Abstract
The additive manufacturing (AM) process is used for joining materials to make objects from 3D model data, usually layer upon layer, contrary to subtractive manufacturing methods. This technology plays a significant role in fabricating orthopedic implants, especially parts of hip implants (HI), such as femoral head, stem, neck, polyethylene linear, acetabular shell, and so on, using biomaterials. These biodegradable resources are those that can be utilized as tissue substitutes since they are accepted by live tissues. Here, the study is to examine the most preferable AM process and biomaterial used for making HI, including its manufacturing methods, compositions, types, advantages, and defects and cross-examining the limitations to bring some new technology in the future. Then we elaborate on the outlook of the most preferable material, followed by evaluating its biocompatibility, detailed application, and structural defects occurring while using it as an HI. Subsequently, the physical characteristics and design constraints are also reviewed in the paper. We assess the current stage of the topology optimization technique (TO) with respect to the characteristics of newly designed implants. The review concludes with future perspectives and directions for research.
1. Introduction
In ancient times, when the joints of the bones were damaged, it was very difficult to replace them. Either treatment had to be performed till the end of life, or any supporting belt was put on. The problem is encountered when some of the elements between the bones are damaged or broken. The invention of biomaterials has created major changes in the medical field [,,,]. Biocompatible materials are generally used to replace or support damaged biological tissues [,,]. These biomaterials are used to produce bone fragments and broken parts [,,,,,,]. They can be applied to hard tissues in orthopedics or orthodontics or to support soft tissues, such as blood vessel or artificial valves in the heart [].
Nowadays, AM plays an important role in the medical field [,,,,,,,,], especially in the production of some internal organs using artificial tissues that are accepted by the natural living tissues of human beings. AM consists of design modeling and production. The 3D model for the production can be designed by the CAD software or a model obtained through CT scan or MRI. We know our body will reject all the unnecessary substance. Therefore, materials that are not harmful and acceptable by living organisms are used. Biomaterials can enhance the quality and longevity of human life [,,,,]. The science and technology associated with the field of biomaterial has now led to a multi-million-dollar business. Biomaterials are used in different parts of the human body as replacement implants in shoulders, hips, knees, and elbows [,,,,]. However, the number of replacements are high in the cases of the spinal area, hip, and knee [].
Approximately 230 million major surgical procedures are performed on a daily basis. Most of these procedures involve repair, reconstruction, or replacement of one or more damaged bones or joints and also tissues and organs. The combination of the medical field and AM has emerged as an alternative technique to manufacture substitute joints and their components to restore, improve, and maintain the function of joints. In orthopedic implants, several biomaterials are used for the construction of joints and components [,,]. Biocompatible metals and alloys, such as Co-Cr alloys, stainless steel, Ti, and its alloys, are the main materials used as implants []. Biodegradable Mg-based alloys are also used for making metal implants [].
Several review papers related to biomaterials have been published in recent years, such as references [,,,,,], in which most of them have concentrated on AM techniques used for the fabrication of different biomaterials [,,]. Some of them have studied their thermal effects and corrosion rate [,], and a few studies have been carried out on surface modification for bioimplants []. Furthermore, some other articles have presented a discussion of specific objectives; for example, Attar et al. [] used metals that are accepted by living tissues. They reported that metals such as surgical-grade stainless steel (316L), Co-Cr alloys, and pure commercial Ti or Ti alloys do not affect negatively the human body and will have good mechanical properties, high strength-to-weight ratio, and excellent corrosion resistance. Mostly these metals are used for joint replacements, dental roots, and orthopedic fixations, for stents and orthopedic implants. Prakasam M et al. and Minagar S et al. [,] studied the main advantage of metal implants. It was revealed that they have brilliant mechanical properties, which make them the most commonly used implant materials in bone surgical repair. Meanwhile, the main disadvantage of metals in their use as biomaterials is their lack of tissue adherence and excess deterioration [,,,,]. This will result in either a second surgery to remove the implant or keeping the implant in the body with the risk of toxicity due to accumulation of metal ions due to rusting.
Other than metals, ceramic materials are biocompatible and have very high resistance to corrosion and compression with low electrical and thermal conductivities [,,]. Habibovic P et al. [] reported that ceramic biomaterials are mostly suited for medical implants; they have very low toxicity and help to achieve higher efficiency in forming new bone tissues. They reported that ceramics can replicate human tissues or can be absorbed back into the body to encourage the restoration of healthy tissues. They are composed of bioactive glass, glass ceramics, calcium silicates, hydroxyapatite, alumina, zirconia radiation glasses, and resorbable calcium phosphates.
Bose S et al. [] mentioned that the most common material used in the 3D printing industry are polymers because of their diversity. They revealed that polymers are widely used more than ceramics and metallic biomaterials. The ease of manufacturing to produce various shapes, ease of secondary processing stages, reasonable cost, and availability with desired physical and mechanical properties are the major advantages of polymers when compared with metallic and ceramic biomaterials [,,,,,]. Devi P et al. [] highlighted the main reasons behind the wide usage of polymers. They are biocompatibility, biodegradability, sterilizability, adequate mechanical and physical properties, and excellent manufacturability because of their low melting points. Karageorgiou et al. [] revealed that many natural polymers benefit from being biocompatible and biodegradable. They stated that polymers can modify their chemical structure due to their flexibility. Jones L et al. [] found that a variety of polymers have been used in medical care, including preventive medicine, clinical inspections, and surgical treatment. They mentioned that the commonly used polymer in orthopedic surgery is polymethyl methacrylate.
Despite many existing review papers, to the best of the authors’ knowledge, there is no study that has reviewed the best material specifically for making implants, its manufacturing process, benefits, defects, and comparison with other biomaterials.
Therefore, it is important to have in-depth knowledge of this subject, and hence, the aim of this review paper is to provide comprehensive information and details of the materials and processes. It is important for review articles such as this one to bring out the intricacies of processes and critical analysis of the materials to better utilize them. This will prove to be of great use to understand suitable biomaterials for orthopedic implants, as well as in-service performance issues, after implanting them into the body. The present article aims to provide an overview of the significant contributions of AM in the biomedical industry. This paper gives the state of the art on current research studies conducted in all biomaterials used for making orthopedic implants using AM. The paper begins with a brief introduction of biomaterials and their types, and then introduces their manufacturing processes. It further discusses the AM process application in orthopedics and its benefits. The main text of this article provides a meticulous review on major areas of orthopedic biomaterial research using the best material. The last section of the paper draws conclusions and trends of the reviewed bodies of research.
2. Biomaterials for Biological Tissue Replacements
Different biomaterials are integrated with tissue replacements. This area summarizes commonly used materials for making biological implants. The most commonly used material will be projected after the following subsections.
2.1. Metals
Metals are the main materials that are used in the replacements of bones or used as a support for broken bones [,,,,,,,,]. Metals that are accepted by living tissues are only used in this case. Using these metals does not affect negatively the human body and will result in good mechanical properties, high strength-to-weight ratio, and remarkable resistance to corrosion []. These required metal parts are manufactured by traditional processing technologies, including casting, machining, and powder metallurgy—all of these processes need more time and energy and have a high metal consumption rate []. However, AM techniques are replacing these conventional methods nowadays []. Mostly the metals are used for biomedical replacement surgeries []. Among these metals, Ti alloy is considered to be the best material for orthopedic implants []. In the case of fracture fixations, the fixation devices used to immobilize the bone are wires, screws, plates, and intramedullary nails. The primary benefit of metal implants is their superior mechanical qualities, which make them the most popular implant type for bone surgical repair [,]. The main disadvantage of metals in being used as a biomaterial is their lack of tissue adherence and low rate of disintegration [,,,,]. This will result either in a second surgery to remove the implant or keeping the implant in the body with the risk of toxicity due to the accumulation of metal ions due to corrosion.
2.2. Ceramics
Generally, ceramic materials are biocompatible and have very high resistance to corrosion and compression with low electrical and thermal conductivities [,,]. Ceramic biomaterials are mostly suited for medical implants; they have very low toxicity, and they help in the higher efficiency of forming new born tissues []. These have the ability to perform like human tissues or to absorb into the body and promote the regeneration of natural tissues. They include radiotherapy glasses, bioactive glass, hydroxyapatite, glass ceramic calcium silicates, alumina and zirconia, and resorbable calcium phosphates []. Mostly ceramics are used for dental implants and some orthopedic implants. The ceramics used in bone replacement and healing are categorized into three types based on the body’s reaction. The first is the bioinert ceramic, which is almost inert in the body and forms a thin nonadherent fibrous layer at the connection. The bioactive ceramic falls under the second type and can firmly attach to bones. Finally, the third type is the bioresorbable ceramic, which progressively deteriorates over time, eventually being replaced by natural bone []. The use of ceramics in medical applications is limited due to their poor tensile strength [].
2.3. Polymers
Polymers are the most common material in the 3D printing industry because of their diversity []. Polymeric biomaterials are considered to be one of the best materials for medical end use. Polymers are widely used more than ceramic biomaterials []. The required mechanical and physical properties, the simplicity of secondary processing stages, the cost, and the ease of manufacturing to manufacture diverse shapes are the major advantages of polymers when compared with metallic and ceramic biomaterials [,,,,,]. The main reasons behind the wide usage of polymers are biocompatibility, biodegradability, sterilizability, adequate mechanical and physical properties, and low melting point, which make them easy to fabricate []. Many natural polymers have the advantage of biodegradability and biocompatibility [] Polymers can modify their chemical structure due to their flexibility. Biodegradable and biocompatible polymers are generally called biopolymers [,,]. In medical care, a variety of polymers have been used, including preventive medicine, clinical inspections, and surgical treatment []. A commonly used polymer in orthopedic surgery is polymethyl methacrylate []. Even though polymers have lot of positives, the use of polymers is confined due to its low load handling capacity and low tissue affinity [].
According to the aforementioned forms of publications, all three types of materials can be employed based on the implant type, location inside the body, size, design, and mechanical demands that they must withstand. The most widely accepted and used biomaterials are metallic since early days. Studies are underway to make ceramics and polymeric materials common substitutes for metals. Hence, this review focuses on metallic biomaterials used for making implants. The next part of the review discusses the AM methods involved in the manufacturing of metallic biomaterials.
3. AM Techniques for Metallic Biomaterials
AM is simply defined as the addition of material in a layer-by-layer fashion. This technology uses data from a computer-aided design (CAD) software or from any 3D object scanners to direct a hardware to deposit the material, layer upon layer, in precise geometric shapes. Currently, there are many types of AM technologies for making metallic biomaterials. The relevant studies classified according to the AM processes used in the manufacturing of biomaterials are as follows:
3.1. Direct Energy Deposition Technique (DED)
Bose S et al. [] explain DED as a process in which a direct source of energy is used to simultaneously deposit, melt, and solidify the material that is fed as either a powder or as a filament. Electron beam or lasers are mainly used as the direct energy source. Yousuf S et al. [] say that the continuous feeding of starting material is needed in the form of powder or filament for the process. Then the feeding material becomes melted by the energy source and becomes deposited in a layerwise pattern. The heat conduction in direct energy deposit processes is more effective because the heat, material, and shield gas are transferred directly from the nozzle to the build area. The laser direct deposition technique is the most popular one that falls under this category []. DED features a nozzle that moves around multiple axes. Davoodi E et al. mention that the DED process can be used for fabricating a wide range of metallic biomaterials. Moreover, this technique has proven its capability of manufacturing porous biomedical implants []. Furthermore, multimaterial structures produced by DED have benefits and can be constructed using existing technology and process management []. DED is faster and less expensive as compared with powder bed fusion when creating midsize metal parts []. Even though DED is a method having various advantages, it has certain disadvantages, such as support structures being necessary for overhangs, limitations in producing complex shapes, and lower accuracy than powder bed fusion AM processes []. The working of DED is shown in Figure 1.
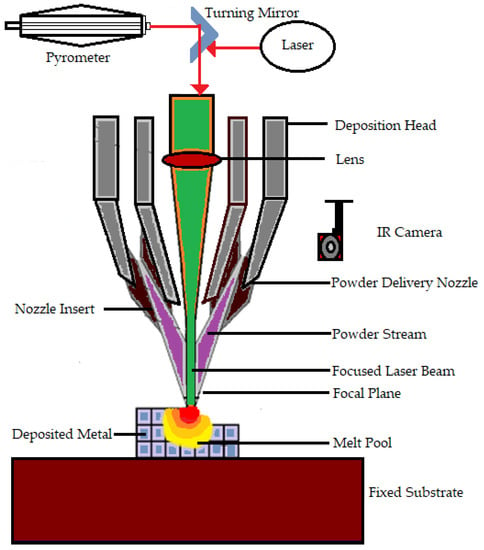
Figure 1.
Working of the direct energy deposition technique.
3.2. Material Extrusion (ME)
Susmita Bose et al. [] define material extrusion as an AM technique that builds three-dimensional models using a continuous filament made of thermoplastic or composite material. The process has a large number of variables that influence the quality of the final model, but when these variables are tightly controlled, they have great potential and viability. Fuse deposition modeling is a common material extrusion process []. Ortega Z et al. [] mention that the material is selectively distributed through a nozzle or orifice using a sliced CAD part model. The filament material is fed to a nozzle using a driving gear in the polymer material extrusion AM. Then, to build the complex 3D structure, the filament is deposited onto the build platform layer by layer. The nozzle temperature must be high to melt the filament material (e.g., 200–230 °C for ABS and 180–200 °C for PLA polymers). To prevent component warping and delamination, heat lamps are utilized to control the temperature in the area surrounding the build platform. This technique is comparatively less expensive and easier to run and has more biomedical applications, including biological cells, ceramics, and polymers []. Adding more parts to a single build did not reduce the processing time per part []. Most material extrusion systems use a spool of plastic filament as the feedstock. Even though material extrusion is a successful method for manufacturing metallic biomaterials Cheuh Y et al. [] found that material extrusion produces metal components with a lower density than laser powder bed fusion. Figure 2 shows the representation of material extrusion process.
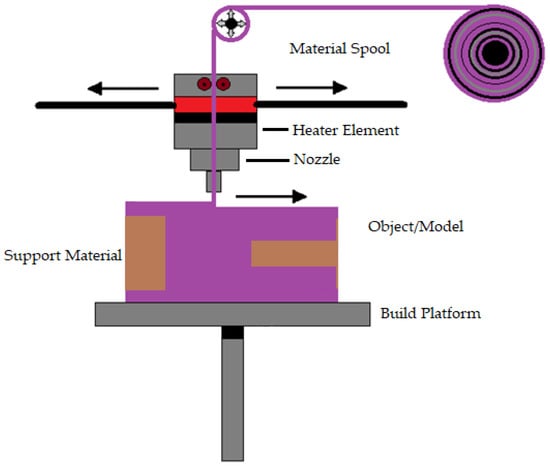
Figure 2.
Representation of material extrusion.
3.3. Powder Bed Fusion (PBF)
In PBF, very fine powders are used as thin layers and are evenly and densely stacked on a platform. The particles in each layer are fused using a laser beam or a binder []. PBF approaches allow for excellent dimensional precision at large relative densities [] often with high repeatability and surface roughness []. Additionally, the process has high porosity when the powder is forced with a binder []. PBF uses thermal energy to fuse certain areas on the powder bed. PBF is one of the first generation of commercialized AM processes []. The printing processes of PBF and binder jetting are similar. N Derimow et al. [] say that an oxidation-free environment is needed for PBF to prevent oxidation of the feedstock powder. A hopper or a reservoir below the side of the bed provides fresh material supply. PBF’s advantages include the fact that it has less material wastage and less cost, improves production development times, enables rapid prototyping and low-volume production, is capable of building functionally graded parts, performs efficient recycling of unmelted powder, and provides elimination of the need for machining fixtures []. In addition, even though the high powder density across the build geometry can act as a self-support [], the PBF approach frequently requires structural support to combat shape distortions of the manufactured sample. In the case of titanium, PBF can create materials with high strength, high detail, and load bearing []. On the other hand, using tiny, spherical metallic powders enables high packing densities, enhances component consolidation, and reduces flaws []. Behzad Fotovvat et al. [] focuses on the PBF–AM technique of direct metal laser sintering for Ti-6Al-4V ELI material’s dimensional precision. The impact of size, geometry, and position on the build platform was studied using a variety of geometric pieces with different thicknesses and sizes. They came to the conclusion that accuracy is not affected by where the components are placed on the build platform and that this method typically produces high-resolution results. They concluded that inaccuracy is not a function of the location of the parts on the build platform, and such process usually shows results with high resolution. Meanwhile, from the findings of Samira et al. [] when compared with laser metal deposition, laser PBF provides the best level of accuracy for dimensions, perpendicularity, and position tolerance. The PBF process is shown in Figure 3.
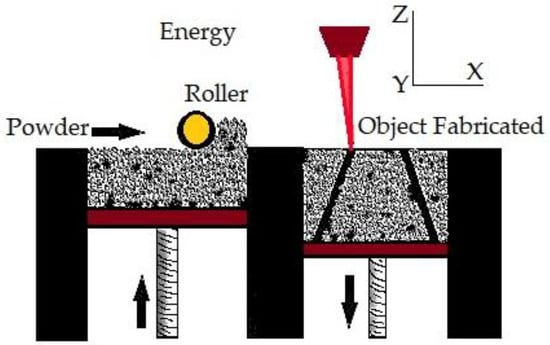
Figure 3.
Powder bed fusion.
3.4. Vat Polymerization (VP)
The first commercially available AM technology is vat polymerization. It produces parts through selectively curing liquid photoreactive polymers []. Vat polymerization is also known as stereolithography (SLA). This process has high accuracy in building parts, superior part quality, and quick construction pace []. This technology is known for its smooth surface finish and highly detailed parts, average build speed, and a wide range of materials []. The SLA process is based on layer-by-layer photosensitive resin polymerization using UV light []. Increasing the number of parts reduces the time per part in vat polymerization. Support structures are essential in the case of vat polymerization []. Biomaterials in large sizes can be manufactured by vat polymerization using different materials, such as metal, ceramics, and polymers. Figure 4 shows the vat polymerization technique.
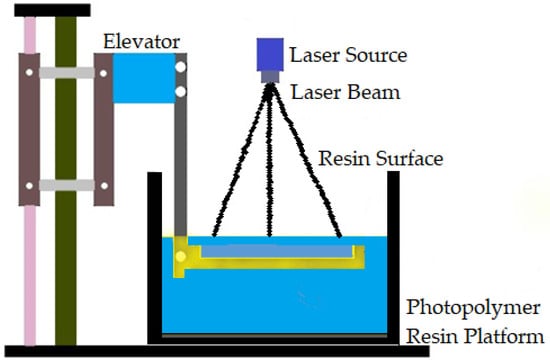
Figure 4.
Vat polymerization technique.
Several studies have been carried out to examine the precision and resolution of the model created using various AM processes. A detailed solidification model was developed by C Zhou et al. [], and it was used to estimate the curing dynamics of the resin used in 3D printing. To guarantee the accuracy of the micro part, the suggested model-based strategy optimizes the photos while taking the light distribution from all closely neighboring pixels into account. When a complex 3D object is printed using SLA, the results demonstrate how dramatically overcured areas are improved by employing optimal grayscale pictures. Actual printing results from the experimental setup confirm the improvements in the accuracy and precision of the printing method. The authors came to the conclusion that the precision and resolution of manufactured components can be considerably impacted by the mask pictures utilized in the construction process. The effectiveness of the suggested strategy to increase the XY resolution of the mask projection over a sizable region in the SLA process has been demonstrated by experimental findings. P Shah et al. [] found that SLA is the most precise of all methods, with a variety of changing tolerances in areas such bossed cube parallelism and perpendicularity.
3.5. Binder Jetting (BJ)
Binder jetting (BJ) is a powder-based AM process that employs a liquid binder, instead of an electron beam or laser source, for locally binding the powders and creating the intricate ceramic, polymeric, and metallic structures []. Shick T M et al. [] mentioned that to form a designed structure layer by layer in the BJ technique, the liquid binding agent is spread to a selected area on the power bed based on the part geometry. The binder acts as an adhesive to bond the powder layers. In a normal BJ system, there are three axes, namely x, y, and z. The x and y axes are responsible for the horizontal position, and the z axis refers to the depth for x and y []. For a successful build, the binder jetting needs an understanding of many process variables. BJ is able to be conducted on dry powders as well as on wet powders. Fine powder BJ can give good surface finishing and low surface roughness []. There are two types of binders: organic and inorganic. Binder jetting is capable of printing a variety of materials, including metals, sands, and ceramics []. A schematic representation of binder jetting is shown in Figure 5.
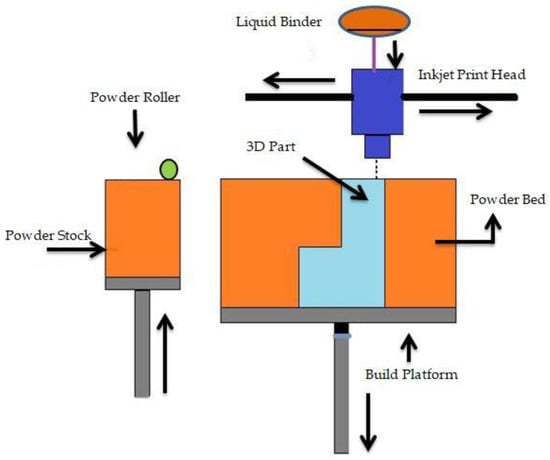
Figure 5.
Schematic representation of binder jetting.
3.6. Material Jetting (MJ)
Material jetting is one of the fastest and more accurate 3D printing technologies. It uses liquid photopolymer droplets to build parts. MJ creates objects in a similar method to a 2D inkjet printer []. MJ builds layer upon a layer until the part is finished. This process is also quite similar to SLA since it uses a UV light source to cure the resin []. MJ is the only AM technology that can combine different print materials within the same 3D-printed model in the same print job. The key feature of MJ is that objects appear perfectly realistic. Even compared with other 3D printing methods that use photopolymers, MJ hands down as print heads is capable of forming objects more accurately []. The photopolymer liquid allows the 3D printer to form smoother parts, which appear shinier when support material is not used. This process can be used for full color printing []. Objects created through this process are rigid, flexible, opaque, and translucent. MJ is also used in medical hospitals and universities to 3D-print full-color anatomical models that look like real body parts. This makes it possible for surgeons to plan and be ready for an operation more quickly than they could with a 2D picture [,]. F Klocke et al. [] found that although the accuracy of the laser-assisted MJ method is equivalent to that of the EBM process, future accuracy increases at high building rates due to enhanced process parameters and automated tool path generation are still very likely.
3.7. Sheet Lamination (SL)
SL processes include ultrasonic AM and laminated object manufacturing []. The ultrasonic AM process uses sheets or ribbons of metal, which are bound together using ultrasonic welding []. The process does require additional CNC machining and removal of inbound metal, often during the process. Laminated object manufacturing uses a similar layer-by-layer approach but uses paper as material and adhesive instead of welding []. Laminated objects are often used for aesthetic and visual models and are not suitable for structural use []. UAM uses metals and includes aluminums, copper, stainless steel, and titanium []. The process has low temperature and allows for internal geometries to be created. The process can bond different materials and requires relatively little energy, as the metal is not melted []. M Salmi et al. [] mention that sheet lamination is only used for medical models or phantoms. They also state that sheet lamination devices are rare, and the vendors have receivership status. Different types of AM technologies and their medical applications are listed in Table 1.

Table 1.
AM technologies and their medical applications.
D. Singh et al. [] highlight that variable temperature and build speed during the build process can affect the precision of the products and are factors in the manufacturing accuracy of different AM methods. Additionally, they discuss potential solutions that could reduce the amount of warpage that the component experiences as a result of the length of time.
From the above literature upon the authors’ perspective, DED is the most efficiently used AM technique for making implants. Among them, the electron beam DED technique is most accepted. Based on the findings of M Salmi et al. [], DED is suitable for metals. Hence, DED can be effectively used for fabricating metallic bioimplants. The percentage contribution of AM methods for making implants on behalf of the above references is shown in Figure 6.
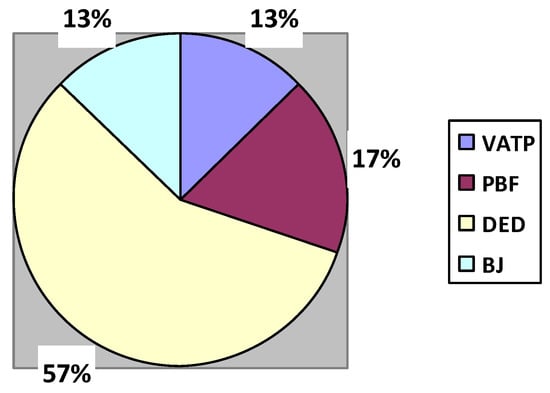
Figure 6.
Percentage contribution of AM methods in the medical industry.
4. Types of Metal Implants
Titanium and its alloys, cobalt-based alloys, stainless steels, tantalum and its alloys, niobium and zirconium alloys, and magnesium alloys are the main materials used as implants in orthopedics [,,,,,]. Among all these metal alloys, titanium alloy is the most used metal alloy for orthopedics and other implants [,]. The unique characteristics of titanium alloy make it different from other materials. Different types of metal alloys are discussed in the following subsections.
4.1. Stainless Steel (SS)
Most of the internal fixation devices are manufactured using SS because of its ease of availability, low price, excellent fabrication property, biocompatibility, and good strength. SS is an alloy mainly made from iron; molybdenum, chromium, and nickel are the main alloying elements. To increase the life expectancy of the implant and to act as a protective coating, SS is coated with transition metal nitrides, such as TiN, VN, and TiAIN []. Austenitic stainless steels are the most widely used materials for orthopedic applications []. Large amounts of type 316L stainless steel are used for implant devices because they are less expensive than Co-Cr alloys, titanium, and its alloys by a factor of one-tenth to one-fifth times. Type 316L stainless steels (16–18% Cr, 12–15% Ni, 2–3% Mo) are austenitic stainless steels []. However, type 316L stainless steel orthopedic implants corrode in the body environment and release iron, chromium, and nickel ions []. SS can be used as fixed implants, such as those for artificial joints to aid in bone repair after undergoing some physical and chemical treatment. However, when we consider corrosion resistance in the human body, titanium alloys and cobalt chromium alloys are much better than SS []. Irene Buj et al. [] stated that SS alloys are prone to deformation and cannot be hardened. Mantripragada V P et al. [] and Sing S L et al. [] mention that SS releases chromium and nickel to the body as a result of ageing.
4.2. Cobalt-Based Alloys
Cobalt-based alloys are mainly used as hip and knee implants []. In knee and hip replacements, cobalt-based alloys are widely used in ball-and-socket joints where motion occurs []. They have high wear resistance and great mechanical properties under static loadings []. ASTM F75 is a widely used cobalt-based alloy. Almanza et al. [] found that the comparison of corrosion results for EBM-fabricated Ti-6Al-4V alloy and ASTM F75 Co-based alloy was similar. This material is also used as implant in dental parts, pacemaker lead casing, tibial trays, acetabular cups, and cardiovascular stents [,]. Bartolo P et al. [] mention that wear and corrosion of cobalt-based alloys can lead to the release of metal ions into the body, and limitations on the complexity of components are their main defects.
4.3. Titanium and Its Alloys
Titanium alloys are one of the most important nonferrous materials that are used as orthopedic implants []. This is because of their high strength, rigidity, and fracture toughness. The oxide layer formed when Ti is implanted allows the titanium implant to integrate with the living bone tissues []. These alloys are commonly utilized in making surface components that do not bear weight, such as femoral necks and stems []. Titanium alloys typically contain traces of aluminum, molybdenum, vanadium, niobium, tantalum, zirconium, manganese, iron, chromium, cobalt, nickel, and copper []. The commonly used Ti-based alloys for making implants are Ti-6Al-7Nb, Ti-6Al-4V, Ti-13Cu-4.5Ni, Ti-25Pd-5Cr, Ti-20Cr-0.2Si, and so on []. They are one of the few materials that do not contain calcium phosphate and have been shown to calcify when exposed to simulate body fluids and, therefore, are expected to calcify in vivo also []. Titanium implants having calcium phosphate coatings on their surface show good fixation to the bone []. Xiaoliang Cheng et al. found that the formation of calcium phosphate deposit on the Ti surface decreased the corrosion rate []. Additionally Ti reduces failure due to infection, because of the increased antimicrobial property []. Lutjering G et al. [] and McGilvray K C et al. [] listed the defects of Ti, such as poor tribological properties, challenging process, unstable creep, and wear debris causing biological reactions. Meanwhile, Liu S et al. [] and Hao Y L et al. [] mention the disadvantages of Ti alloys, such as low stress shielding, high price, and poor tribological properties. However, the low elastic modulus of Ti alloys is well appreciable as it reduces the stress shielding effect []. Ti alloys are damage tolerant because of their mechanical and physical properties []. Taniguchi et al. [] found the bone ingrowth of various pore sizes of Ti implants using SLM. Their intention was to find the best implant having the property of osseointegration, and they succeeded in their work. Later, Fousova M et al. [] stated that SLM technology can achieve a mimicking of almost 60% porosity of the human bone.
4.4. Tantalum and Its Alloys
Tantalum is a transition metal. It remains relatively inert in vivo []. Tantalum-based implants have shown an exceptional biocompatibility and safety record in orthopedics []. Tantalum can also be alloyed with other elements, such as niobium- and nickel-based superalloys, to increase its temperature resistance and strength. Adding niobium to tantalum reduces weight and saves cost while maintaining good corrosion resistance []. Tantalum needs a bonelike apatite layer on the surface first in order to bond with bones []. It has the capacity to integrate with tissue, enabling the development of bone tissue; it has good biomechanical properties and excellent chemical stability []. It is currently seen to be an essential part of many of the best systems for enhancing the mechanical strength of the bone–implant interface and joint performance []. Alvarez K et al. [] and Sporer S et al. [] tell that the cost of Ta and its alloys is really high. Additionally, they mention that Ta has a high melting point, and this creates challenges while manufacturing implants.
4.5. Niobium and Zirconium Alloy
Niobium (Nb) and zirconium (Zr) alloys are new to this biomedical application field. Nb and Zr, which are recently introduced, have shown the ability to resist corrosion and exhibit crack propagation and fatigue strength in simulated body fluids []. When compared with TiN coating, it supports high adhesion []. Due to a large volume of the omega phase, the Zr-9Nb alloy was found to be brittle []. Zr has low magnetic susceptibility and low cytotoxicity [].
4.6. Mg Alloys
Magnesium alloys have been using as biomaterial for many years []. The mechanical characteristics of magnesium are comparable to those of the bones []. Since magnesium is the fourth most abundant element in the human body, the degraded product of magnesium alloy can be stored in either fracture callus or new bone or can be eliminated into the blood to be excreted by urine without causing hypermagnesium []. Even though magnesium has its own advantages, its disadvantages highlighted by Elkaiam L et al. [] Prasadh S et al. [] withdraw researchers from trust Mg as a successful implant material. They point out defects, such as inadequate corrosion resistance, low elastic modulus, higher cost, moderate strength, and flammable character.
4.7. Other Metals and Their Alloys
Metals such as iron, zinc, and copper are also used in a few biomedical applications [,]. Their alloys are also available. However, due to several reasons, they are not used in a larger amount. For instance, iron cannot be hardened [], zinc has low toughness and is brittle [], and copper is expensive and difficult to print []. Even though aluminum oxidizes only until a passivated layer and has high oxidation rate [], it has an impact on the acceleration and stimulation of Alzheimer’s disease []. Similar to these metals, gold and its alloys, which have high cost [], and nickel and its alloys, having average biocompatibility [], are also very less preferred for making implants. M Niinomi et al. [] and Q Chen et al. [] say that several studies have concluded that Ba, Fe, and Cr are toxic elements, while Ti and its alloys and stainless steel and so on are nontoxic and resistant metallic biomaterials. The combination of nickel–titanium shape-memory alloys called nitinol is highly biocompatible, which makes them materials used as orthopedic implants [,,]. The shape-memory property of nitinol means that it has the potential to be used for applying mechanical stimuli for improved bone regeneration, or it can be used to provide a better fit into bone defects. However, a major concern with nitinol is the toxicity of nickel and potential carcinogenicity []. Moreover, it has lower strength especially after smaller expansions []. Furthermore, nitinol may form defective surface layers and lattices during fabrication []. Table 2 shows the properties of biomaterials used for making orthopedic implants.

Table 2.
Properties of biomaterials used for making orthopedic implants.
From the authors’ perspective, the most promising and widely used materials for medical implants are Ti and its alloys. The section below expands the role of Ti alloys in the field of medical implants and their expansions.
5. Outlook of Titanium and Its Alloys
In this section, studies dealing with various outlooks of Ti and its alloys are reviewed based on the following classification scheme:
From the authors’ perspective, reviewing the literature related to the biomaterials shows that most studies discuss Ti and its alloys in making medical implants, such as in [,]. While few studies discuss structural defects in AM implants, such as in [,], and for design methodologies for implant AM, there are very few researchers studying the topology optimization of implants [,]. However, there are relevant studies available related to the involvement of AM for making implants using Ti and alloys [,].
Objectives Functions:
The following objective functions are reported in the current relevant literature:
Objective 1: AM application in orthopedics;
Objective 2: Titanium alloy in medical implants;
Objective 3: Biocompatibility of titanium and its alloys;
Objective 4: Use of titanium and its alloy as lower-limb prostheses;
Objective 5: Structural defects in AM implants;
Objective 6: Topology optimization of implants.
Using this classification scheme, Table 3 chronologically lists studies related to Ti and its alloys in making orthopedic implants. Relevant studies classified according to the AM processes used in the manufacturing of biomaterials are also listed in Table 3.

Table 3.
List of the studies related to Ti and its alloys in making orthopedic implants.
5.1. AM Application in Orthopedics
Upon the authors’ view, there is a high demand of orthopedic implants in the current world. Normal wear and accidents and aging force patients to use orthopedic implants [,]. Javaid et al. [] say that AM is used in orthopedics for various uses, such as replication of the bone that aids in many bone therapies. In orthopedics, the patient-specific analysis is essential to have accurate medical imaging data of the individual patient. M Javaid et al. [] mention that the first role of the 3D-printed model is to closely mirror the instances seen in the clinic and provide the surgeon with a thorough review. The basic application of such model is for testing the surgical procedures in advance for the surgeon, which provides to the surgeon a feeling of the mechanical response of a real bone. The application of AM in orthopedic surgery is very useful when compared with the traditional surgical techniques [,]. Better surgical management and accurate diagnosis are provided by this. It provides reproducible, reliable, and credible models that improve patient outcomes and reduce operating time. AM develops personalized prostheses and implants, which is more valuable in the field of orthopedics []. K Wong et al. [] reveal that with a real 3D-printed physical model, the surgeon and the patient can now understand easily the medical conditions, which helps the surgeon obtain a tactile and visual expertise of the disease and anatomy unique to the patient. The application of AM in orthopedics is helpful for giving training to medical students and even for the doctor for a better understanding of the numerous types of fractures []. After the evolution of metal AM technologies, the orthopedic field is one of the biomedical fields that have received the most attention [,]. With the AM technology, the bone 3D images can be reconstructed, and by the layer-by-layer technique, a bone prototype can be obtained. Therefore, this plays an important role in medical and orthopedic surgery. Elahinia et al. [] reveal that this technology has the ability to minimize the weight of the implant by changing the properties of the raw material; thus the patient feels comfortable. AM produces more precise implants, which will reduce the risk of the operation []. Patient-specific instruments can be made by this technology. The orthopedic surgeon can readily achieve a high-quality product that meets the necessary standards [,]. The benefits of metal AM for orthopedic implants include quick prototyping on the same platform as the production, aid in the rapid progression of novel designs and features [], advanced mechanical properties [], support for a customized health care [], and economical feasibility as the specific implant of the patient can be manufactured in one build [,]. A case study was conducted by Anatoliy Popovich et al. [] using selective laser melting (SLM) for making a custom-made hip implant. They used Ti-6Al-4V for the experiment. The authors reported that the SLM created a textured surface, which potentially improved osseointegration by areas with high specific surface. They also mention that SLM materials showed higher tensile strength but lower elongation to break as compared with the properties of Ti-6Al-4V obtained using EBM. This might due to the higher cooling rates during SLM, and this results in the formation of finer microstructures. Based on the findings of M. Vignesh et al. [], the bioimplant manufacturing sector is categorized into four: laser or electron beam melting, paste extrusion technology, friction stir process, and photopolymerization. The authors believe that in less than 20 years, a fully functioning heart printed through AM will be available in the market. Moreover, they are expecting the possibilities of collecting stem cells from an infant’s teeth and using them for his entire life for organ and tissue replacements through AM.
The benefits and descriptions of AM technologies in orthopedics are shown in Table 4. Following this, the next session goes into the selection of Ti and its alloys in making medical implants.

Table 4.
Benefits and descriptions of AM in orthopedics.
5.2. Titanium Alloy in Medical Implants
It is evident that a lot of studies take place on the development of Ti and its alloys in biomedical applications. The most widely used Ti in medical field is pure Ti and alloy Ti-6AI-4V [,]. Ti alloys are widely used in medical implants because of their excellent biocompatibility and outstanding mechanical characteristics [,]. They are typically employed in implants that swap out hard tissue [,]. According to Yuan Bin et al. [], NiTi alloys can change their shape after implantation. The beta-type Ti alloy used for biomedical application consists of nontoxic and affordable common metals, such as Mn, Fe, Si, and Sn, which have high strength, are highly corrosion resistant, and have low Young’s modulus []. Due to the high oxygen solubility of Ti, the surface of the Ti components can be hardened easily through inward diffusion of oxygen by heating in air. This will also result in the formation of a TiO2 surface passivation layer and increase the biocompatibility and make the Ti implants more corrosion resistive [,]. Titanium alloys are safe for both humans and animals. The first-generation Ti alloys have shown allergies. Therefore, the second-generation beta Ti alloys are far better than the first generation []. These alloys are much harder []. The chemical composition, surface roughness, internal pore size, and porosity of titanium and its alloys have a direct impact on osteoblast migration, proliferation, adhesion, and differentiation [].
H Matsuno et al. [] say that Ti alloys have substantially higher stiffness values than the cortical bone. Among all the metallic biomaterials, Ti is the only metal that can form a bond with bones [,]. Due to its advantageous characteristics of high strength, stiffness, fracture toughness, and dependable mechanical performance as replacements for hard tissues, it continues to be one of the most crucial components utilized in orthopedic implant devices. It is stable and insoluble in vivo [,]. D. Mccutchen et al. [] conducted a study of the wear properties and friction of alpha titanium and showed poor wear characteristics for unalloyed titanium and common Ti alloys. From the view of Z Liu et al. [], Ti alloys are still under development. Meanwhile, porous Ti alloys are used widely as orthopedic implants because of their property of good biological fixation with bone tissue ingrowth into the porous network [,]. They allow close apposition of the bone under proper conditions, as bioinert material. This procedure is called osseointegration. Titanium grade 2 is mainly used in orthopedics and prosthesis implants [,].
Niinomi et al. [] mentioned that β-type Ti alloys have low stiffness, great cold workability, and high strength. As a result, low-rigidity β-type titanium alloys have a high scope of research and development. For the purpose of justifying the benefit of reduced stiffness for bone remodeling and repair, Hatori T et al. carried out some experiments on rabbits to evaluate the bone tissue reaction to new β-type titanium alloys. Utilizing rabbits, an experimental tibia fracture was generated by oscillating a saw at a point slightly below the tibial tuberosity. An intramedullary rod made of low-rigidity Ti-29Nb-13Ta-4.6Zr, Ti–6Al–4V ELI, or SUS 316L SS was inserted into the intramedullary canal to fix the fracture. Bone remodeling, atrophy, and healing were observed every 2 weeks by X-ray image transmission up to 24 weeks. The results are shown in Figure 7 [,].
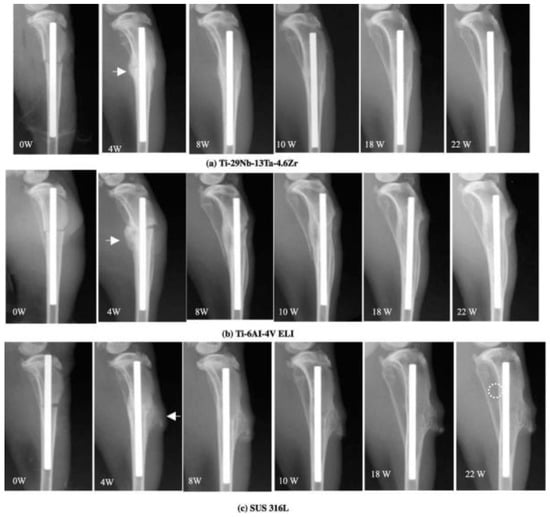
Figure 7.
X-ray used to track the healing of a bone fracture from the time of surgery until 22 weeks later. Callus development is shown by the arrow, and atrophic alteration is indicated by the dotted round mark. Adapted with permission from [] 2022.
In Ti-29Nb-13Ta-4.6Zr, it was found that the fracture callus pattern was extremely smooth with bone remodeling. A similar behavior was seen in Ti-6Al-4V ELI and SUS 316L after 8 weeks. In Ti–29Nb–13Ta–4.6Zr, the amount of callus fracture was relatively small and decreased steadily after 6 weeks, and then no signs of fracture were found after 10 weeks after fixation. No difference could be detected after 10 weeks, up to 18 weeks. However, a little atrophic shift in the posterior tibial bone was noticed after 20 weeks. The callus development and bone remodeling in Ti-6Al-4V ELI were slower than in Ti-29Nb-13Ta-4.6Zr, but they were virtually identical to those in Ti-29Nb-13Ta-4.6Zr. A little atrophic shift was reportedly seen at 18 weeks. A large amount of the fracture callus was observed in SUS 316L stainless steel and remained until the end of the current cycle. At 10 weeks, bone atrophy appeared to occur in the posterior proximal tibial bone, which became evident every 2 weeks. At 24 weeks, the posterior tibial bone started to become quite thin. Therefore, it was observed that the load transfer issue of the existing metal implants with high stiffness may be improved by the low-rigidity Ti alloy and Ti-29Nb-13Ta-4.6Zr. This proves the mechanical biocompatibility of low-rigidity Ti alloys for biomedical applications.
5.3. Biocompatibility of Titanium and Its alloy
The mechanical biocompatibility of Ti metal implants should be enhanced for long-term usage [,,]. Studies and developments are taking place in order to increase the biocompatibility of Ti alloys. For achieving excellent blood compatibility, V. Manivasagam et al. [] mentioned that biopolymer surface improvisations are to be carried out on Ti alloys. They also revealed that this surface modification is performed via chemical bonding, and if this surface modification is conducted successfully, then a high-endurance extended life scaffold for artificial organs may be created through incorporation with tissue engineering.
Niinomi et al. [,] say that in order to perform this surface modification, phosphate-calcium-type ceramics, such as HAP phosphate calcium, are mainly coated on the surface of titanium to get the formation of HAP, which increases the biocompatibility of titanium. The biocompatibility of the implanted surface depends highly on the initial immobilization and possible structural changes of mediating proteins at the implant surface [,]. The surface modification process not only helps in increasing the biocompatibility, but also helps in shortening the healing time after implantation and also helps in the improved bone bonding of the implant. Sobieszczyk et al. [], based on their findings, have proven that oxygen and helium implantation can improve the biocompatibility of titanium and its alloys. Kulkarni et al. [] state that the surface modification process for titanium can be performed by various methods, such as chemical, physical, and mechanical methods. Mohammed et al. [] list some surface modification processes used to increase the biocompatibility and other features of Ti, such as chemical vapor deposition, laser cladding, thermal oxidation and thermal spraying, plasma spray, ion implantation, micro-arc oxidation, sand blasting, and electrochemical treatment. From the observations, it was found that the biocompatibility of Ti and its alloys is appreciable. Hence, the use of Ti and its alloys can be promoted in making lower-limb prostheses.
5.4. Use of Titanium and Its Alloy as Lower-Limb Prostheses
The majority of limb salvage surgeries can be completed with the use of modular prostheses; however, customized prostheses are still required []. Cronskar et al. [] made Ti-6Al-4V hip stems using EBM. The authors found that the fatigue limit is reduced as compared with the conventional machining process. M. Galati et al. [] created a full factorial design of an experiment model and made a deep investigation of surface roughness to obtain analytical predictive models. The proposed models are useful for predicting the surface roughness of Ti-6Al-4V components manufactured using the EBM method. They concluded that the final surface texture is greatly influenced by the surface design, which also has a substantial impact on heat transport. Lebea et al. [] performed an experimental investigation into the effect of surface roughness and the mechanical properties of 3D-printed Ti alloy after heat treatment. They compared the performance of implants manufactured by EBM and direct metal laser sintering methods. It was observed that higher surface roughness results in lower mechanical properties, ultimately leading to decreased implant life and poor performance. Riwa et al. [] reviewed the mechanical properties and characterization of laser-based powder bed–fused Ti lattice structures. They used bending, compression, and tensile tests for obtaining the results. The authors observed that the lattice structures have low weight, good mechanical properties, and energy absorption capabilities that make them suitable in the fields of biomedicine. Murr et al. [] and Liu et al. [] used EBM porous structures for knee replacements and found Ti alloy as a suitable material for the purpose. Ruppert et al. [] compared femoral heads made using EBM and SLM and found that coarse EBM implants have higher removal torque as compared with fine implants made using SLM. Moreover, a study conducted by Goswami et al. [] found that a femoral stem made of Ti alloys with elliptical and circular cross sections had reduced stresses over materials, such as Cr-Co-Mo and stainless steel 316L. The next section gives a brief idea about the structural defects in AM implants.
5.5. Structural Defects in AM Implants
There are certain critical regions in HIs that tend to become cracked and fail in the future. Identification of these regions and redesigning them is a challenge for many researchers. According to C Gao et al. [], even though normal prostheses have had significant success in orthopedics, several issues still need to be resolved, such as implant osseointegration issues and the inability to completely repair bone defects. They may cause implant failure and even implant revision []. The implants can be customized to overcome bone defect according to the 3D data. This can be treated by a CAD software for the model and TO []. Tanisha Pereira et al. [] concluded that AM-made implants have less structural defects as compared with other traditional methods. Among the researcher groups, Babic et al. [] investigated by applying linear elastic finite element analysis the maximum tensile and compressive stresses in HI. The CAD model was prepared by 3D scanning, and loads were taken as per ISO 7206-4 standards for investigation. They found that the lateral side of HI has maximum tensile stresses, while compressive stress is maximum on the anterior medial side in which the fatigue cracks are being developed. A study carried out by Sedmak et al. [] analyzed the number of cycles a Ti-6Al-4V HI endures after a crack first appears by performing normal walking. Loading conditions of 2.040 and 3.845 KN were taken as the values for slow and normal walking to analyze the finite element analysis of the prosthesis. The results showed that after 80,022,270 cycles, an initial crack growth of 0.5 mm was created, leading to a fracture point growth of 18.5 mm. Pimenta et al. [] investigated the failure of total hip replacement in a 62-year-old patient with the help of finite element, mechanical, and microstructural analysis. The pits present in the implant surface were the reason for the fatigue that initiated the fracture of HI. Nadia Koura et al. [] talk about the CT scanning method that gives pertinent information about faults with AM acetabular hip prostheses such as fractures and porosity in the main part of the body and about powder trapped inside the porous structure. They mention that these defects can be identified during the preparation of image processing. The method described in their paper concentrates on the porous structure of AM acetabular hip prosthesis with image processing. They presented the frequency of occurrences of each pore and strut size in histograms, and it seems that the results are appreciable. AM Vilardell et al. [] found small pores up to 0.5 mm and total porosity up to 1%, which makes no difference in the strength of AM implants. The authors came to the conclusion that failure mechanisms are mostly controlled by the microstructure and that the component shape and surface roughness are more significant factors. Small porosity can occasionally be present without harming the strength or ductility under static loading circumstances. The strength and ductility decrease with increasing porosity, and failures are more likely to start at the biggest pores []. In most studies, it was visible that porosity has a greater impact on ductility than strength. Based on the findings of A Du Plessis et al. [], X-ray tomography makes it simple to determine the types and sizes of pores, and only the largest pores are really important. To overcome the structural defects, it is a better option to make a defect-free CAD model before the AM process. The next session deals with the TO technique, which helps in making error-free models of HI for the AM process.
5.6. Topology Optimization (TO) of Implants
TO is used for the shape optimization of mechanical structures []. This optimization method is suitable for the design stage of a new part since it can investigate novel ideas and solutions in regions where researchers meet difficulties []. It takes a 3D design space to reduce the material within it to achieve the most efficient design []. N. Gardan et al. [] say that for the most part, with TOs, it is the case that we want to maximize stiffness while reducing the model’s volume. By reducing the strain energy in the model, the stiffness is increased. It is also feasible to focus optimization on different goals, such as certain eigenfrequencies or local displacements. Naichao Wu et al. [] define TO as a technique that can provide more efficient material distribution according to the objective function under the special load and boundary conditions. They mention that many researchers have paid close attention to TO for the optimal design of orthopedic implants. Moreover, development of AM helps to make the complex structure of the TO design easily. In their study, M. Belwanshi et al. [] say that finite element analysis enables the design and optimization of the HI model to improve its strength by optimizing stress and strain distribution. They found that the TO technique was taken by some authors for achieving results, as shown in Figure 8.
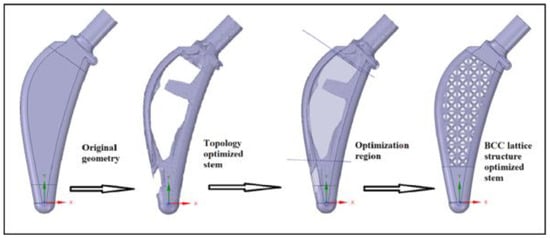
Figure 8.
The process of hip stem topology optimization with a BCC lattice structure. Adapted with permission from [] 2022.
It was observed that many researchers followed TO, such as Milovanovic et al. []. They investigated five Ti-6Al-4V HIs having various neck diameters for reducing the stresses that result in crack formation and then by failure of implants by reducing the neck thickness. The results showed that a neck having 9 mm thickness has reduced stress as compared with other samples. From the study, it can be easily mentioned that the reverse engineering process helps in the design and optimization of implants having better mechanical properties. Meanwhile, N Kladovasilakis et al. [] conducted the TO of HI; then they modelled bioinspired lattice structures. The name of the lattice structures is as follows: Voronoi, Gyroid, and Schwarz diamond. Finite element analysis was carried out for finding the mechanical strength. The experiment shows that the Schwarz diamond lattice was the best suitable lattice having the least amount of stress. In a study conducted by Nathanael Tan et al. [], the focus of their paper was to develop a multiple-load-case TO approach for a hip implant design, rather than to produce a specific “best” design. Two proof-of-concept designs were created using a TO method that was devised and applied to the construction of the femoral hip stem. These designs are drastically different from one another, yet they both reduce stiffness in comparison with a conventional solid implant. It was observed that more load transfer in femur with reduced risk of strain shielding can be made available by designing HIs with the TO technique.
6. Discussion
Reviewing publications related to AM and HIs shows that the majority of the studies investigated the possibility of using Ti and its alloys for making ideal hip prosthesis. A superior biocompatibility and osseointegration property make the material more attractive. However, the life and mechanical properties of HIs need further improvisation by reducing the wear. Here, the critical regions that initiate crack propagation and fatigue failure can be redesigned and optimized for improved efficiency. The most relevant findings of this study is TO, which can be used to design implants with increased compliance for reduced stress. The fixation devices that help to reduce implant failure can also be studied as part of future investigations.
Other research groups have found that:
- The most recent manufacturing process is electron beam melting [,,,,]. It is a useful method to directly manufacture customized orthopedic implants of Ti. It can improve the effectiveness of limb salvage surgery for sarcomas in usual sites [].
- The mainly used AM technology in the manufacture of metal implants is DED [] and PBF []. However, laser PBF, which is also called SLM, is widely used for making Ti and other metal alloys for implants, because this process provides an advantage of manufacturing complex structures with a customized design [].
- AM is recently used for a number of biomedical applications, such as printing of biodegradable tissue and planning of surgical operations, but most importantly, it is used more in the development of orthopedic implants [].
- Most of the studies say that titanium possesses greater mechanical properties and stiffness compared with natural bone, which may result in the failure of an implant []. Hence, alloying is necessarily important. Even though titanium is more expensive than other metals and it has poor wear resistance, it is mainly used as a metal implant because it adapts well to the human body when it is implanted [].
- Some surface modifications are needed for Ti and its alloys in order to achieve better bonding with the human body. Surface chemistry, surface potential, surface roughness, surface conductivity, and surface energy are the needed modifications. These modifications cause protein adhesion and biofilm formation on the implants, which will lead to the change in biocompatibility and then lead to the ultimate success of the implant [,].
7. Conclusions
Reviews on state-of-art studies on the AM-associated design and fabrication of HIs lead to the following conclusions.
- According to the general agreement on the results, AM is a successful method used in orthopedics mainly for the replication of bones. AM improves patient outcome and creates reproducible models with reduced operating time. Even though ME, PBF, VP, BJ, MJ, and SL each have their own advantages, from the authors’ perspective, DED is the most efficiently used AM technique for making metallic implants. DED has the ability to control the grain structure, so the process is highly recommended for the repair of high-quality functional parts. The laser and electron beam can be controlled very precisely. The DED process also allows for the creation of components with composition gradients or hybrid structures using multiple materials with differing compositions.
- Ti and its alloys are suitable for making HIs using the AM process. Their biocompatibility and biological inertness are appreciable. However, notch sensitivity and poor wear resistance, when compared with other metals, make Ti and its alloys remain hurdles in a successful implant osseointegration.
- According to the major observations of the researchers, the reverse engineering process helps in the design and optimization of implants with better mechanical properties.
- This review reveals that increasing the porosity inside the HI results in the reduction of strength and ductility, and therefore, failures are likely to initiate at the largest pores. Such defects can be overcome by using an optimization method, such as TO, for the design of HIs.
From the analysis, it can be concluded that Ti-based HI made using AM holds a brilliant promise in the field of bone replacements, in particular, regarding process efficiency and product quality. Therefore, an extensive study is required to understand the ability to produce lightweight HIs with design improvements and will be performed in future research.
Author Contributions
Conceptualization, A.A. and J.E.A.Q.; methodology, A.A. and J.E.A.Q.; software, A.A.; validation, A.K. and A.K.V.; formal analysis, A.A. and J.E.A.Q.; investigation, A.A. and A.K.; resources, A.A., J.E.A.Q. and A.K.; writing—original draft preparation, A.A. and J.E.A.Q.; writing—review and editing, A.A., J.E.A.Q. and A.K.; visualization, A.A. and A.K.V.; supervision, A.A. and J.E.A.Q.; project administration, J.E.A.Q. and A.A.; funding acquisition, J.E.A.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Affairs Office at UAE University, grant number 31N424.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jon Goldberg, A.; Kuhn, L.T. Biomaterials. In Regenerative Engineering; CRC Press: Boca Raton, FL, USA, 2013; ISBN 9781482216837. [Google Scholar]
- O’Brien, F.J. Biomaterials & Scaffolds for Tissue Engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Nair, L.S.; Laurencin, C.T. Biodegradable Polymers as Biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The Path Forward for Biofuels and Biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef]
- Vert, M.; Doi, Y.; Hellwich, K.H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for Biorelated Polymers and Applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Hench, L.L. Biomaterials: A Forecast for the Future. Biomaterials 1998, 19, 1419–1423. [Google Scholar] [CrossRef]
- Hastings, G.W. Bio-Materials Science and Technology. Biomater. Med. Devices Artif. Organs 1979, 7, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; de Groot, K. Calcium Phosphate Biomaterials: An Overview. In Learning from Nature How to Design New Implantable Biomaterialsis: From Biomineralization Fundamentals to Biomimetic Materials and Processing Routes; Springer: Dordrecht, The Netherlands, 2004; pp. 37–57. [Google Scholar]
- Lin, K.; Xia, L.; Li, H.; Jiang, X.; Pan, H.; Xu, Y.; Lu, W.W.; Zhang, Z.; Chang, J. Enhanced Osteoporotic Bone Regeneration by Strontium-Substituted Calcium Silicate Bioactive Ceramics. Biomaterials 2013, 34, 10028–10042. [Google Scholar] [CrossRef] [PubMed]
- Kashirina, A.; Yao, Y.; Liu, Y.; Leng, J. Biopolymers as Bone Substitutes: A Review. Biomater. Sci. 2019, 7, 3961–3983. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Bioceramics of Calcium Orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef]
- Jones, F.H. Teeth and Bones: Applications of Surface Science to Dental Materials and Related Biomaterials. Surf. Sci. Rep. 2001, 42, 75–205. [Google Scholar] [CrossRef]
- Zeng, R.; Dietzel, W.; Witte, F.; Hort, N.; Blawert, C. Progress and Challenge for Magnesium Alloys as Biomaterials. Adv. Eng. Mater. 2008, 10, B3–B14. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological Design and Additive Manufacturing of Porous Metals for Bone Scaffolds and Orthopaedic Implants: A Review; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 83, pp. 127–141. [Google Scholar]
- Hänzi, A.C.; Gerber, I.; Schinhammer, M.; Löffler, J.F.; Uggowitzer, P.J. On the in Vitro and in Vivo Degradation Performance and Biological Response of New Biodegradable Mg-Y-Zn Alloys. Acta Biomater. 2010, 6, 1824–1833. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Advincula, R.C. Mechanical Characterization of 3D-Printed Polymers. Addit. Manuf. 2018, 20, 44–67. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies: 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Javaid, M.; Haleem, A. Additive Manufacturing Applications in Medical Cases: A Literature Based Review. Alex. J. Med. 2018, 54, 411–422. [Google Scholar] [CrossRef]
- Jyothish Kumar, L.; Pandey, P.M.; Wimpenny, D.I. 3D Printing and Additive Manufacturing Technologies; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 9789811303050. [Google Scholar]
- Li, C.; Liu, Z.Y.; Fang, X.Y.; Guo, Y.B. Residual Stress in Metal Additive Manufacturing. Procedia Cirp. 2018, 71, 348–353. [Google Scholar] [CrossRef]
- Culmone, C.; Smit, G.; Breedveld, P. Additive Manufacturing of Medical Instruments: A State-of-the-Art Review. Addit. Manuf. 2019, 27, 461–473. [Google Scholar] [CrossRef]
- Salmi, M.; Paloheimo, K.S.; Tuomi, J.; Wolff, J.; Mäkitie, A. Accuracy of Medical Models Made by Additive Manufacturing (Rapid Manufacturing). J. Cranio-Maxillofac. Surg. 2013, 41, 603–609. [Google Scholar] [CrossRef]
- Zadpoor, A.A.; Malda, J. Additive Manufacturing of Biomaterials, Tissues, and Organs. Ann. Biomed. Eng. 2017, 45, 1–11. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti Based Biomaterials, the Ultimate Choice for Orthopaedic Implants—A Review; Elsevier Ltd.: Amsterdam, The Netherlands, 2009; Volume 54, pp. 397–425. [Google Scholar]
- Ekdahl, K.N.; Lambris, J.D.; Elwing, H.; Ricklin, D.; Nilsson, P.H.; Teramura, Y.; Nicholls, I.A.; Nilsson, B. Innate Immunity Activation on Biomaterial Surfaces: A Mechanistic Model and Coping Strategies. Adv. Drug Deliv. Rev. 2011, 63, 1042–1050. [Google Scholar] [CrossRef]
- Davies, J.E. Bone Bonding at Natural and Biomaterial Surfaces. Biomaterials 2007, 28, 5058–5067. [Google Scholar] [CrossRef]
- Patel, N.; Gohil, P. A Review on Biomaterials: Scope, Applications & Human Anatomy Significance. Int. J. Emerg. Technol. Adv. Eng. 2012, 2, 91–101. [Google Scholar]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Development of Biomaterial Scaffold for Nerve Tissue Engineering: Biomaterial Mediated Neural Regeneration. J. Biomed. Sci. 2009, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Bellamkonda, R.V. Biomaterials for the Central Nervous System. J. R. Soc. Interface 2008, 5, 957–975. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for Tissue Engineering: Scaffold Design Variables and Applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Webber, M.J.; Appel, E.A.; Meijer, E.W.; Langer, R. Supramolecular Biomaterials. Nat. Mater. 2016, 15, 13–26. [Google Scholar] [CrossRef]
- Huebsch, N.; Mooney, D.J. Inspiration and Application in the Evolution of Biomaterials. Nature 2009, 462, 426–432. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and Its Alloys as Orthopedic Biomaterials: A Review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Matsushita, T.; Takahashi, H. Orthopedic Applications of Metallic Biomaterials. In Metals for Biomedical Devices; Woodhead Publishing: Cambridge, UK, 2019; ISBN 9780081026663. [Google Scholar]
- Jin, W.; Chu, P.K. Orthopedic Implants. In Encyclopedia of Biomedical Engineering; Elsevier, 2019; Available online: http://www.cityu.edu.hk/phy/appkchu/Publications/2017/17.108.pdf (accessed on 1 November 2022).
- Ebramzadeh, E.; Chiesa, R.; Sangiorgio, S.N.; Longjohn, D.B. Orthopedic Biomaterials. In Successful Techniques for Total Hip Replacement; Future Medicine Ltd.: London, UK, 2014; ISBN 9781780844718. [Google Scholar]
- Mansfield, B.; Torres, S.; Yu, T.; Wu, D. A Review on Additive Manufacturing of Ceramics. In Proceedings of the ASME 2019 14th International Manufacturing Science and Engineering Conference. American Society of Mechanical Engineers 2019, Erie, PA, USA, 10–14 June 2019; pp. 609–614. [Google Scholar] [CrossRef]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg Alloys for Orthopedic Implants—A Review. J. Magnes. Alloy. 2021, 9, 1884–1905. [Google Scholar] [CrossRef]
- Novikova, G.E. Introduction to Corrosion of Bioimplants. Prot. Met. Phys. Chem. Surf. 2011, 47, 372–380. [Google Scholar] [CrossRef]
- Kurella, A.; Dahotre, N.B. Review Paper: Surface Modification for Bioimplants: The Role of Laser Surface Engineering. J. Biomater. Appl. 2005, 20, 5–50. [Google Scholar] [CrossRef]
- Kang, C.W.; Fang, F.Z. State of the Art of Bioimplants Manufacturing: Part I. Adv. Manuf. 2018, 6, 20–40. [Google Scholar] [CrossRef]
- Lazzi, G. Thermal Effects of Bioimplants. IEEE Eng. Med. Biol. Mag. 2005, 24, 75–81. [Google Scholar] [CrossRef]
- Ho, C.M.B.; Ng, S.H.; Yoon, Y.J. A Review on 3D Printed Bioimplants. Int. J. Precis. Eng. Manuf. 2015, 16, 1035–1046. [Google Scholar] [CrossRef]
- Attar, H.; Calin, M.; Zhang, L.C.; Scudino, S.; Eckert, J. Manufacture by Selective Laser Melting and Mechanical Behavior of Commercially Pure Titanium. Mater. Sci. Eng. A 2014, 593, 170–177. [Google Scholar] [CrossRef]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable Materials and Metallic Implants-A Review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Minagar, S.; Berndt, C.C.; Wang, J.; Ivanova, E.; Wen, C. A Review of the Application of Anodization for the Fabrication of Nanotubes on Metal Implant Surfaces. Acta Biomater. 2012, 8, 2875–2888. [Google Scholar] [CrossRef]
- Rohman, G. Materials Used in Biomaterial Applications. In Biomaterials; Wiley Online Library John Wiley & Sons, Inc., 2014; Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119043553.ch3 (accessed on 1 November 2022).
- Jayaraj, K.; Pius, A. Biocompatible Coatings for Metallic Biomaterials. In Fundamental Biomaterials: Metals; Woodhead Publishing: Cambridge, UK, 2018. [Google Scholar] [CrossRef]
- Dewidar, M.M.; Yoon, H.C.; Lim, J.K. Mechanical Properties of Metals for Biomedical Applications Using Powder Metallurgy Process: A Review. Met. Mater. Int. 2006, 12, 193–206. [Google Scholar] [CrossRef]
- Das, D.; Zhang, Z.; Winkler, T.; Mour, M.; Gunter, C.; Morlock, M.; Machens, H.G.; Schilling, A.F. Bioresorption and Degradation of Biomaterials. Adv. Biochem. Eng. Biotechnol. 2012, 126, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Arango, S.; Peláez-Vargas, A.; García, C. Coating and Surface Treatments on Orthodontic Metallic Materials. Coatings 2013, 3, 1–15. [Google Scholar] [CrossRef]
- Li, R.W.K.; Chow, T.W.; Matinlinna, J.P. Ceramic Dental Biomaterials and CAD/CAM Technology: State of the Art. J. Prosthodont. Res. 2014, 58, 208–216. [Google Scholar] [CrossRef]
- Pina, S.; Reis, R.L.; Oliveira, J.M. Ceramic Biomaterials for Tissue Engineering. In Fundamental Biomaterials: Ceramics; Elsevier, 2018; Available online: https://hdl.handle.net/1822/51774 (accessed on 1 November 2022).
- Habibovic, P.; Yuan, H.; Van Der Valk, C.M.; Meijer, G.; Van Blitterswijk, C.A.; De Groot, K. 3D Microenvironment as Essential Element for Osteoinduction by Biomaterials. Biomaterials 2005, 26, 3565–3575. [Google Scholar] [CrossRef]
- Kamitakahara, M.; Ohtsuki, C.; Miyazaki, T. Review Paper: Behavior of Ceramic Biomaterials Derived from Tricalcium Phosphate in Physiological Condition. J. Biomater. Appl. 2008, 23, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Ke, D.; Sahasrabudhe, H.; Bandyopadhyay, A. Additive Manufacturing of Biomaterials. Prog. Mater. Sci. 2018, 93, 45–111. [Google Scholar] [CrossRef] [PubMed]
- Frazar, E.M.; Shah, R.A.; Dziubla, T.D.; Hilt, J.Z. Multifunctional Temperature-Responsive Polymers as Advanced Biomaterials and Beyond. J. Appl. Polym. Sci. 2020, 137, 48770. [Google Scholar] [CrossRef]
- Backes, E.H.; Pires, L.D.N.; Beatrice, C.A.G.; Costa, L.C.; Passador, F.R.; Pessan, L.A. Fabrication of Biocompatible Composites of Poly(Lactic Acid)/Hydroxyapatite Envisioning Medical Applications. Polym. Eng. Sci. 2020, 60, 636–644. [Google Scholar] [CrossRef]
- Demina, T.S.; Frolova, A.; Istomin, A.V.; Kotova, S.L.; Piskarev, M.S.; Bardakova, K.N.; Yablokov, M.Y.; Altynov, V.A.; Kravets, L.I.; Gilman, A.B.; et al. Coating of Polylactide Films by Chitosan: Comparison of Methods. J. Appl. Polym. Sci. 2020, 13, 48287. [Google Scholar] [CrossRef]
- Dellaquila, A.; Greco, G.; Campodoni, E.; Mazzocchi, M.; Mazzolai, B.; Tampieri, A.; Pugno, N.M.; Sandri, M. Optimized Production of a High-Performance Hybrid Biomaterial: Biomineralized Spider Silk for Bone Tissue Engineering. J. Appl. Polym. Sci. 2020, 137, 48739. [Google Scholar] [CrossRef]
- Calleros, E.L.; Simonovsky, F.I.; Garty, S.; Ratner, B.D. Crosslinked, Biodegradable Polyurethanes for Precision-Porous Biomaterials: Synthesis and Properties. J. Appl. Polym. Sci. 2020, 137, 48943. [Google Scholar] [CrossRef]
- Newswire, P.R. Dental Bone Graft Substitutes and Other Biomaterials Market (Natural, Ceramic, Composite and Polymer)—Global Industry Analysis, Size, Share, Growth, Trends and Forecast 2014–2020. NY-Reportlinker. 2014. Available online: http://www.Prnewswire.com (accessed on 12 March 2022).
- Devi, P.R.S. Biomaterial and Its Medical. Int. J. Adv. Res. Innov. Ideas Educ. 2017, 3, 489–498. [Google Scholar]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Jones, L.C.; Timmie Topoleski, L.D.; Tsao, A.K. Biomaterials in Orthopaedic Implants; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081002865. [Google Scholar]
- Gallo, J.; Gibon, E.; Goodman, S.B. Implants for Joint Replacement of the Hip and Knee. In Materials and Devices for Bone Disorders; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Evans, E.M.; Freeman, M.A.R.; Miller, A.J.; Vernon Roberts, B. Metal Sensitivity as a Cause of Bone Necrosis and Loosening of the Prosthesis in Total Joint Replacement. J. Bone Jt. Surg.—Ser. B 1974, 56-B, 626–642. [Google Scholar] [CrossRef]
- Ghosh, S.; Sanghavi, S.; Sancheti, P. Metallic Biomaterial for Bone Support and Replacement. In Fundamental Biomaterials: Metals; Woodhead Publishing: Cambridge, UK, 2018. [Google Scholar] [CrossRef]
- Shayesteh Moghaddam, N.; Taheri Andani, M.; Amerinatanzi, A.; Haberland, C.; Huff, S.; Miller, M.; Elahinia, M.; Dean, D. Metals for Bone Implants: Safety, Design, and Efficacy. Biomanuf. Rev. 2016, 1, 1–16. [Google Scholar] [CrossRef]
- Simske, S.J.; Ayers, R.A.; Bateman, T.A. Porous Materials for Bone Engineering. Mater. Sci. Forum 1997, 250, 151–182. [Google Scholar] [CrossRef]
- Manakari, V.; Parande, G.; Gupta, M. Selective Laser Melting of Magnesium and Magnesium Alloy Powders: A Review. Metals 2017, 7, 2. [Google Scholar] [CrossRef]
- Bansiddhi, A.; Sargeant, T.D.; Stupp, S.I.; Dunand, D.C. Porous NiTi for Bone Implants: A Review. Acta Biomater. 2008, 4, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Katti, K.S. Biomaterials in Total Joint Replacement. Colloids Surf. B Biointerfaces 2004, 39, 133–142. [Google Scholar] [CrossRef]
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication Methods of Porous Metals for Use in Orthopaedic Applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef] [PubMed]
- Bommala, V.K.; Krishna, M.G.; Rao, C.T. Magnesium Matrix Composites for Biomedical Applications: A Review. J. Magnes. Alloy. 2019, 7, 72–79. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A. Additive Manufacturing Applications in Orthopaedics: A Review. J. Clin. Orthop. Trauma 2018, 9, 202–206. [Google Scholar] [CrossRef]
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical Implants: Corrosion and Its Prevention-a Review. Recent Pat. Corros. Sci. 2010, 2, 40–54. [Google Scholar] [CrossRef]
- Mantripragada, V.P.; Lecka-Czernik, B.; Ebraheim, N.A.; Jayasuriya, A.C. An Overview of Recent Advances in Designing Orthopedic and Craniofacial Implants. J. Biomed. Mater. Res.—Part A 2013, 101, 3349–3364. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, A.; Rivera, E.; Brostow, W.; Castaño, V.M. Ceramic Biomaterials: An Introductory Overview. J. Mater. Educ. 1999, 21, 267–276. [Google Scholar]
- Kulkarni, S.V.; Nemade, A.C.; Sonawwanay, P.D. An Overview on Metallic and Ceramic Biomaterials. Recent Adv. Manuf. Process. Syst. 2022, 149–165. [Google Scholar]
- Festas, A.J.; Ramos, A.; Davim, J.P. Medical Devices Biomaterials–A Review. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2020, 234, 218–228. [Google Scholar] [CrossRef]
- Andrade, C.T.; Rojas, E.G.A. Biopolymers. In Biopolymer Engineering in Food Processing; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9781439844953. [Google Scholar]
- Jahnke, A. Could Biopolymers Answer the Single-Use Problem? Reinf. Plast. 2020, 64, 317–319. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I. Production of Poly-Hydroxyalkanoate as Secondary Metabolite with Main Focus on Sustainable Energy. Renew. Sustain. Energy Rev. 2017, 72, 95–104. [Google Scholar] [CrossRef]
- Suzuki, S.; Ikada, Y. Biomaterials for Surgical Operation; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; ISBN 1617795704. [Google Scholar]
- Yusuf, S.M.; Cutler, S.; Gao, N. Review: The Impact of Metal Additive Manufacturing on the Aerospace Industry. Metals 2019, 9, 1286. [Google Scholar] [CrossRef]
- Sing, S.L.; Yeong, W.Y.; Wiria, F.E.; Tay, B.Y.; Zhao, Z.; Zhao, L.; Tian, Z.; Yang, S. Direct Selective Laser Sintering and Melting of Ceramics: A Review. Rapid Prototyp. J. 2017, 23, 611–623. [Google Scholar] [CrossRef]
- Krishna, B.V.; Xue, W.; Bose, S.; Bandyopadhyay, A. Functionally Graded Co–Cr–Mo Coating on Ti–6Al–4V Alloy Structures. Acta Biomater. 2008, 4, 697–706. [Google Scholar] [CrossRef]
- Feenstra, D.R.; Banerjee, R.; Fraser, H.L.; Huang, A.; Molotnikov, A.; Birbilis, N. Critical Review of the State of the Art in Multi-Material Fabrication via Directed Energy Deposition. Curr. Opin. Solid State Mater. Sci. 2021, 25, 100924. [Google Scholar] [CrossRef]
- Vartanian, K.; Brewer, L.; Manley, K.; Cobbs, T. Powder Bed Fusion vs. Directed Energy Deposition Benchmark Study: Mid-Size Part with Simple Geometry. Optomec Tech. Rep 2016, 1, 1–4. [Google Scholar]
- Del Val, J.; Arias-González, F.; Barro, O.; Riveiro, A.; Comesaña, R.; Penide, J.; Lusquiños, F.; Bountinguiza, M.; Quintero, F.; Pou, J. Functionally Graded 3D Structures Produced by Laser Cladding. Procedia Manuf. 2017, 13, 169–176. [Google Scholar] [CrossRef]
- Huotilainen, E.; Salmi, M.; Chekurov, S.; Flores Ituarte, I. Effect of Build Orientation in 3D Printing Production for Material Extrusion, Material Jetting, Binder Jetting, Sheet Object Lamination, Vat Photopolymerisation, and Powder Bed Fusion. Int. J. Collab. Enterp. 2016, 5, 218. [Google Scholar] [CrossRef]
- Wasti, S.; Adhikari, S. Use of Biomaterials for 3D Printing by Fused Deposition Modeling Technique: A Review. Front. Chem. 2020, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Ortega, Z.; Alemán, M.E.; Benítez, A.N.; Monzón, M.D. Theoretical–Experimental Evaluation of Different Biomaterials for Parts Obtaining by Fused Deposition Modeling. Measurement 2016, 89, 137–144. [Google Scholar] [CrossRef]
- Li, B.; Webster, T. Orthopedic Biomaterials Progress in Biology, Manufacturing, and Industry Perspectives; Springer: Dordrecht, The Netherlands, 2018; ISBN 9783319895420. [Google Scholar]
- Chueh, Y.-H.; Wei, C.; Zhang, X.; Li, L. Integrated Laser-Based Powder Bed Fusion and Fused Filament Fabrication for Three-Dimensional Printing of Hybrid Metal/Polymer Objects. Addit. Manuf. 2020, 31, 100928. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Mellor, S.; Hao, L.; Zhang, D. Additive Manufacturing: A Framework for Implementation. Int. J. Prod. Econ. 2014, 149, 194–201. [Google Scholar] [CrossRef]
- Singh, D.D.; Mahender, T.; Reddy, A.R. Powder Bed Fusion Process: A Brief Review. Mater. Today Proc. 2021, 46, 350–355. [Google Scholar] [CrossRef]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Advances in Powder Bed Fusion 3D Printing in Drug Delivery and Healthcare. Adv. Drug Deliv. Rev. 2021, 174, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Gibson, I.; Rosen, D.; Stucker, B. Powder Bed Fusion Processes. In Additive Manufacturing Technologies; Springer: Berlin/Heidelberg, Germany, 2015; pp. 107–145. [Google Scholar]
- Derimow, N.; Hrabe, N. Oxidation in Reused Powder Bed Fusion Additive Manufacturing Ti-6Al-4V Feedstock: A Brief Review. Jom 2021, 73, 3618–3638. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic Implant Biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Romero, C.; Yang, F.; Bolzoni, L. Fatigue and Fracture Properties of Ti Alloys from Powder-Based Processes—A Review. Int. J. Fatigue 2018, 117, 407–419. [Google Scholar] [CrossRef]
- Fotovvati, B.; Asadi, E. Size Effects on Geometrical Accuracy for Additive Manufacturing of Ti-6Al-4V ELI Parts. Int. J. Adv. Manuf. Technol. 2019, 104, 2951–2959. [Google Scholar] [CrossRef]
- Gruber, S.; Grunert, C.; Riede, M.; López, E.; Marquardt, A.; Brueckner, F.; Leyens, C. Comparison of Dimensional Accuracy and Tolerances of Powder Bed Based and Nozzle Based Additive Manufacturing Processes. J. Laser Appl. 2020, 32, 32016. [Google Scholar] [CrossRef]
- Alammar, A.; Kois, J.C.; Revilla-León, M.; Att, W. Additive Manufacturing Technologies: Current Status and Future Perspectives. J. Prosthodont. 2022, 31, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Starosolski, Z.A.; Kan, J.H.; Rosenfeld, S.D.; Krishnamurthy, R.; Annapragada, A. Application of 3-D Printing (Rapid Prototyping) for Creating Physical Models of Pediatric Orthopedic Disorders. Pediatr. Radiol. 2014, 44, 216–221. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, Y. Additive Manufacturing Based on Optimized Mask Video Projection for Improved Accuracy and Resolution. J. Manuf. Process. 2012, 14, 107–118. [Google Scholar] [CrossRef]
- Shah, P.; Racasan, R.; Bills, P. Comparison of Different Additive Manufacturing Methods Using Computed Tomography. Case Stud. Nondestruct. Test. Eval. 2016, 6, 69–78. [Google Scholar] [CrossRef]
- Fayazfar, H.; Salarian, M.; Rogalsky, A.; Sarker, D.; Russo, P.; Paserin, V.; Toyserkani, E. A Critical Review of Powder-Based Additive Manufacturing of Ferrous Alloys: Process Parameters, Microstructure and Mechanical Properties. Mater. Des. 2018, 144, 98–128. [Google Scholar] [CrossRef]
- Shick, T.M.; Abdul Kadir, A.Z.; Ngadiman, N.H.A.; Ma’aram, A. A Review of Biomaterials Scaffold Fabrication in Additive Manufacturing for Tissue Engineering. J. Bioact. Compat. Polym. 2019, 34, 415–435. [Google Scholar] [CrossRef]
- Dini, F.; Ghaffari, S.A.; Jafar, J.; Hamidreza, R.; Marjan, S. A Review of Binder Jet Process Parameters; Powder, Binder, Printing and Sintering Condition. Met. Powder Rep. 2020, 75, 95–100. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. Binder Jetting. In Additive Manufacturing Technologies; Springer: Berlin/Heidelberg, Germany, 2021; pp. 237–252. [Google Scholar]
- Yuan, J.; Chen, C.; Yao, D.; Chen, G. 3D Printing of Oil Paintings Based on Material Jetting and Its Reduction of Staircase Effect. Polymers 2020, 12, 2536. [Google Scholar] [CrossRef]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Klocke, F.; Klink, A.; Veselovac, D.; Aspinwall, D.K.; Soo, S.L.; Schmidt, M.; Schilp, J.; Levy, G.; Kruth, J.-P. Turbomachinery Component Manufacture by Application of Electrochemical, Electro-Physical and Photonic Processes. CIRP Ann. 2014, 63, 703–726. [Google Scholar] [CrossRef]
- Salmi, M. Additive Manufacturing Processes in Medical Applications. Materials 2021, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.; Carou, D.; Rubio, E.M.; Teti, R. Current Advances in Additive Manufacturing. Procedia CIRP 2020, 88, 439–444. [Google Scholar] [CrossRef]
- Katampe, I. Innovative Applications of Polymer Materials for 3D Printing. In Defense Innovation Handbook: Guidelines, Strategies, and Techniques; CRC Press: Boca Raton, FL, USA, 2018; pp. 375–387. [Google Scholar]
- Singer, F.; Deisenroth, D.C.; Hymas, D.M.; Ohadi, M.M. Additively Manufactured Copper Components and Composite Structures for Thermal Management Applications. In Proceedings of the 2017 16th IEEE Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems (ITherm), Orlando, FL, USA, 30 May–2 June 2017; pp. 174–183. [Google Scholar]
- Feygin, M.; Hsieh, B. Laminated Object Manufacturing: A Simpler Process. In Proceedings of the 1991 International Solid Freeform Fabrication Symposium, Austin, TX, USA, 12–14 August 1991; pp. 123–130. [Google Scholar]
- Wysocki, B.; Maj, P.; Sitek, R.; Buhagiar, J.; Kurzydłowski, K.J.; Świeszkowski, W. Laser and Electron Beam Additive Manufacturing Methods of Fabricating Titanium Bone Implants. Appl. Sci. 2017, 7, 657. [Google Scholar] [CrossRef]
- Trevisan, F.; Calignano, F.; Aversa, A.; Marchese, G.; Lombardi, M.; Biamino, S.; Ugues, D.; Manfredi, D. Additive Manufacturing of Titanium Alloys in the Biomedical Field: Processes, Properties and Applications. J. Appl. Biomater. Funct. Mater. 2018, 16, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, G.J.; Hansell, R.G. Direct Tool Steel Injection Mould Inserts through the Arcam EBM Free-Form Fabrication Process. Assem. Autom. 2005, 25, 300–305. [Google Scholar] [CrossRef]
- Salmi, M.; Tuomi, J.; Paloheimo, K.S.; Björkstrand, R.; Paloheimo, M.; Salo, J.; Kontio, R.; Mesimäki, K.; Mäkitie, A.A. Patient-Specific Reconstruction with 3D Modeling and DMLS Additive Manufacturing. Rapid Prototyp. J. 2012, 18, 209–214. [Google Scholar] [CrossRef]
- Grecchi, F.; Zecca, P.A.; Macchi, A.; Mangano, A.; Riva, F.; Grecchi, E.; Mangano, C. Full-Digital Workflow for Fabricating a Custom-Made Direct Metal Laser Sintering (Dmls) Mandibular Implant: A Case Report. Int. J. Environ. Res. Public Health 2020, 17, 2693. [Google Scholar] [CrossRef]
- Gale, J.; Achuhan, A. Application of Ultrasonic Peening during DMLS Production of 316L Stainless Steel and Its Effect on Material Behavior. Rapid Prototyp. J. 2017, 23, 1185–1194. [Google Scholar] [CrossRef]
- Deckers, J.P.; Shahzad, K.; Cardon, L.; Rombouts, M.; Vleugels, J.; Kruth, J.P. Shaping Ceramics through Indirect Selective Laser Sintering. Rapid Prototyp. J. 2016, 22, 544–558. [Google Scholar] [CrossRef]
- Deckers, J.; Shahzad, K.; Vleugels, J.; Kruth, J.P. Isostatic Pressing Assisted Indirect Selective Laser Sintering of Alumina Components. Rapid Prototyp. J. 2012, 18, 409–419. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, Y.; Yang, Z.; Khoshnevis, B. Digital Material Fabrication Using Mask-Image-Projection-Based Stereolithography. Rapid Prototyp. J. 2013, 19, 153–165. [Google Scholar] [CrossRef]
- Bens, A.; Seitz, H.; Bermes, G.; Emons, M.; Pansky, A.; Roitzheim, B.; Tobiasch, E.; Tille, C. Non-Toxic Flexible Photopolymers for Medical Stereolithography Technology. Rapid Prototyp. J. 2007, 13, 38–47. [Google Scholar] [CrossRef]
- Kan, C.W.; Yuen, C.W.M. Digital Ink-Jet Printing on Textiles. Res. J. Text. Appar. 2012, 16, 1–24. [Google Scholar] [CrossRef]
- El-Wahab, H.A.; El-Molla, M.M.; Lin, L. Preparation and Characterisation of Ink Formulations for Jet Printing on Nylon Carpet. Pigment Resin Technol. 2010, 39, 163–169. [Google Scholar] [CrossRef]
- Yuan, J.; Zhu, M.; Xu, B.; Chen, G. Review on Processes and Color Quality Evaluation of Color 3D Printing. Rapid Prototyp. J. 2018, 24, 409–415. [Google Scholar] [CrossRef]
- Mançanares, C.G.; de S. Zancul, E.; Cavalcante da Silva, J.; Cauchick Miguel, P.A. Additive Manufacturing Process Selection Based on Parts’ Selection Criteria. Int. J. Adv. Manuf. Technol. 2015, 80, 1007–1014. [Google Scholar] [CrossRef]
- Guo, Y.; Patanwala, H.S.; Bognet, B.; Ma, A.W.K. Inkjet and Inkjet-Based 3D Printing: Connecting Fluid Properties and Printing Performance. Rapid Prototyp. J. 2017, 23, 562–576. [Google Scholar] [CrossRef]
- Singh, D.; Orellana, C.F.; Hu, Y.; Jones, C.D.; Liu, Y.; Chiang, D.Y.; Liu, J.; Prins, J.F. FDM: A Graph-Based Statistical Method to Detect Differential Transcription Using RNA-Seq Data. Bioinformatics 2011, 27, 2633–2640. [Google Scholar] [CrossRef] [PubMed]
- Spoerke, E.D.; Murray, N.G.; Li, H.; Brinson, L.C.; Dunand, D.C.; Stupp, S.I. A Bioactive Titanium Foam Scaffold for Bone Repair. Acta Biomater. 2005, 1, 523–533. [Google Scholar] [CrossRef]
- Bencharit, S.; Byrd, W.C.; Altarawneh, S.; Hosseini, B.; Leong, A.; Reside, G.; Morelli, T.; Offenbacher, S. Development and Applications of Porous Tantalum Trabecular Metal-Enhanced Titanium Dental Implants. Clin. Implant Dent. Relat. Res. 2014, 16, 817–826. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of Titanium Surface Modification Techniques and Coatings for Antibacterial Applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Hussein, M.A.; Mohammed, A.S.; Al-Aqeeli, N. Wear Characteristics of Metallic Biomaterials: A Review. Materials 2015, 8, 2749–2768. [Google Scholar] [CrossRef]
- Yuan, L.; Ding, S.; Wen, C. Additive Manufacturing Technology for Porous Metal Implant Applications and Triple Minimal Surface Structures: A Review. Bioact. Mater. 2019, 4, 56–70. [Google Scholar] [CrossRef]
- Sansone, V.; Pagani, D.; Melato, M. The Effects on Bone Cells of Metal Ions Released from Orthopaedic Implants. A Review. Clin. Cases Miner. Bone Metab. 2013, 10, 34. [Google Scholar] [CrossRef]
- Taddei, E.B.; Henriques, V.A.R.; Silva, C.R.M.; Cairo, C.A.A. Production of New Titanium Alloy for Orthopedic Implants. Mater. Sci. Eng. C 2004, 24, 683–687. [Google Scholar] [CrossRef]
- Talha, M.; Behera, C.K.; Sinha, O.P. A Review on Nickel-Free Nitrogen Containing Austenitic Stainless Steels for Biomedical Applications. Mater. Sci. Eng. C 2013, 33, 3563–3575. [Google Scholar] [CrossRef]
- Sumita, M.; Hanawa, T.; Teoh, S.H. Development of Nitrogen-Containing Nickel-Free Austenitic Stainless Steels for Metallic Biomaterials. Mater. Sci. Eng. C 2004, 24, 753–760. [Google Scholar] [CrossRef]
- Nielsen, K. Corrosion of Metallic Implants. Br. Corros. J. 1987, 22, 272–278. [Google Scholar] [CrossRef]
- Godbole, N.; Yadav, S.; Ramachandran, M.; Belemkar, S. A Review on Surface Treatment of Stainless Steel Orthopedic Implants. Int. J. Pharm. Sci. Rev. Res. 2016, 36, 190–194. [Google Scholar]
- Buj-Corral, I.; Tejo-Otero, A.; Fenollosa-Artés, F. Development of Am Technologies for Metals in the Sector of Medical Implants. Metals 2020, 10, 686. [Google Scholar] [CrossRef]
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and Electron-beam Powder-bed Additive Manufacturing of Metallic Implants: A Review on Processes, Materials and Designs. J. Orthop. Res. 2016, 34, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Aherwar, A.; Singh, A.K.; Patnaik, A. Cobalt Based Alloy: A Better Choice Biomaterial for Hip Implants. Trends Biomater. Artif. Organs 2016, 30. [Google Scholar]
- Hussain, O.; Saleem, S.; Ahmad, B. Implant Materials for Knee and Hip Joint Replacement: A Review from the Tribological Perspective. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Tamilnadu, India, 2019; Volume 561, p. 12007. [Google Scholar]
- Ghalme, S.G.; Mankar, A.; Bhalerao, Y. Biomaterials in Hip Joint Replacement. Int. J. Mater. Sci. Eng. 2016, 4, 113–125. [Google Scholar]
- Almanza, E.; Pérez, M.J.; Rodríguez, N.A.; Murr, L.E. Corrosion Resistance of Ti-6Al-4V and ASTM F75 Alloys Processed by Electron Beam Melting. J. Mater. Res. Technol. 2017, 6, 251–257. [Google Scholar]
- Rudarstvo, R.Z.A.; Geologijo, M.I.N. Rmz—Materiali in Geookolje. Period. Mining Metall. Geol. 2010, 57, 1–158. [Google Scholar]
- Bartolo, P.; Kruth, J.-P.; Silva, J.; Levy, G.; Malshe, A.; Rajurkar, K.; Mitsuishi, M.; Ciurana, J.; Leu, M. Biomedical Production of Implants by Additive Electro-Chemical and Physical Processes. CIRP Ann. 2012, 61, 635–655. [Google Scholar] [CrossRef]
- Wang, W.; Khoon, C. Titanium Alloys in Orthopaedics. Titan. Alloy.—Adv. Prop. Control 2013, 1–20. [Google Scholar] [CrossRef][Green Version]
- Codell, M.; Norwitz, G.; Mikula, J.J. Analytical Chemistry of Titanium Alloys. Anal. Chem. 1955, 27, 1379–1383. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Schmuki, P.; Iglič, A.; Seifalian, A. Biomaterial Surface Modification of Titanium and Titanium Alloys for Medical Applications. Nanomedicine 2014, 111, 111. [Google Scholar]
- Narayanan, R.; Seshadri, S.K.; Kwon, T.Y.; Kim, K.H. Calcium Phosphate-Based Coatings on Titanium and Its Alloys. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2008, 85, 279–299. [Google Scholar] [CrossRef]
- Cheng, X.; Roscoe, S.G. Corrosion Behavior of Titanium in the Presence of Calcium Phosphate and Serum Proteins. Biomaterials 2005, 26, 7350–7356. [Google Scholar] [CrossRef]
- Kirmanidou, Y.; Sidira, M.; Drosou, M.E.; Bennani, V.; Bakopoulou, A.; Tsouknidas, A.; Michailidis, N.; Michalakis, K. New Ti-Alloys and Surface Modifications to Improve the Mechanical Properties and the Biological Response to Orthopedic and Dental Implants: A Review. Biomed Res. Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Ltjering, G.; Williams, J.C. Titanium, Engineering Materials and Processes; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- McGilvray, K.C.; Easley, J.; Seim, H.B.; Regan, D.; Berven, S.H.; Hsu, W.K.; Mroz, T.E.; Puttlitz, C.M. Bony Ingrowth Potential of 3D-Printed Porous Titanium Alloy: A Direct Comparison of Interbody Cage Materials in an in Vivo Ovine Lumbar Fusion Model. Spine J. 2018, 18, 1250–1260. [Google Scholar]
- Liu, S.; Shin, Y.C. Additive Manufacturing of Ti6Al4V Alloy: A Review. Mater. Des. 2019, 164, 107552. [Google Scholar] [CrossRef]
- Hao, Y.-L.; Li, S.-J.; Yang, R. Biomedical Titanium Alloys and Their Additive Manufacturing. Rare Met. 2016, 35, 661–671. [Google Scholar] [CrossRef]
- Hon, Y.-H.; Wang, J.-Y.; Pan, Y.-N. Composition/Phase Structure and Properties of Titanium-Niobium Alloys. Mater. Trans. 2003, 44, 2384–2390. [Google Scholar] [CrossRef]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of Pore Size on Bone Ingrowth into Porous Titanium Implants Fabricated by Additive Manufacturing: An In Vivo Experiment. Mater. Sci. Eng. C 2016, 59, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Fousová, M.; Vojtěch, D.; Kubásek, J.; Jablonská, E.; Fojt, J. Promising Characteristics of Gradient Porosity Ti-6Al-4V Alloy Prepared by SLM Process. J. Mech. Behav. Biomed. Mater. 2017, 69, 368–376. [Google Scholar] [CrossRef]
- Levine, B.; Sporer, S.; Della Valle, C.J.; Jacobs, J.J.; Paprosky, W. Porous Tantalum in Reconstructive Surgery of the Knee–A Review. J. Knee Surg. 2007, 20, 185–194. [Google Scholar] [PubMed]
- Levine, B.R.; Sporer, S.; Poggie, R.A.; Della Valle, C.J.; Jacobs, J.J. Experimental and Clinical Performance of Porous Tantalum in Orthopedic Surgery. Biomaterials 2006, 27, 4671–4681. [Google Scholar] [CrossRef]
- Donachie, M.J.; Donachie, S.J. Superalloys: A Technical Guide; ASM International: Almere, The Netherlands, 2002; ISBN 1615030646. [Google Scholar]
- Balla, V.K.; Bose, S.; Davies, N.M.; Bandyopadhyay, A. Tantalum—A Bioactive Metal for Implants. Jom 2010, 62, 61–64. [Google Scholar]
- Lewallen, E.A.; Riester, S.M.; Bonin, C.A.; Kremers, H.M.; Dudakovic, A.; Kakar, S.; Cohen, R.C.; Westendorf, J.J.; Lewallen, D.G.; van Wijnen, A.J. Biological Strategies for Improved Osseointegration and Osteoinduction of Porous Metal Orthopedic Implants. Tissue Eng. Part B Rev. 2015, 21, 218–230. [Google Scholar] [CrossRef]
- Alvarez, K.; Nakajima, H. Metallic Scaffolds for Bone Regeneration. Materials 2009, 2, 790–832. [Google Scholar]
- Kondo, R.; Nomura, N.; Suyalatu; Tsutsumi, Y.; Doi, H.; Hanawa, T. Microstructure and Mechanical Properties of As-Cast Zr-Nb Alloys. Acta Biomater. 2011, 7, 4278–4284. [Google Scholar] [CrossRef] [PubMed]
- Gomez, I.J.; Arnaiz, B.; Cacioppo, M.; Arcudi, F.; Prato, M. Nitrogen-Doped Carbon Nanodots for Bioimaging and Delivery of Paclitaxel. J. Mater. Chem. B 2018, 6, 5540–5548. [Google Scholar] [CrossRef]
- Suyalatu; Kondo, R.; Tsutsumi, Y.; Doi, H.; Nomura, N.; Hanawa, T. Effects of Phase Constitution on Magnetic Susceptibility and Mechanical Properties of Zr-Rich Zr-Mo Alloys. Acta Biomater. 2011, 7, 4259–4266. [Google Scholar] [CrossRef]
- Zhuang, H.; Han, Y.; Feng, A. Preparation, Mechanical Properties and in Vitro Biodegradation of Porous Magnesium Scaffolds. Mater. Sci. Eng. C 2008, 28, 1462–1466. [Google Scholar] [CrossRef]
- Elkaiam, L.; Hakimi, O.; Goldman, J.; Aghion, E. The Effect of Nd on Mechanical Properties and Corrosion Performance of Biodegradable Mg-5% Zn Alloy. Metals 2018, 8, 438. [Google Scholar] [CrossRef]
- Prasadh, S.; Ratheesh, V.; Manakari, V.; Parande, G.; Gupta, M.; Wong, R. The Potential of Magnesium Based Materials in Mandibular Reconstruction. Metals 2019, 9, 302. [Google Scholar] [CrossRef]
- Oliveri, V. Biomedical Applications of Copper Ionophores. Coord. Chem. Rev. 2020, 422, 213474. [Google Scholar] [CrossRef]
- Arun Kumar, S.; Balasubramaniam, B.; Bhunia, S.; Jaiswal, M.K.; Verma, K.; Khademhosseini, A.; Gupta, R.K.; Gaharwar, A.K. Two-dimensional Metal Organic Frameworks for Biomedical Applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1674. [Google Scholar] [CrossRef]
- Su, Y.; Cockerill, I.; Wang, Y.; Qin, Y.-X.; Chang, L.; Zheng, Y.; Zhu, D. Zinc-Based Biomaterials for Regeneration and Therapy. Trends Biotechnol. 2019, 37, 428–441. [Google Scholar] [CrossRef]
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-Dimensional Printing of High-Content Graphene Scaffolds for Electronic and Biomedical Applications. ACS Nano 2015, 9, 4636–4648. [Google Scholar] [CrossRef]
- Subramanian, B.; Muraleedharan, C.V.; Ananthakumar, R.; Jayachandran, M. A Comparative Study of Titanium Nitride (TiN), Titanium Oxy Nitride (TiON) and Titanium Aluminum Nitride (TiAlN), as Surface Coatings for Bio Implants. Surf. Coat. Technol. 2011, 205, 5014–5020. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Raaen, S. Development of Plasma Electrolytic Oxidation for Improved Ti6Al4V Biomaterial Surface Properties. Int. J. Adv. Manuf. Technol. 2016, 85, 2425–2437. [Google Scholar] [CrossRef]
- Awasthi, A.; Saxena, K.K.; Dwivedi, R.K. An Investigation on Classification and Characterization of Bio Materials and Additive Manufacturing Techniques for Bioimplants. Mater. Today Proc. 2021, 44, 2061–2068. [Google Scholar] [CrossRef]
- Widu, F.; Drescher, D.; Junker, R.; Bourauel, C. Corrosion and Biocompatibility of Orthodontic Wires. J. Mater. Sci. Mater. Med. 1999, 10, 275–281. [Google Scholar] [CrossRef]
- Niinomi, M. Recent Metallic Materials for Biomedical Applications. Metall. Mater. Trans. A 2002, 33, 477–486. [Google Scholar] [CrossRef]
- Chaudhari, R.; Vora, J.J.; Parikh, D.M. A Review on Applications of Nitinol Shape Memory Alloy. Recent Adv. Mech. Infrastruct. 2021, 123–132. [Google Scholar]
- Aihara, H.; Zider, J.; Fanton, G.; Duerig, T. Combustion Synthesis Porous Nitinol for Biomedical Applications. Int. J. Biomater. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Gorgin Karaji, Z.; Speirs, M.; Dadbakhsh, S.; Kruth, J.-P.; Weinans, H.; Zadpoor, A.A.; Amin Yavari, S. Additively Manufactured and Surface Biofunctionalized Porous Nitinol. ACS Appl. Mater. Interfaces 2017, 9, 1293–1304. [Google Scholar] [CrossRef]
- Duerig, T.; Pelton, A.; Stöckel, D. An Overview of Nitinol Medical Applications. Mater. Sci. Eng. A 1999, 273, 149–160. [Google Scholar] [CrossRef]
- Shabalovskaya, S.; Anderegg, J.; Van Humbeeck, J. Critical Overview of Nitinol Surfaces and Their Modifications for Medical Applications. Acta Biomater. 2008, 4, 447–467. [Google Scholar] [CrossRef]
- Liu, Y.; Enggist, L.; Kuffer, A.F.; Buser, D.; Hunziker, E.B. The Influence of BMP-2 and Its Mode of Delivery on the Osteoconductivity of Implant Surfaces during the Early Phase of Osseointegration. Biomaterials 2007, 28, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Jinno, T.; Goldberg, V.M.; Davy, D.; Stevenson, S. Osseointegration of Surface-Blasted Implants Made of Titanium Alloy and Cobalt-Chromium Alloy in a Rabbit Intramedullary Model. J. Biomed. Mater. Res. 1998, 42, 20–29. [Google Scholar] [CrossRef]
- Levine, B.; Della Valle, C.J.; Jacobs, J.J. Applications of Porous Tantalum in Total Hip Arthroplasty. J. Am. Acad. Orthop. Surg. 2006, 14, 646–655. [Google Scholar] [CrossRef]
- Sidambe, A.T. Biocompatibility of Advanced Manufactured Titanium Implants—A Review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef] [PubMed]
- Morgan, N.B. Medical Shape Memory Alloy Applications—The Market and Its Products. Mater. Sci. Eng. A 2004, 378, 16–23. [Google Scholar] [CrossRef]
- Kourra, N.; Warnett, J.M.; Attridge, A.; Dibling, G.; McLoughlin, J.; Muirhead-Allwood, S.; King, R.; Williams, M.A. Computed Tomography Metrological Examination of Additive Manufactured Acetabular Hip Prosthesis Cups. Addit. Manuf. 2018, 22, 146–152. [Google Scholar] [CrossRef]
- Wycisk, E.; Solbach, A.; Siddique, S.; Herzog, D.; Walther, F.; Emmelmann, C. Effects of Defects in Laser Additive Manufactured Ti-6Al-4V on Fatigue Properties. Phys. Procedia 2014, 56, 371–378. [Google Scholar] [CrossRef]
- Moussa, A.; Melancon, D.; El Elmi, A.; Pasini, D. Topology Optimization of Imperfect Lattice Materials Built with Process-Induced Defects via Powder Bed Fusion. Addit. Manuf. 2021, 37, 101608. [Google Scholar] [CrossRef]
- Gebisa, A.W.; Lemu, H.G. A Case Study on Topology Optimized Design for Additive Manufacturing. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Stavanger, Norway, 2017; Volume 276, p. 12026. [Google Scholar]
- Popovich, A.; Sufiiarov, V.; Polozov, I.; Borisov, E.; Masaylo, D. Producing Hip Implants of Titanium Alloys by Additive Manufacturing. Int. J. Bioprinting 2016, 2, 78–84. [Google Scholar] [CrossRef]
- Rahmati, S.; Abbaszadeh, F.; Farahmand, F. An Improved Methodology for Design of Custom-made Hip Prostheses to Be Fabricated Using Additive Manufacturing Technologies. Rapid Prototyp. J. 2012, 18, 389–400. [Google Scholar] [CrossRef]
- Guo, N.; Leu, M.C. Additive Manufacturing: Technology, Applications and Research Needs. Front. Mech. Eng. 2013, 8, 215–243. [Google Scholar] [CrossRef]
- De Viteri, V.S.; Fuentes, E. Titanium and Titanium Alloys as Biomaterials, Tribology—Fundamentals and Advancements. Tribol.—Fundam. Adv. 2013, 55, 561–565. [Google Scholar] [CrossRef]
- Lele, A. Additive Manufacturing (AM). Smart Innov. Syst. Technol. 2019, 132, 101–109. [Google Scholar] [CrossRef]
- Gardan, N.; Schneider, A. Topological Optimization of Internal Patterns and Support in Additive Manufacturing. J. Manuf. Syst. 2015, 37, 417–425. [Google Scholar] [CrossRef]
- Attallah, P.M.M. Metals and Alloys for Implants & Medical Devices; 2017; Available online: http://www.cost-newgen.org/wp-content/uploads/2017/06/BioMatSchool_ATTALLAH.pdf (accessed on 1 November 2022).
- Cui, C.; Hu, B.M.; Zhao, L.; Liu, S. Titanium Alloy Production Technology, Market Prospects and Industry Development. Mater. Des. 2011, 32, 1684–1691. [Google Scholar] [CrossRef]
- Wong, T.M.; Jin, J.; Lau, T.W.; Fang, C.; Yan, C.H.; Yeung, K.; To, M.; Leung, F. The use of three-dimensional printing technology in orthopaedic surgery: A Review. J. Orthop. Surg. 2017, 25, 2309499016684077. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D. Additive Manufacturing Technologies; Springer: New York, NY, USA, 2014; ISBN 9781493921126. [Google Scholar]
- Mohammed, M.T. ScienceDirect Development of a New Metastable Beta Titanium Alloy for Biomedical Applications. Karbala Int. J. Mod. Sci. 2017, 3, 224–230. [Google Scholar] [CrossRef]
- Niinomi, M. Titanium Alloys for Biomedical, Dental, and Healthcare Applications. Ti-2007 Sci. Technol. 2007, 2, 1417–1424. [Google Scholar]
- Moussa, A.; Rahman, S.; Xu, M.; Tanzer, M.; Pasini, D. Topology Optimization of 3D-Printed Structurally Porous Cage for Acetabular Reinforcement in Total Hip Arthroplasty. J. Mech. Behav. Biomed. Mater. 2020, 105, 103705. [Google Scholar] [CrossRef]
- Diah, M. Evidence of the Effect of Darwin Topiramate in the Treatment of Bipolar Disorder. Available online: http://dlib.sbmu.ac.ir/site/catalogue/28667 (accessed on 1 November 2022).
- Hamweendo, A. Fabrication of Porous Structures Using Cold Gas Dynamic Spray. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2017. [Google Scholar]
- Jiang, J.; Xu, X.; Stringer, J. Support Structures for Additive Manufacturing: A Review. J. Manuf. Mater. Process. 2018, 2, 64. [Google Scholar] [CrossRef]
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in Orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [PubMed]
- Lal, H.; Patralekh, M.K. 3D Printing and Its Applications in Orthopaedic Trauma: A Technological Marvel. J. Clin. Orthop. Trauma 2018, 9, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Chartier, T.; Chaput, C.; Doreau, F.; Loiseau, M. Stereolithography of Structural Complex Ceramic Parts. J. Mater. Sci. 2002, 7, 3141–3147. [Google Scholar] [CrossRef]
- Series, I.O.P.C.; Science, M. Research and Development of Ti and Ti Alloys: Past, Present and Future. IOP Conf. Ser. Mater. Sci. Eng. 2018, 430, 012007. [Google Scholar] [CrossRef]
- Why Is Titanium the Metal of Choice for Medical Applications from Head to Toe? Unique Properties Make Titanium the Ultimate Biomedical Choice; 1950; Available online: http://titaniumthemetal.org/Resources/DataSheetMedical.pdf (accessed on 1 November 2022).
- Brar, H.S.; Platt, M.O.; Sarntinoranont, M.; Martin, P.I.; Manuel, M.V. Magnesium as a Biodegradable and Bioabsorbable Material for Medical Implants. Jom 2009, 61, 31–34. [Google Scholar] [CrossRef]
- Duz, V.; Matviychuk, M.; Klevtsov, A.; Moxson, V. Industrial Application of Titanium Hydride Powder. Met. Powder Rep. 2017, 72, 30–38. [Google Scholar] [CrossRef]
- Cheng, L.; Liang, X.; Bai, J.; Chen, Q.; Lemon, J.; To, A. On Utilizing Topology Optimization to Design Support Structure to Prevent Residual Stress Induced Build Failure in Laser Powder Bed Metal Additive Manufacturing. Addit. Manuf. 2019, 27, 290–304. [Google Scholar] [CrossRef]
- Yang, D.; Shao, H.; Guo, Z.; Lin, T.; Fan, L. Preparation and Properties of Biomedical Porous Titanium Alloys by Gelcasting. Biomed. Mater. 2011, 6, 045010. [Google Scholar] [CrossRef]
- Murr, L.E.; Gaytan, S.M.; Martinez, E.; Medina, F.; Wicker, R.B. Next Generation Orthopaedic Implants by Additive Manufacturing Using Electron Beam Melting. Int. J. Biomater. 2012, 2012, 251. [Google Scholar] [CrossRef]
- Wu, N.; Li, S.; Zhang, B.; Wang, C.; Chen, B.; Han, Q.; Wang, J. The Advances of Topology Optimization Techniques in Orthopedic Implants: A Review. Med. Biol. Eng. Comput. 2021, 59, 1673–1689. [Google Scholar] [CrossRef] [PubMed]
- Oldani, C.; Dominguez, A. Titanium as a Biomaterial for Implants. Recent Adv. Arthroplast. 2012, 218, 149–162. [Google Scholar] [CrossRef]
- Zaman, H.A.; Sharif, S.; Idris, M.H.; Kamarudin, A. Metallic Biomaterials for Medical Implant Applications: A Review. Appl. Mech. Mater. 2015, 735, 19–25. [Google Scholar] [CrossRef]
- Veiga, C.; Davim, J.P.; Loureiro, A.J.R. Review on Machinability of Titanium Alloys: The Process Perspective. Rev. Adv. Mater. Sci. 2013, 34, 148–164. [Google Scholar]
- Iqbal, T.; Wang, L.; Li, D.; Dong, E.; Fan, H.; Fu, J.; Hu, C. A General Multi-Objective Topology Optimization Methodology Developed for Customized Design of Pelvic Prostheses. Med. Eng. Phys. 2019, 69, 8–16. [Google Scholar] [CrossRef]
- Hendra, H.; Dadan, R.; Hermawan, H.; Ramdan, D.; Djuansjah, J.R.P. Metals for Biomedical Applications. Biomed. Eng.—Theory Appl. 2011, 1, 411–431. [Google Scholar]
- Issue, S.; Biomaterials, T. Special Issue: Ti-Based Biomaterials: Synthesis. Materials 2020, 13, 1696. [Google Scholar]
- Sodian, R.; Weber, S.; Markert, M.; Loeff, M.; Lueth, T.; Weis, F.C. Pediatric Cardiac Transplantation: Three-Dimensional Printing of Anatomic Models for Surgical Planning of Heart Transplantation in Patients with Univentricular Heart. J. Thorac. Cardiovasc. Surg. 2008, 1098–1099. [Google Scholar] [CrossRef]
- Rouhi, G.; Hamedani, M. A Brief Introduction into Orthopaedic Implants: Screws, Plates, and Nails. 2012. Available online: https://www.researchgate.net/publication/309923041 (accessed on 1 November 2022).
- Zhang, J.; Yanagimoto, J. Topology Optimization of Microlattice Dome with Enhanced Stiffness and Energy Absorption for Additive Manufacturing. Compos. Struct. 2021, 255, 112889. [Google Scholar] [CrossRef]
- Niemeyer, T.C.; Grandini, C.R.; Pinto, L.M.C.; Angelo, A.C.D.; Schneider, S.G. Corrosion Behavior of Ti—13Nb—13Zr Alloy Used as a Biomaterial. J. Alloys Compd. 2009, 476, 172–175. [Google Scholar] [CrossRef]
- Okazaki, Y. Corrosion Resistance and Corrosion Fatigue Strength of New Titanium Alloys for Medical Implants without V and A1. Mater. Sci. Eng. A 1996, 213, 138–147. [Google Scholar] [CrossRef]
- Kladovasilakis, N.; Tsongas, K.; Tzetzis, D. Finite Element Analysis of Orthopedic Hip Implant with Functionally Graded Bioinspired Lattice Structures. Biomimetics 2020, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Nakai, M. Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone. Int. J. Biomater. 2011, 2011, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Subaşi, M.; Karataş, Ç. Titanyum ve Titanyum Alaşımlarından Yapılan İmplantlar Üzerine İnceleme A Review on Implants Made of Titanium and Titanium Alloys. Politeknik Dergisi 2012, 15, 87–103. [Google Scholar]
- Mahyudin, F.; Widhiyanto, L.; Hermawan, H. Biomaterials in Orthopaedics. In Biomaterials and Medical Devices; Springer: Berlin/Heidelberg, Germany, 2016; pp. 161–181. [Google Scholar]
- Atesok, K.; Mabrey, J.D.; Jazrawi, L.M.; Egol, K.A. Surgical Simulation in Orthopaedic Skills Training. JAAOS-J. Am. Acad. Orthop. Surg. 2012, 20, 410–422. [Google Scholar]
- Gao, C.; Wang, C.; Jin, H.; Wang, Z.; Li, Z.; Shi, C.; Leng, Y.; Yang, F.; Liu, H.; Wang, J. Additive Manufacturing Technique-Designed Metallic Porous Implants for Clinical Application in Orthopedics. RSC Adv. 2018, 8, 25210–25227. [Google Scholar] [CrossRef]
- Wong, K.C. 3D-Printed Patient-Specific Applications in Orthopedics. Orthop. Res. Rev. 2016, 8, 57. [Google Scholar] [CrossRef]
- Harun, W.S.W.; Kamariah, M.; Muhamad, N.; Ghani, S.A.C.; Ahmad, F.; Mohamed, Z. A Review of Powder Additive Manufacturing Processes for Metallic Biomaterials. Powder Technol. 2018, 327, 128–151. [Google Scholar] [CrossRef]
- Elahinia, M.H.; Hashemi, M.; Tabesh, M.; Bhaduri, S.B. Manufacturing and Processing of NiTi Implants: A Review. Prog. Mater. Sci. 2012, 57, 911–946. [Google Scholar] [CrossRef]
- Martelli, N.; Serrano, C.; van den Brink, H.; Pineau, J.; Prognon, P.; Borget, I.; El Batti, S. Advantages and Disadvantages of 3-Dimensional Printing in Surgery: A Systematic Review. Surgery 2016, 159, 1485–1500. [Google Scholar] [CrossRef]
- Vaezi, M.; Seitz, H.; Yang, S. A Review on 3D Micro-Additive Manufacturing Technologies. Int. J. Adv. Manuf. Technol. 2013, 67, 1721–1754. [Google Scholar] [CrossRef]
- Da Silva, L.R.R.; Sales, W.F.; Campos, F.d.A.R.; de Sousa, J.A.G.; Davis, R.; Singh, A.; Coelho, R.T.; Borgohain, B. A Comprehensive Review on Additive Manufacturing of Medical Devices. Prog. Addit. Manuf. 2021, 6, 517–553. [Google Scholar] [CrossRef]
- Emelogu, A.; Marufuzzaman, M.; Thompson, S.M.; Shamsaei, N.; Bian, L. Additive Manufacturing of Biomedical Implants: A Feasibility Assessment via Supply-Chain Cost Analysis. Addit. Manuf. 2016, 11, 97–113. [Google Scholar] [CrossRef]
- Vignesh, M.; Ranjith Kumar, G.; Sathishkumar, M.; Manikandan, M.; Rajyalakshmi, G.; Ramanujam, R.; Arivazhagan, N. Development of Biomedical Implants through Additive Manufacturing: A Review. J. Mater. Eng. Perform. 2021, 30, 4735–4744. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A. Current Status and Challenges of Additive Manufacturing in Orthopaedics: An Overview. J. Clin. Orthop. Trauma 2019, 10, 380–386. [Google Scholar] [CrossRef]
- Gospodinov, D.; Ferdinandov, N.; Dimitrov, S. Classification, Properties and Application of Titanium and Its Alloys. Proc. Univ. Ruse 2016, 55, 27–32. [Google Scholar]
- Abdel-Hady Gepreel, M.; Niinomi, M. Biocompatibility of Ti-Alloys for Long-Term Implantation. J. Mech. Behav. Biomed. Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef]
- Yuan, B.; Zhu, M.; Chung, C.Y. Biomedical Porous Shape Memory Alloys for Hard-Tissue Replacement Materials. Materials 2018, 11, 1716. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical Biocompatibilities of Titanium Alloys for Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef]
- Kunčická, L.; Kocich, R.; Lowe, T.C. Advances in Metals and Alloys for Joint Replacement. Prog. Mater. Sci. 2017, 88, 232–280. [Google Scholar] [CrossRef]
- Do Prado, R.F.; Rabêlo, S.B.; de Andrade, D.P.; Nascimento, R.D.; Henriques, V.A.R.; Carvalho, Y.R.; Cairo, C.A.A.; de Vasconcellos, L.M.R. Porous Titanium and Ti–35Nb Alloy: Effects on Gene Expression of Osteoblastic Cells Derived from Human Alveolar Bone. J. Mater. Sci. Mater. Med. 2015, 26, 1–11. [Google Scholar] [CrossRef]
- Matsuno, H.; Yokoyama, A.; Watari, F.; Uo, M.; Kawasaki, T. Biocompatibility and Osteogenesis of Refractory Metal Implants, Titanium, Hafnium, Niobium, Tantalum and Rhenium. Biomaterials 2001, 22, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, R.; Watari, F.; Takashi, N.; Tanimura, Y.; Uo, M.; Totsuka, Y. Effects of Ti Ions and Particles on Neutrophil Function and Morphology. Biomaterials 2002, 23, 3757–3764. [Google Scholar] [CrossRef] [PubMed]
- McCutchen, D.; Dibble, E.; Blount, M.M. Phonemic Effects in Reading Comprehension and Text Memory. Appl. Cogn. Psychol. 1994, 8, 597–611. [Google Scholar] [CrossRef]
- Liu, Z.; He, B.; Lyu, T.; Zou, Y. A Review on Additive Manufacturing of Titanium Alloys for Aerospace Applications: Directed Energy Deposition and beyond Ti-6Al-4V. Jom 2021, 73, 1804–1818. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef]
- Agapovichev, A.; Sotov, A.; Kokareva, V.; Smelov, V. Possibilities and Limitations of Titanium Alloy Additive Manufacturing. MATEC Web Conf. 2018, 224, 01064. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Knowles, J.C. Physical and Biocompatibility Studies of Novel Titanium Dioxide Doped Phosphate-Based Glasses for Bone Tissue Engineering Applications. J. Mater. Sci. Mater. Med. 2008, 19, 377–386. [Google Scholar] [CrossRef]
- Niinomi, M. Recent Research and Development in Titanium Alloys for Biomedical Applications and Healthcare Goods. Sci. Technol. Adv. Mater. 2003, 4, 445. [Google Scholar] [CrossRef]
- Hatori, T.; Morikawa, K.; Niwa, S.; Niinomi, M.; Suzuki, A. Bone Tissue Reaction to New b Titanium Low Rigidity Alloy: Rabit Study on Bone Healing Remodeling and Atrophy in Intramedullary Fracture Fixation. In Proceedings of the International Conference on Biomechanics Combined with the Annual Scientific Meeting of Taiwanese Society of Biomechanics, Taipei, Taiwan, 9–11 November 2001. [Google Scholar]
- Hattori, T.; Morikawa, K.; Niwa, S.; Sato, K.; Niinomi, M.; Suzuki, A. Material Rigidity of Fracture Fixation Device and Bone Tissue Reaction—Experimantal Study on Intramedullary Fixation with Different Materials. J. Jpn. Clin. Biomech. 2002, 23, 299–304. [Google Scholar]
- Ratha, I.; Datta, P.; Balla, V.K.; Nandi, S.K.; Kundu, B. Effect of Doping in Hydroxyapatite as Coating Material on Biomedical Implants by Plasma Spraying Method: A Review. Ceram. Int. 2021, 47, 4426–4445. [Google Scholar] [CrossRef]
- Manivasagam, V.K.; Sabino, R.M.; Kantam, P.; Popat, K. Surface Modification Strategies to Improve Titanium Hemocompatibility: A Comprehensive Review. Mater. Adv. 2021, 2, 5824–5842. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Biologically and Mechanically Biocompatible Titanium Alloys. Mater. Trans. 2008, 49, 2170–2178. [Google Scholar] [CrossRef]
- Why Is Titanium Biocompatible? Available online: http://www.ors.org/Transactions/58/0981.pdf (accessed on 1 November 2022).
- Sobieszczyk, S. Surface Modifications of Ti and Its Alloys. Adv. Mater. Sci. 2010, 10, 29. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Schmuki, P.; Iglič, A. Applications Outline: 111–136. Available online: https://www.researchgate.net/publication/265292794 (accessed on 1 November 2022).
- Mohammed, M.T.; Khan, Z.A.; Siddiquee, A.N. Surface Modifications of Titanium Materials for Developing Corrosion Behavior in Human Body Environment: A Review. Procedia Mater. Sci. 2014, 6, 1610–1618. [Google Scholar] [CrossRef]
- Cronskär, M.; Bäckström, M.; Rännar, L. Production of Customized Hip Stem Prostheses–a Comparison between Conventional Machining and Electron Beam Melting (EBM). Rapid Prototyp. J. 2013, 19, 365–372. [Google Scholar] [CrossRef]
- Galati, M.; Rizza, G.; Defanti, S.; Denti, L. Surface Roughness Prediction Model for Electron Beam Melting (EBM) Processing Ti6Al4V. Precis. Eng. 2021, 69, 19–28. [Google Scholar] [CrossRef]
- Lebea, L.; Ngwangwa, H.M.; Desai, D.; Nemavhola, F. Experimental Investigation into the Effect of Surface Roughness and Mechanical Properties of 3D-Printed Titanium Ti-64 ELI after Heat Treatment. Int. J. Mech. Mater. Eng. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- Murr, L.E.; Amato, K.N.; Li, S.J.; Tian, Y.X.; Cheng, X.Y.; Gaytan, S.M.; Martinez, E.; Shindo, P.W.; Medina, F.; Wicker, R.B. Microstructure and Mechanical Properties of Open-Cellular Biomaterials Prototypes for Total Knee Replacement Implants Fabricated by Electron Beam Melting. J. Mech. Behav. Biomed. Mater. 2011, 4, 1396–1411. [Google Scholar] [CrossRef]
- Liu, P.; Yang, Y.; Liu, R.; Shu, H.; Gong, J.; Yang, Y.; Sun, Q.; Wu, X.; Cai, M. A Study on the Mechanical Characteristics of the EBM-Printed Ti-6Al-4V LCP Plates in Vitro. J. Orthop. Surg. Res. 2014, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, D.S.; Harrysson, O.L.A.; Marcellin-Little, D.J.; Abumoussa, S.; Dahners, L.E.; Weinhold, P.S. Osseointegration of Coarse and Fine Textured Implants Manufactured by Electron Beam Melting and Direct Metal Laser Sintering. 3D Print. Addit. Manuf. 2017, 4, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, A.L.; Goswami, T. Hip Implants VII: Finite Element Analysis and Optimization of Cross-Sections. Mater. Des. 2008, 29, 1438–1446. [Google Scholar] [CrossRef]
- Bashiri, A.; Sallam, H.E.M.; Abd-Elhady, A.A. Progressive Failure Analysis of a Hip Joint Based on Extended Finite Element Method. Eng. Fail. Anal. 2020, 117, 104829. [Google Scholar] [CrossRef]
- Sutradhar, A.; Park, J.; Carrau, D.; Nguyen, T.H.; Miller, M.J.; Paulino, G.H. Designing Patient-Specific 3D Printed Craniofacial Implants Using a Novel Topology Optimization Method. Med. Biol. Eng. Comput. 2016, 54, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.; Kennedy, J.V.; Potgieter, J. A Comparison of Traditional Manufacturing vs Additive Manufacturing, the Best Method for the Job. Procedia Manuf. 2019, 30, 11–18. [Google Scholar] [CrossRef]
- Babić, M.; Verić, O.; Božić, Ž.; Sušić, A. Finite Element Modelling and Fatigue Life Assessment of a Cemented Total Hip Prosthesis Based on 3D Scanning. Eng. Fail. Anal. 2020, 113, 104536. [Google Scholar] [CrossRef]
- Sedmak, A.; Čolić, K.; Grbović, A.; Balać, I.; Burzić, M. Numerical Analysis of Fatigue Crack Growth of Hip Implant. Eng. Fract. Mech. 2019, 216, 106492. [Google Scholar] [CrossRef]
- Pimenta, A.R.; Tavares, S.S.M.; Dias, D.F.; Correa, S.R.; Sobreiro, A.L.; Diniz, M.G. Failure Analysis of a Titanium Hip Prosthesis. J. Fail. Anal. Prev. 2021, 21, 28–35. [Google Scholar] [CrossRef]
- Vilardell, A.M.; Fredriksson, G.; Yadroitsev, I.; Krakhmalev, P. Fracture Mechanisms in the As-Built and Stress-Relieved Laser Powder Bed Fusion Ti6Al4V ELI Alloy. Opt. Laser Technol. 2019, 109, 608–615. [Google Scholar] [CrossRef]
- Du Plessis, A.; le Roux, S.G. Standardized X-ray Tomography Testing of Additively Manufactured Parts: A Round Robin Test. Addit. Manuf. 2018, 24, 125–136. [Google Scholar] [CrossRef]
- Du Plessis, A.; Yadroitsava, I.; Yadroitsev, I. Effects of Defects on Mechanical Properties in Metal Additive Manufacturing: A Review Focusing on X-ray Tomography Insights. Mater. Des. 2020, 187, 108385. [Google Scholar]
- Sigmund, O.; Maute, K. Topology Optimization Approaches. Struct. Multidiscip. Optim. 2013, 48, 1031–1055. [Google Scholar]
- Eschenauer, H.A.; Olhoff, N. Topology Optimization of Continuum Structures: A Review. Appl. Mech. Rev. 2001, 54, 331–390. [Google Scholar] [CrossRef]
- Belwanshi, M.; Jayaswal, P.; Aherwar, A. A Study on Finite Element Analysis Methodologies and Approaches Used for Total Hip Arthroplasty. Mater. Today Proc. 2022, 56, 2596–2604. [Google Scholar] [CrossRef]
- Milovanović, A.; Sedmak, A.; Grbović, A.; Mijatović, T.; Čolić, K. Design Aspects of Hip Implant Made of Ti-6Al-4V Extra Low Interstitials Alloy. Procedia Struct. Integr. 2020, 26, 299–305. [Google Scholar] [CrossRef]
- Tan, N.; van Arkel, R.J. Topology Optimisation for Compliant Hip Implant Design and Reduced Strain Shielding. Materials 2021, 14, 7184. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Meta-Biomaterials. Biomater. Sci. 2020, 8, 18–38. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Zhang, W.; Li, Y. Fabrication and Characterization of Porous Ti6Al4V Parts for Biomedical Applications Using Electron Beam Melting Process. Mater. Lett. 2009, 63, 403–405. [Google Scholar] [CrossRef]
- Wang, Q.S.; Li, S.J.; Hou, W.T.; Wang, S.G.; Hao, Y.L.; Yang, R.; Misra, R.D.K. Mechanistic Understanding of Compression-Compression Fatigue Behavior of Functionally Graded Ti–6Al–4V Mesh Structure Fabricated by Electron Beam Melting. J. Mech. Behav. Biomed. Mater. 2020, 103, 103590. [Google Scholar] [CrossRef]
- Chua, C.K.; Leong, K.F.; An, J. Introduction to Rapid Prototyping of Biomaterials. In Rapid Prototyping of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–15. [Google Scholar]
- Sing, S.L.; Tey, C.F.; Tan, J.H.K.; Huang, S.; Yeong, W.Y. 3D Printing of Metals in Rapid Prototyping of Biomaterials: Techniques in Additive Manufacturing. In Rapid Prototyping of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–40. [Google Scholar]
- Fan, H.; Fu, J.; Li, X.; Pei, Y.; Li, X.; Pei, G.; Guo, Z. Implantation of Customized 3-D Printed Titanium Prosthesis in Limb Salvage Surgery: A Case Series and Review of the Literature. World J. Surg. Oncol. 2015, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Gong, C.; Chen, X.; Sun, Y.; Zhang, J.; Cai, L. Additive Manufacturing of Customized Metallic Orthopedic Implants: Materials, Structures, and Surface Modifications. Metals 2019, 9, 1004. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, P.; Voshage, M.; Jauer, L.; Qin, Y.; Schleifenbaum, J.H.; Poprawe, R. Laser Additive Manufacturing of Zn Metal Parts for Biodegradable Implants: Effect of Gas Flow on Evaporation and Formation Quality Laser Additive Manufacturing of Zn Metal Parts for Biodegradable Implants: Effect of Gas Fl Ow on Evaporation and Formatio. J. Laser Appl. 2019, 31, 022304. [Google Scholar] [CrossRef]
- Mahmoud, D.; Elbestawi, M. Lattice Structures and Functionally Graded Materials Applications in Additive Manufacturing of Orthopedic Implants: A Review. J. Manuf. Mater. Process. 2017, 1, 13. [Google Scholar] [CrossRef]
- Shahali, H.; Jaggessar, A.; Yarlagadda, P.K.D.V. Recent Advances in Manufacturing and Surface Modification of Titanium Orthopaedic Applications. Procedia Eng. 2017, 174, 1067–1076. [Google Scholar] [CrossRef]
- Hagberg, K.; Häggström, E.; Jönsson, S.; Rydevik, B.; Brånemark, R. Prosthetic Limbs. 131–132. Available online: https://journals.sagepub.com/doi/pdf/10.1177/0309364611409003 (accessed on 1 November 2022).
- Khorasani, A.M.; Goldberg, M.; Doeven, E.H.; Littlefair, G. Titanium in Biomedical Applications—Properties and Fabrication: A Review. J. Biomater. Tissue Eng. 2015, 5, 593–619. [Google Scholar] [CrossRef]
- Majumdar, T.; Eisenstein, N.; Frith, J.E.; Cox, S.C.; Birbilis, N. Additive Manufacturing of Titanium Alloys for Orthopedic Applications: A Materials Science Viewpoint. Adv. Eng. Mater. 2018, 20, 1800172. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).