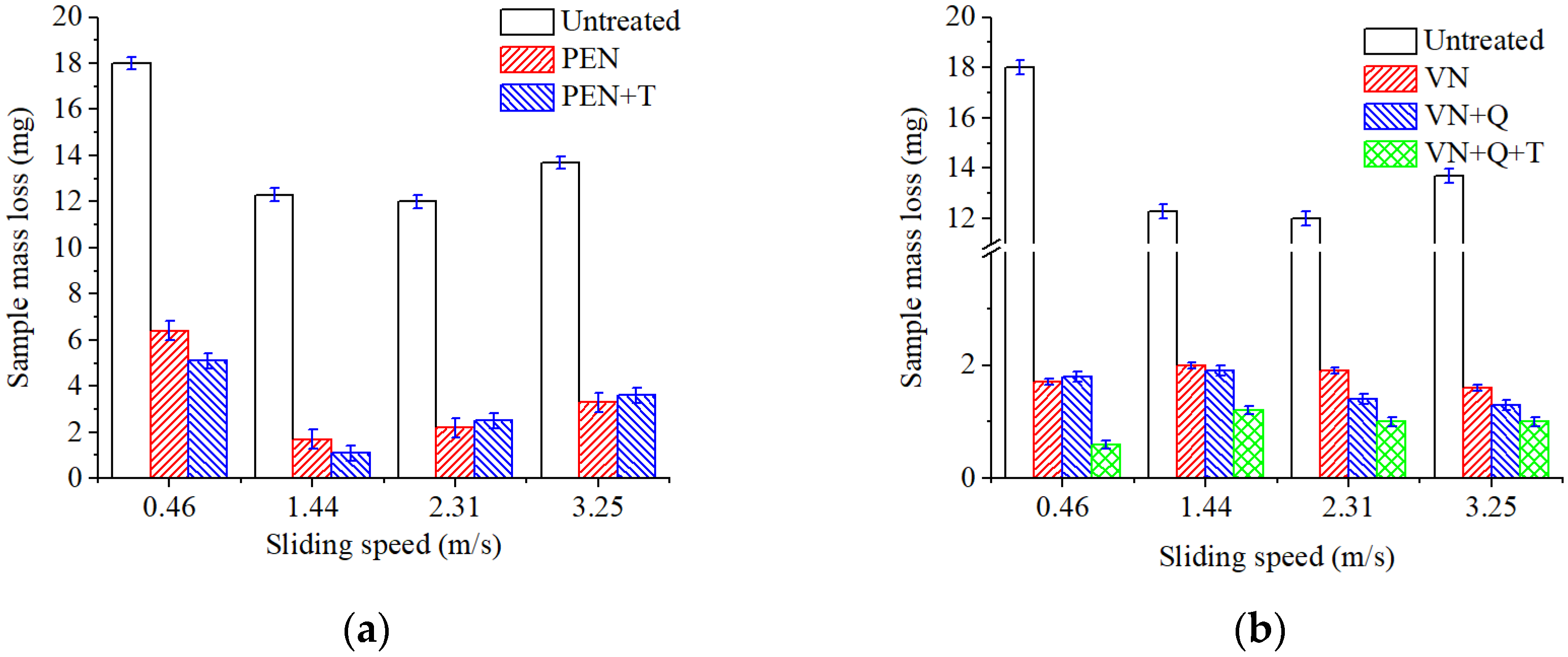
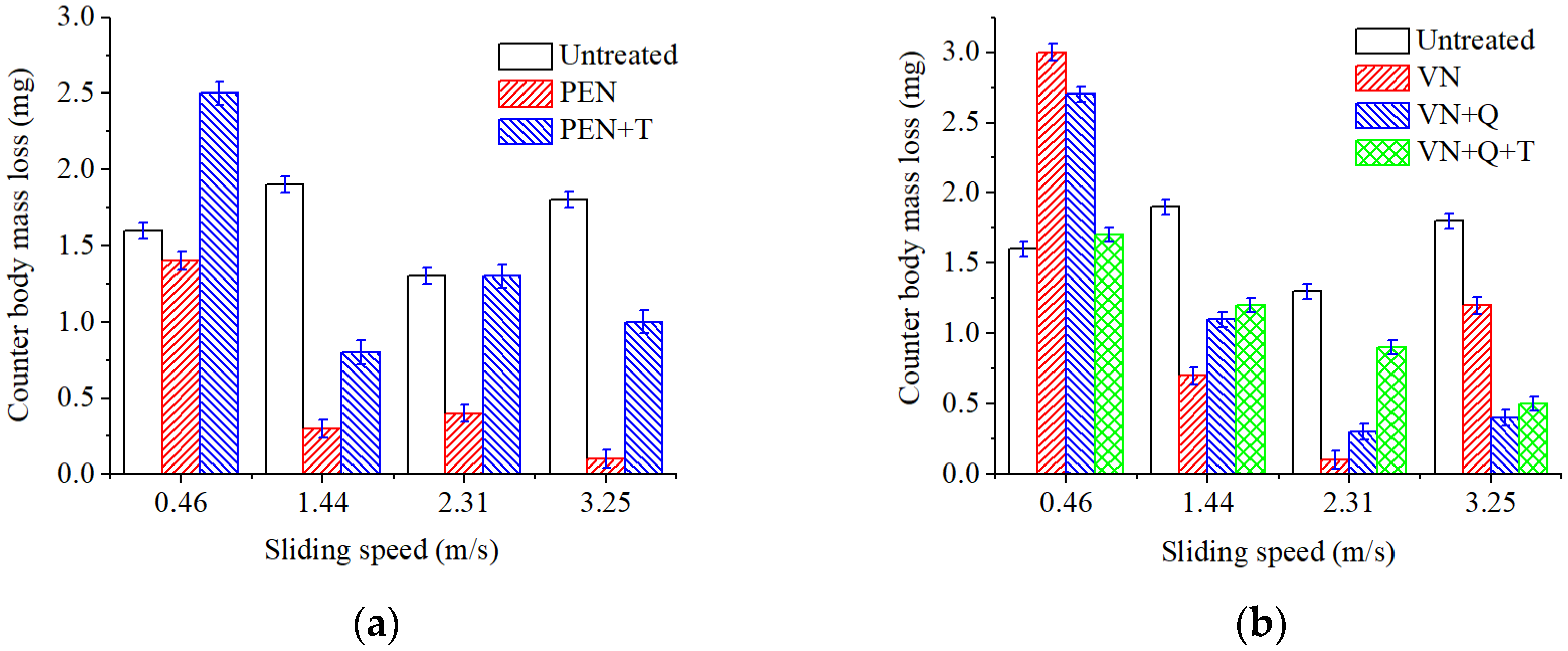
Evaluation of the tribological efficiency of plasma electrolytic and vacuum nitriding processes showed that samples nitrided by both methods provide friction without microcutting and seizures when operating under dry friction conditions without lubrication. Depending on the nitriding mode and subsequent heat treatment, mass loss during friction is reduced by 6–29 times. Increased wear resistance is determined by the structural state of the deformation zone during friction. Hardened particles of chromium and vanadium nitrides are located in a softer substrate of residual austenite. The applied load during friction is transferred to the hard components of the chromium and vanadium nitride deformation zone, and the substrate of residual austenite has a damping effect. It should be noted that both the compound layer and the diffusion zone affect surface quality from a tribological point of view. Iron nitrides Fe2-3N also have a favorable effect on the tribological characteristics of frictional conjugation.
Chromium and vanadium nitrides formed during VN and chromium nitrides formed during PEN are the result of segregations of alloying elements within the diffusion zone structure. Therefore, such nitrides do not form a coherent bond with the iron matrix. Incoherent nitride particles ensure the formation of a low level of microdeformations in the crystal lattice of the matrix metal. According to [
49], the wear resistance of steel increases with the degree of disruption of the coherent bond between nitrides and the matrix in the nitrided layer.
The calculation of contact deformations (plasticity criterion in Equation (7); relative penetration of roughness protrusions of compressed surfaces through the ratio of Equations (7) and (5) in
Table 2) made it possible to determine the wear mechanism depending on the sliding speed of the sample along the counter body.
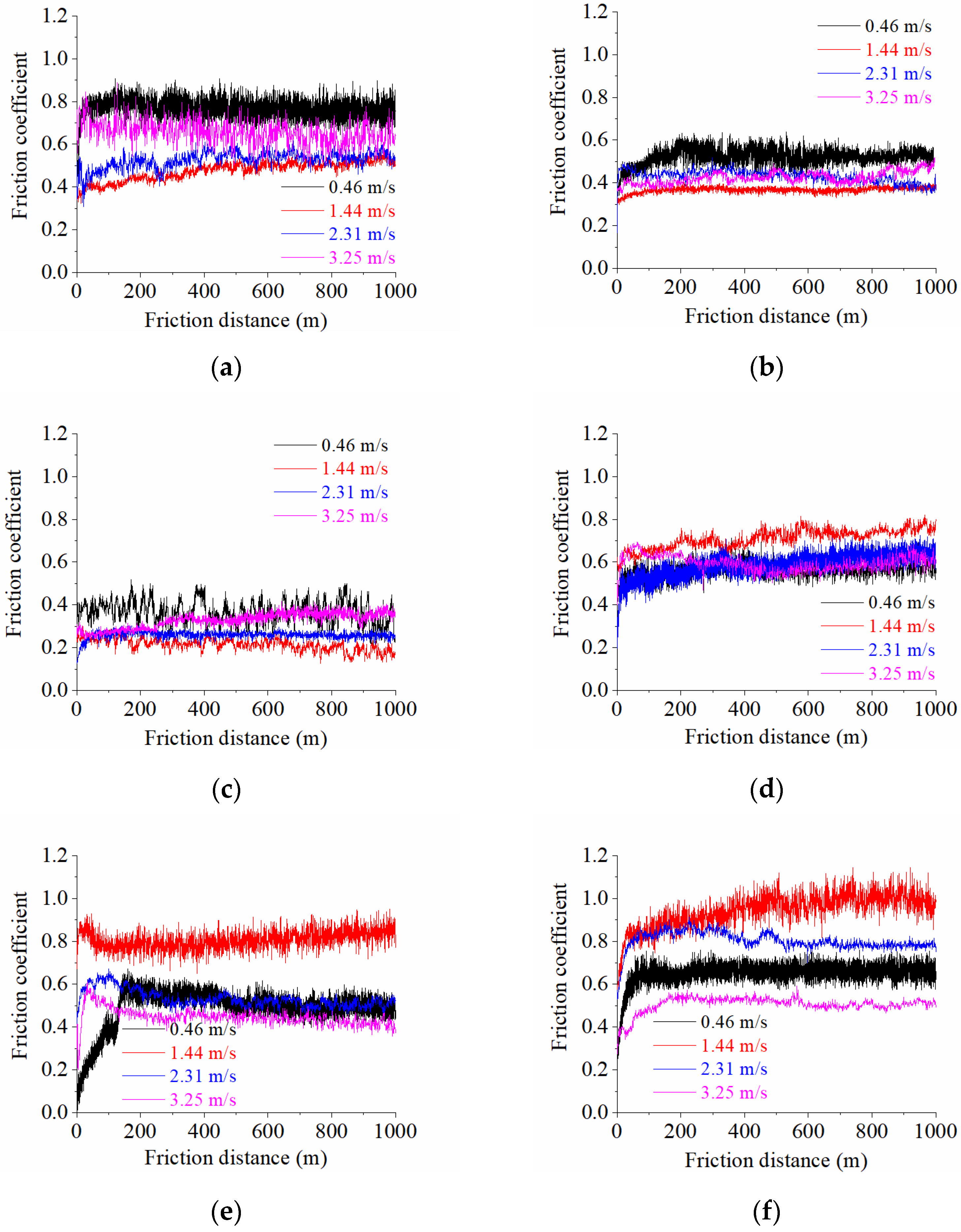
At a low sliding speed of 0.46 m/s for all cases of processing in a tribological conjugation, the leading mechanism of destruction of the emerging frictional bonds is the plastic displacement of the sample material by the roughness protrusions of the counter body (
h/
r < 0.1;
Kp > 3 in
Table 2). Under the influence of plastic deformation, the sample surface areas are intensively strengthened and, after the plasticity reserve has expired, weight shear processes begin in them, leading to the appearance of submicroscopic cracks and brittle fatigue failure (
Figure 16a).
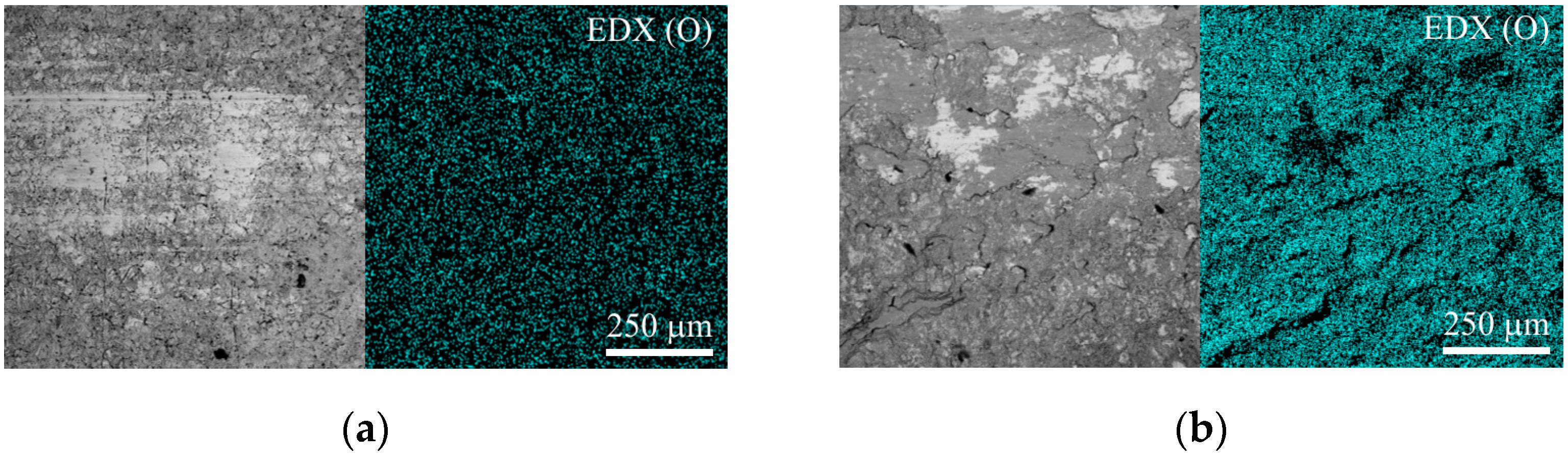
Increasing the sliding speed to 1.44 m/s activates oxidation processes in the tribocontact. During friction, the surface oxide films are seized and destroyed (
Figure 16b). The surface is destroyed by cutting the oxide film, and the leading wear mechanism is oxidative wear. This type of wear is favorable since the destruction processes are localized in the thinnest near-surface layers, which is what causes the minimum wear among all the cases studied.
A further increase in the sliding speed of the sample on the counter body to 2.31 m/s leads to more intense plastic deformations. The relative convergence
h/
r, determined by the ratio of Equation (7) to Equation (5) and calculated in
Table 2, in this case, is greater than 0.1, which indicates the implementation of a plastic contact in the triboconjugation, not an elastic one. Plastic deformations destroy the surface oxide films, and fatigue wear during plastic contact is realized at the points of contact between the film-free metal surfaces of the sample and the counter body. On a portion of the friction track, the oxide films have time to grow again after destruction (
Figure 16c); here, the oxidative wear mechanism is realized. Both oxide film wear and mechanical wear take place.
Increasing the sliding speed to 3.25 m/s further intensifies plastic deformations in the surface layer; as a result, the deformation rate becomes higher than the rate of growth of a new oxide film, and friction occurs mainly on metal surfaces (
Figure 16d). The leading type of wear becomes fatigue wear, with a predominance of plastic deformations in tribocontact. The predominance of plastic deformations over elastic ones is proven by calculating the plasticity criterion and the relative penetration of rough surfaces according to Equations (5)–(7), as indicated in
Table 2.
5. Conclusions
For the first time, the prospects of combining vacuum low-temperature nitriding with subsequent heat treatment (quenching) by the plasma electrolytic method are shown. Such a combination allows for creating thicker diffusion layers with a higher nitrogen concentration by vacuum nitriding than with plasma electrolytic nitriding due to the limited duration of the latter’s treatment. At the same time, subsequent plasma electrolytic treatment can allow controlled hardening.
The possibility of nitriding M2 high-speed tool steel by plasma electrolytic treatment with anodic polarity of the processed products and vacuum nitriding is studied. The results of structural–phase changes are presented, as well as tribological tests of samples processed by a traditional method for each technology and a combination of vacuum nitriding with subsequent quenching under plasma electrolytic heating conditions. The main conclusions of this work are as follows:
(1) The composition of modified layers on the surface of M2 steel after PEN was determined. High-temperature oxidation results in the formation of FeO, Fe2O3, and Fe3O4 oxides. The oxide layers have a porous structure, which ensures the transport of iron ions into the electrolyte during anodic dissolution and N atoms into the surface of the treated steel during diffusion saturation. Immediately beneath the oxide layer, the ε-phase Fe2-3N and chromium nitride CrN with residual austenite are formed, and acicular nitrogen martensite is formed below. The composition of the modified surface zone after VN was determined; in addition to the nitrides detected after PEN, it includes vanadium nitride. A higher percentage of nitrides in the surface layer and a deeper occurrence of nitrogen are shown after vacuum diffusion saturation compared to accelerated plasma diffusion saturation.
(2) Wear resistance was shown to be a structure-sensitive characteristic. The structure of the friction deformation zone depends on the initial structure of the nitrided layer. Modeling the topology of worn surfaces revealed the predominance of plastic deformation in the tribological interface zone during friction after both plasma electrolyte and vacuum nitriding. Surface plastic deformation alters the structure and properties of the friction deformation zone material, creating a friction structure that controls the degree of wear.
(3) The relationship between the tribological characteristics of the structural state of the deformation zone under friction and the structural state of the nitrided layer was determined. Hardened chromium and vanadium nitride particles are embedded in a softer substrate of retained austenite. During friction, the applied load is transferred to the hard components, while the retained austenite substrate acts as a damper. Chromium and vanadium nitride particles, formed at the segregated alloying elements, create low levels of microstrain in the crystal lattice, which also contributes to increased wear resistance.
Further research may focus on the use of plasma electrolytic polishing of surfaces as a finishing treatment after vacuum nitriding and plasma electrolytic hardening. Plasma electrolytic saturation of metal surfaces after vacuum nitriding with carbon, boron, and possibly sulfur atoms appears promising. In this case, a pre-nitrided layer with a high nitrogen concentration and a large nitrided layer depth will be saturated with carbon, boron, and sulfur, forming a unique structural and phase composition that is impossible to achieve using each method separately.