Hydrophobic Material: Effect of Alkyl Chain Length on the Surface Roughness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Acid-Catalyzed SiO2-TiO2-Alkylsilane Sol
2.3. Preparation of Coatings
2.4. Characterization
2.5. Anti-Dirt Test
3. Results and Discussion
3.1. Wetting Properties of Coatings with Various Compositions of SiO2 and TiO2
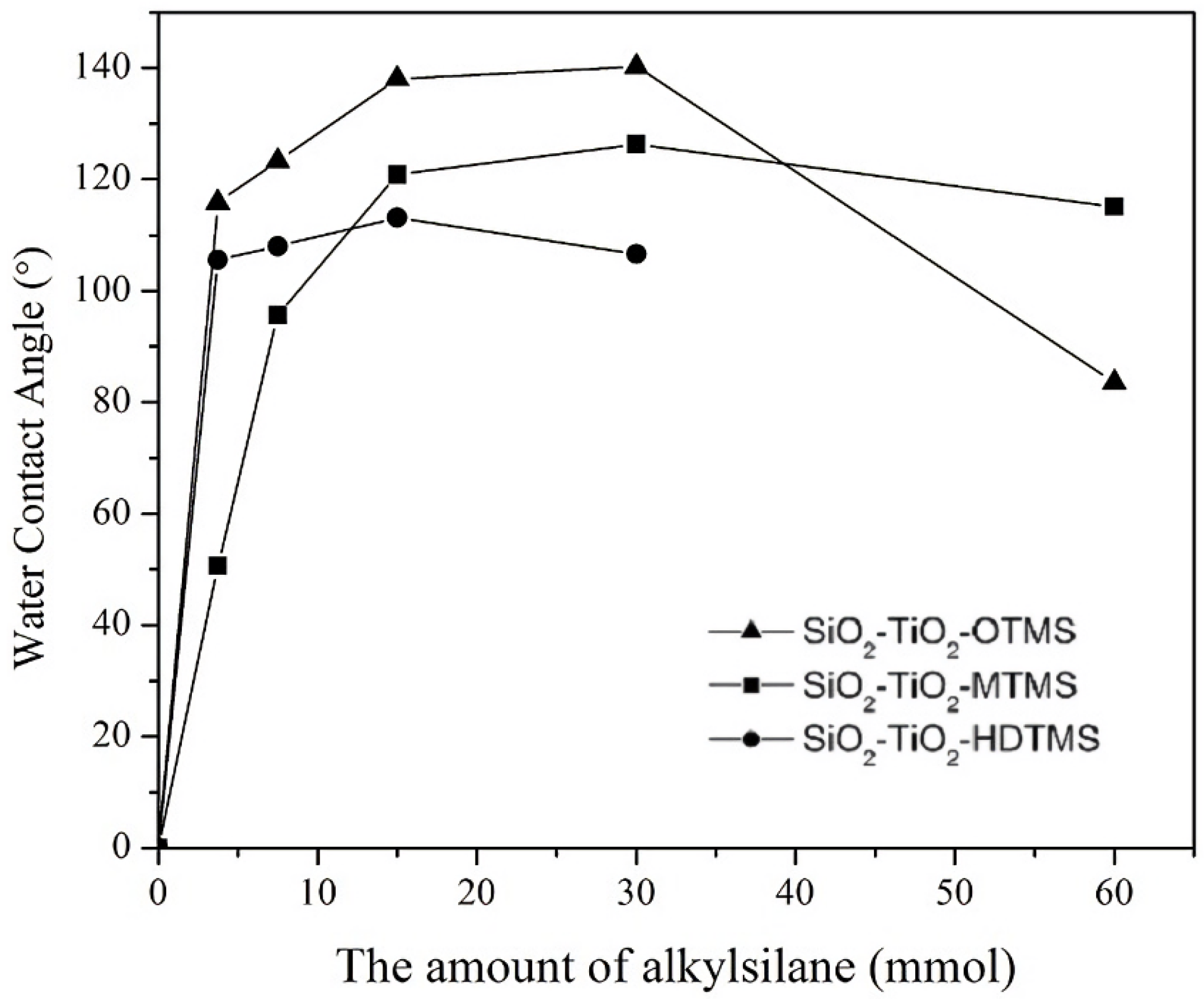
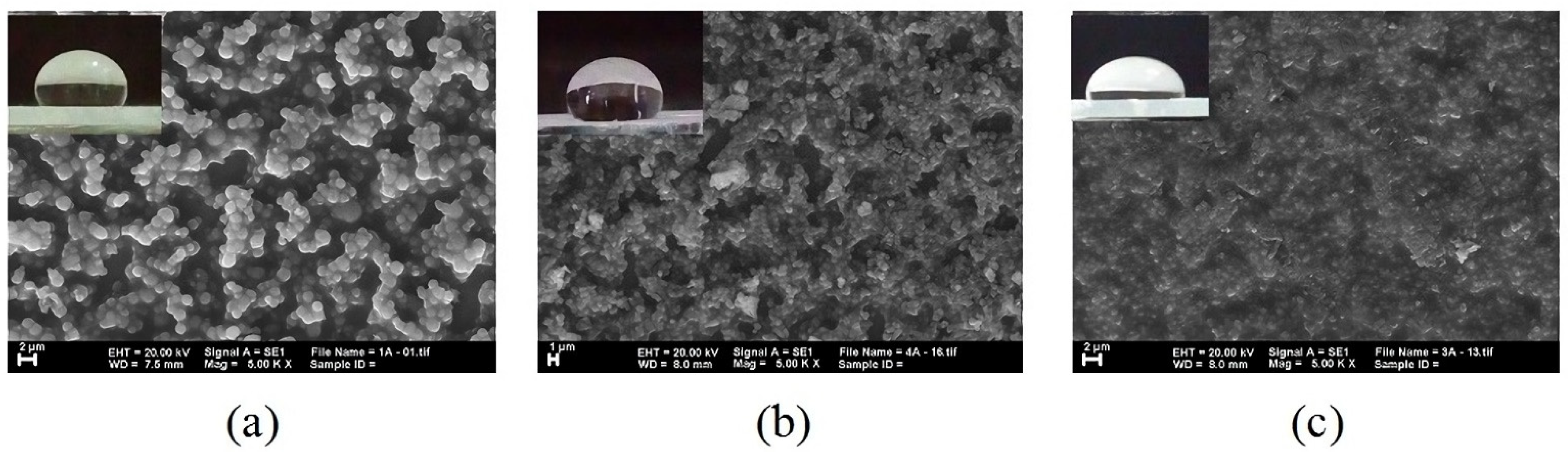
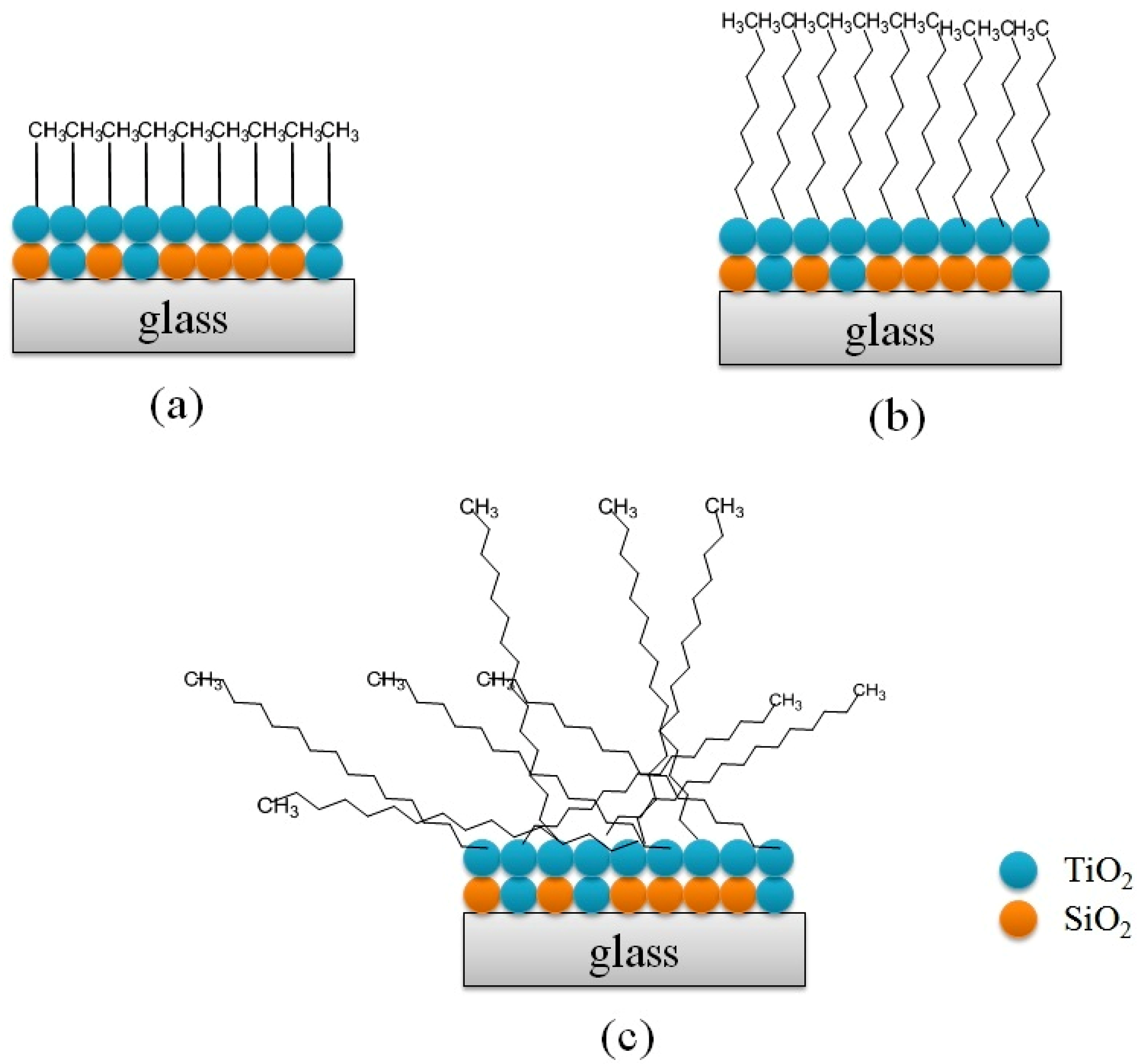
3.2. The Influence of Composition and the Alkyl Chain Length of Silane on Hydrophobicity
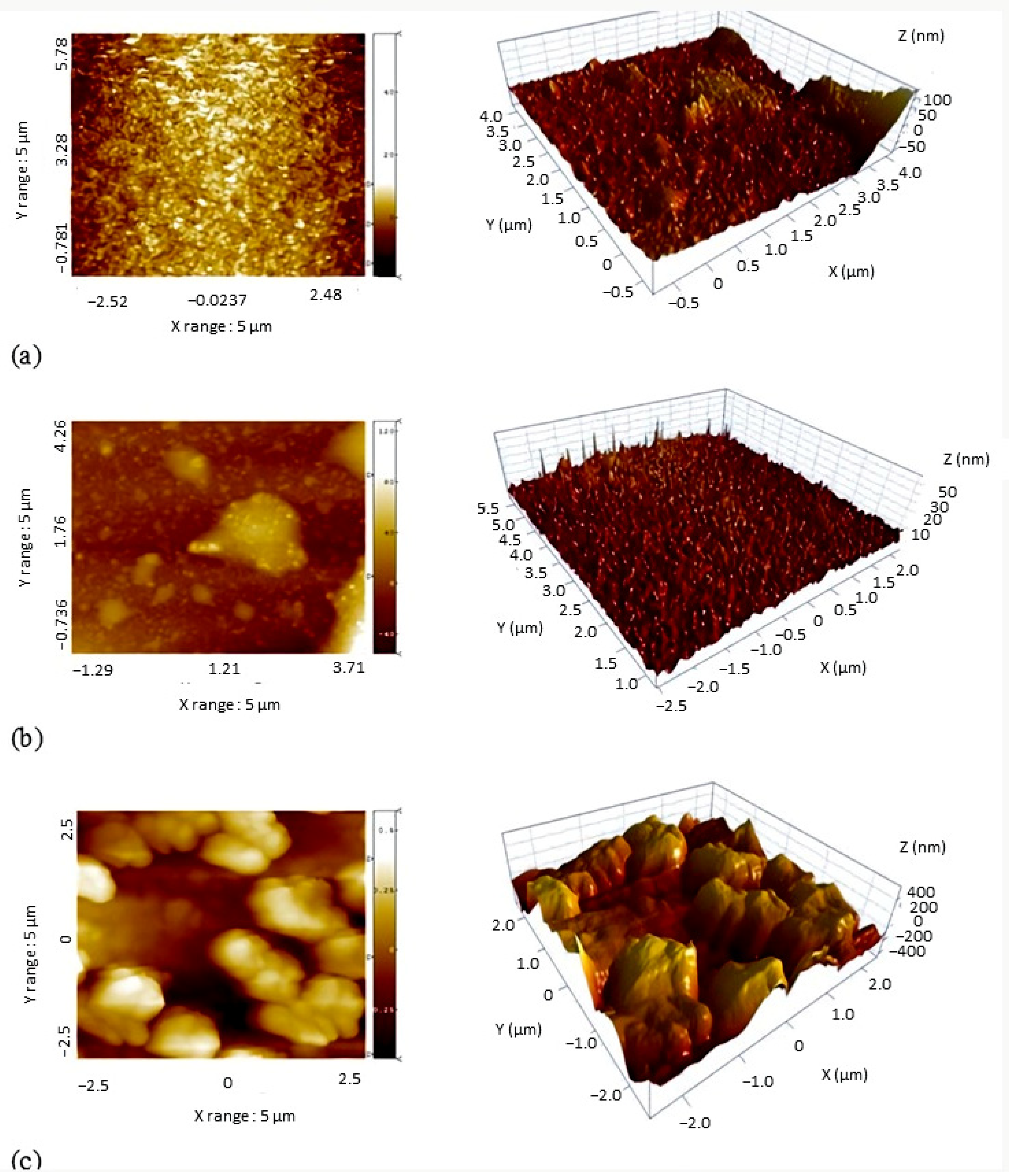
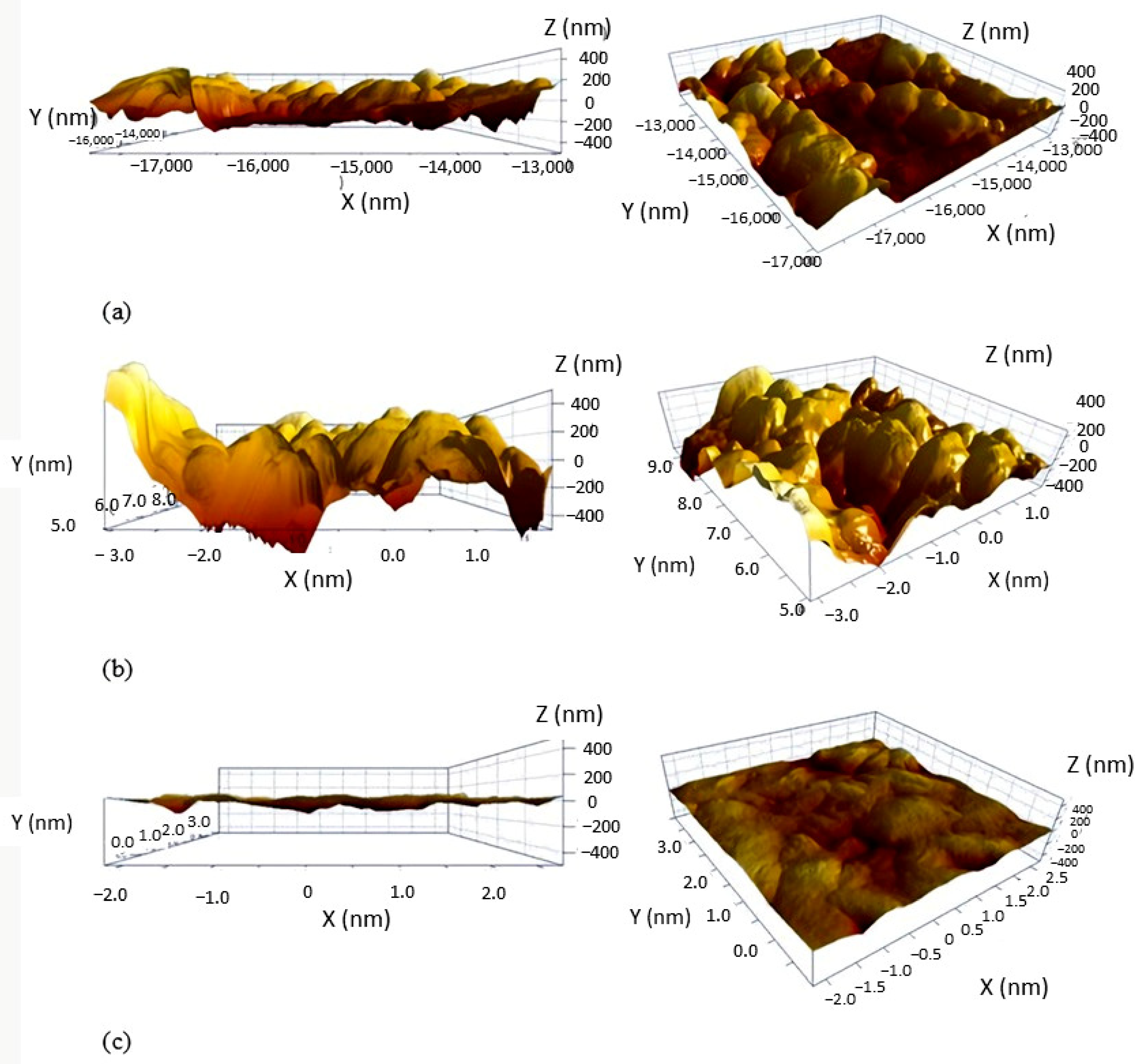
3.3. The Influence of Surface Roughness on Hydrophobicity
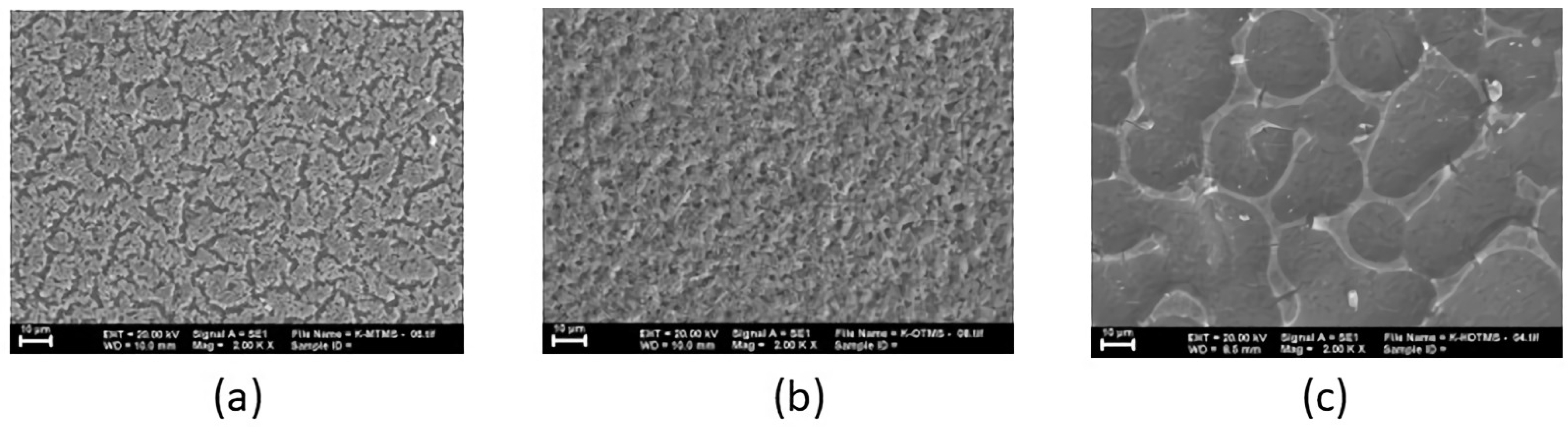
3.4. The Influence of Coating Technique on Hydrophobic Coatings
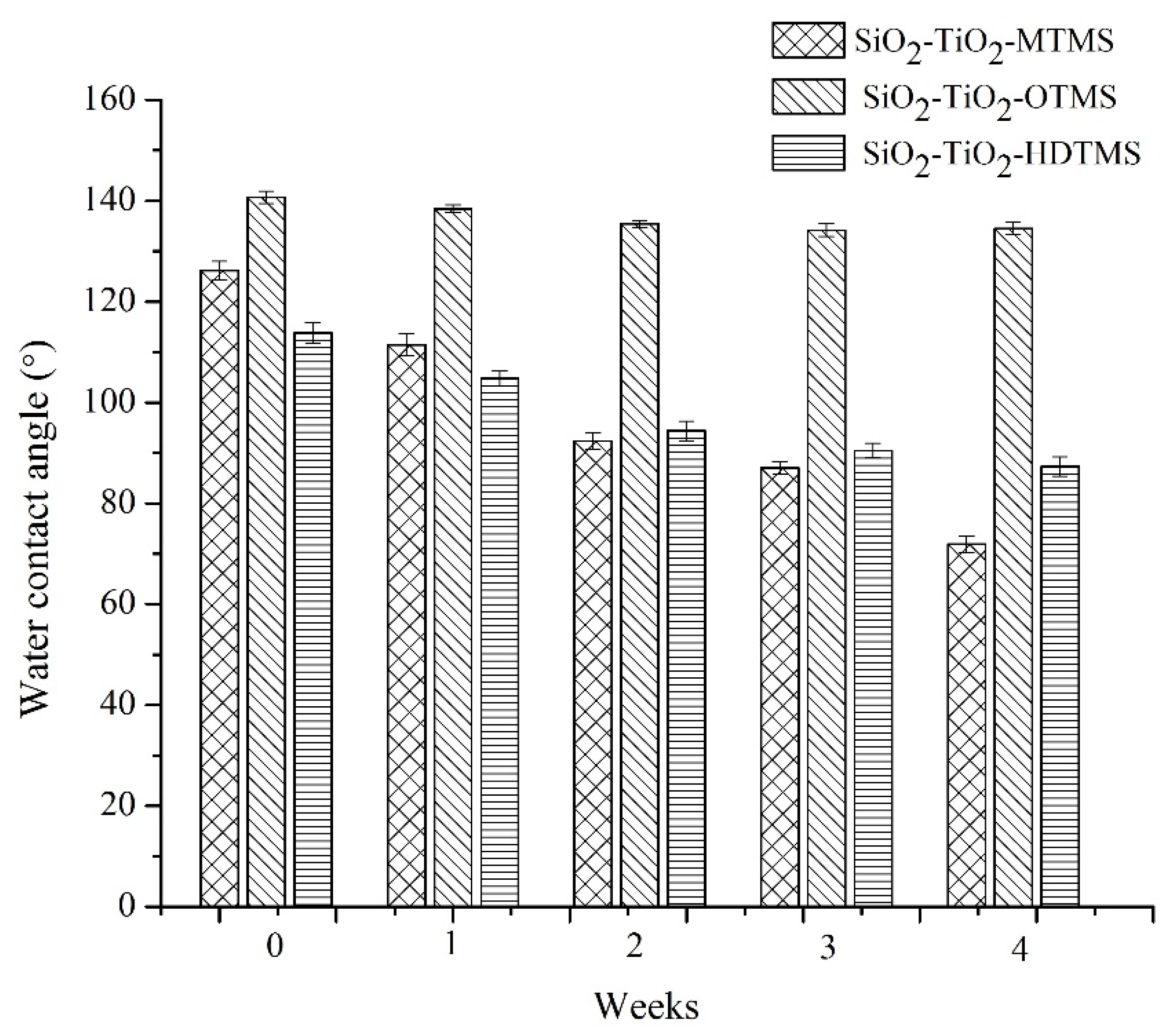
3.5. Stability of Coatings
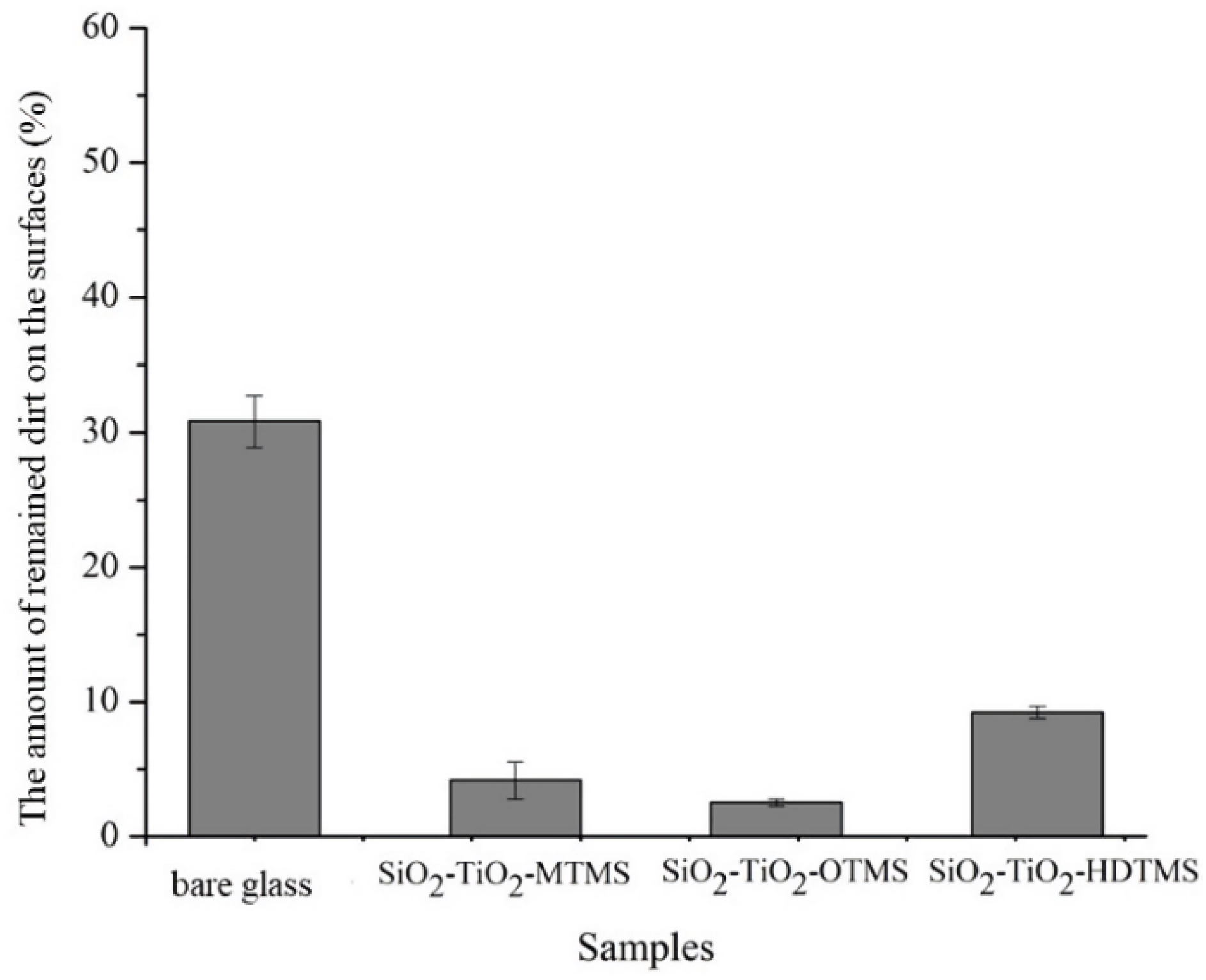
3.6. Anti-Dirt Performance of Coatings
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quan, Y.Y.; Zhang, L.Z.; Qi, R.H.; Cai, R.R. Self-cleaning of Surfaces: The role of surface wettability and dust types. Sci. Rep. 2016, 6, 38239. [Google Scholar] [CrossRef]
- Kumar, D.; Wu, X.; Fu, Q.; Ho, J.W.C.; Kanhere, P.D.; Li, L.; Chen, Z. Hydrophobic sol-gel coatings based on polydimethylsiloxane for self-cleaning applications. Mater. Des. 2015, 86, 855–862. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wang, H.J.; Liang, Y.H.; Li, X.J.; Ren, L.Q.; Cui, Z.Q.; Luo, C. One-step fabrication of robust superhydrophobic and superoleophilic surfaces with self-cleaning and oil/water separation function. Sci. Rep. 2018, 8, 3869. [Google Scholar] [CrossRef]
- Norgaard, A.W.; Hansen, J.S.; Sorli, J.B.; Levin, M.; Wolkoff, P.; Nielsen, G.D.; Larsen, S.T. Pulmonary Toxicity of Perfluorinated Silane-Based Nanofilm Spray Products: Solvent Dependency. Toxicol. Sci. 2013, 137, 180–188. [Google Scholar] [CrossRef]
- Pan, G.; Zhou, Q.; Luan, X.; Fu, Q.S. Distribution of perfluorinated compounds in lake taihu (China): Impact to human health and water standards. Sci. Total Environ. 2014, 487, 778–784. [Google Scholar] [CrossRef]
- Song, J.; Rojas, O.J. Approaching super-hydrophobicity from cellulosic materials: A review. Nord. Pulp Pap. Res. J. 2013, 28, 216–238. [Google Scholar] [CrossRef]
- Lin, J.; Chen, H.; Fei, T.; Zhang, J. Highly transparent superhydrophobic organic-inorganic nanocoating from the aggregation of silica nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 421, 51–62. [Google Scholar] [CrossRef]
- Hwang, J.; Ahn, Y. Fabrication of superhydrophobic silica nanoparticles and nanocomposite coating on glass surfaces. Bull. Korean Chem. Soc. 2015, 36, 391–394. [Google Scholar] [CrossRef]
- Petcu, C.; Purcar, V.; Spătaru, C.; Alexandrescu, E.; Şomoghi, R.; Trică, B.; Niţu, S.G.; Panaitescu, D.M.; Donescu, D.; Jecu, M.L. The influence of new hydrophobic silica nanoparticles on the surface properties of the films obtained from bilayer hybrids. Nanomaterials 2017, 7, 47. [Google Scholar] [CrossRef]
- Widati, A.A.; Nuryono, N.; Kartini, I.; Martino, N.D. Silica-methyltrimethoxysilane based hydrophobic coatings on a glass substrate. J. Chem. Technol. Metall. 2017, 52, 1123–1128. [Google Scholar]
- Widati, A.A.; Nuryono, N.; Aryanti, D.P.; Wibowo, M.A.; Kunarti, E.S.; Kartini, I.; Rusdiarso, B. Preparation of water repellent layer on glass using hydrophobic compound modified rice hull ash silica. Indones. J. Chem. 2018, 18, 587–593. [Google Scholar] [CrossRef]
- Ramírez-García, R.E.; González-Rodríguez, J.A.; Arroyo-Ortega, M.; Pérez-García, S.A.; Licea-Jiménez, L. Engineered TiO2 and SiO2-TiO2 films on silica-coated glass for increased thin film durability under abrasive conditions. Int. J. Appl. Ceram. Technol. 2017, 14, 39–49. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Latthe, S.S.; Gao, L.; An, S.; Yoon, S.S.; Liu, B.; Xing, R. Self-cleaning transparent superhydrophobic coatings through simple sol-gel processing of fluoroalkylsilane. Appl. Surf. Sci. 2015, 351, 897–903. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Yang, H. TEOS/Silane-Coupling Agent Composed Double Layers Structure: A Novel Super-hydrophilic Surface. Energy Procedia 2015, 75, 349–354. [Google Scholar]
- Spataru, C.I.; Purcǎr, V.; Donescu, D.; Ghiurea, M.; Cintezǎ, O.; Miclǎuš, T. Preparation of hydrophobic surface based on hybrid silica films by sol-gel process. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2013, 75, 117–128. [Google Scholar]
- Tang, Z.; Li, H.; Hess, D.; Breedveld, V. Effect of chain length on the wetting properties of alkyltrichlorosilane coated cellulose-based paper. Cellulose 2016, 23, 1401–1413. [Google Scholar] [CrossRef]
- Dong, S.; Wang, Z.; Wang, Y.; Bai, X.; Fu, Y.Q.; Guo, B.; Tan, C.; Zhang, J.; Hu, P. Rol-to-roll manufacturing of robust superhydrophobic coating on metallic engineering materials. ACS Appl. Mater. Interfaces 2018, 10, 2174–2184. [Google Scholar] [CrossRef] [PubMed]
- Prakash, P.; Satheesh, U.; Devaprakasam, D. Study of High Temperature Thermal Behavior of Alkyl and Perfluoroalkylsilane Molecules Self-Assembled on Titanium Oxide Nanoparticles. arXiv 2014, arXiv:1409.6823. [Google Scholar]
- Farzaneh, M.; Kulinich, S.A. Hydrophobic properties of surface coated with fluoroalkylsilane and alkylsiloxane monolayers. Surf. Sci. 2004, 573, 379–390. [Google Scholar]
- Kulinich, S.A.; Farzaneh, M. Alkylsilane self-assembled monolayers: Modeling their wetting characteristics. Appl. Surf. Sci. 2004, 230, 232–240. [Google Scholar] [CrossRef]
- Hu, J.; Pan, C.; Li, H.; Shen, P.; Sun, H.; Duan, H.; Lanza, M. Improvement of the electrical contact resistance at rough interfaces using two dimensional materials. J. Appl. Phys. 2015, 118, 215301. [Google Scholar] [CrossRef]
- Kavale, M.S.; Mahadik, D.B.; Parale, V.G.; Wagh, P.B.; Gupta, S.C.; Rao, A.V.; Barshilia, H.C. Optically transparent, superhydrophobic methyltrimethoxysilane based silica coatings without silylating reagent. Appl. Surf. Sci. 2011, 258, 158–162. [Google Scholar] [CrossRef]









| Sample | x (mmol) | Water Contact Angle (°) | Roughness Value (nm) |
|---|---|---|---|
| x SiO2, 7.5 mmol TiO2, 15 mmol OTMS | 7.5 | 99.09 ± 1.73 | 11.2 |
| 15 | 121.75 ± 1.71 | 157 | |
| 30 | 98.79 ± 1.21 | 12.9 | |
| 15 mmol SiO2, x TiO2, 15 mmol OTMS | 7.5 | 121.75 ± 1.71 | 163 |
| 15 | 136.92 ± 2.45 | 235 | |
| 30 | 114.77 ± 2.15 | 87 |
| Sol | Composition (mmol) | The Ratio of Peak Areas (A) at- | ||
|---|---|---|---|---|
| 3400 cm−1 | 1450 cm−1 | |||
| SiO2 | 15 | 71.32 | 7.074 | 10.082 |
| 30 | 42.50 | 4.12 | 10.315 | |
| TiO2 | 15 | 64.12 | 12.97 | 4.94 |
| 30 | 100.47 | 15.85 | 6.33 | |
| Composition (mmol) | Water Contact Angle | ||
|---|---|---|---|
| SiO2 | TiO2 | MTMS | |
| 30 | - | 30 | 63.73 ± 1.58° |
| - | 30 | 30 | 80.03 ± 1.45° |
| 15 | 15 | 30 | 126.18 ± 1.86° |
| Composition (mmol) | The Ratio of Peak Areas (A) at- | ||||
|---|---|---|---|---|---|
| 3400 cm−1 | 1450 cm−1 | 1100 cm−1 | |||
| 15 | 108.1 | 18.59 | 32.29 | 3.35 | 0.57 |
| 30 | 93.6 | 18.61 | 40.54 | 2.31 | 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widati, A.A.; Fahmi, M.Z.; Sakti, S.C.W.; Budiastanti, T.A.; Purbaningtias, T.E. Hydrophobic Material: Effect of Alkyl Chain Length on the Surface Roughness. J. Manuf. Mater. Process. 2022, 6, 110. https://doi.org/10.3390/jmmp6050110
Widati AA, Fahmi MZ, Sakti SCW, Budiastanti TA, Purbaningtias TE. Hydrophobic Material: Effect of Alkyl Chain Length on the Surface Roughness. Journal of Manufacturing and Materials Processing. 2022; 6(5):110. https://doi.org/10.3390/jmmp6050110
Chicago/Turabian StyleWidati, Alfa Akustia, Mochamad Zakki Fahmi, Satya Candra Wibawa Sakti, Titah Aldila Budiastanti, and Tri Esti Purbaningtias. 2022. "Hydrophobic Material: Effect of Alkyl Chain Length on the Surface Roughness" Journal of Manufacturing and Materials Processing 6, no. 5: 110. https://doi.org/10.3390/jmmp6050110
APA StyleWidati, A. A., Fahmi, M. Z., Sakti, S. C. W., Budiastanti, T. A., & Purbaningtias, T. E. (2022). Hydrophobic Material: Effect of Alkyl Chain Length on the Surface Roughness. Journal of Manufacturing and Materials Processing, 6(5), 110. https://doi.org/10.3390/jmmp6050110






