Energy Efficient Jet Polishing via Electrolytic Plasma Enhances Corrosion Resistance in Stainless Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Material and Preparation
2.2. Material Characterization
2.3. Electrolytic Polishing
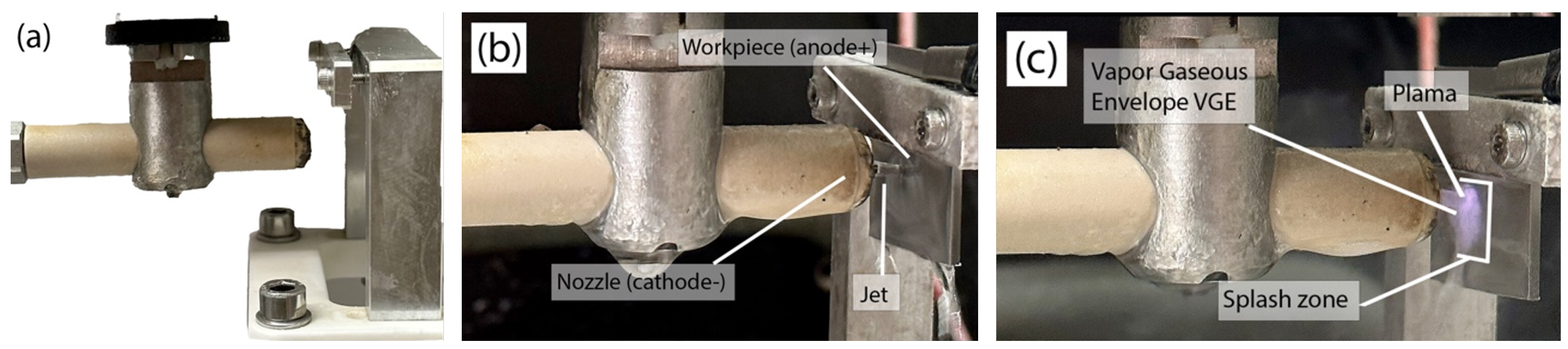
2.4. Polishing Process
3. Results
3.1. Energy Consumption
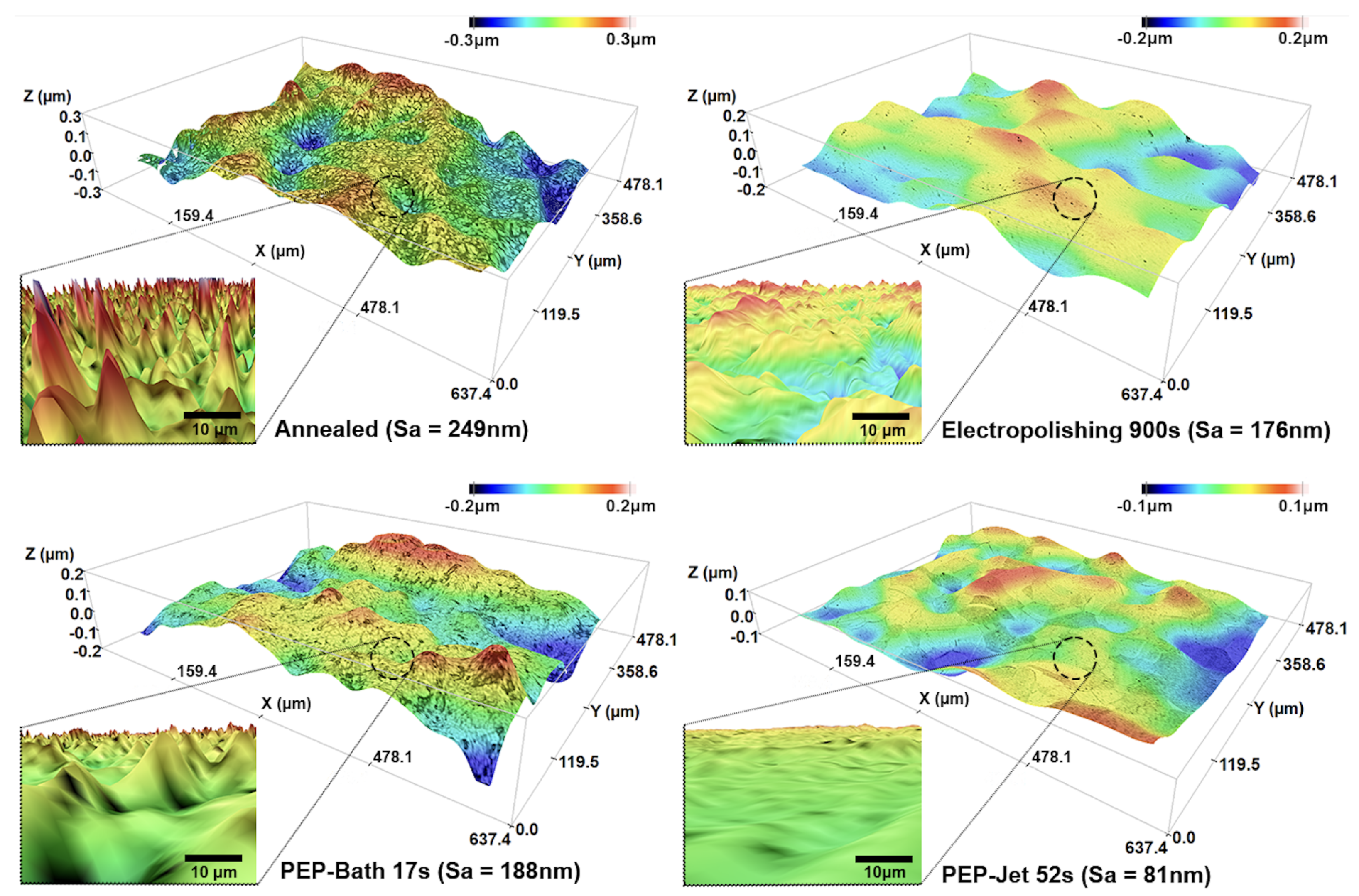
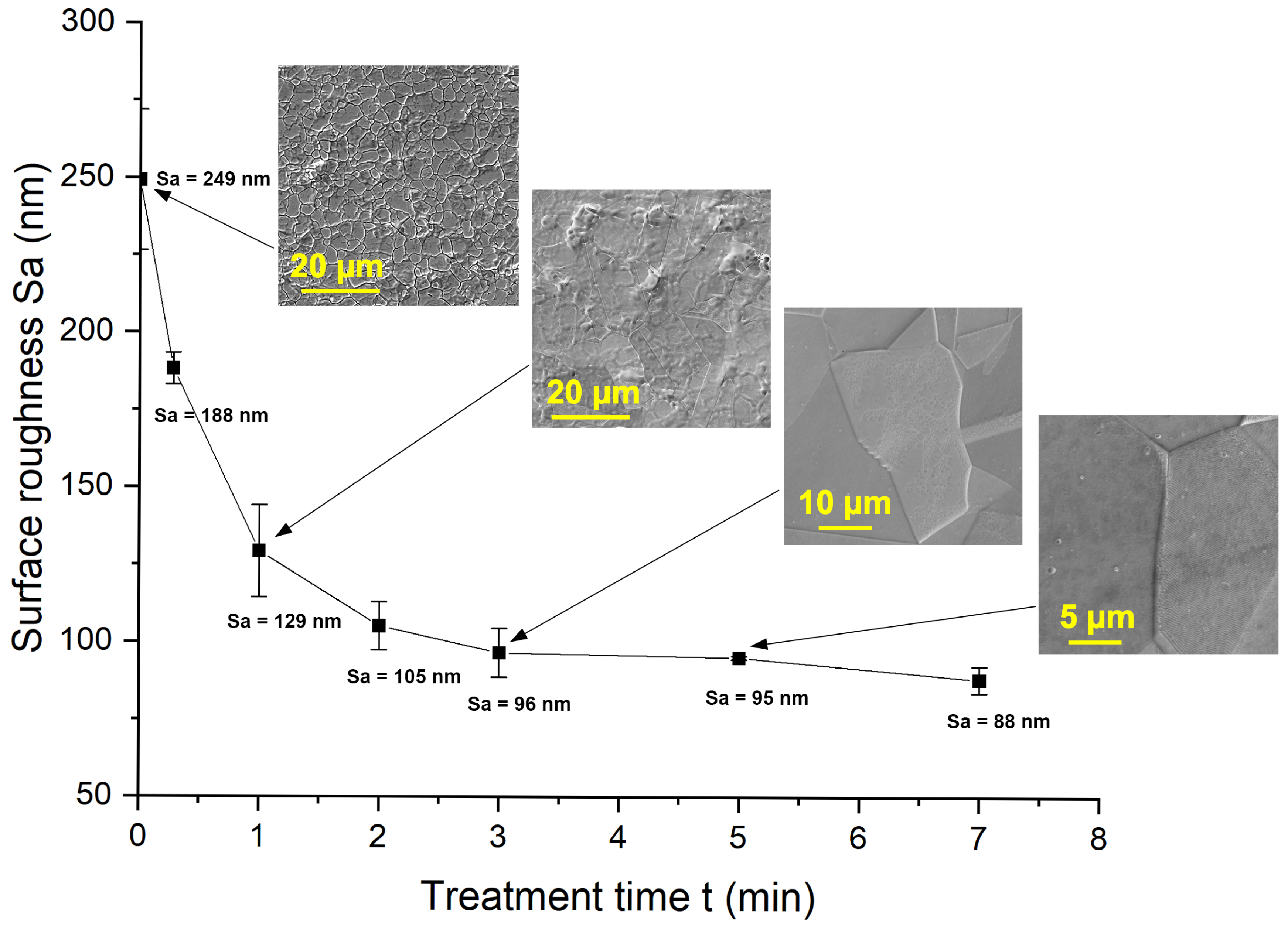
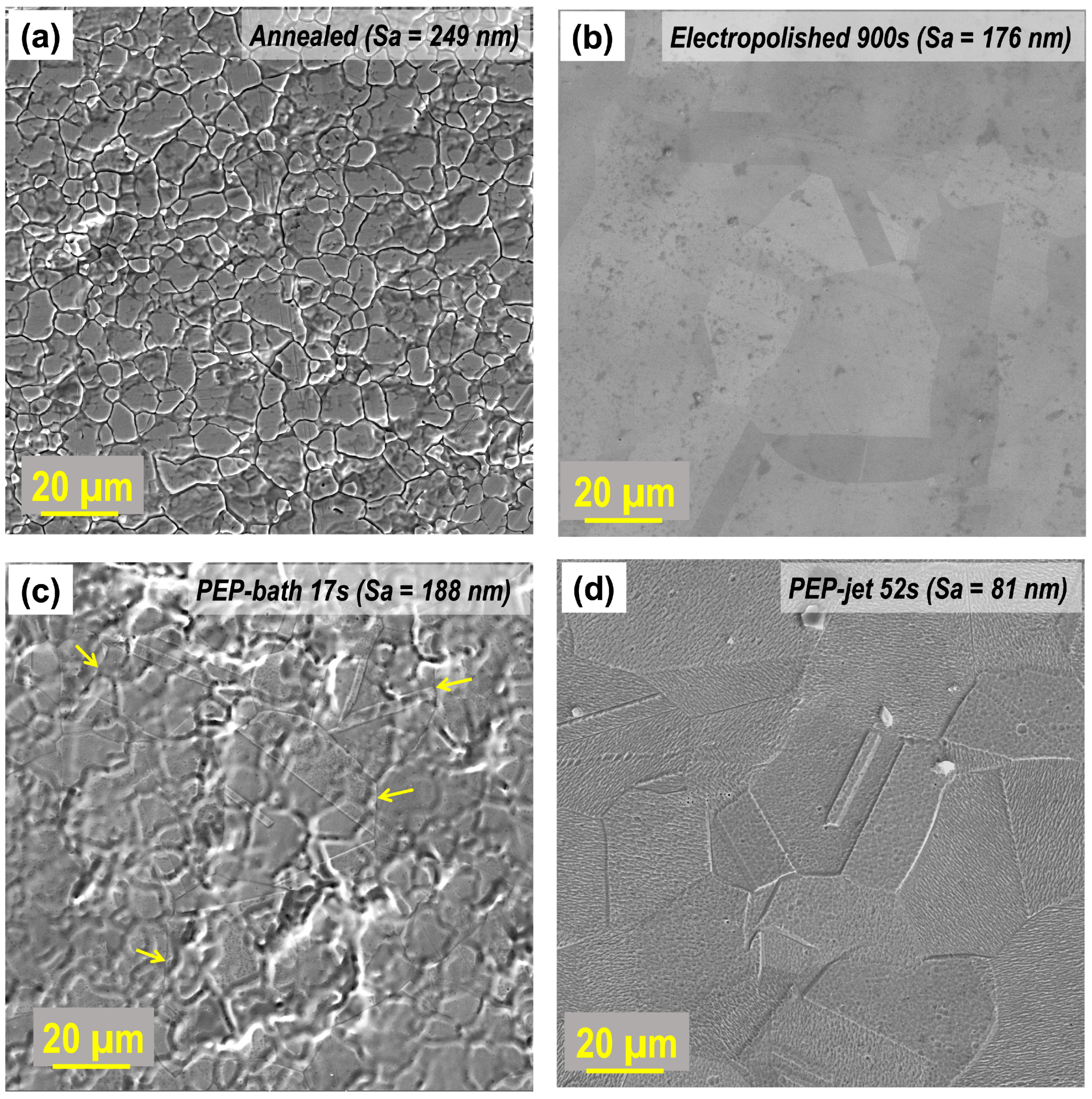
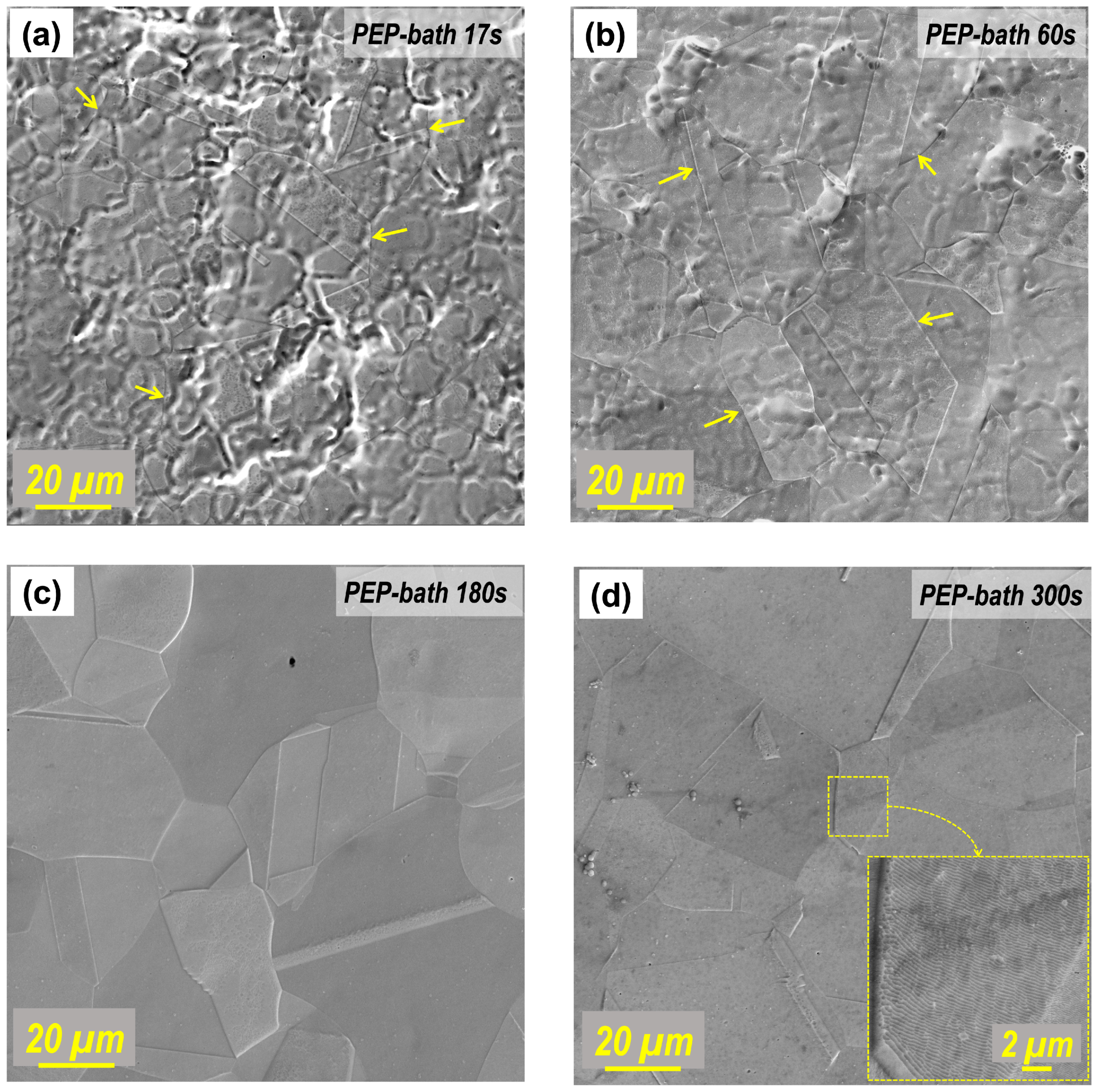
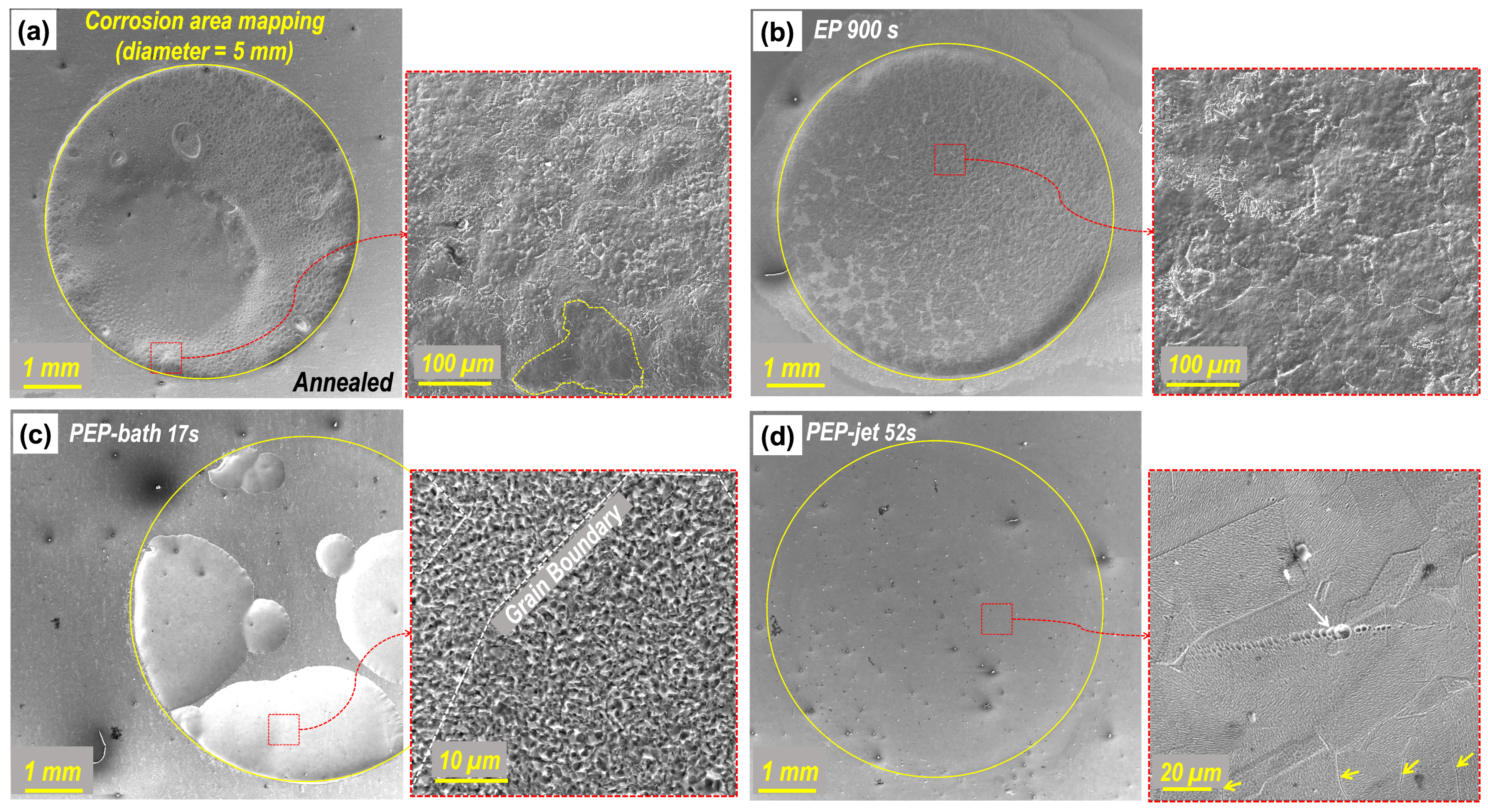
3.2. Characterization of Surface and Microstructure
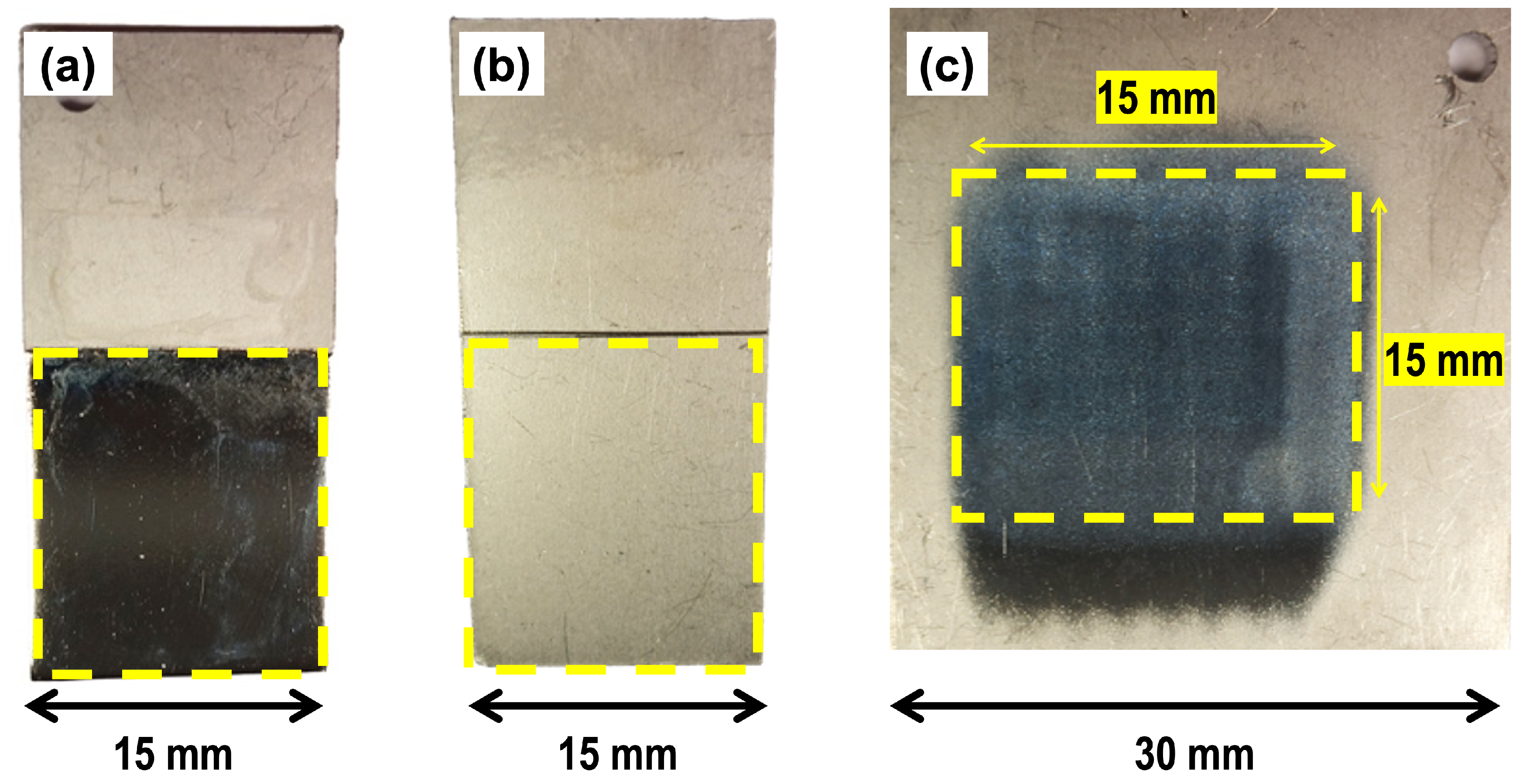
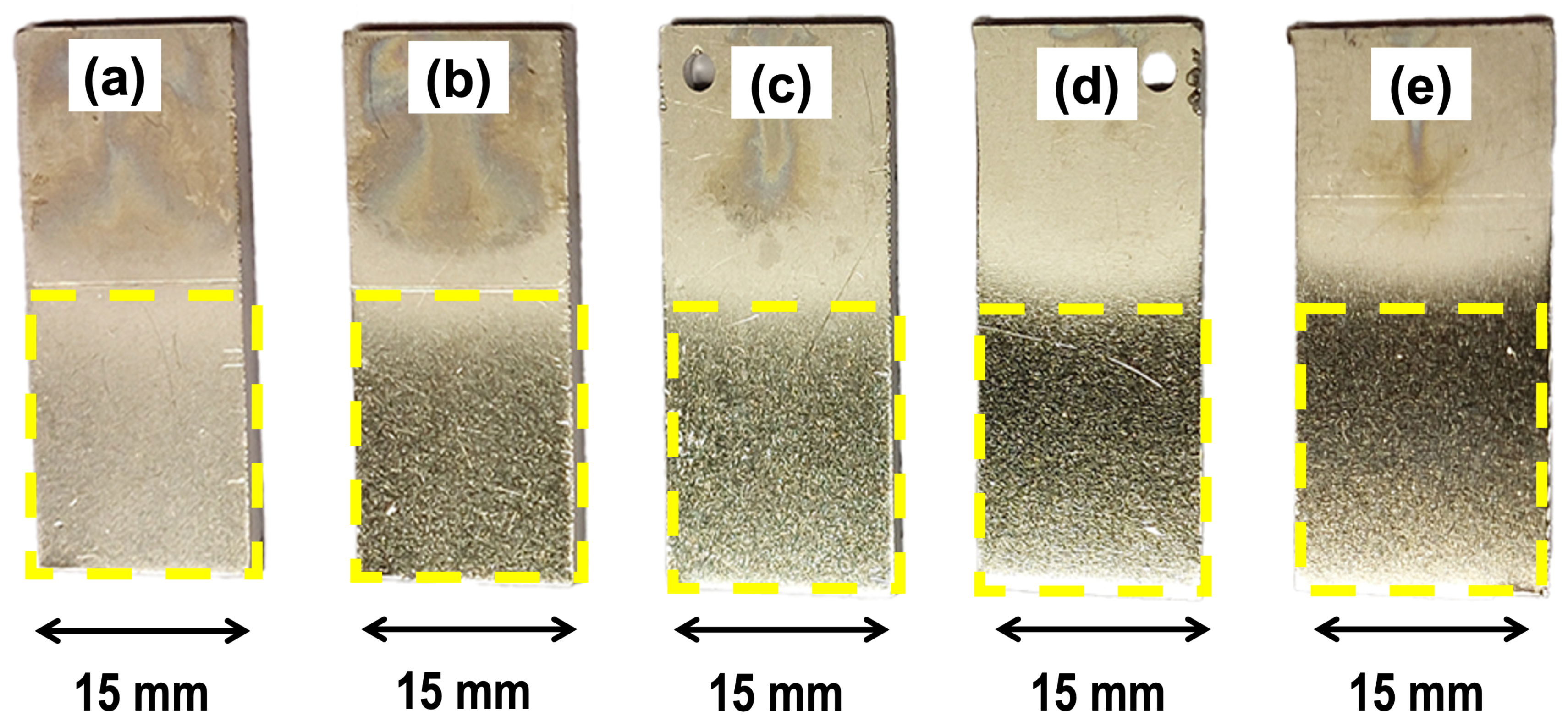
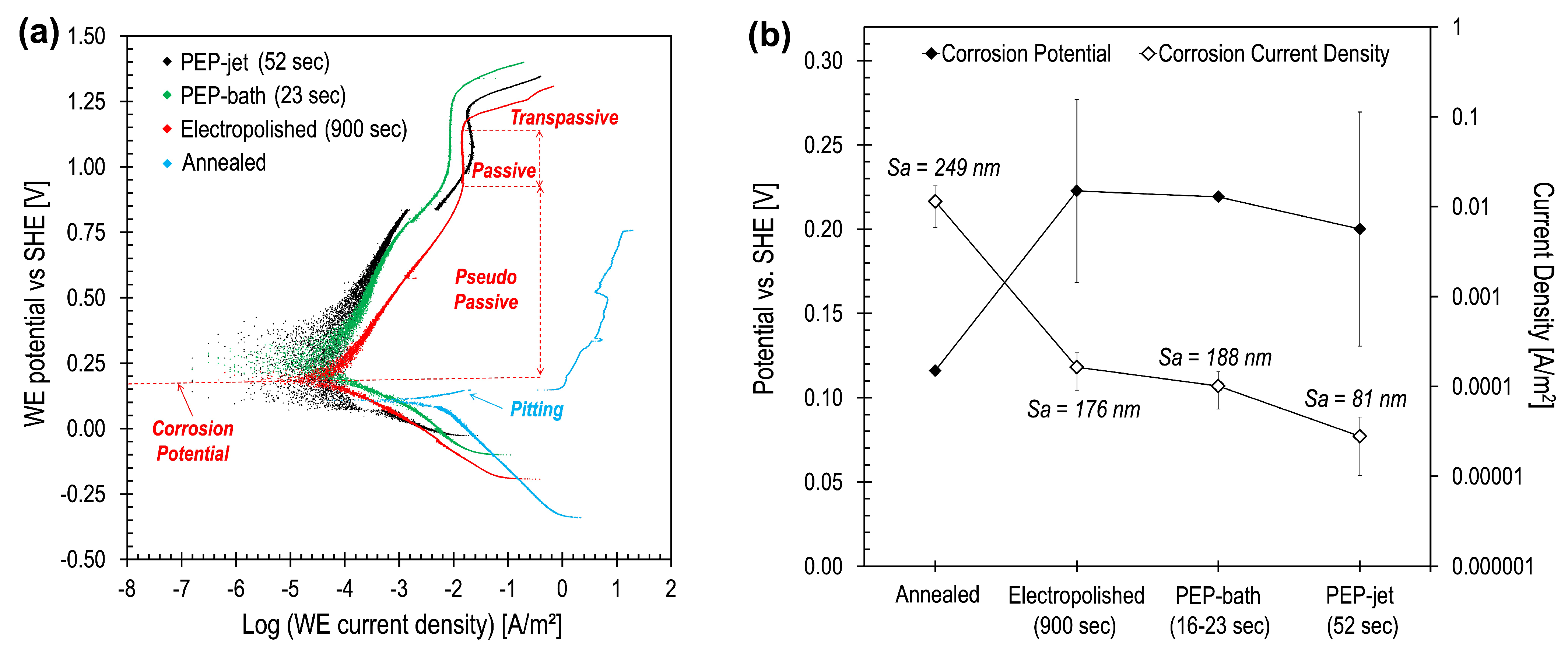
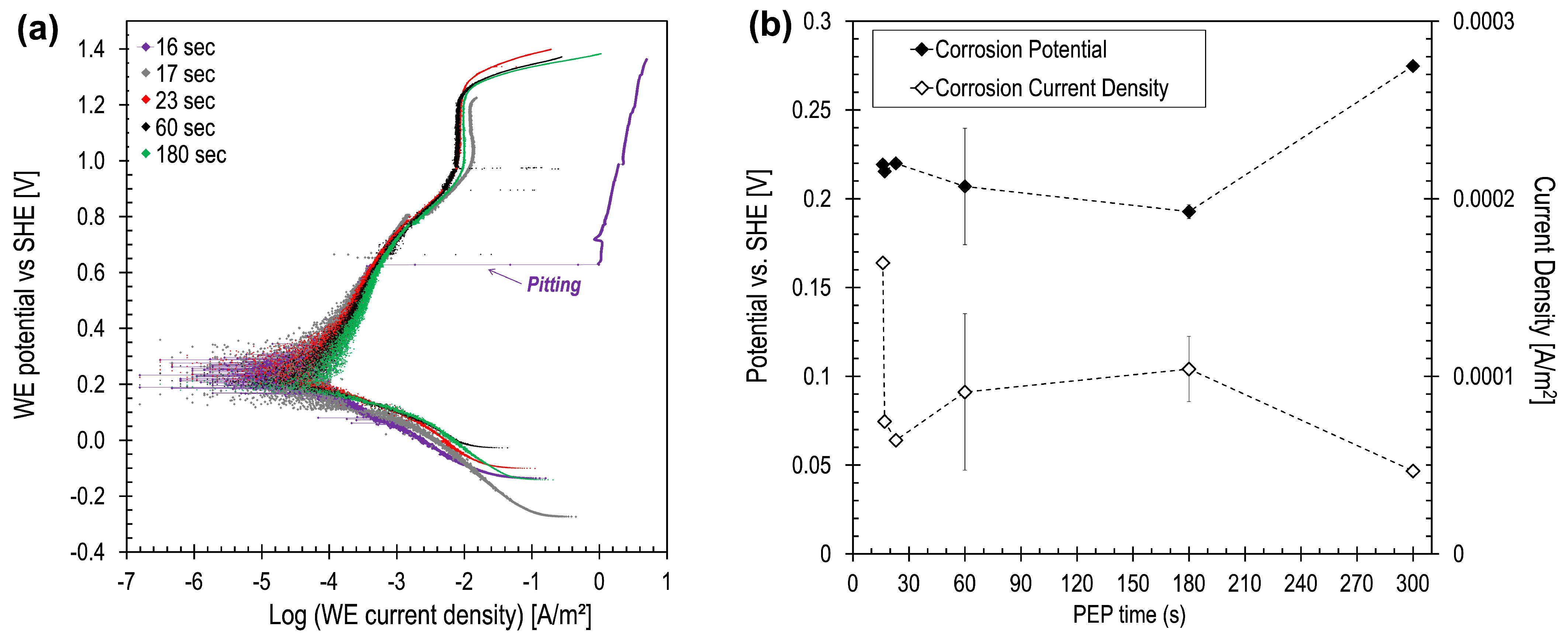
3.3. Corrosion Rate Analysis
4. Discussion
Surface Quality and Corrosion Resistance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heng, L.; Kim, J.S.; Song, J.H.; Mun, S.D. A Review on Surface Finishing Techniques for Difficult-to-Machine Ceramics by Non-Conventional Finishing Processes. Materials 2022, 15, 1227. [Google Scholar] [CrossRef] [PubMed]
- Núñez, P.; García-Plaza, E.; Hernando, M.; Trujillo, R. Characterization of Surface Finish of Electropolished Stainless Steel AISI 316L with Varying Electrolyte Concentrations. Procedia Eng. 2013, 63, 771–778. [Google Scholar] [CrossRef]
- Quitzke, S.; Martin, A.S.A. Localized surface functionalization of steel by jet-plasma electrolytic polishing. In Proceedings of the 9th International Workshop on Thin Films and New Ideas for Pushing the Limits of RF Superconductivity, Virtual, 15–18 May 2021. [Google Scholar]
- Edyta, Ł.W.; Paweł, L.; Ginter, N. Electrochemical Polishing of Austenitic Stainless Steels. Materials 2020, 13, 2557. [Google Scholar] [CrossRef]
- Chaghazardi, Z.; Wüthrich, R. Review—Electropolishing of Additive Manufactured Metal Parts. J. Electrochem. Soc. 2022, 169, 043510. [Google Scholar] [CrossRef]
- Belkin, P.; Kusmanov, S.; Parfenov, E. Mechanism and technological opportunity of plasma electrolytic polishing of metals and alloys surfaces. Appl. Surf. Sci. Adv. 2020, 1, 100016. [Google Scholar] [CrossRef]
- Danilov, I.; Hackert-Oschätzchen, M.; Zinecker, M.; Meichsner, G.; Edelmann, J.; Schubert, A. Process Understanding of Plasma Electrolytic Polishing through Multiphysics Simulation and Inline Metrology. Micromachines 2019, 10, 214. [Google Scholar] [CrossRef]
- Chyhyrynets, E.; Stivanello, F.; Pira, C.; Caforio, R.; García, V.; Zanierato, M. Plasma electrolytic polishing as a promising treatment replacement of electropolishing in the copper and niobium substrate preparation for SRF. SRF Technol. 2021, 718, 718. [Google Scholar] [CrossRef]
- Nestler, K.; Böttger-Hiller, F.; Adamitzki, W.; Glowa, G.; Zeidler, H.; Schubert, A. Plasma Electrolytic Polishing—An Overview of Applied Technologies and Current Challenges to Extend the Polishable Material Range. Proc. CIRP 2016, 42, 503–507. [Google Scholar] [CrossRef]
- Gangqiang, J.; Huanwu, S.; Haidong, D.; Dongliang, Y.; Jinyan, S. Effect of electrolytic plasma polishing on microstructural evolution and tensile properties of 316L stainless steel. Surf. Coat. Technol. 2021, 420, 127330. [Google Scholar] [CrossRef]
- Kalenchukova, O.V.; Nagula, P.K.; Tretinnikov, D.L. About changes in the chemical composition of the electrolyte in the process of electrolytic-plasma treatment of materials. Int. Sci. Publ. 2015, 9, 404–413. [Google Scholar]
- Ma, G.; Ma, L.; Wu, L. Effect of the gas layer evolution on electrolytic plasma polishing of stainless steel. Sci. Rep. 2024, 14, 22099. [Google Scholar] [CrossRef]
- Ushomirskaya, L.; Gerasimov, A. Technological Possibilities of Using a Jet Flow of Electrolyte During Electrolyte-Plasma Polishing; Metalloobrab: Moscow, Russia, 2015. [Google Scholar]
- Volenko, A.; Boychenko, O.; Chirkunova, N. Introduction of technology of electrolytic-plasma polishing of metal goods. Front. Mater. Technol. 2016, 1, 11–16. [Google Scholar] [CrossRef]
- Zeidler, H.; Boettger-Hiller, F.; Edelmann, J.; Schubert, A. Surface Finish Machining of Medical Parts Using Plasma Electrolytic Polishing. Proc. CIRP 2016, 49, 83–87. [Google Scholar] [CrossRef]
- Quitzke, S.; Kröning, O.; Safranchik, D.; Zeidler, H.; Danilov, I.; Martin, A.; Falko Böttger-Hiller, S.E.; Schubert, A. Design and setup of a jet-based technology for localized small scale Plasma electrolytic Polishing. J. Manuf. Process. 2022, 75, 1123–1133. [Google Scholar] [CrossRef]
- Küenzi, A.; Goetschi, M.; Nelis, T.; Bessire, C. Jet Application of Plasma Electrolyte Polishing. Proc. CIRP 2022, 113, 525–529. [Google Scholar] [CrossRef]
- Zatkalíková, V.; Podhorský, Š.; Štrbák, M.; Liptáková, T.; Markovičová, L.; Kuchariková, L. Plasma Electrolytic Polishing—An Ecological Way for Increased Corrosion Resistance in Austenitic Stainless Steels. Materials 2022, 15, 4223. [Google Scholar] [CrossRef]
- Kestel, B.J. A jet electropolishing solution for silicon, germanium, tantalum, niobium, and tungsten-rhenium. Ultramicroscopy 1982, 9, 379–383. [Google Scholar] [CrossRef]
- Kestel, B.J. Improved Methods and Novel Techniques for Jet Electropolishing of TEM Foils. MRS Proc. 1990, 199, 51. [Google Scholar] [CrossRef]
- Speidel, A.; Bisterov, I.; Saxena, K.K.; Zubayr, M.; Reynaerts, D.; Natsu, W.; Clare, A.T. Electrochemical jet manufacturing technology: From fundamentals to application. Int. J. Mach. Tools Manuf. 2022, 180, 103931. [Google Scholar] [CrossRef]
- Lochyński, P.; Charazińska, S.; Łyczkowska-Widłak, E.; Sikora, A. Electropolishing of Stainless Steel in Laboratory and Industrial Scale. Metals 2019, 9, 854. [Google Scholar] [CrossRef]
- Yang, D.; Sun, H.; Ji, G.; Xiang, Y.; Wang, J. Effect of Electrolytic Plasma Polishing on Surface Properties of Titanium Alloy. Coatings 2024, 14, 615. [Google Scholar] [CrossRef]
- García de Andrés, C.; Caballero, F.; Capdevila, C.; San Martín, D. Revealing austenite grain boundaries by thermal etching: Advantages and disadvantages. Mater. Charact. 2002, 49, 121–127. [Google Scholar] [CrossRef]
- Otieno-Alego, V.; Hope, G.; Flitt, H.; Cash, G.; Schweinsberg, D. The effect of potential scan rate on the parameters used to synthesize anodic polarization curves. Corros. Sci. 1992, 33, 1719–1734. [Google Scholar] [CrossRef]
- Cornelsen, M.; Deutsch, C.; Seitz, H. Electrolytic Plasma Polishing of Pipe Inner Surfaces. Metals 2017, 8, 12. [Google Scholar] [CrossRef]
- Parfenov, E.; Farrakhov, R.; Mukaeva, V.; Gusarov, A.; Nevyantseva, R.; Yerokhin, A. Electric field effect on surface layer removal during electrolytic plasma polishing. Surf. Coat. Technol. 2016, 307, 1329–1340. [Google Scholar] [CrossRef]
- Mu, J.; Sun, T.; Leung, C.L.A.; Oliveira, J.; Wu, Y.; Wang, H.; Wang, H. Application of electrochemical polishing in surface treatment of additively manufactured structures: A review. Prog. Mater. Sci. 2023, 136, 101109. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, C.; Ding, F.; Yang, Y.; Zhang, T.; He, X.; Zheng, L.; Li, N. Principle, process, and application of metal plasma electrolytic polishing: A review. Int. J. Adv. Manuf. Technol. 2021, 114, 1893–1912. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, L.; Wikman, S.; Cui, D.; Shen, Z. Intragranular cellular segregation network structure strengthening 316L stainless steel prepared by selective laser melting. J. Nucl. Mater. 2016, 470, 170–178. [Google Scholar] [CrossRef]
- Casati, R.; Lemke, J.; Vedani, M. Microstructure and Fracture Behavior of 316L Austenitic Stainless Steel Produced by Selective Laser Melting. J. Mater. Sci. Technol. 2016, 32, 738–744. [Google Scholar] [CrossRef]
- Voisin, T.; Shi, R.; Zhu, Y.; Qi, Z.; Wu, M.; Sen-Britain, S.; Zhang, Y.; Qiu, S.R.; Wang, Y.M.; Thomas, S.; et al. Pitting Corrosion in 316L Stainless Steel Fabricated by Laser Powder Bed Fusion Additive Manufacturing: A Review and Perspective. JOM 2022, 74, 1668–1689. [Google Scholar] [CrossRef]
- Tsai, S.P.; Makineni, S.K.; Gault, B.; Kawano-Miyata, K.; Taniyama, A.; Zaefferer, S. Precipitation formation onΣ5 and Σ7 grain boundaries in 316L stainless steel and their roles on intergranular corrosion. Acta Mater. 2021, 210, 116822. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Blanquet, A.; Staiger, M.P.; Dias, G.J.; Woodfield, T.B.F. On the role of surface roughness in the corrosion of pure magnesium in vitro. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Evgeny, B.; Hughes, T.; Eskin, D. Effect of surface roughness on corrosion behaviour of low carbon steel in inhibited 4 M hydrochloric acid under laminar and turbulent flow conditions. Corros. Sci. 2016, 103, 196–205. [Google Scholar] [CrossRef]
- Toloei, A.S.; Stoilov, V.; Northwood, D.O. The effect of different surface topographies on the corrosion behaviour of nickel. In Proceedings of the WIT Transactions on Engineering Sciences, Los Angeles, CA, USA, 31 July 2013; pp. 193–204. [Google Scholar] [CrossRef]
- Syafira, T.; Achmad, T.L.; Dilasari, B. Effect of steel surface roughness on the performance of organic inhibitors in hydrochloric acid solution. In Proceedings of the Advances in metallurgy and engineering materials: Characterizations and innovation, Depok, Indonesia, 16–17 November 2020; p. 080001. [Google Scholar] [CrossRef]
- Kranhold, C.; Kröning, O.; Schulze, H.P.; Herzig, M.; Zeidler, H. Investigation of stable boundary conditions for the Jet-electrolytic Plasma Polishing (Jet-ePP). Proc. CIRP 2020, 95, 987–992. [Google Scholar] [CrossRef]
- Lu, J.; Zhan, S.; Liu, B.; Zhao, Y. Plasma-enabled electrochemical jet micromachining of chemically inert and passivating material. Int. J. Extrem. Manuf. 2022, 4, 045101. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Wang, B.; Jin, H.L.; Dong, S. Development of material removal function in atmospheric pressure plasma jet machining processing. Eng. Rev. 2014, 34, 103–108. [Google Scholar]
- Kim, Y.; Yoo, M.; Moon, M. Effects of Surface Roughness on the Electrochemical Properties and Galvanic Corrosion Behavior of CFRP and SPCC Alloy. Materials 2020, 13, 4211. [Google Scholar] [CrossRef]
- Lee, J.S.; Kawano, T.; Ishii, T.; Kitagawa, Y.; Nakanishi, T.; Hasegawa, Y.; Fushimi, K. Initiation of Localized Corrosion of Ferritic Stainless Steels by Using the Liquid-Phase Ion Gun Technique. J. Electrochem. Soc. 2017, 164, C1–C7. [Google Scholar] [CrossRef]
- Alvarez, R.B.; Martin, H.J.; Horstemeyer, M.; Chandler, M.; Williams, N.; Wang, P.T.; Ruiz, A. Corrosion relationships as a function of time and surface roughness on a structural AE44 magnesium alloy. Corros. Sci. 2010, 52, 1635–1648. [Google Scholar] [CrossRef]
- Parfenov, E.; Mukaeva, V.; Farrakhov, R. Plasma electrolytic treatments for advanced surface finishing technologies. Mater. Technol. Des. 2014, 1, 34–41. [Google Scholar]
| Polishing Technique | EP | PEP-Bath | PEP-Jet |
|---|---|---|---|
| Mean time (s) | 905 | 17 | 52 |
| Mean voltage (V) | 7 | 340 | 340 |
| Mean current (A) | 2.4 | 2.8 | 0.4 |
| Mean power (W) | 17 | 935 | 124 |
| Total Energy (Wh) | 4.2 | 4.3 | 1.8 |
| Energy density (Ws/mm2) | 30 | 30 | 29 |
| PEP-Bath Sample | 1 min | 2 min | 3 min | 5 min | 7 min |
|---|---|---|---|---|---|
| Process time (s) | 61 | 121 | 180 | 305 | 423 |
| Total Energy (Wh) | 15.5 | 28.9 | 38.3 | 60.3 | 81.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghezri, A.; Pratama, K.; Scholl, Y.; Küenzi, A.; Nelis, T.; Burger, J.; Bessire, C. Energy Efficient Jet Polishing via Electrolytic Plasma Enhances Corrosion Resistance in Stainless Steel. J. Manuf. Mater. Process. 2024, 8, 289. https://doi.org/10.3390/jmmp8060289
Ghezri A, Pratama K, Scholl Y, Küenzi A, Nelis T, Burger J, Bessire C. Energy Efficient Jet Polishing via Electrolytic Plasma Enhances Corrosion Resistance in Stainless Steel. Journal of Manufacturing and Materials Processing. 2024; 8(6):289. https://doi.org/10.3390/jmmp8060289
Chicago/Turabian StyleGhezri, Adel, Killang Pratama, Yan Scholl, Alexander Küenzi, Thomas Nelis, Jürgen Burger, and Cedric Bessire. 2024. "Energy Efficient Jet Polishing via Electrolytic Plasma Enhances Corrosion Resistance in Stainless Steel" Journal of Manufacturing and Materials Processing 8, no. 6: 289. https://doi.org/10.3390/jmmp8060289
APA StyleGhezri, A., Pratama, K., Scholl, Y., Küenzi, A., Nelis, T., Burger, J., & Bessire, C. (2024). Energy Efficient Jet Polishing via Electrolytic Plasma Enhances Corrosion Resistance in Stainless Steel. Journal of Manufacturing and Materials Processing, 8(6), 289. https://doi.org/10.3390/jmmp8060289





