Effect of Powder Recycling on the Surface and Selected Technological Properties of M300 Maraging Steel Produced via the SLM Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Powder Characteristics
2.2. Three-Dimensional Printing and Printing Parameters
2.3. Microstructure and Metallography Observation
2.4. Corrosion Test
2.5. Surface Wettability
2.6. Two-Dimensional Surface Roughness
3. Results
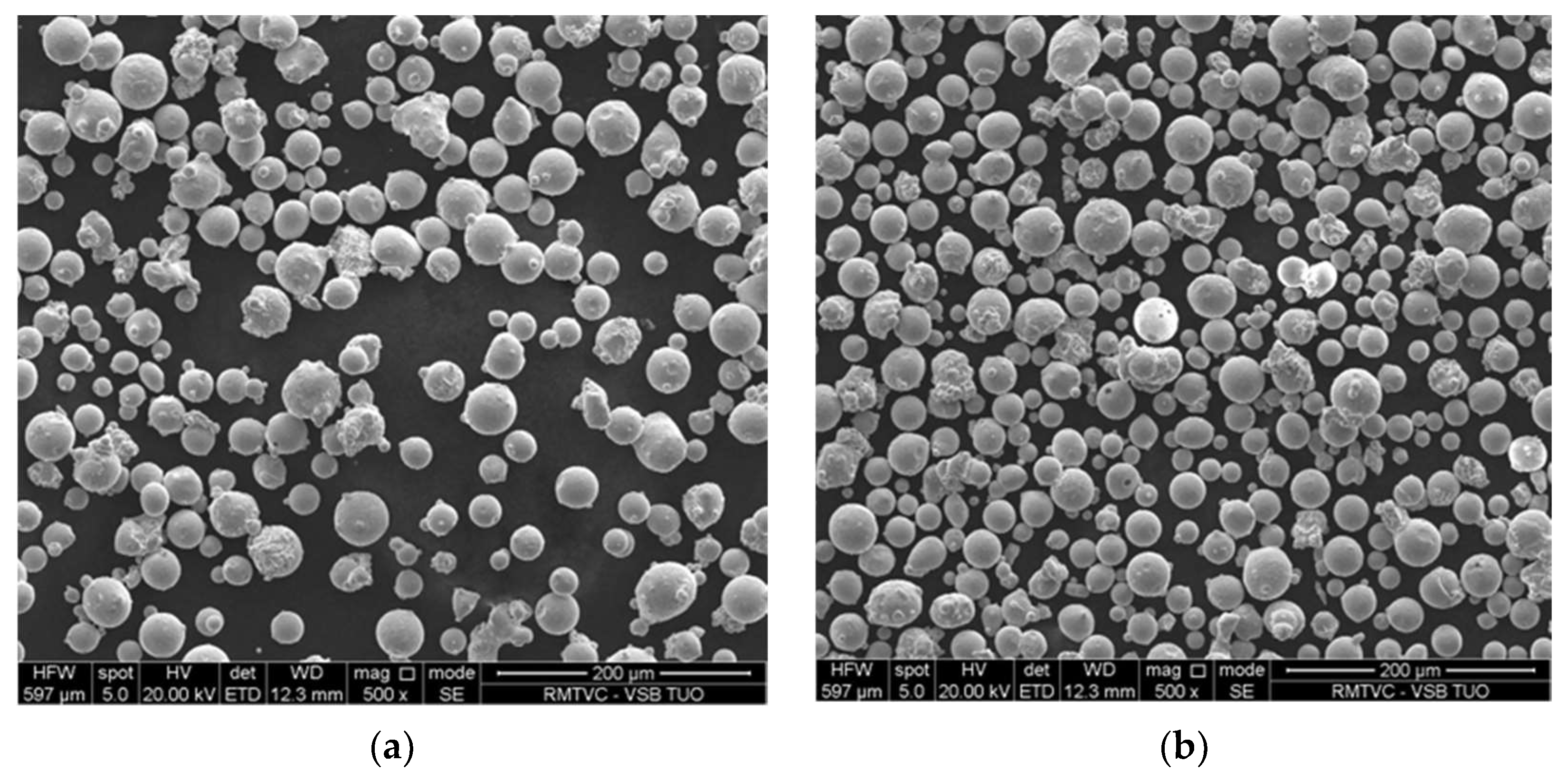
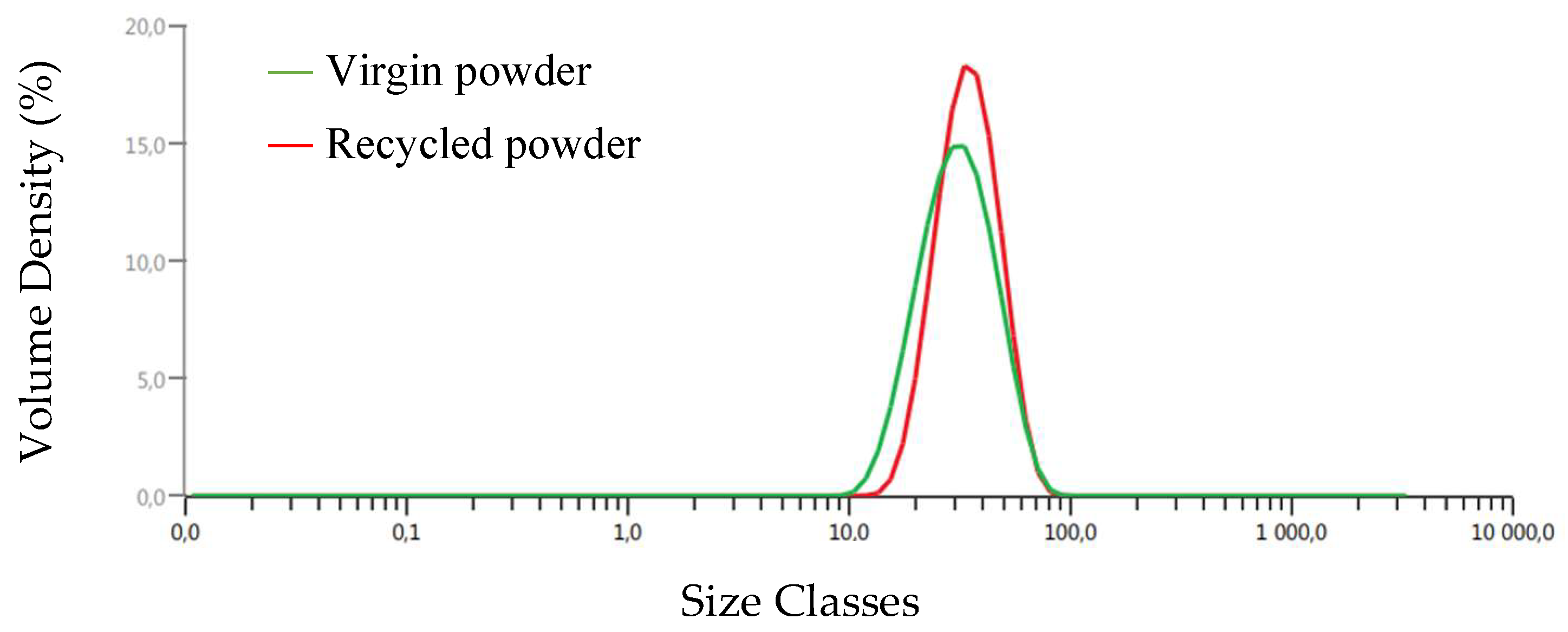
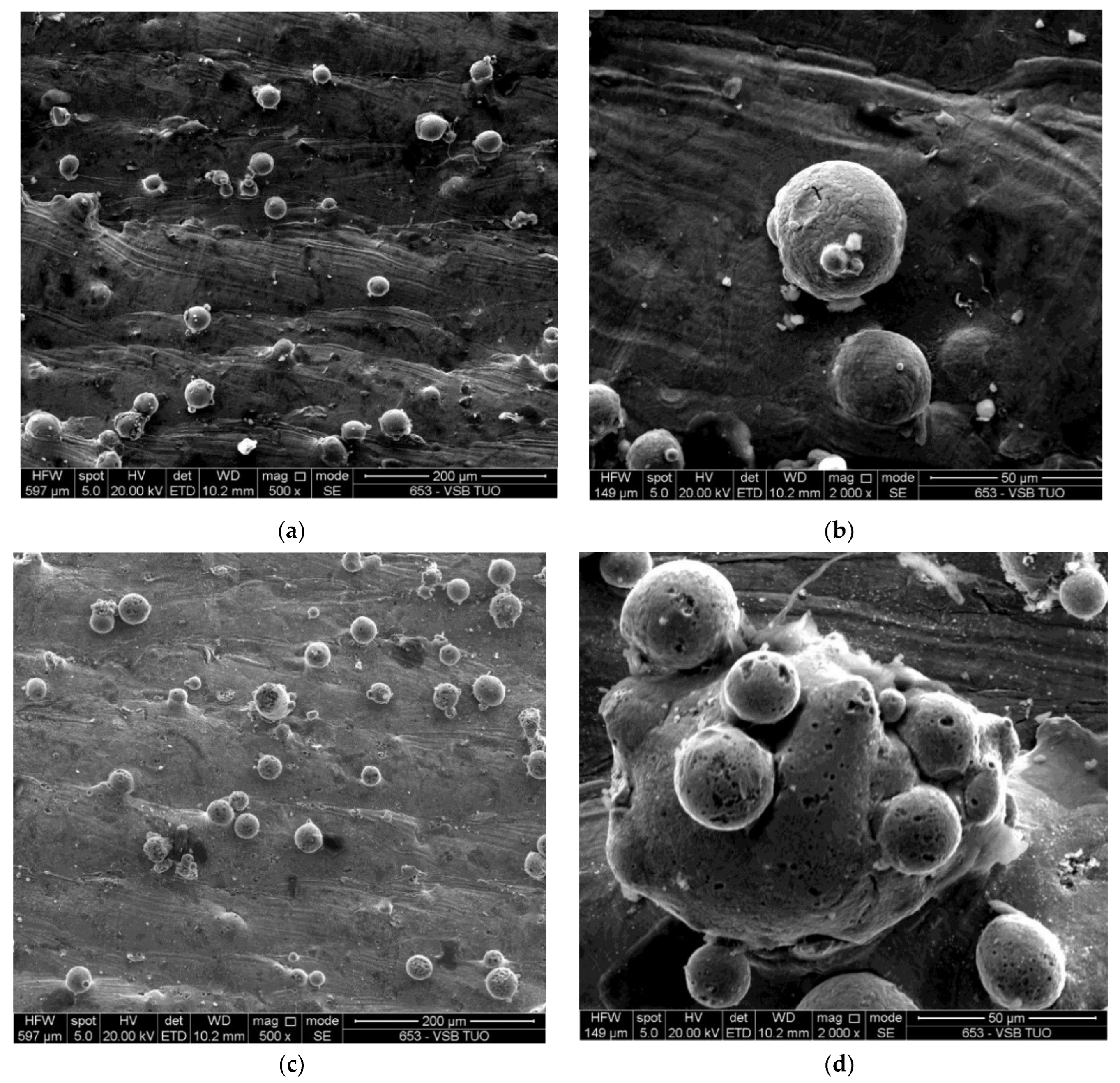
3.1. Morphology of the Powder
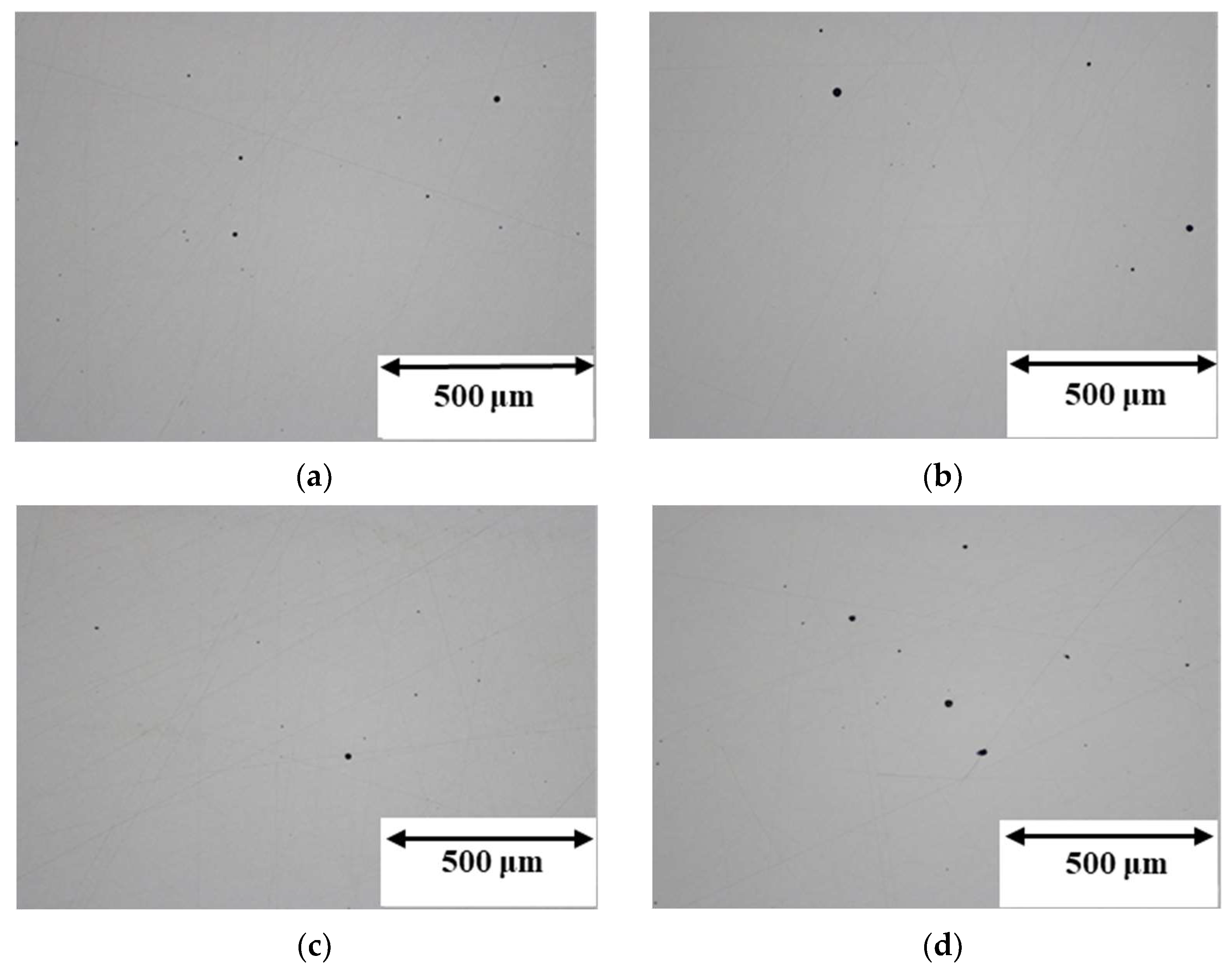
3.2. Porosity
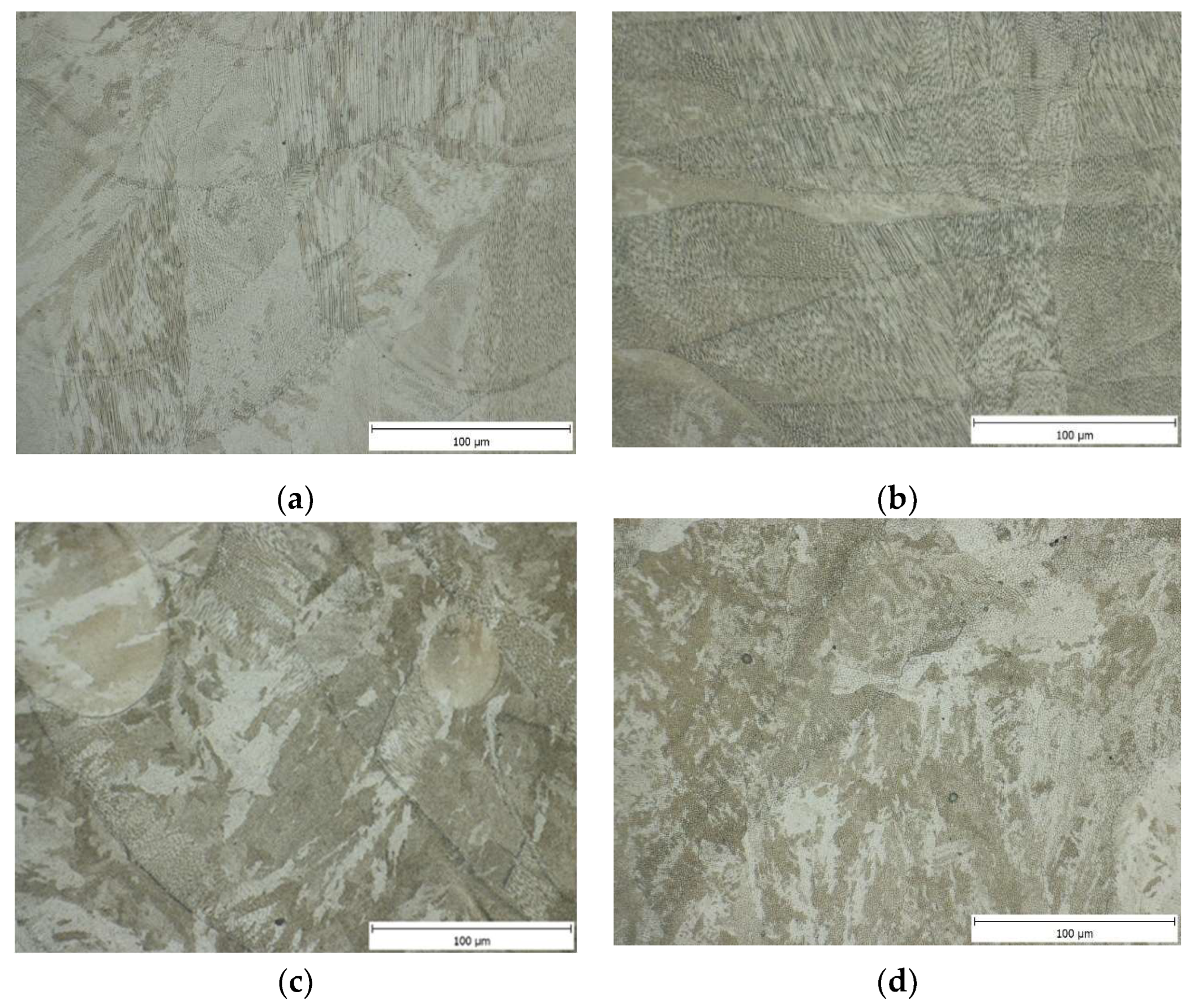
3.3. Microstructure
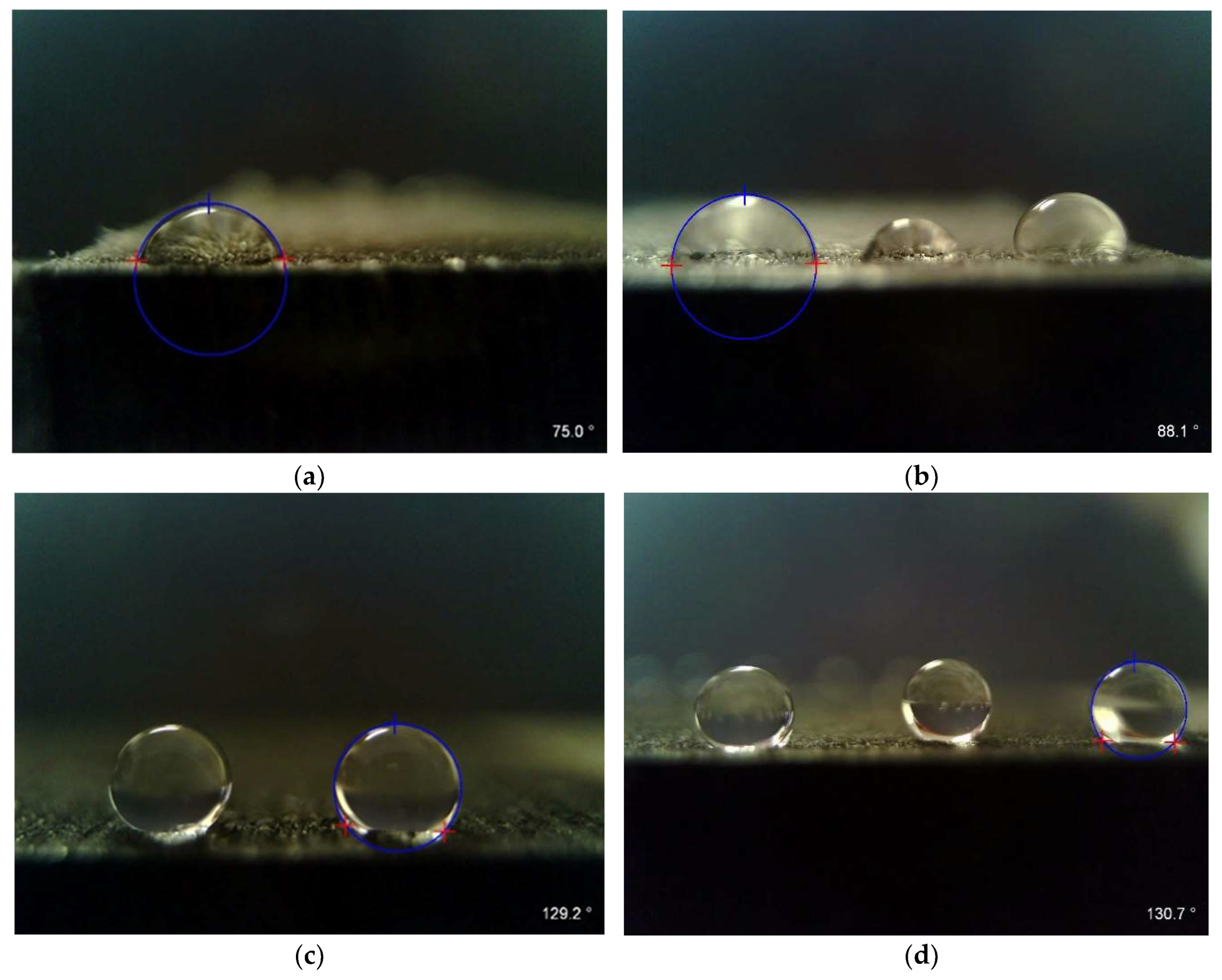
3.4. Surface Wettability and 2D Roughness Measurements
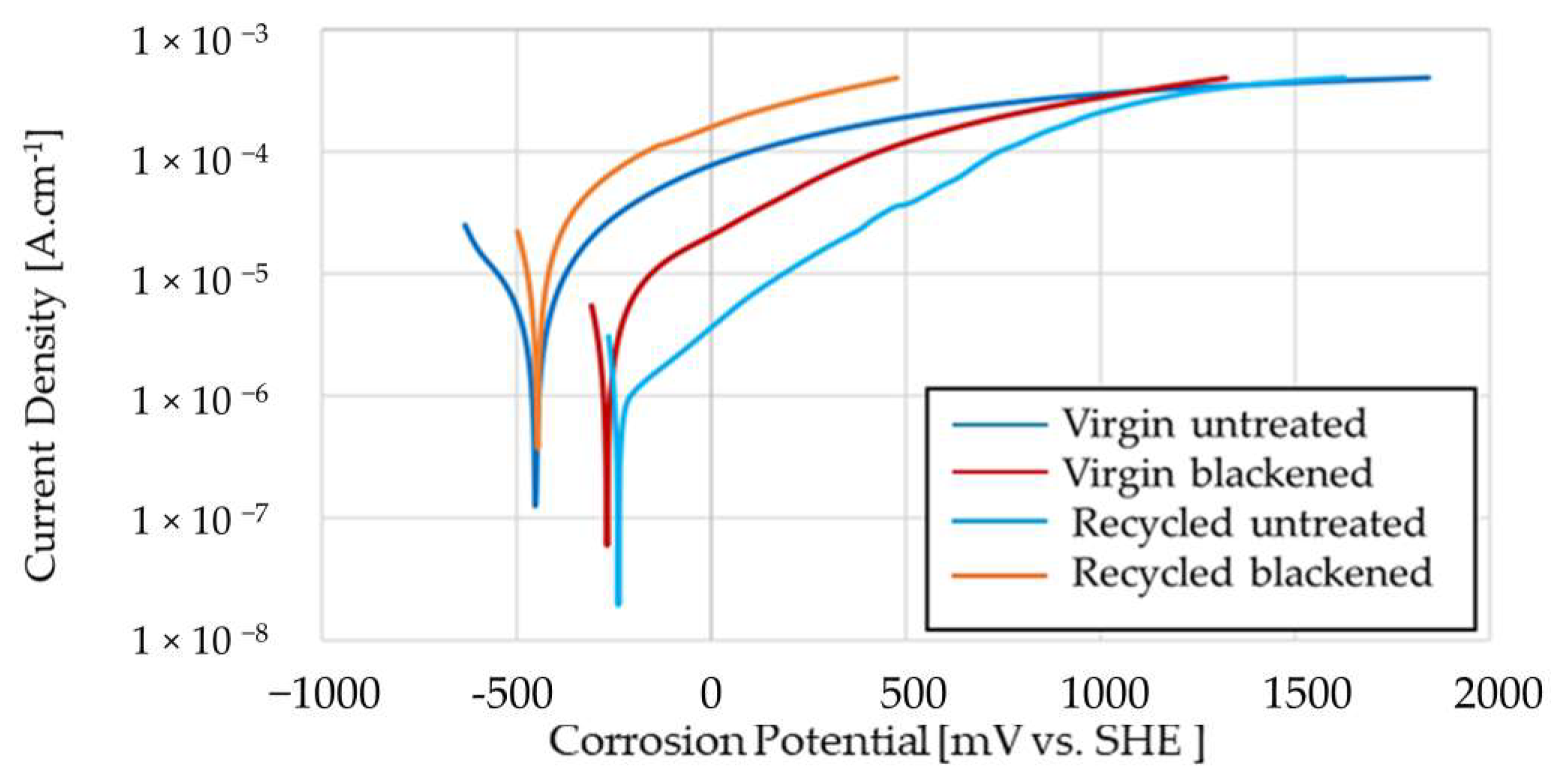
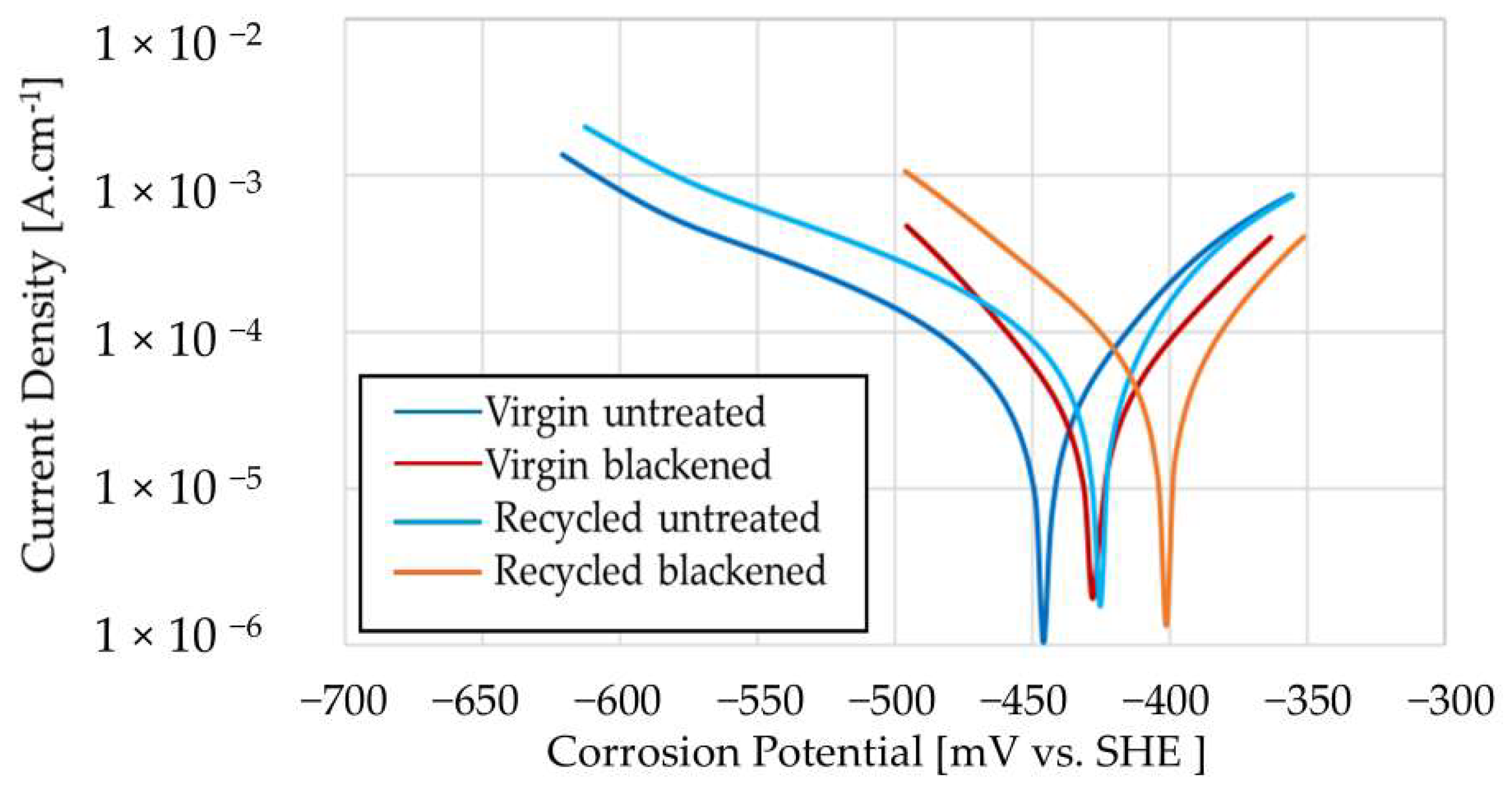
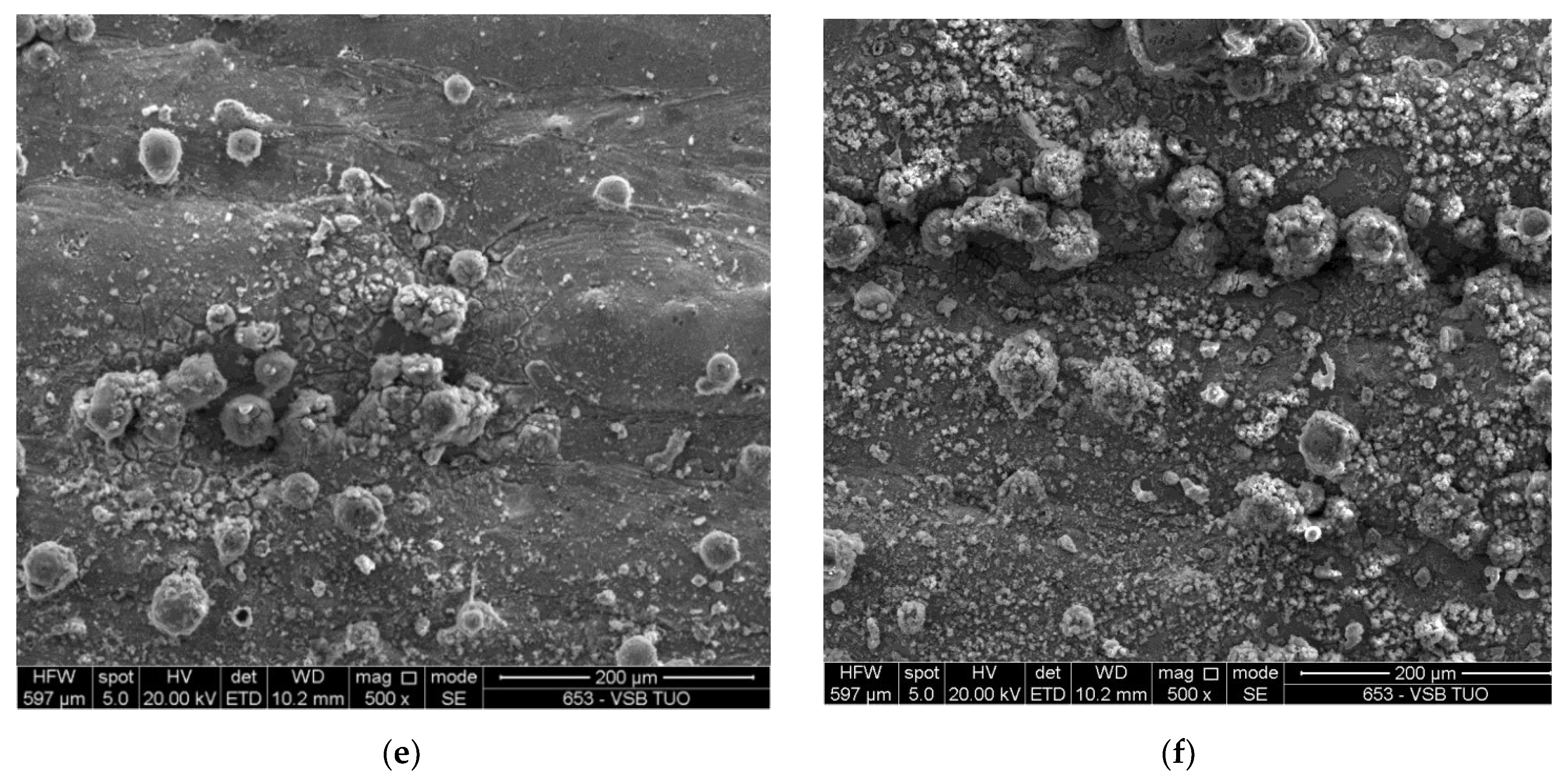
3.5. Corrosion
4. Discussion
5. Conclusions
- The chemical composition of the recycled powder is comparable to the unused powder, if any, and the printing chamber is free of all powder residues from previous prints. The same applies also to the chemical composition of the printed objects. In the case of this work, the powder was contaminated with aluminum and silicon particles. These elements subsequently influenced the chemical compositions of the prints from the recycled powder.
- The particles for both the virgin and used powders were discovered to have mostly a spherical shape, with a notable proportion of satellites sticking to the surfaces of the particles. The analysis of both powders indicates that the gas atomization process results in a non-ideal spherical form for minor amounts of particles.
- The particle size distribution (PSD) showed that the size of the particles for the virgin powders varied from 10 to 90 µm, and it ranged from 15 to 90 µm in the case of the recycled powder. This was considered to be the most suitable distribution for the given application.
- The type of powder used does not affect the resulting microstructure of the material.
- Reusing the powder does not affect the resulting porosity of the objects. The virgin powder samples exhibited a mean value of 0.07% for both parallel and perpendicular sections, while values of 0.09% and 0.10% were found in the case of the used powder.
- The measurement of the roughness on untreated and blackened surfaces of both types of samples showed that the type of powder or surface treatment by blackening does not affect the surface roughness parameters.
- The corrosion resistance showed variability and was influenced by the surface treatments. Blackened surfaces exhibited partial corrosion, independently of whether the virgin or recycled powder was used. This suggests that a recycled powder may not consistently maintain its corrosion resistance under all conditions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jin, L.; Zhai, X.; Wang, K.; Zhang, K.; Wu, D.; Nazir, A.; Jiang, J.; Liao, W.-H. Big data, machine learning, and digital twin assisted additive manufacturing: A review. Mater. Des. 2024, 244, 113086. [Google Scholar]
- Khan, N.; Riccio, A. A systematic review of design for additive manufacturing of aerospace lattice structures: Current trends and future directions. Prog. Aerosp. Sci. 2024, 149, 101021. [Google Scholar] [CrossRef]
- Dong, C.; Petrovic, M.; Davies, I.J. Applications of 3D printing in medicine: A review. Ann. 3D Print. Med. 2024, 14, 100149. [Google Scholar] [CrossRef]
- Ranjan, N.; Kumar, R.; Kumar, S.; Kumar, D.; Saxena, K.K. Innovative high-performance metal reinforced polymers composites for 3D printing applications: A review. Adv. Mater. Process. Technol. 2023, 10, 1800–1813. [Google Scholar] [CrossRef]
- Yang, J.; Li, B.; Liu, J.; Tu, Z.; Wu, X. Application of Additive Manufacturing in the Automobile Industry: A Mini Review. Processes 2024, 12, 1101. [Google Scholar] [CrossRef]
- Kolomy, S.; Maly, M.; Sedlak, J.; Zouhar, J.; Slany, M.; Hrabec, P.; Kouril, K. Machinability of extruded H13 tool steel: Effect of cutting parameters on cutting forces, surface roughness, microstructure, and residual stresses. Alex. Eng. J. 2024, 99, 394–407. [Google Scholar] [CrossRef]
- Saini, R.S.; Gurumurthy, V.; Quadri, S.A.; Bavabeedu, S.S.; Abdelaziz, K.M.; Okshah, A.; Alshadidi, A.A.F.; Yessayan, L.; Mosaddad, S.A.; Heboyan, A. The flexural strength of 3D-printed provisional restorations fabricated with different resins: A systematic review and meta-analysis. BMC Oral Health 2024, 24, 66. [Google Scholar] [CrossRef]
- Dancausa Millán, M.G.; Millán Vázquez de la Torre, M.G. 3D food printing: Technological advances, personalization and future challenges in the food industry. Int. J. Gastron. Food Sci. 2024, 37, 100963. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, D.; Wang, P.; Yan, M.; Yang, C.; Chen, Z.; Lu, J.; Lu, Z. Additive manufacturing of metals: Microstructure evolution and multistage control. J. Mater. Sci. Technol. 2022, 100, 224–236. [Google Scholar] [CrossRef]
- Kolomy, S.; Benc, M.; Harant, M.; Sedlak, J.; Jopek, M. Effect of different strain rates on mechanical behavior and structure of Inconel 718 produced by powder bed fusion. Int. J. Mech. Mater. Des. 2024, 1–16. [Google Scholar] [CrossRef]
- Aufa, A.N.; Hassan, M.Z.; Ismail, Z.; Ramlie, F.; Jamaludin, K.R.; Md Daud, M.Y.; Ren, J. Current trends in additive manufacturing of selective laser melting for biomedical implant applications. J. Mater. Res. Technol. 2024, 31, 213–243. [Google Scholar] [CrossRef]
- Bochnia, J.; Kozior, T.; Zyz, J. The Mechanical Properties of Direct Metal Laser Sintered Thin-Walled Maraging Steel (MS1) Elements. Materials 2023, 16, 4699. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.; Hajnys, J.; Mesicek, J.; Nag, A.; Ma, Q.-P.; Petru, J. Effect of Build Position on Surface Roughness of SLM Printed Inconel 718. MM Sci. J. 2024, 4, 7469. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, K.S.; Dip, T.M.; Chowdhury, M.F.M.; Debnath, S.R.; Hasan, S.M.d.M.; Sakib, M.d.S.; Saha, T.; Padhye, R.; Houshyar, S. A review on nanomaterial-based additive manufacturing: Dynamics in properties, prospects, and challenges. Prog. Addit. Manuf. 2024, 9, 1197–1224. [Google Scholar] [CrossRef]
- Karimzadeh, M.; Basvoju, D.; Vakanski, A.; Charit, I.; Xu, F.; Zhang, X. Machine Learning for Additive Manufacturing of Functionally Graded Materials. Materials 2024, 17, 3673. [Google Scholar] [CrossRef]
- Khan, I.; Barsoum, I.; Abas, M.; Al Rashid, A.; Koç, M.; Tariq, M. A review of extrusion-based additive manufacturing of multi-materials-based polymeric laminated structures. Compos. Struct. 2024, 349–350, 118490. [Google Scholar] [CrossRef]
- Kolomy, S.; Jopek, M.; Sedlak, J.; Benc, M.; Zouhar, J. Study of dynamic behaviour via Taylor anvil test and structure observation of M300 maraging steel fabricated by the selective laser melting method. J. Manuf. Process 2024, 125, 283–294. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, M.; Li, Q.; Liu, C.; Li, L.; Li, X.; Liu, Z. A Review on Energy Consumption and Efficiency of Selective Laser Melting Considering Support: Advances and Prospects. Int. J. Precis. Eng. Manuf. Green. Technol. 2024, 11, 259–276. [Google Scholar] [CrossRef]
- Zou, Z.; Liu, Z.; Yang, L.; Tang, Y.; Qiao, Y.; Lu, D. Corrosion behavior of different building planes of selective laser melting 316L stainless steel in 0.1 M HCl solution. J. Mater. Res. Technol. 2024, 28, 4738–4753. [Google Scholar] [CrossRef]
- Měsíček, J.; Čegan, T.; Ma, Q.-P.; Halama, R.; Skotnicová, K.; Hajnyš, J.; Juřica, J.; Krpec, P.; Pagáč, M. Effect of artificial aging on the strength, hardness, and residual stress of SLM AlSi10Mg parts prepared from the recycled powder. Mater. Sci. Eng. A 2022, 855, 143900. [Google Scholar] [CrossRef]
- Bai, G.; Duan, M.; Ming, Z.; Peng, Y.; Zhou, H.; Zhang, M.; Wang, W.; Wang, K. Segregation-dependent mechanical stability of reverted austenite and resultant tensile properties of Co-free maraging steel fabricated by wire-arc directed energy deposition. Mater. Sci. Eng. A 2024, 901, 146569. [Google Scholar] [CrossRef]
- Casalino, G.; Campanelli, S.L.; Contuzzi, N.; Ludovico, A.D. Experimental investigation and statistical optimisation of the selective laser melting process of a maraging steel. Opt. Laser Technol. 2015, 65, 151–158. [Google Scholar] [CrossRef]
- Mateev, V.; Sinico, M.; Gorji Ghalamestani, S.; Van Hooreweder, B. Magnetic properties of as-built and heat treated M789 and M300 maraging steels produced via laser powder bed fusion. Next Mater. 2025, 6, 100287. [Google Scholar] [CrossRef]
- Jeong, J.; No, G.W.; Bae, H.J.; Yoo, S.K.; Choi, I.-C.; Kim, H.S.; Seol, J.B.; Kim, J.G. Mechanical properties of lamellar-structured 18Ni300 maraging steel manufactured via directed energy deposition. Mater. Sci. Eng. A 2024, 892, 146031. [Google Scholar] [CrossRef]
- Brytan, Z.; Król, M.; Benedyk, M.; Pakieła, W.; Tański, T.; Dagnaw, M.J.; Snopiński, P.; Pagáč, M.; Czech, A. Microstructural and Mechanical Properties of Novel Co-Free Maraging Steel M789 Prepared by Additive Manufacturing. Materials 2022, 15, 1734. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J.; Li, S.; Wang, S.; Ren, Y.; Yang, K.; Wang, Y.-D. The influence of post-aging treatment on the microstructure and micromechanical behaviors of additively manufactured maraging steel investigated by in situ high-energy X-ray diffraction. J. Mater. Sci. Technol. 2024, 200, 1–12. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, J.; Lu, B.; Liu, Y.; Shi, W.; Liu, M.; Wang, G.; Yan, X. Powder bed fusion pure tantalum and tantalum alloys: From original materials, process, performance to applications. Opt. Laser Technol. 2024, 177, 111057. [Google Scholar] [CrossRef]
- Kolomy, S.; Sedlak, J.; Zouhar, J.; Slany, M.; Benc, M.; Dobrocky, D.; Barenyi, I.; Majerik, J. Influence of Aging Temperature on Mechanical Properties and Structure of M300 Maraging Steel Produced by Selective Laser Melting. Materials 2023, 16, 977. [Google Scholar] [CrossRef]
- Foti, P.; Mocanu, L.P.; Razavi, N.; Bellini, C.; Borrelli, R.; Cocco, V.D.; Franchitti, S.; Iacoviello, F.; Berto, F. Effect of recycling powder on the fatigue properties of AM Ti6Al4V. Procedia Struct. Integr. 2022, 42, 1436–1441. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, R.; Xiao, M.; Wei, X.; Yin, Y.; Qu, Y.; Li, H.; Liu, P.; Qiu, X.; Guo, T. A comprehensive characterization of virgin and recycled 316L powders during laser powder bed fusion. J. Mater. Res. Technol. 2022, 18, 2292–2309. [Google Scholar] [CrossRef]
- Mechali, A.; Mesicek, J.; Ma, Q.-P.; Hajnys, J.; Gautam, P.; Blaha, R.; Krisak, D.; Petru, J. Abrasive Surface Treatment of Alsi10mg Parts Made by L-PBF. MM Sci. J. 2024, 1, 7165. [Google Scholar] [CrossRef]
- Mesicek, J.; Ma, Q.-P.; Hajnys, J.; Zelinka, J.; Pagac, M.; Petru, J.; Mizera, O. Abrasive Surface Finishing on SLM 316L Parts Fabricated with Recycled Powder. Appl. Sci. 2021, 11, 2869. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.; Shen, J.; Zhang, X.; Li, S.; Wang, Z. Research progress of the metal powder reuse for powder bed fusion additive manufacturing technology. Powder Technol. 2024, 441, 119815. [Google Scholar] [CrossRef]
- Lanzutti, A.; Marin, E. The Challenges and Advances in Recycling/Re-Using Powder for Metal 3D Printing: A Comprehensive Review. Metals 2024, 14, 886. [Google Scholar] [CrossRef]
- Talebi, F.A.; Haydari, Z.; Salehi, H.; Mehrabi, M.; Gardy, J.; Bradley, M.; Bayly, A.E.; Hassanpour, A. Spreadability of powders for additive manufacturing: A critical review of metrics and characterisation methods. Particuology 2024, 93, 211–234. [Google Scholar] [CrossRef]
- Muñiz-Lerma, J.A.; Nommeots-Nomm, A.; Waters, K.E.; Brochu, M. A Comprehensive Approach to Powder Feedstock Characterization for Powder Bed Fusion Additive Manufacturing: A Case Study on AlSi7Mg. Materials 2018, 11, 2386. [Google Scholar] [CrossRef]
- Hlinka, J.; Kraus, M.; Hajnys, J.; Pagac, M.; Petrů, J.; Brytan, Z.; Tański, T. Complex Corrosion Properties of AISI 316L Steel Prepared by 3D Printing Technology for Possible Implant Applications. Materials 2020, 13, 1527. [Google Scholar] [CrossRef]
- Rońda, N.; Grzelak, K.; Polański, M.; Dworecka-Wójcik, J. The Influence of Layer Thickness on the Microstructure and Mechanical Properties of M300 Maraging Steel Additively Manufactured by LENS® Technology. Materials 2022, 15, 603. [Google Scholar] [CrossRef]
- Sun, H.; Chu, X.; Liu, Z.; Gisele, A.; Zou, Y. Selective Laser Melting of Maraging Steels Using Recycled Powders: A Comprehensive Microstructural and Mechanical Investigation. Metall. Mater. Trans. A 2021, 52, 1714–1722. [Google Scholar] [CrossRef]
- Cooke, S.; Ahmadi, K.; Willerth, S.; Herring, R. Metal additive manufacturing: Technology, metallurgy and modelling. J. Manuf. Process 2020, 57, 978–1003. [Google Scholar] [CrossRef]
- Sames, W.J.; List, F.A.; Pannala, S.; Dehoff, R.R.; Babu, S.S. The metallurgy and processing science of metal additive manufacturing. Int. Mater. Rev. 2016, 61, 315–360. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Bai, Q. Defect Formation Mechanisms in Selective Laser Melting: A Review. Chin. J. Mech. Eng. 2017, 30, 515–527. [Google Scholar] [CrossRef]
- Mechali, A.; Sadilek, M.; Mrkvica, I.; Hlinka, J.; Jahan, I.; Pagac, M.; Gautam, P.; Mesick, J.; Hajnys, J.; Petru, J. Research about M300 Maraging Steel Metallic Powder for Use in SLM 3D Printing. MM Sci. J. 2024, 4, 7569. [Google Scholar] [CrossRef]
- Mechali, A.; Hlinka, J.; Mesicek, J.; Hajnys, J.; Zelinka, J.; Krisak, D.; Cepova, L.; Blaha, R.; Cep, R.; Petru, J. Effect of Tumbling on the M300 Maraging Steel Parts Made by Selective Laser Melting. MM Sci. J. 2024, 4, 7491. [Google Scholar] [CrossRef]
- Mohd Yusuf, S.; Choo, E.; Gao, N. Comparison between Virgin and Recycled 316L SS and AlSi10Mg Powders Used for Laser Powder Bed Fusion Additive Manufacturing. Metals 2020, 10, 1625. [Google Scholar] [CrossRef]
- Cordova, L.; Campos, M.; Tinga, T. Revealing the Effects of Powder Reuse for Selective Laser Melting by Powder Characterization. JOM 2019, 71, 1062–1072. [Google Scholar] [CrossRef]
- Giganto, S.; Martínez-Pellitero, S.; Barreiro, J.; Zapico, P. Influence of 17-4 PH stainless steel powder recycling on properties of SLM additive manufactured parts. J. Mater. Res. Technol. 2022, 16, 1647–1658. [Google Scholar] [CrossRef]
- Rayan, O.; Brousseau, J.; Belzile, C.; Ouafi, A.E. Maraging steel powder recycling effect on the tensile and fatigue behavior of parts produced through the laser powder bed fusion (L-PBF) process. Int. J. Adv. Manuf. Technol. 2023, 127, 1737–1754. [Google Scholar] [CrossRef]
- Sukal, J.; Palousek, D.; Koutny, D. The Effect of Recycling Powder Steel on Porosity and Surface Roughness of SLM Parts. MM Sci. J. 2018, 12, 2643–2647. [Google Scholar] [CrossRef]
- Barile, C.; Casavola, C.; Paramsamy Kannan, V.; Renna, G. The influence of AlSi10Mg recycled powder on corrosion-resistance behaviour of additively manufactured components: Mechanical aspects and acoustic emission investigation. Arch. Civil. Mech. Eng. 2022, 22, 52. [Google Scholar] [CrossRef]






| Parameter | Value |
|---|---|
| Laser power | 250 W |
| Printing strategy | Stripe |
| Hatch spacing | 0.11 mm |
| Scan speed | 650 mm/s |
| Preheat temperature | Ambient |
| Layer thickness | 50 μm |
| Element | Al | Ni | Co | Mo | Ti | V | Si | Fe |
|---|---|---|---|---|---|---|---|---|
| Manufacturer | - | 17–19 | 7–10 | 4.50–5.20 | 0.30–1.20 | - | 0.08 | Bal |
| Zone 1 | 0.71 | 17.20 | 9.04 | 4.13 | 0.78 | 0.27 | 0.22 | 67.65 |
| Zone 2 | 0.73 | 16.86 | 9.32 | 3.85 | 0.88 | - | 0.35 | 68.01 |
| Zone 3 | 0.72 | 17.40 | 9.43 | 3.50 | 0.80 | 0.13 | 0.22 | 67.82 |
| Zone 4 | 0.83 | 12.37 | 11.33 | 1.43 | 0.76 | - | - | 73.27 |
| Element | Al | Ni | Co | Mo | Ti | V | Si | Fe |
|---|---|---|---|---|---|---|---|---|
| Manufacturer | - | 17–19 | 7–10 | 4.50–5.20 | 0.30–1.20 | - | 0.08 | Bal |
| Zone 1 | 1.39 | 17.90 | 9.12 | 4.55 | 0.80 | 0.10 | 0.24 | 65.92 |
| Zone 2 | 89.25 | - | - | - | - | - | 10.75 | - |
| Zone 3 | 89.80 | - | - | - | - | - | 10.20 | - |
| Zone 4 | 1.01 | 15.86 | 10.21 | 3.25 | - | - | - | 69.67 |
| Parameter | Virgin Powder | Used Powder |
|---|---|---|
| Laser obscuration (%) | 0.69 | 1.34 |
| Dv (10) μm | 18.4 | 22.6 |
| Dv (50) μm | 30.6 | 34.3 |
| Dv (90) μm | 50.0 | 51.1 |
| Sample | Average Porosity (%) |
|---|---|
| Virgin section perpendicular | 0.07 ± 0.02 |
| Virgin section parallel | 0.07 ± 0.03 |
| Used section perpendicular | 0.09 ± 0.04 |
| Used section parallel | 0.10 ± 0.05 |
| Sample | Contact Angle (◦) | Surface Energy (mJ·m−2) | Ra | Rz | Wetting Behavior |
|---|---|---|---|---|---|
| Virgin untreated | 76.58 ± 10.94 | 37.64 | 4.68 ± 0.51 | 21.72 ± 1.99 | Wetting |
| Virgin blackened | 83.70 ± 9.89 | 33.15 | 5.33 ± 0.16 | 24.17 ± 0.99 | Wetting |
| Recycled untreated | 127.72 ± 7.75 | 7.86 | 5.62 ± 1.42 | 25.31 ± 0.61 | No wetting |
| Recycled blackened | 131.60 ± 4.00 | 6.21 | 5.48 ± 0.47 | 24.07 ± 1.86 | No wetting |
| pH 3 | E (i = 0) | Corrosion Current | Rp | Corrosion Rate |
|---|---|---|---|---|
| mV | μA/cm2 | kOhm·cm2 | μm/Year | |
| Virgin untreated | −478.60 | 12.30 | 3.46 | 144.20 |
| Virgin blackened | −475.20 | 67.27 | 0.84 | 788.90 |
| Recycled untreated | −493.20 | 16.97 | 3.04 | 198.90 |
| Recycled blackened | −454.60 | 21.67 | 2.32 | 254.10 |
| pH 11 | E (i = 0) | Corrosion Current | Rp | Corrosion Rate |
|---|---|---|---|---|
| mV | μA/cm2 | kOhm·cm2 | μm/Year | |
| Virgin untreated | −440.50 | 4.37 | 8.60 | 51.20 |
| Virgin blackened | −259.70 | 5.02 | 9.60 | 58.90 |
| Recycled untreated | −242.10 | 0.83 | 44.50 | 9.70 |
| Recycled blackened | −436.90 | 17.26 | 2.80 | 202.40 |
| 3.5% NaCl | E (i = 0) | Corrosion Current | Rp | Corrosion Rate |
|---|---|---|---|---|
| mV | μA/cm2 | kOhm·cm2 | μm/Year | |
| Virgin untreated | −435.50 | 21.31 | 423.40 | 249.90 |
| Virgin blackened | −392.30 | 39.13 | 312.30 | 458.80 |
| Recycled untreated | −414.00 | 61.40 | 273.90 | 720.00 |
| Recycled blackened | −391.10 | 45.90 | 277.30 | 537.90 |
| Element | O | Se | AL | Si | W | Mo | Ti | V | Fe | Ni | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone 1 | 7.77 | 6.94 | 2.87 | 0.51 | 1.08 | 3.94 | 3.76 | 0.09 | 49.99 | 12.33 | 2.32 |
| Zone 2 | 11.03 | 12.63 | - | 0.37 | - | 4.13 | 0.77 | - | 49.45 | 12.33 | 2.32 |
| Element | O | Se | Na | Er | Mo | Cl | Ti | Fe | Co | Ni | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone 1 | 15.22 | - | 1.17 | 13.81 | 2.88 | 0.7 | 2.67 | 41.99 | 6.12 | 12.14 | 3.39 |
| Zone 2 | 31.81 | 1.75 | 4.1 | - | 1.55 | 0.9 | 0.89 | 45.89 | 5.55 | 7.55 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechali, A.; Hlinka, J.; Kresta, M.; Petrovic, M.; Mesicek, J.; Jahan, I.; Hajnys, J.; Petru, J. Effect of Powder Recycling on the Surface and Selected Technological Properties of M300 Maraging Steel Produced via the SLM Method. J. Manuf. Mater. Process. 2024, 8, 267. https://doi.org/10.3390/jmmp8060267
Mechali A, Hlinka J, Kresta M, Petrovic M, Mesicek J, Jahan I, Hajnys J, Petru J. Effect of Powder Recycling on the Surface and Selected Technological Properties of M300 Maraging Steel Produced via the SLM Method. Journal of Manufacturing and Materials Processing. 2024; 8(6):267. https://doi.org/10.3390/jmmp8060267
Chicago/Turabian StyleMechali, Abdesselam, Josef Hlinka, Michal Kresta, Marin Petrovic, Jakub Mesicek, Ibrahim Jahan, Jiri Hajnys, and Jana Petru. 2024. "Effect of Powder Recycling on the Surface and Selected Technological Properties of M300 Maraging Steel Produced via the SLM Method" Journal of Manufacturing and Materials Processing 8, no. 6: 267. https://doi.org/10.3390/jmmp8060267
APA StyleMechali, A., Hlinka, J., Kresta, M., Petrovic, M., Mesicek, J., Jahan, I., Hajnys, J., & Petru, J. (2024). Effect of Powder Recycling on the Surface and Selected Technological Properties of M300 Maraging Steel Produced via the SLM Method. Journal of Manufacturing and Materials Processing, 8(6), 267. https://doi.org/10.3390/jmmp8060267










