The Mechanical Properties of a Transient Liquid Phase Diffusion Bonded SSM-ADC12 Aluminum Alloy with a ZnAl4Cu3 Zinc Alloy Interlayer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
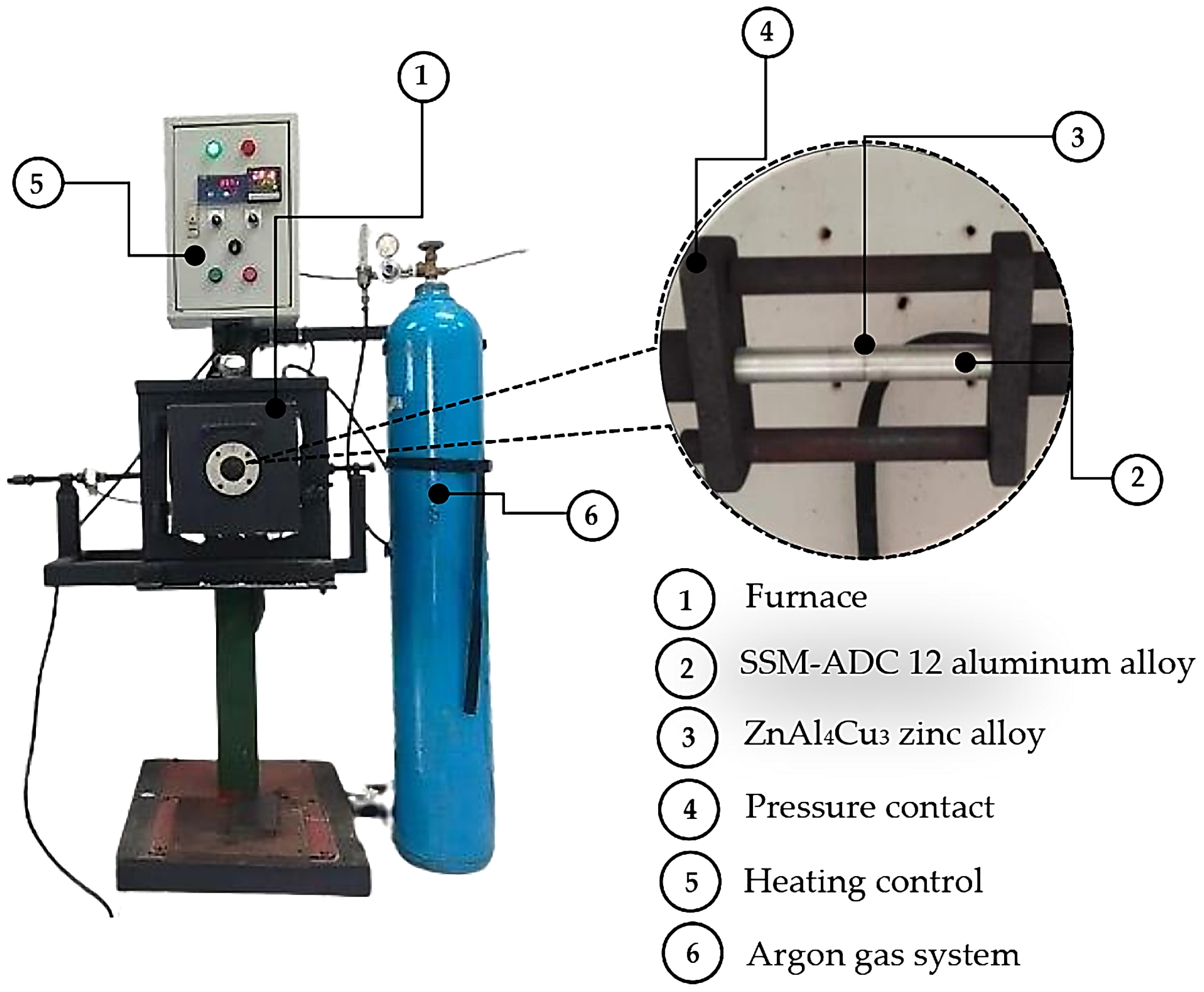
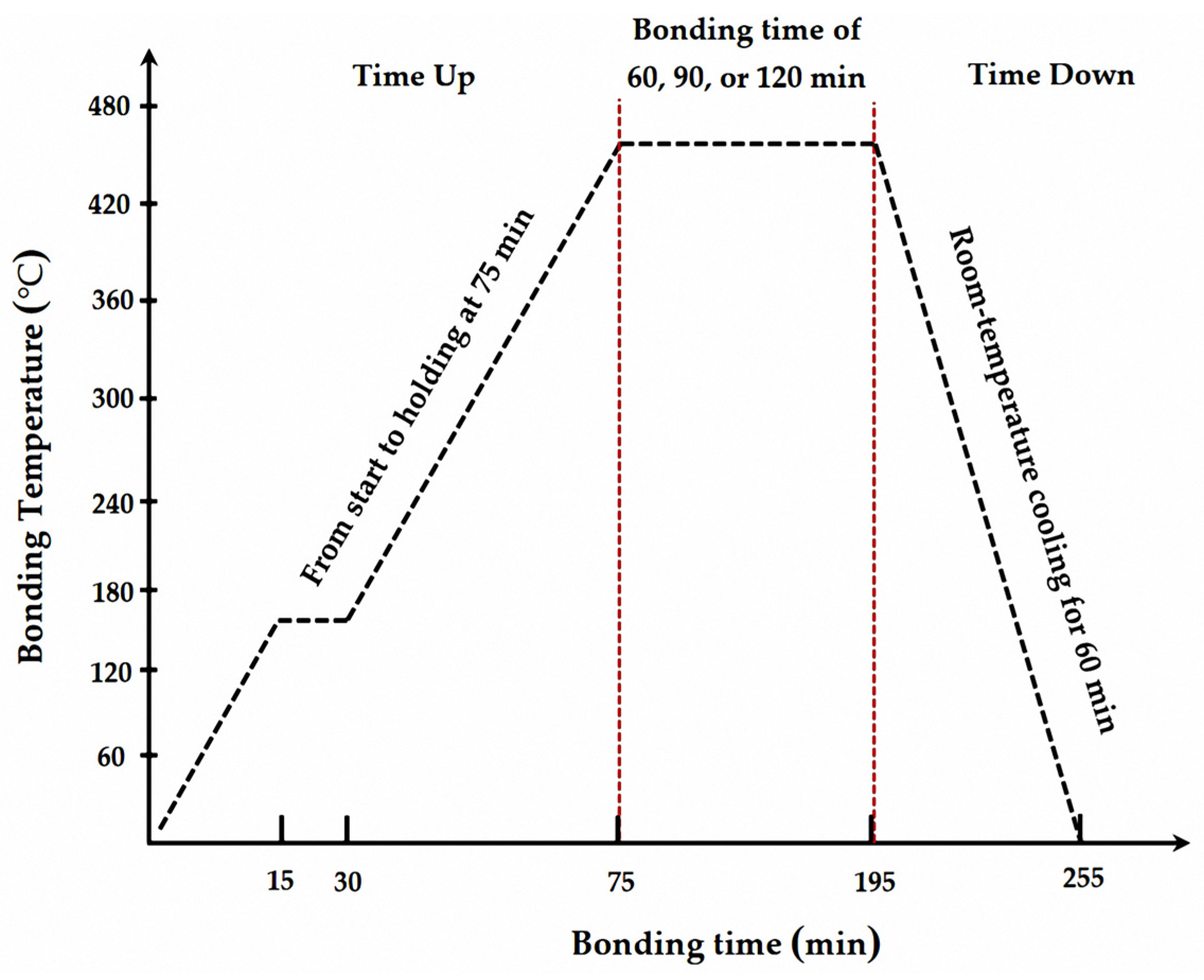
2.2. TLPDB Process
2.3. Fatigue Testing
2.4. Metallurgy and Mechanical Testing
3. Results and Discussion
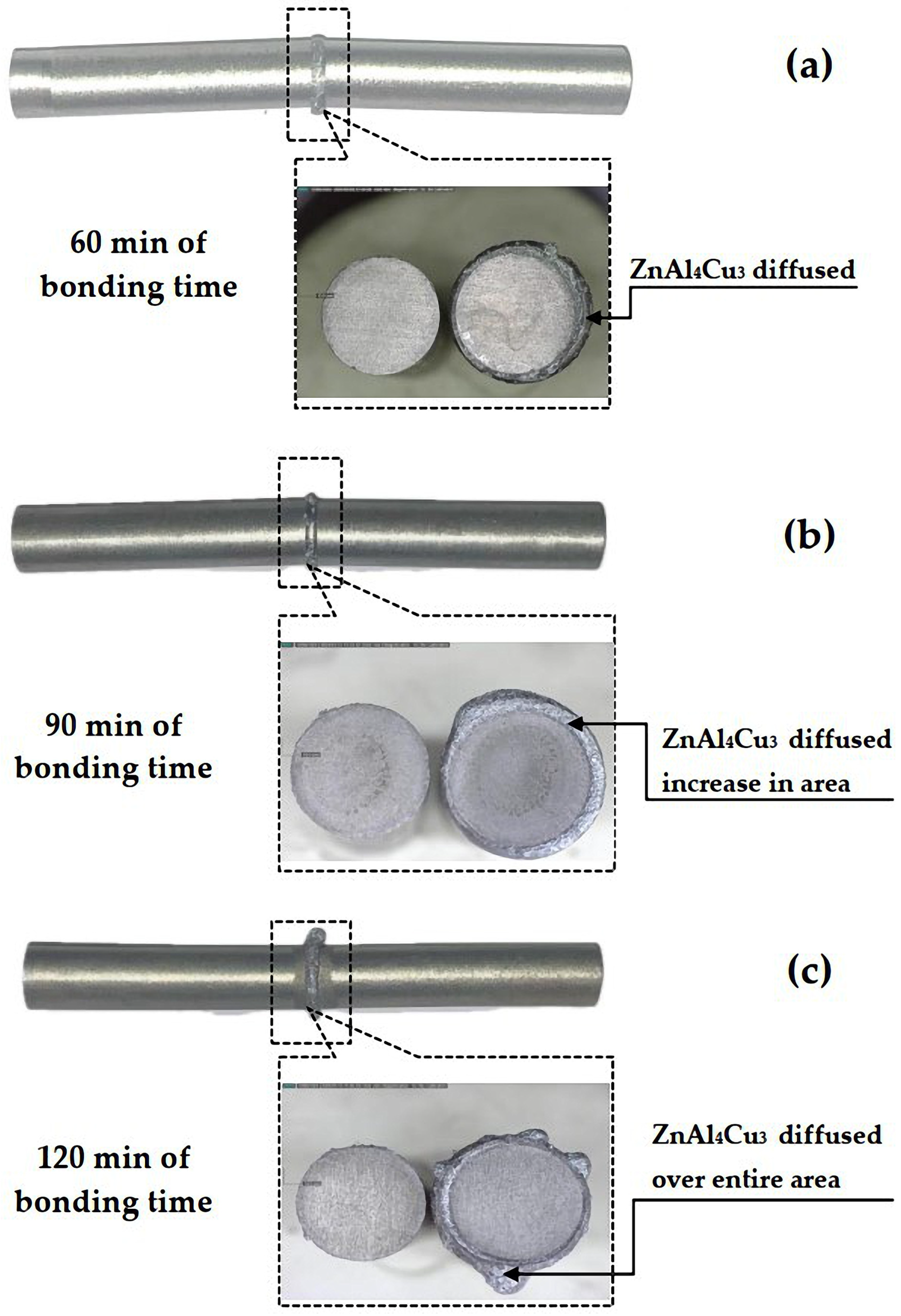
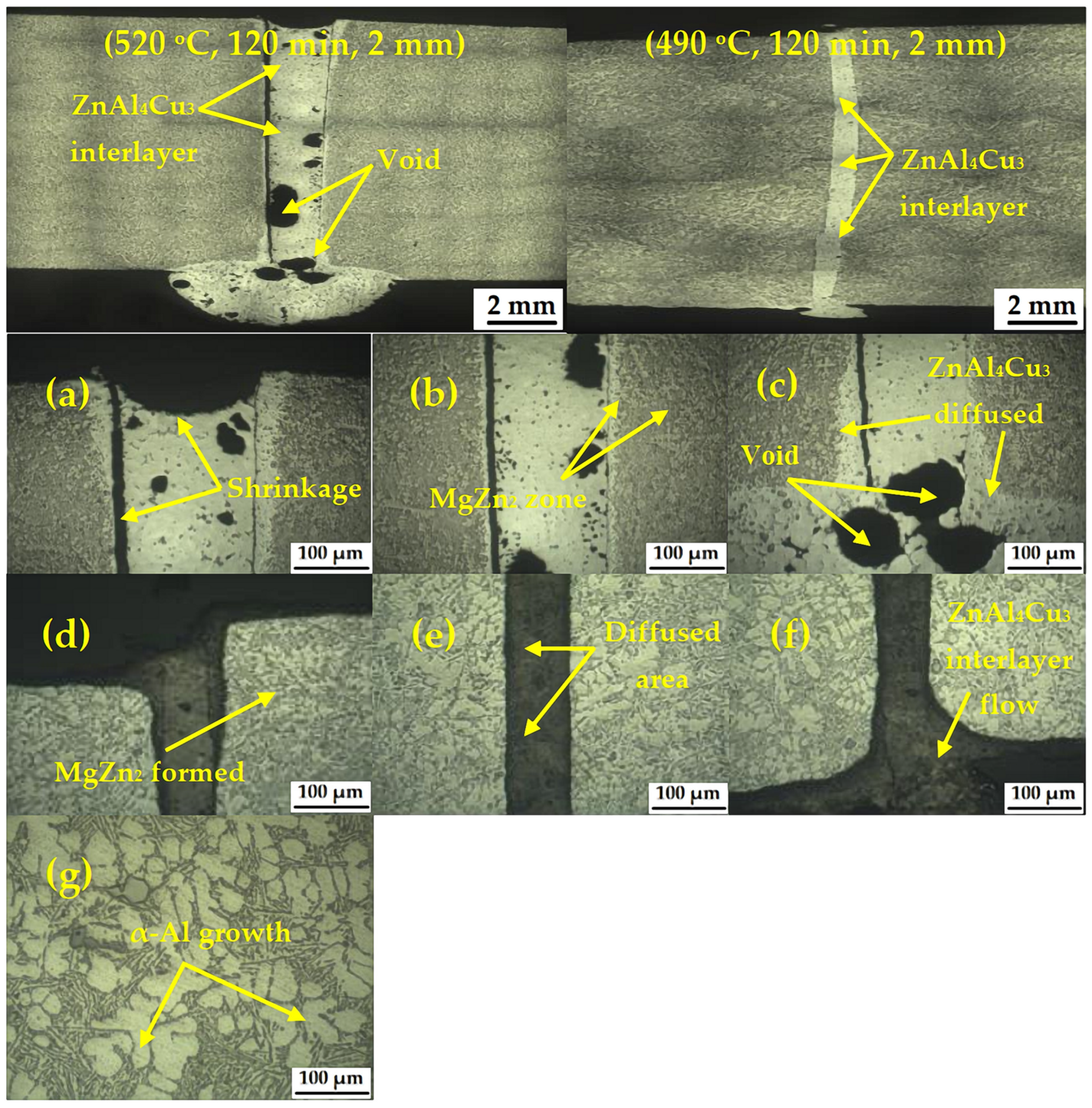
3.1. Characteristics of the TLPDB Samples
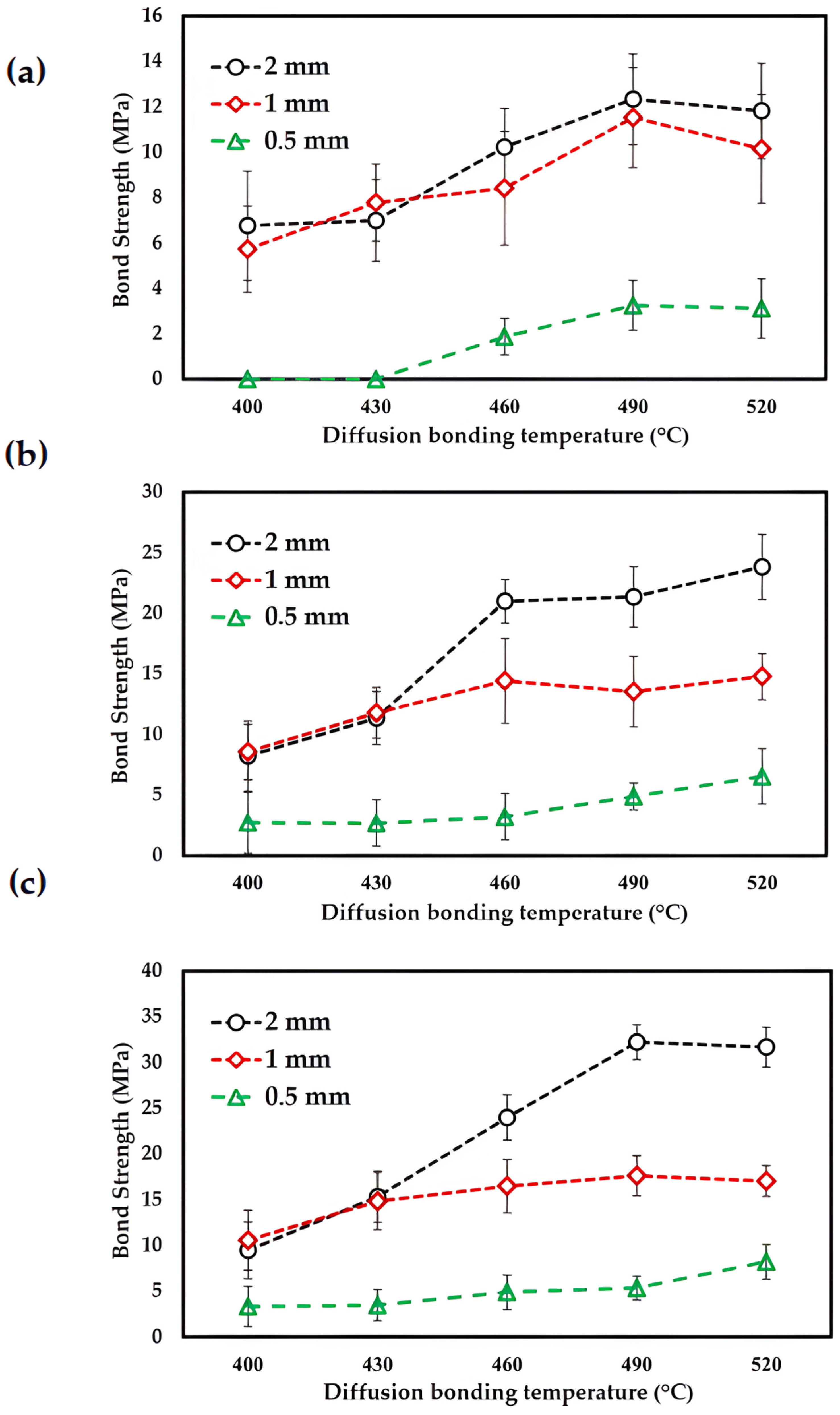
3.2. Bonding Strength Analysis
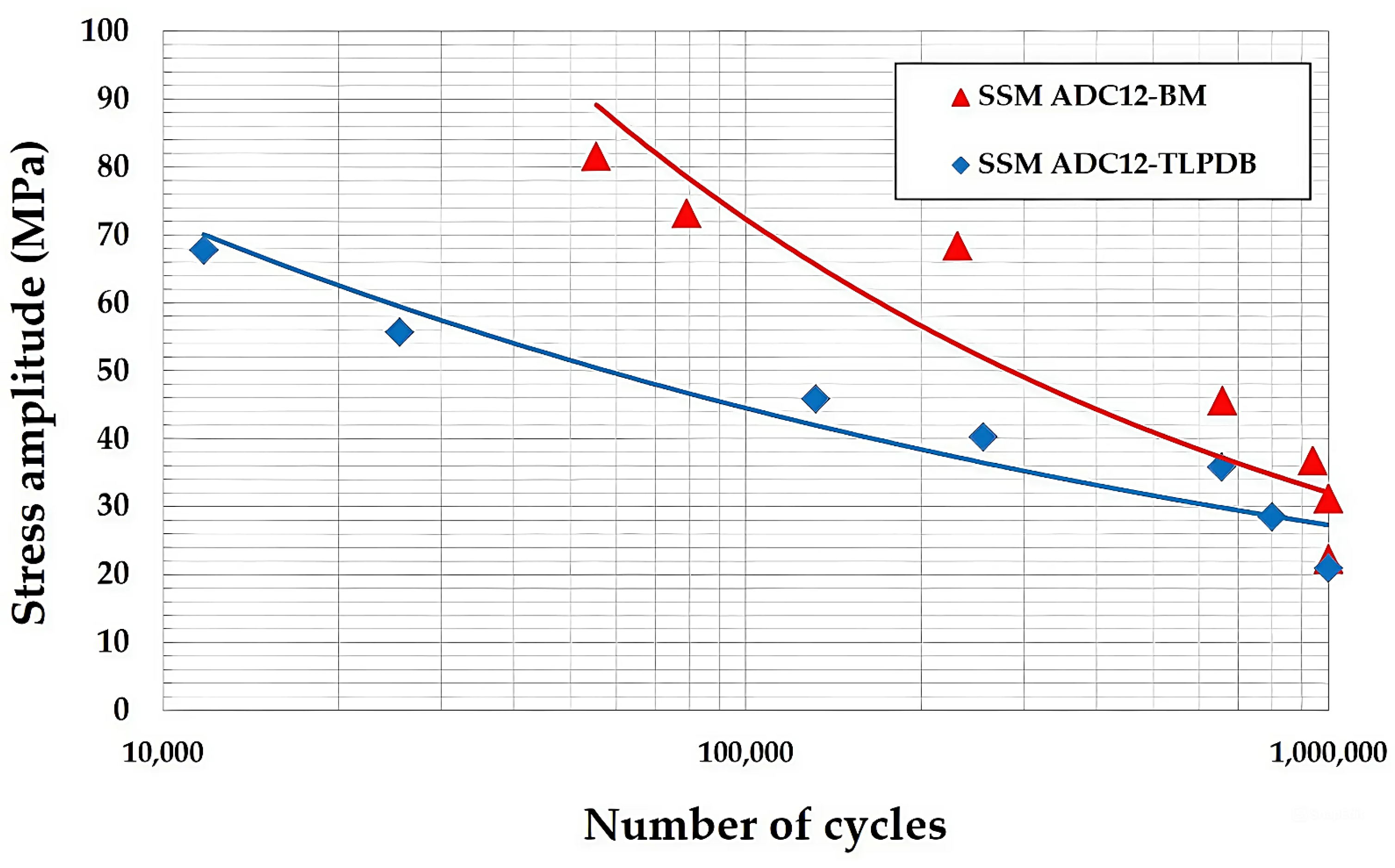
3.3. Fatigue Analysis
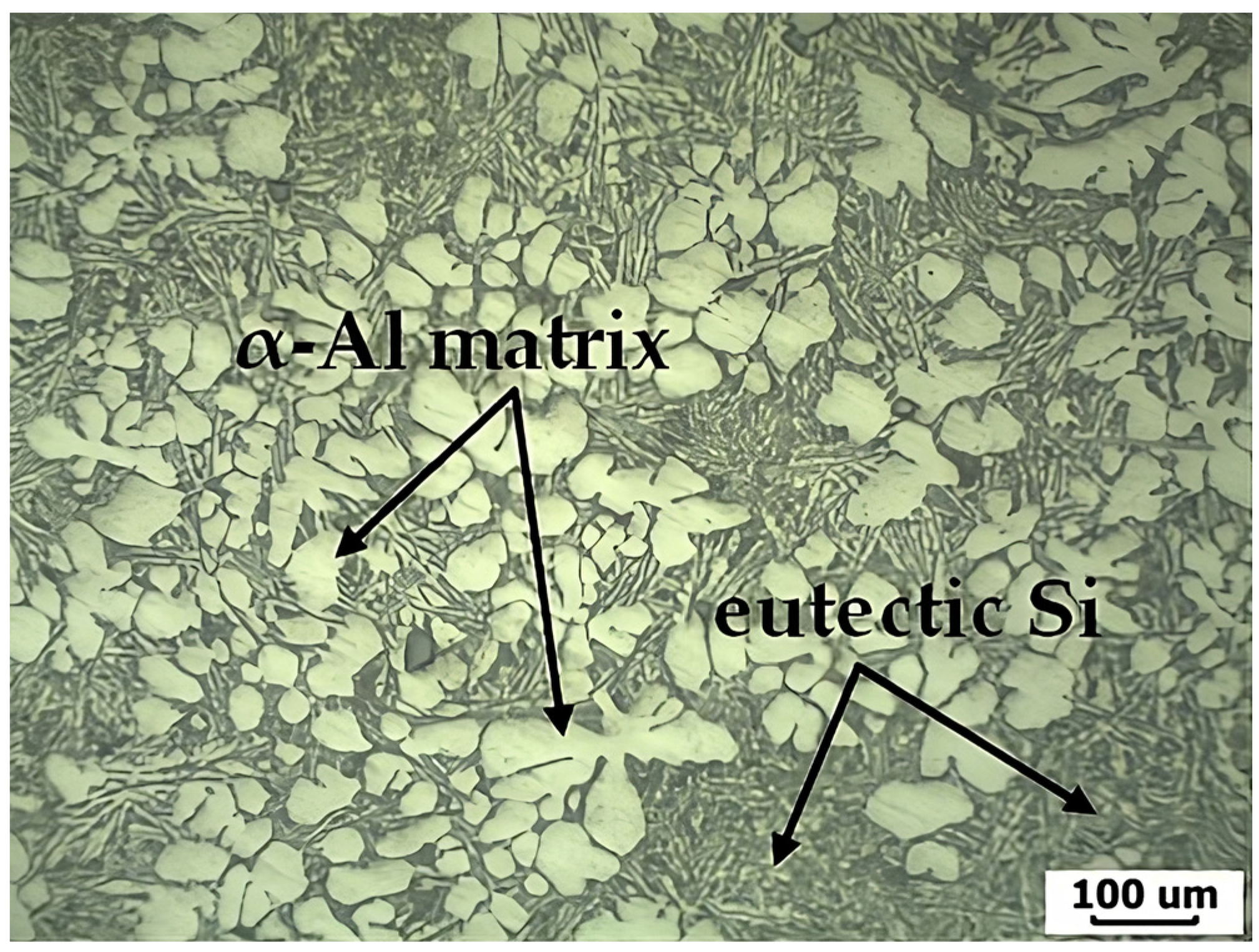
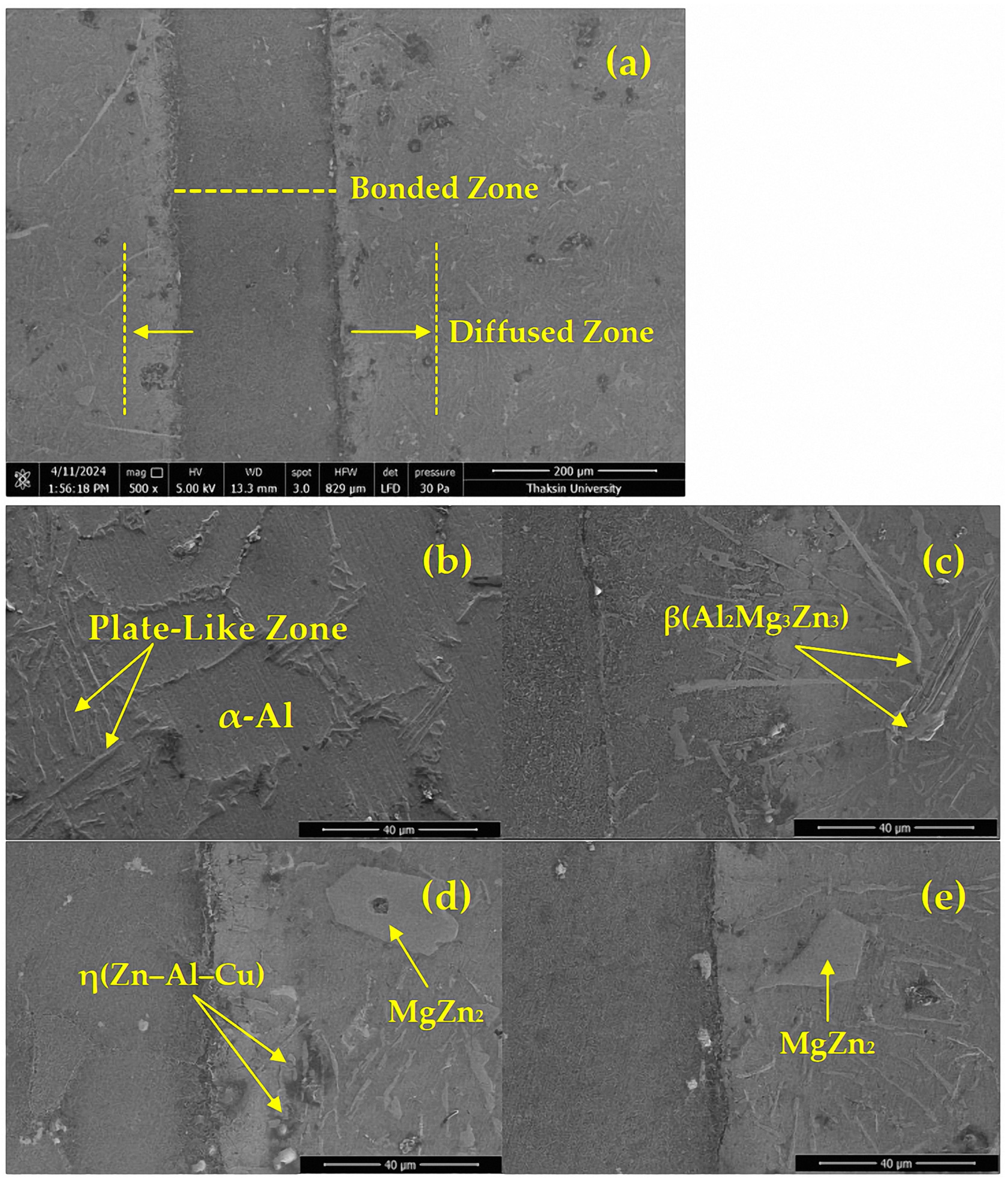
3.4. Microstructure of the TLPDB Material of the SSM-ADC12 Aluminum Alloy
3.5. Vickers Microhardness
4. Conclusions
- Different parameters directly affect the mechanical properties. An average maximum bonding strength of 32.21 MPa was obtained at a bonding temperature of 490 °C, a bonding time of 120 min, and a ZnAl4Cu3 zinc alloy interlayer material thickness of 2.0 mm.
- After the TLPDB material was evaluated, crack, void, and deformation defects could be detected in this experiment.
- The fatigue tests for the TLPDB material of the SSM-ADC12 aluminum alloy with ZnAl4Cu3 zinc alloy interlayer materials revealed amplitude fatigue similar to the base material (BMs), and the endurance limits of the TLPDB material and BMs were 20.29 and 31.12 MPa, respectively.
- The maximum Vickers microhardness value obtained with 120 min of bonding, a bonding temperature of 520 °C, and a ZnAl4Cu3 zinc alloy interlayer that was 2.0 mm thick was 83.20 HV. Meanwhile, η(Zn–Al–Cu), β(Al2Mg3Zn3), and MgZn2 intermetallic compounds (IMCs) led to increases in hardness.
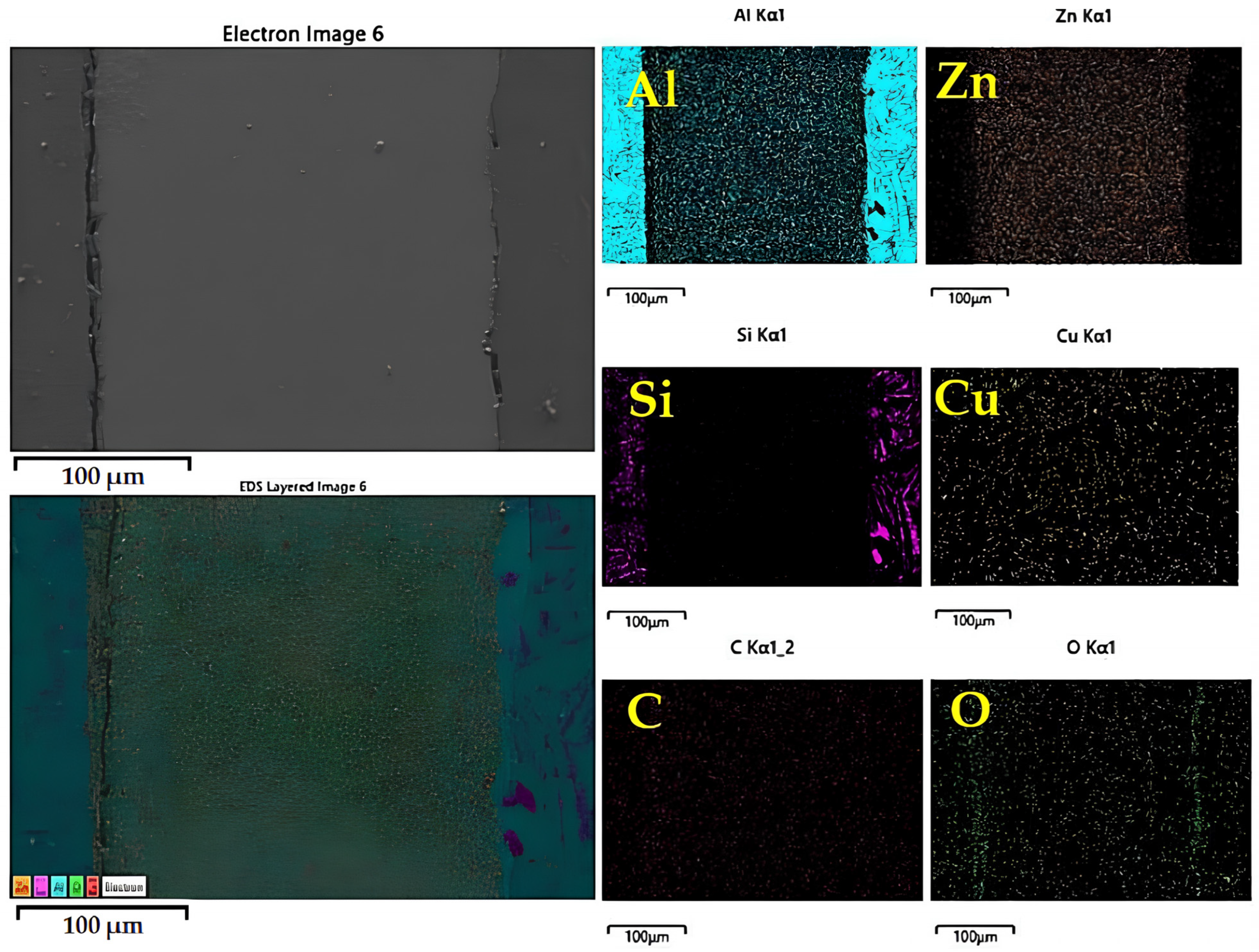
- A MgZn2 phase formed in the microstructure and precipitation at and near the bonded line led to improved mechanical properties. A transformation of the α-primary matrix with β-eutectic Si IMCs to form an η(Zn–Al–Cu) phase was also observed at the bonded line. Evaluation using optical microscopy showed that the precipitation changed from globular to coarse structures with larger grains, whilst SEM evaluation showed that β-eutectic Si IMCs diffused into β(Al2Mg3Zn3) and MgZn2 IMCs with an average width of 19–29 µm and an average length of 12–27 µm. Finally, EDX mapping at the joint showed that Mg, Si, and Al were able to move freely.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, H.; Yoon, J.; Yoo, J. Manufacturing of Aerospace Parts with Diffusion Bonding Technology. Appl. Mech. Mater. 2011, 87, 182–185. [Google Scholar] [CrossRef]
- Pola, A.; Tocci, M.; Kapranos, P. Microstructure and Properties of Semi-Solid Aluminum Alloys: A Literature Review. Metals 2018, 8, 181. [Google Scholar] [CrossRef]
- Vinith, S.; Uthayakumar, A.; Rajan, S. Fluidity of ADC12 alloy based on theoretical and computational fluid dynamics. Mech. Eng. 2015, 4, 996–999. [Google Scholar]
- JIS H 5302:Japan; Japanese Industrial Standard, Aluminum Alloys Die Castings (ADC 12). Japanese Industrial Standard: Tokyo, Japan, 2000; p. 10.
- Wannasin, J.; Janudom, S.; Rattanochaikul, T.; Canyook, R.; Burapa, R.; Chucheep, T.; Thanabumrungkul, S. Research and development of gas induced semi-solid process for industrial applications. Trans. Nonferr. Met. Soc. China 2010, 20, 1010–1015. [Google Scholar] [CrossRef]
- Janudom, S.; Rattanochaikul, T.; Burapa, R.; Wisutmethangoon, S.; Wannasin, J. Feasibility of semi-solid die casting of ADC12 aluminum alloy. Trans. Nonferr. Met. Soc. China 2010, 20, 1756–1762. [Google Scholar] [CrossRef]
- Gautam, S.K.; Singh, B.K. Investigation on the effects of isothermal holding temperature and time on the coarsening mechanism and rheological properties of ADC12 Al semi-solid slurry. Mater Chem Phys. 2024, 314, 128813. [Google Scholar] [CrossRef]
- Simões, S. Diffusion Bonding and Brazing of Advanced Materials. Metals 2018, 8, 959. [Google Scholar] [CrossRef]
- Zhang, L.X.; Chang, Q.; Sun, Z.; Xue, Q.; Feng, J.C. Effects of boron and silicon on microstructural evolution and mechanical properties of transient liquid phase bonded GH3039/IC10 joints. J. Manuf. Process. 2019, 38, 167–173. [Google Scholar] [CrossRef]
- Jiao, Y.J.; Sheng, G.M.; Zhang, Y.T.; Xu, C.; Yuan, X.J. Transient liquid phase bonding of Inconel 625 with Mar-M247 superalloy using Ni-Cr-B interlayer: Microstructure and mechanical properties. Mater. Sci. Eng. A 2022, 831, 142204. [Google Scholar] [CrossRef]
- Yuan, L.; Ren, J.; Xiong, J.T.; Zhao, W.; Shi, J.M.; Li, J.L. Transient liquid phase bonding of Ni3Al based superalloy using Mn-Ni-Cr filler. J. Mater. Res. Technol. 2021, 11, 1583–1593. [Google Scholar] [CrossRef]
- Vatnalmath, M.; Auradi, V.; Murthy, B.V.; Nagaral, M.; Pandian, A.A.; Islam, S.; Khan, M.S.; Anjinappa, C.; Razak, A. Impact of Bonding Temperature on Microstructure, Mechanical, and Fracture Behaviors of TLP Bonded Joints of Al2219 with a Cu Interlayer. ACS Omega 2023, 8, 26332–26339. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.I.; Roven, H.J.; Khan, T.I.; Iveland, T. Transient Liquid Phase Bonding of Al-6063 to Steel Alloy UNSS32304. J. Manuf. Mater. Process. 2018, 2, 58. [Google Scholar]
- Dong, J.H.; Liu, H.; Ji, S.D.; Yan, D.J.; Zhao, H.X. Diffusion Bonding of Al-Mg-Si Alloy and 301L Stainless Steel by Friction Stir Lap Welding Using a Zn Interlaye. Materials 2022, 15, 696. [Google Scholar] [CrossRef]
- Muhamed, M.N.; Omar, M.Z.; Abdullah, S.; Sajuri, Z.; Zamri, W.F.H.W. Al-Si-Zn Behaviouron Interface of AR500 Steel and AA7075 Aluminium Alloy BrazedJoint. J. Phys. Conf. Ser. 2020, 1532, 012006. [Google Scholar] [CrossRef]
- Zhang, H.; Niu, T.; Yuan, M. Influence of High Magnetic Field-Thermal Coupling Processing on Diffusion Bonding Properties and Element Diffusion of 1420 Al-Li Alloy. Crystals 2022, 12, 1508. [Google Scholar] [CrossRef]
- Canyook, R.; Wannasin, J.; Wisutmethangoon, S.; Flemings, M.C. Characterization of the microstructure evolution of a semi-solid metal slurry during the early stages. Acta Mater. 2012, 60, 3501–3510. [Google Scholar] [CrossRef]
- Chainarong, S.; Pitakaso, R.; Sirirak, W.; Srichok, T.; Khonjun, S.; Sethanan, K.; Sangthean, T. Multi-Objective Variable Neighborhood Strategy Adaptive Search for Tuning Optimal Parameters of SSM-ADC12 Aluminum Friction Stir Welding. J. Manuf. Mater. Process. 2021, 5, 123. [Google Scholar] [CrossRef]
- Seah, K.H.W.; Sharma, S.C.; Girish, B.M. Mechanical properties of cast ZA-27 graphite particulate composites. Mater. Des. 1996, 16, 271–275. [Google Scholar] [CrossRef]
- Kittima, S.; Yoshiharu, M.; Yukio, M.; Nobushiro, S. Fatigue Strength Estimation Based on Local Mechanical Properties for Aluminum Alloy FSW Joints. Metals 2017, 10, 186. [Google Scholar]
- ASTM E466-15; Standard Practice for Conducting Force Controlled Constant Amplitude Axial Fatigue Tests of Metallic Materials. Designation. ASTM International Standards: West Conshohocken, PA, USA, 2015.
- ASTM E8; Standard Test Methods for Tension Testing of Metallic Materials. Designation. ASTM International Standards: West Conshohocken, PA, USA, 2015.
- Malekan, A.; Farvizi, M.; Mirsalehi, S.E.; Saito, N.; Nakashima, K. Holding Time Influence on Creep Behavior of Transient Liquid Phase Bonded Joints of Hastelloy X. Mater. Sci. Eng. A 2020, 772, 138694. [Google Scholar] [CrossRef]
- Al Hazaa, A.; Haneklaus, N.; Almutairi, Z. Impulse pressure-assisted diffusion bonding (IPADB): Review and outlook. Metals 2021, 11, 323. [Google Scholar] [CrossRef]
- Venugopal, S.; Seeman, M.; Seetharaman, R.; Jayaseelan, V. The effect of bonding process parameters on the microstructure and mechanical properties of AA5083 diffusion-bonded joints. Int. J. Ligh. Mater. Manuf. 2022, 5, 555–563. [Google Scholar] [CrossRef]
- Yu, W.; Zhao, H.; Huang, Z.; Chen, X.; Aman, Y.; Li, S.; Zhai, H.; Guo, Z.; Xiong, S. Microstructure evolution and bonding mechanism of Ti2SnC-Ti6Al4V joint by using Cu pure foil interlayer. Mater. Charact. 2017, 127, 53–55. [Google Scholar] [CrossRef]
- Silva, M.; Ramos, A.; Vieira, M.; Simões, S. Diffusion Bonding of Ti6Al4V to Al2O3 Using Ni/Ti Reactive Multilayers. Metals 2021, 11, 655. [Google Scholar] [CrossRef]
- Ben-Haroush, M.; Mittelman, B.; Priel, R.S.E. The Influence of Time, Atmosphere and Surface Roughness on the Interface Strength and Microstructure of AA6061–AA1050 Diffusion Bonded Components. Materials 2023, 16, 769. [Google Scholar] [CrossRef] [PubMed]
- Meengam, C.; Dunyakul, Y.; Maunkhaw, D.; Chainarong, S. Transient Liquid Phase Bonding of Semi-Solid Metal 7075 Aluminum Alloy Using ZA27 Zinc Alloy Interlayer. Metals 2018, 8, 637. [Google Scholar] [CrossRef]
- Alhazaa, A.; Khan, T.; ul Haq, I. Transient liquid phase (TLP) bonding of Al7075 to Ti–6Al–4V alloy. Mater. Charact. 2010, 61, 312–317. [Google Scholar] [CrossRef]
- Wen, Z.; Li, Q.; Liu, F.; Dong, Y.; Zhang, Y.; Hu, W.; Li, L.; Gao, H. Transient Liquid Phase Diffusion Bonding of Ni3Al Superalloy with Low-Boron Nickel-Base Powder Interlayer. Materials 2023, 16, 2554. [Google Scholar] [CrossRef]
- Kejanli, H.; Taşkin, M.; Kolukisa, S.; Topuz, P. Transient liquid phase (TPL) diffusion bonding of Ti45Ni49Cu6 P/M components using Cu interlayer. Int. J. Adv. Manuf. Technol. 2009, 44, 695–699. [Google Scholar] [CrossRef]
- Meengam, C.; Dunyakul, Y.; Chainarong, S.; Maunkhaw, D. The Influence of Diffuse Element in Solid-State on Dissimilar Joint between Semi-Solid Cast 7075 with 6061 Al Alloy by Diffusion Welding. Solid State Phenom. 2022, 330, 71–76. [Google Scholar] [CrossRef]
- Maity, J.; Pal, T.K.; Maiti, R. Transient liquid phase diffusion bonding of 6061-15 wt % SiCp in argon environment. J. Mater. Process. Technol. 2009, 209, 3568–3580. [Google Scholar] [CrossRef]
- Seyyed Afghahi, S.S.; Ekrami, A.; Farahany, S.; Jahangiri, A. Fatigue properties of temperature gradient transient liquid phase diffusion bonded Al7075-T6 alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 1073–1079. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Liu, S.; Xiao, S.; Sun, Y.; Wang, X. Effect of unbonded areas around hole on the fatigue crack growth life of diffusion bonded titanium alloy laminates. Eng. Fract. Mech. 2016, 1, 176–188. [Google Scholar] [CrossRef]
- He, X.; Liu, W.; Sun, Y.; Wang, X. Fatigue crack growth characteristic for diffusion bonded laminates of titanium alloy with centered hole. Eng. Mech. 2015, 4, 244–249. [Google Scholar]
- Syed, A.K.; Zhang, X.; Moffatt, J.E.; Maziarz, R.; Castelletti, L.; Fitzpatrick, M.E. Fatigue performance of bonded crack retarders in the presence of cold worked holes and interference-fit fasteners. Int. J. Fatigue 2017, 105, 111–118. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S. Experimental Research on Fatigue Crack Growth Behavior of Diffusion-Bonded Titanium Alloy Laminates with Preset Unbonded Areas. Materials 2022, 15, 5224. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xuan, F.; Tu, S. Fatigue damage of stainless steel diffusion-bonded joints. Mater. Sci. Eng. A 2008, 480, 125–129. [Google Scholar] [CrossRef]
- Abdolvand, R.; Atapour, M.; Shamanian, M.; Allafchian, A. The effect of bonding time on the microstructure and mechanical properties of transient liquid phase bonding between SAF 2507. J. Manuf. Process. 2017, 25, 172–180. [Google Scholar] [CrossRef]
- Norouzi, E.; Atapour, M.; Shamanian, M.; Allafchian, A. Effect of bonding temperature on the microstructure and mechanical properties of Ti-6Al-4V to AISI 304 transient liquid phase bonded joint. Mater. Des. 2016, 99, 543–551. [Google Scholar] [CrossRef]
- Habisch, S.; Peter, S.; Grund, T.; Mayr, P. The Effect of Interlayer Materials on the Joint Properties of Diffusion-Bonded Aluminium and Magnesium. Metals 2018, 8, 138. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.; Zhang, Y.; Xu, F.; Jian, Q.; Lu, W. Microstructure evolution and mechanical properties of the In-Sn-20Cu composite particles TLP bonding solder joints. Appl. Phys. A 2020, 126, 343. [Google Scholar] [CrossRef]
- Kim, S.H.; Cha, J.-H.; Jang, C.; Sah, I. Microstructure and Tensile Properties of Diffusion Bonded Austenitic Fe-Base Alloys—Before and After Exposure to High Temperature Supercritical-CO2. Metals 2020, 10, 480. [Google Scholar] [CrossRef]
- Ghaderi, S.; Karimzadeh, F.; Ashra, A. Evaluation of microstructure and mechanical properties of transient liquid phase bonding of Inconel 718 and nano/ultrafine-grained 304L stainless steel. J. Manuf. Process. 2020, 49, 162–174. [Google Scholar] [CrossRef]
- Peng, Y.Y.; Li, C.; Li, H.J.; Liu, Y.C. Effect of interlayer on microstructure and mechanical properties of diffusional-bonded Ni3Al-based superalloy/S31042 steel joint. J. Manuf. Process. 2021, 72, 252–261. [Google Scholar] [CrossRef]

| Materials | Bonding Temperature | Bonding Time | Bonding Pressure | Interlayer Materials | Recommended Parameters | Reference |
|---|---|---|---|---|---|---|
| Al2219 | 480, 500, and 520 °C | 30 min | 2 MPa | Cu | A maximum shear strength of 18.75 MPa was produced at 520 °C, with a maximum hardness value of 723 HV. | [12] |
| Al6063 and UNS S32304 | 550, 555, 560, and 570 °C | 90 min | 0.2 KN | Copper foil | A defect-free joint was produced at 570 °C, and IMCs (Al2Cu) were found at the interface. | [13] |
| Al-Mg-Si Alloy and 301L Stainless Steel | 485 °C | 10 and 30 min | Not specified | Sn-based material | TLPDB is particularly important for the joining of semiconductor chips with expensive die-attached materials during low-temperature sintering. | [14] |
| AR500 Steel and AA7075 | 425 and 477 °C | 1, 2, and 5 min | Not specified | Al–Si–Zn | The highest shear load was 6460 N, which was produced at a brazing temperature of 477 °C, and the hardness of the aluminum base metal was decreased by 1 and 2 min flame times. | [15] |
| 1420 Al-Li Alloy | 440–560 °C | 60 min | 7 MPa | Not specified | The diffusion bonding temperature promotes the atomic diffusion of Mg in pure aluminum. The bonding temperature is an important factor affecting the quality of the bonding interface and the bonding strength. | [16] |
| SSM-ADC12 | 400, 430, 460, 490, and 520 °C | 60, 90, and 120 min | 3.4 MPa | ZnAl4Cu3 zinc alloy | This research represents a new concept for GISS materials. The maximum bonding strength value was high at 32.21 MPa. This was generated at a bonding temperature of 490 °C, with a bonding time of 120 min and a ZnAl4Cu3 zinc alloy that was 2.0 mm thick, which had never been studied before. | Present work |
| Element (wt.%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Materials | Si | Fe | Cu | Mn | Mg | Zn | Sn | Other | Al |
| SSM-ADC12 | 11.99 | 0.93 | 1.75 | 0.12 | 0.07 | 0.78 | 0.03 | 0.03 | Rest |
| ZnAl4Cu3 | 0.81 | 0.01 | 3.22 | 0.91 | 0.82 | 89.30 | - | - | 4.20 |
| Materials | Vickers hardness (HV) | Yield strength (MPa) | Ultimate tensile strength (MPa) | Elongation (%) | |||||
| SSM-ADC12 | 96.70 | 163 | 319 | 10–12 | |||||
| ZnAl4Cu3 | 79.12 | 97 | 125 | 5–7 | |||||
| Material | Optimal TLPDB Parameters | Maximum Bonding Strength (MPa) | Reference |
|---|---|---|---|
| SSM7075 | Bonding time of 120 min Temperature of 540 °C | 17.44 | [29] |
| Al7075 to Ti–6Al–4V | Bonding time of 30 min Temperature of 540 °C | 19.50 | [30] |
| Ni3Al superalloy | Bonding time of 6 h Temperature of 1250 °C | 860.84 | [31] |
| Ti45Ni49Cu6 | Bonding time of 60 min Temperature of 970 °C | 193.00 | [32] |
| SSM-ADC12 | Bonding time of 120 min Temperature of 490 °C | 32.21 | Present work |
| Stroke (mm) | SSM-ADC12 (BMs) | SSM-ADC12 (TLPDB) | ||
|---|---|---|---|---|
| Stress (MPa) | Number of Cycles | Stress (MPa) | Number of Cycles | |
| 0.35 | 22.21 | 1,000,000 * | 20.92 | 1,000,000 * |
| 0.40 | 31.12 | 1,000,000 * | 28.45 | 800,000 |
| 0.45 | 36.72 | 940,470 | 35.79 | 655,334 |
| 0.50 | 45.52 | 657,134 | 40.24 | 255,560 |
| 0.60 | 68.31 | 230,780 | 45.81 | 131,835 |
| 0.70 | 73.10 | 79,104 | 55.66 | 25,465 |
| 0.80 | 81.52 | 55,360 | 67.72 | 11,747 |
| Material | Life Equation at 106 Cycles | Endurance Limit (MPa) |
|---|---|---|
| SSM-ADC12 (BMs) | σ = 257.32x−0.131 | 31.12 |
| SSM-ADC12 (TLPDB) | σ = 188.08x−0.127 | 20.29 |
| Element | Line Type | Apparent Concentration | k Ratio | wt% | wt% Sigma | Atomic % | Standard Label |
|---|---|---|---|---|---|---|---|
| C | K series | 0.44 | 0.00939 | 9.73 | 0.69 | 9.94 | C Vit |
| Si | K series | 0.22 | 0.00467 | 4.55 | 0.41 | 5.68 | SiO2 |
| Cu | K series | 0.12 | 0.00168 | 1.46 | 0.19 | 2.34 | CuO |
| Al | K series | 17.13 | 0.08453 | 65.25 | 0.92 | 49.92 | Al2O3 |
| Zn | K series | 5.11 | 0.03453 | 19.01 | 0.32 | 32.12 | ZnO3 |
| Total: | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meengam, C.; Dunyakul, Y.; Maunkhaw, D. The Mechanical Properties of a Transient Liquid Phase Diffusion Bonded SSM-ADC12 Aluminum Alloy with a ZnAl4Cu3 Zinc Alloy Interlayer. J. Manuf. Mater. Process. 2024, 8, 184. https://doi.org/10.3390/jmmp8050184
Meengam C, Dunyakul Y, Maunkhaw D. The Mechanical Properties of a Transient Liquid Phase Diffusion Bonded SSM-ADC12 Aluminum Alloy with a ZnAl4Cu3 Zinc Alloy Interlayer. Journal of Manufacturing and Materials Processing. 2024; 8(5):184. https://doi.org/10.3390/jmmp8050184
Chicago/Turabian StyleMeengam, Chaiyoot, Yongyuth Dunyakul, and Dech Maunkhaw. 2024. "The Mechanical Properties of a Transient Liquid Phase Diffusion Bonded SSM-ADC12 Aluminum Alloy with a ZnAl4Cu3 Zinc Alloy Interlayer" Journal of Manufacturing and Materials Processing 8, no. 5: 184. https://doi.org/10.3390/jmmp8050184
APA StyleMeengam, C., Dunyakul, Y., & Maunkhaw, D. (2024). The Mechanical Properties of a Transient Liquid Phase Diffusion Bonded SSM-ADC12 Aluminum Alloy with a ZnAl4Cu3 Zinc Alloy Interlayer. Journal of Manufacturing and Materials Processing, 8(5), 184. https://doi.org/10.3390/jmmp8050184






