Advanced Processing and Machining of Tungsten and Its Alloys
Abstract
1. Introduction
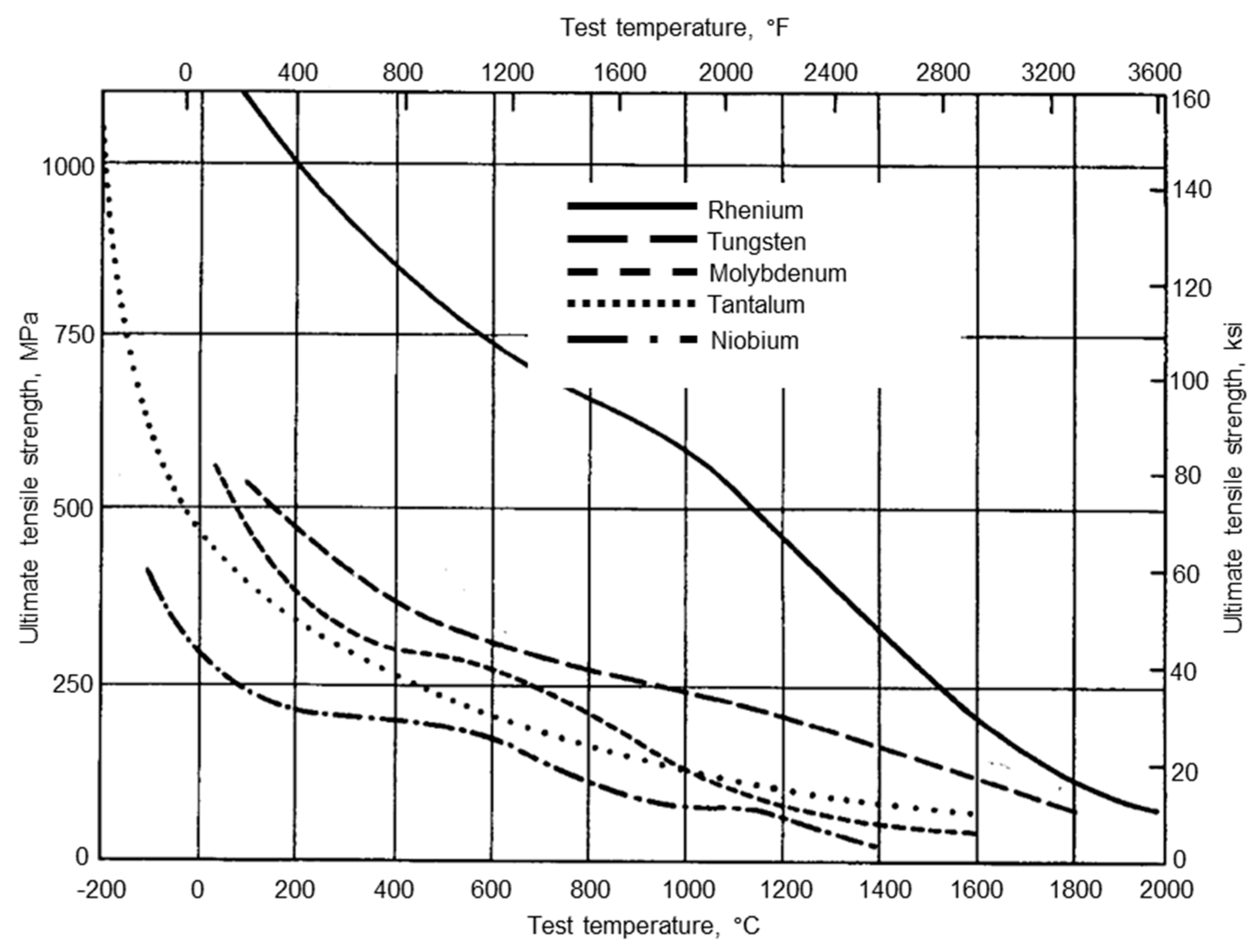
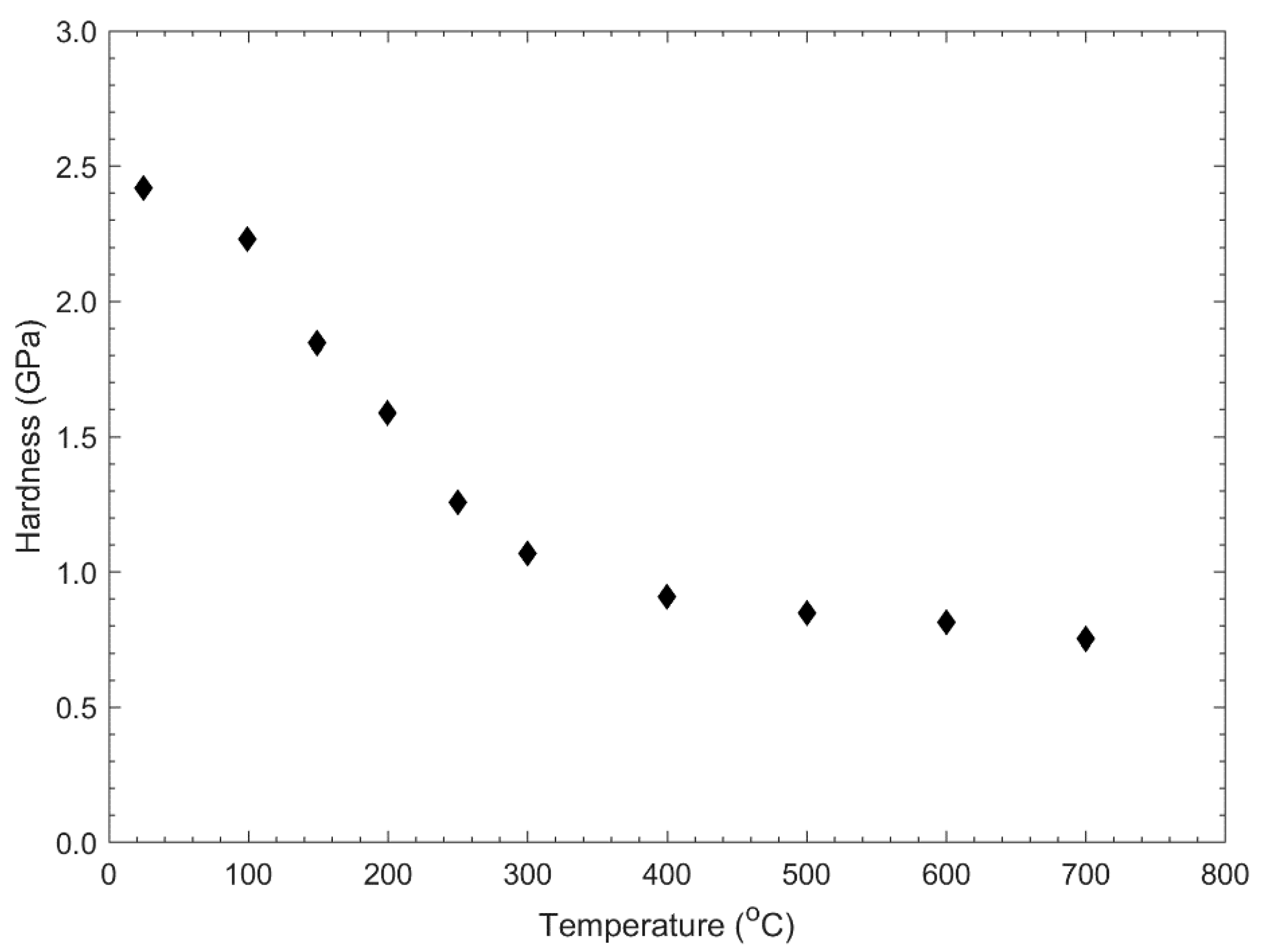
2. Microstructure and Properties of Tungsten
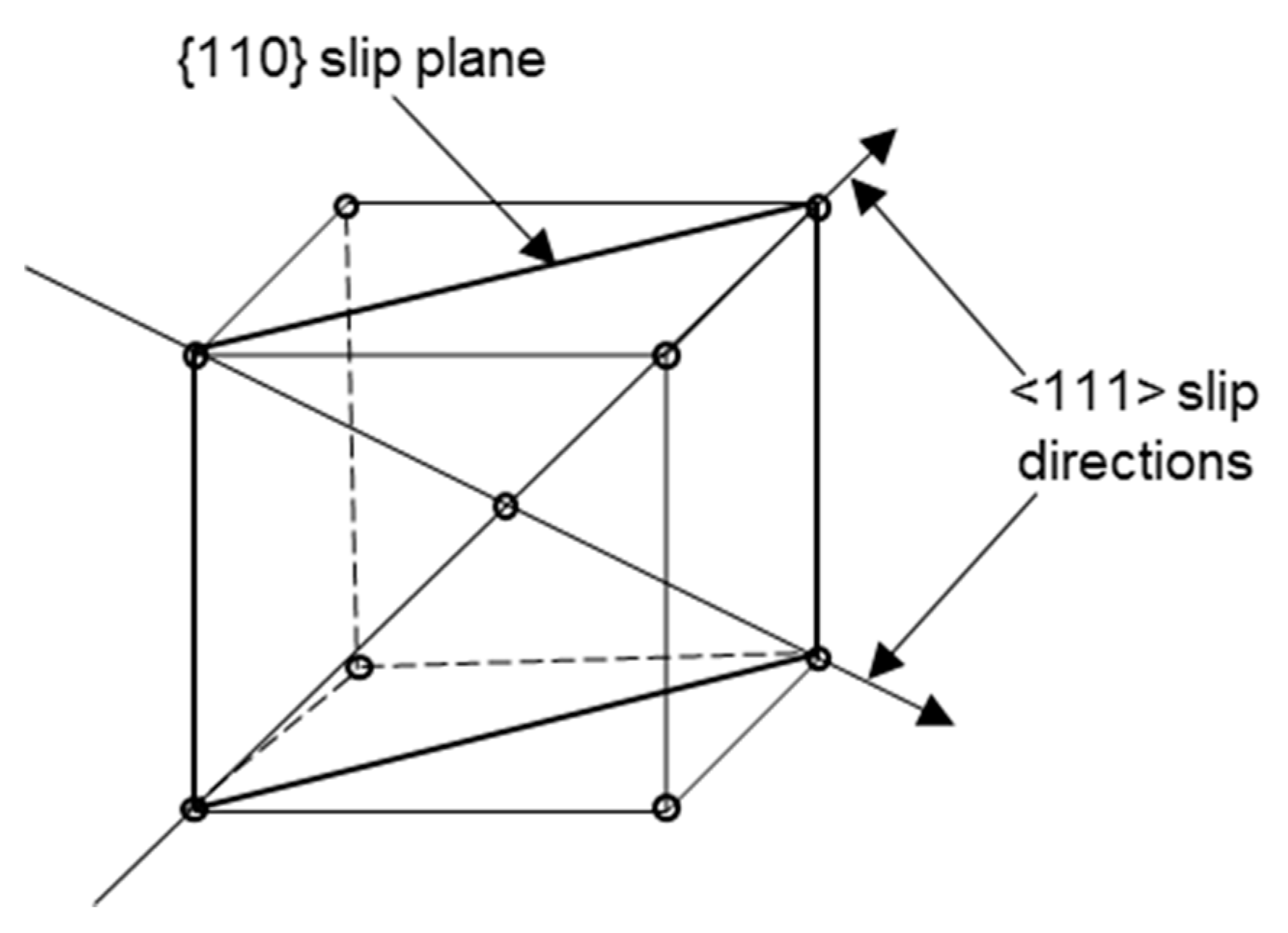
2.1. Microstructure of Tungsten
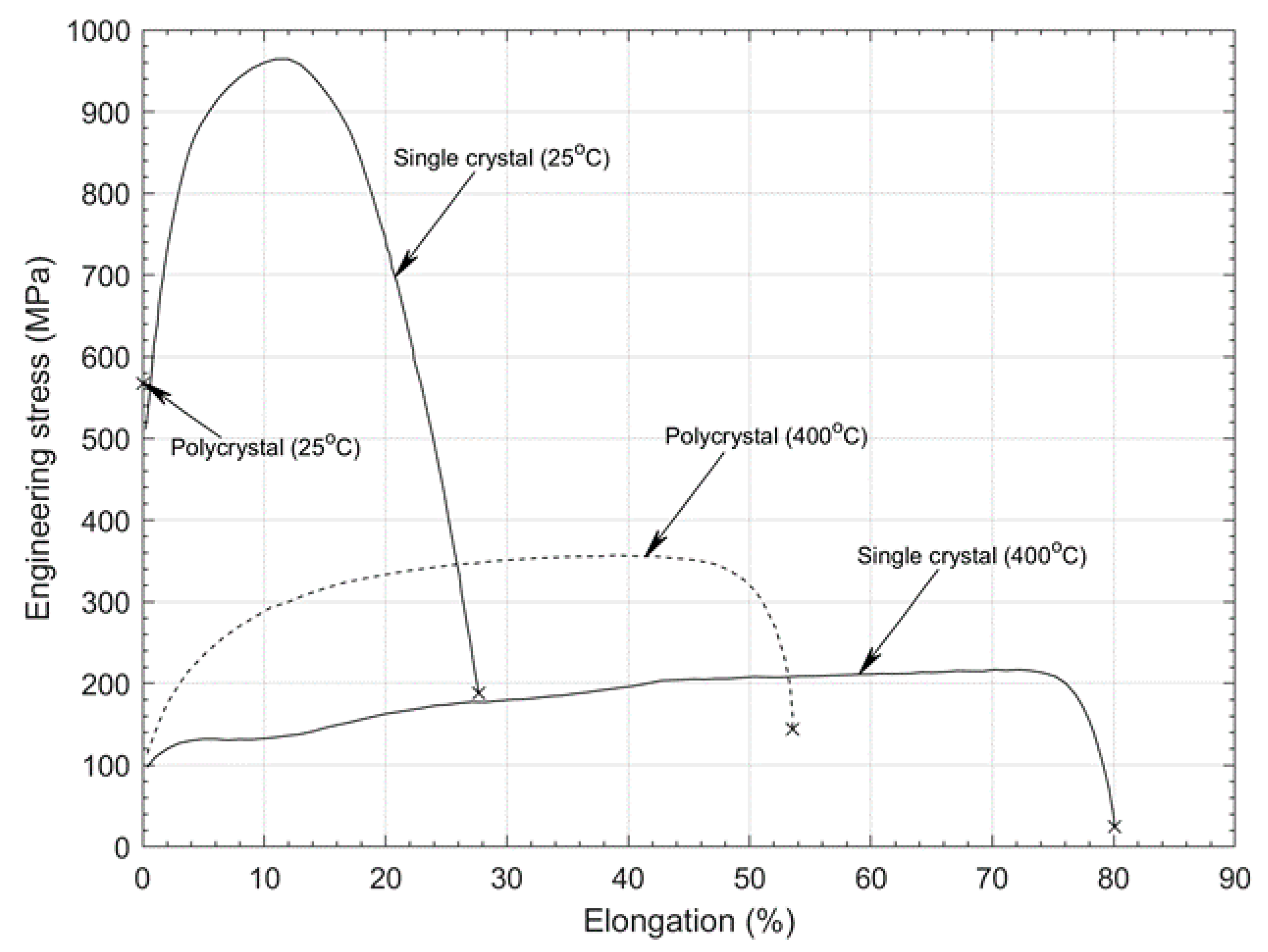
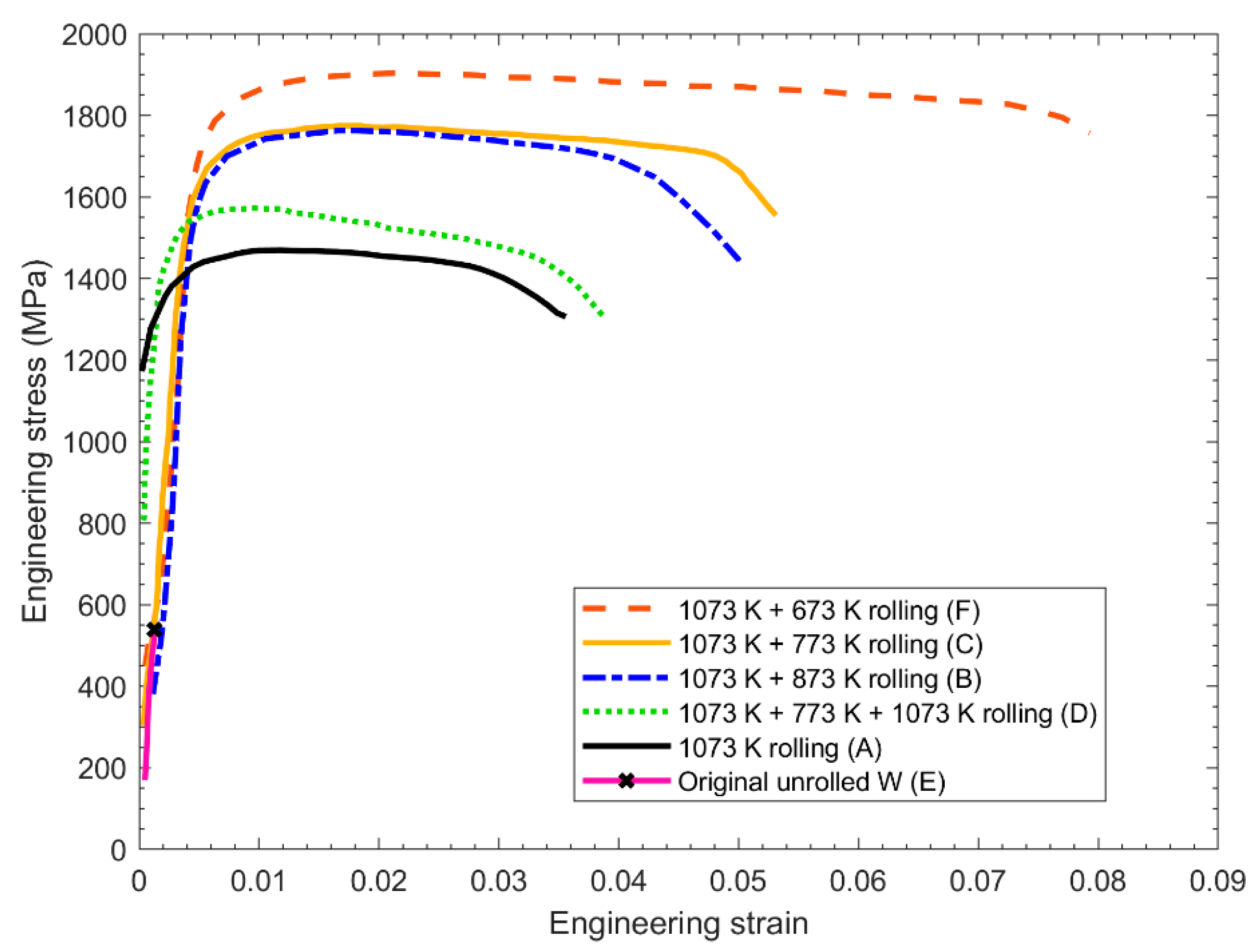
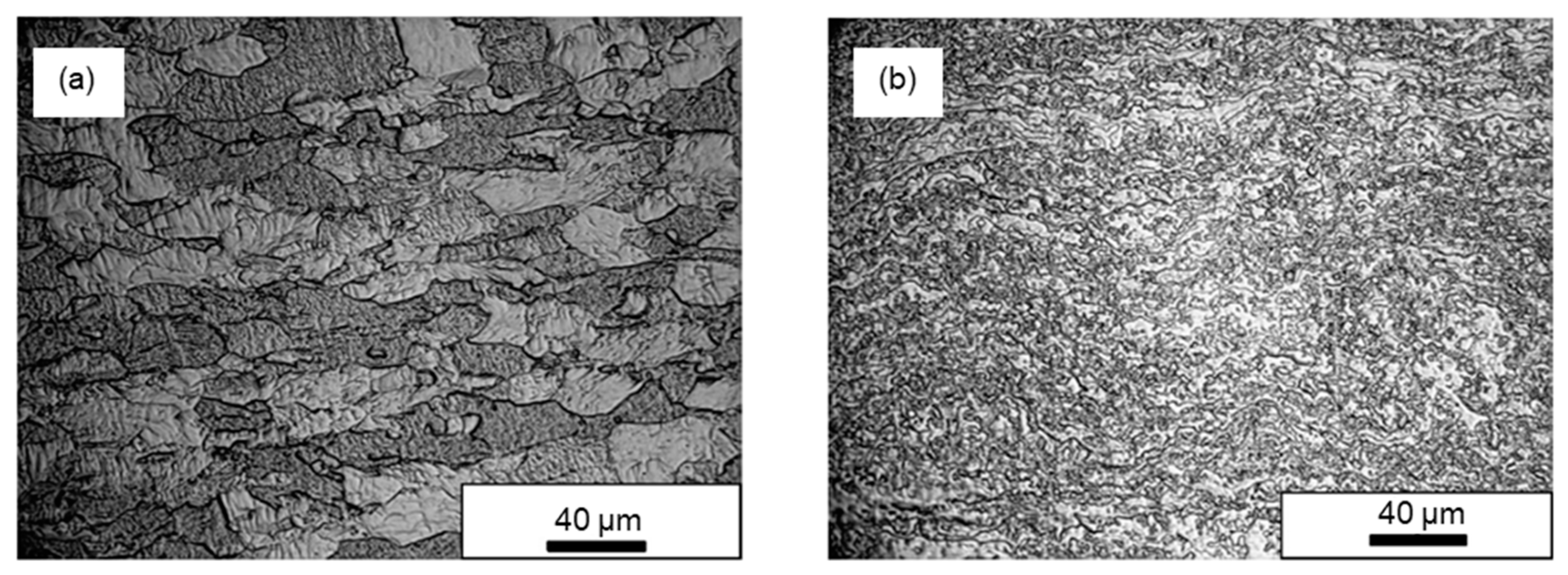
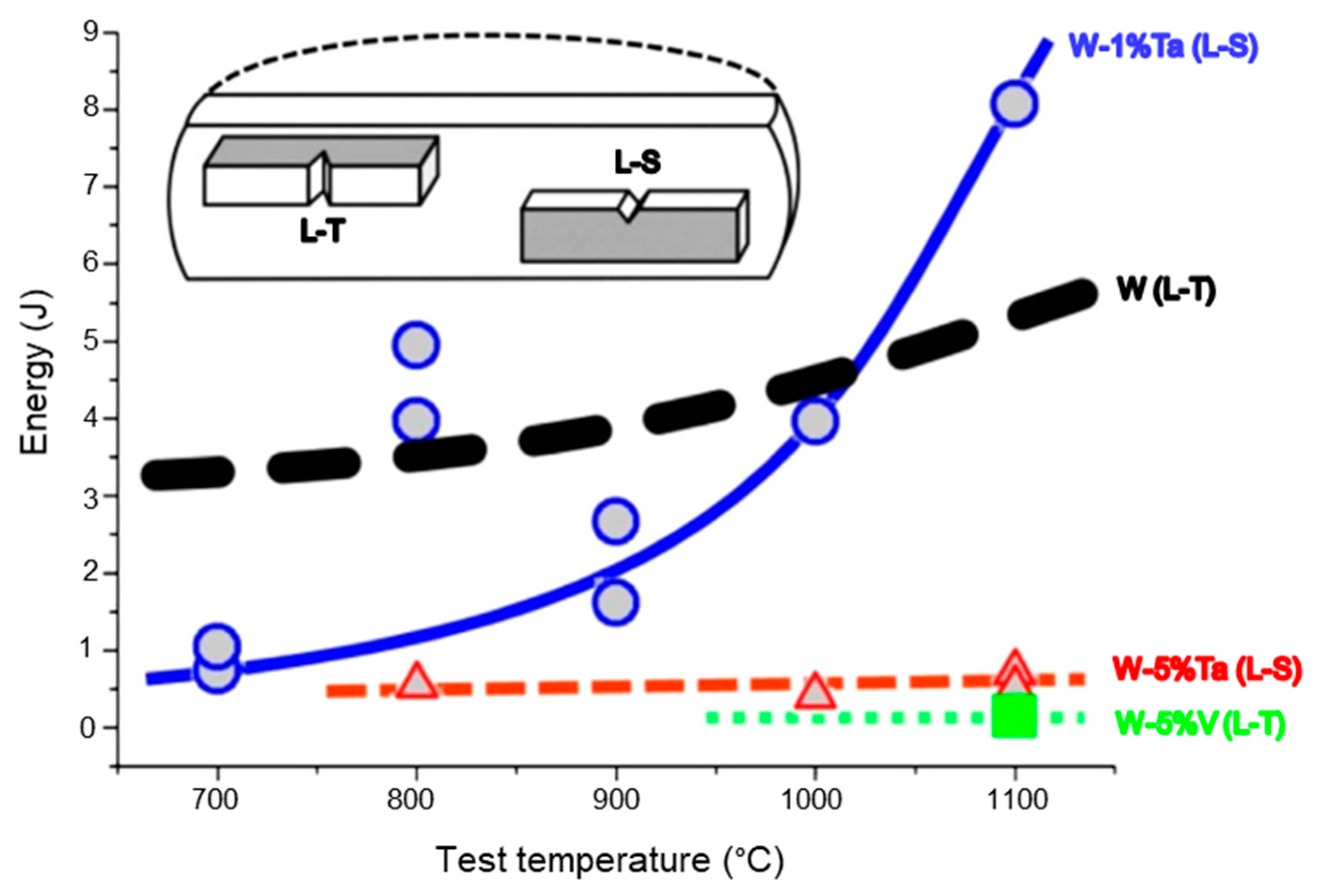
2.2. Methods for Improving the Ductility of Tungsten
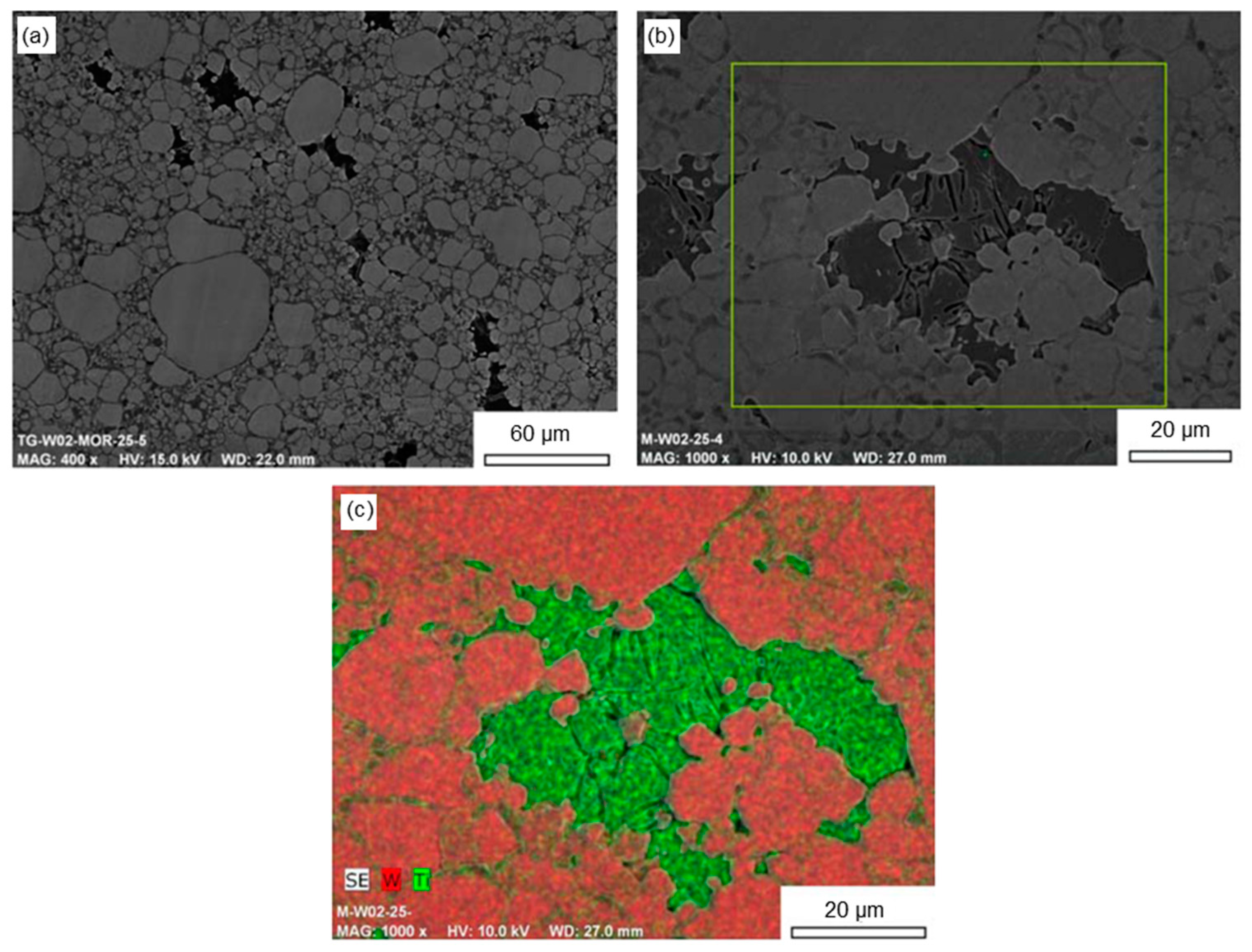
3. Powder Metallurgy for Tungsten Production
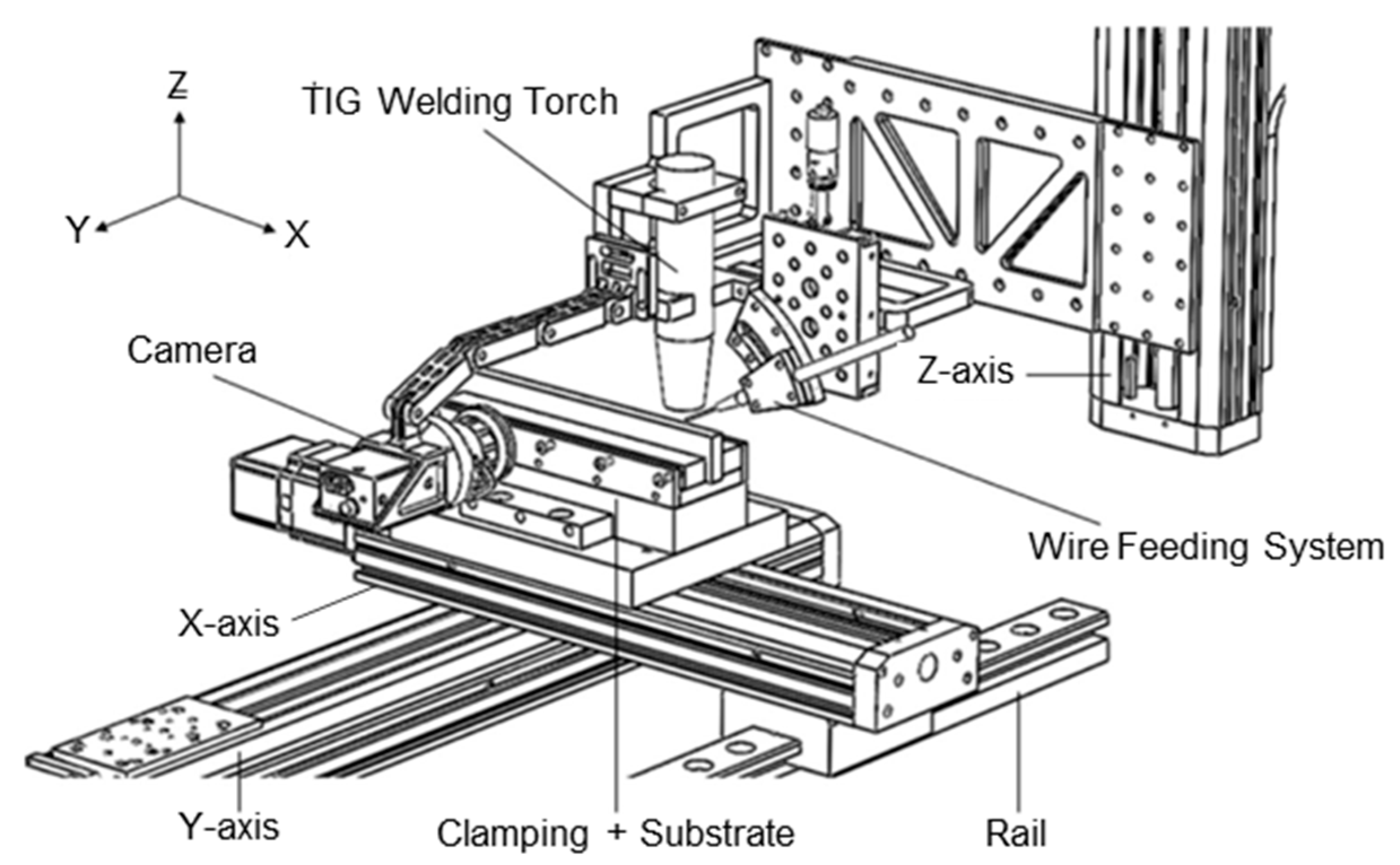
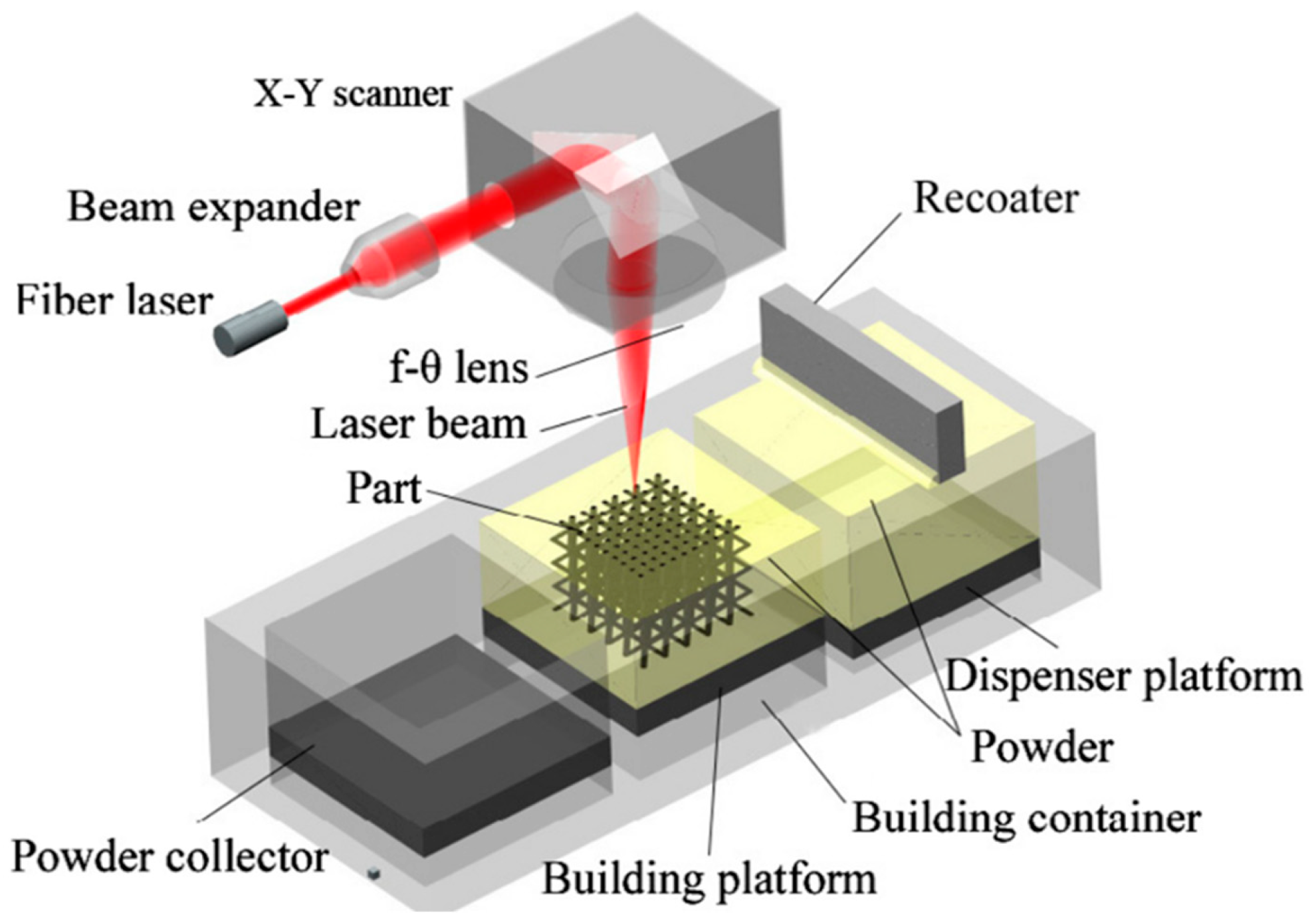
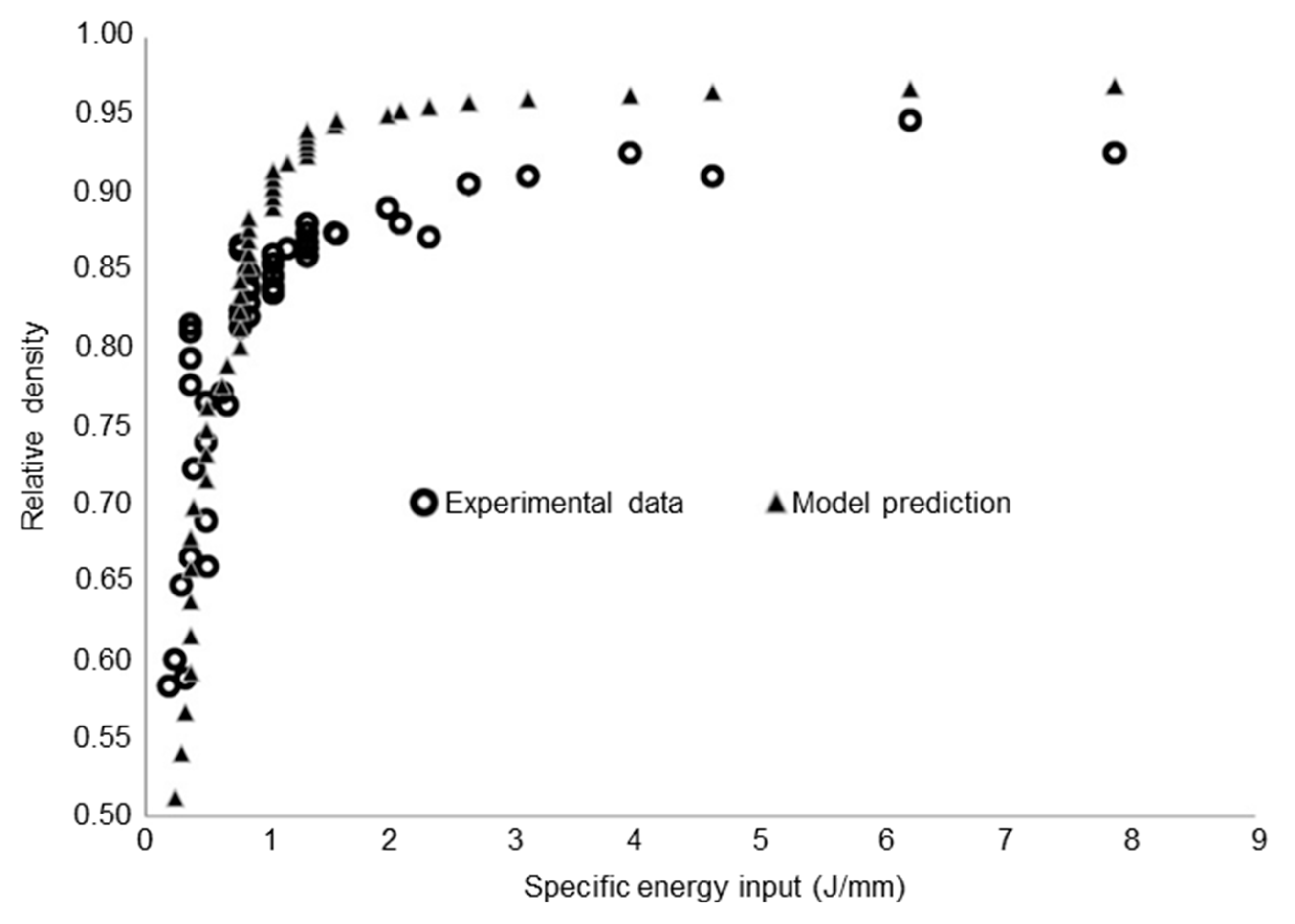
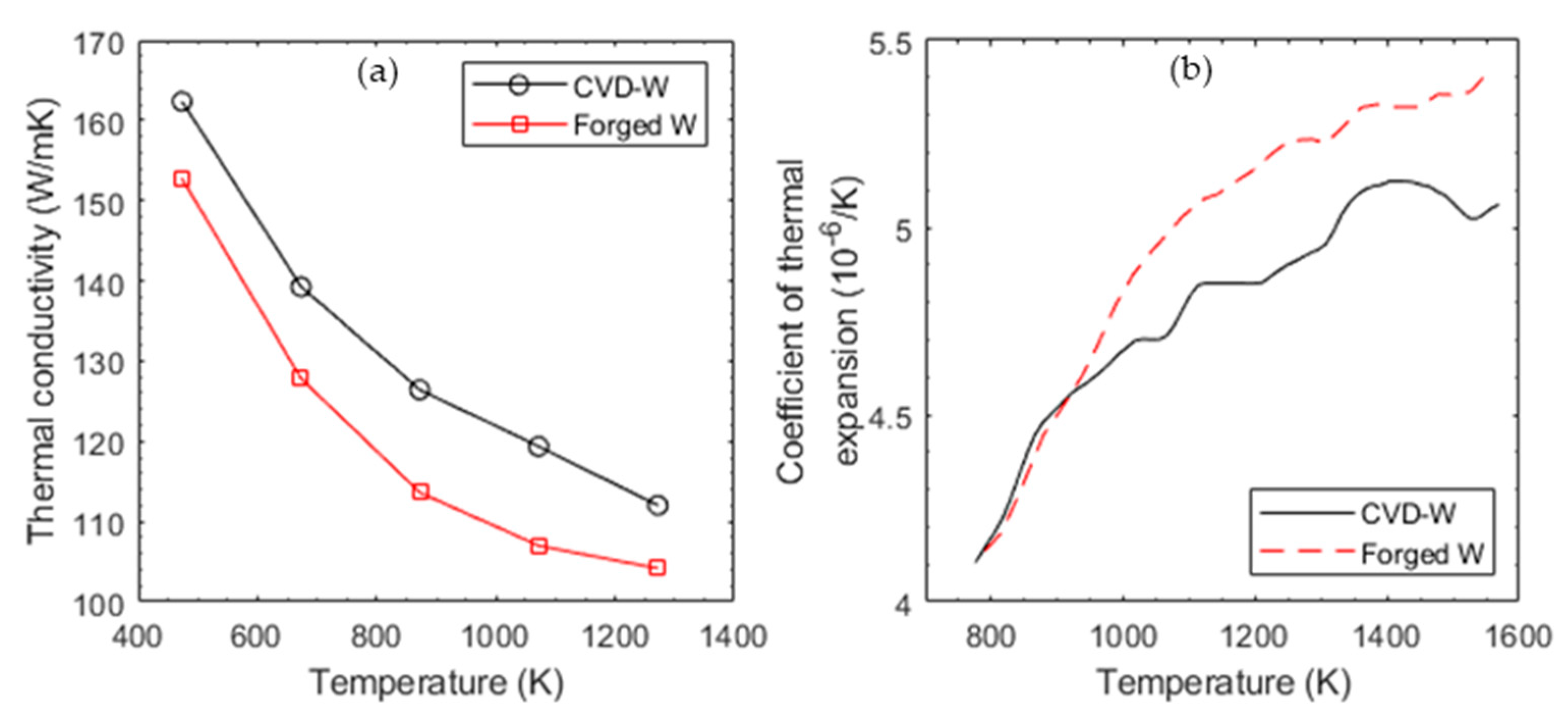
4. Additive Manufacturing of Tungsten
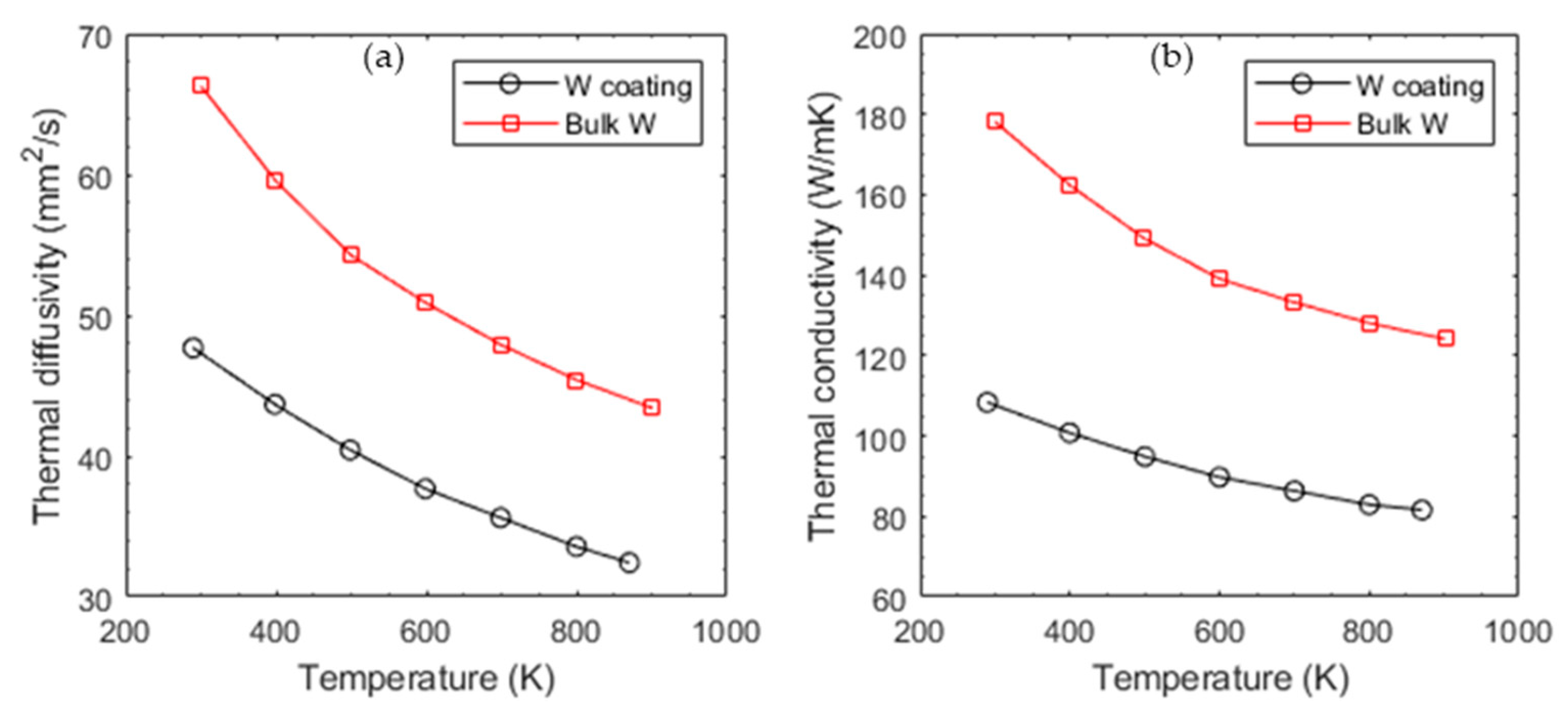
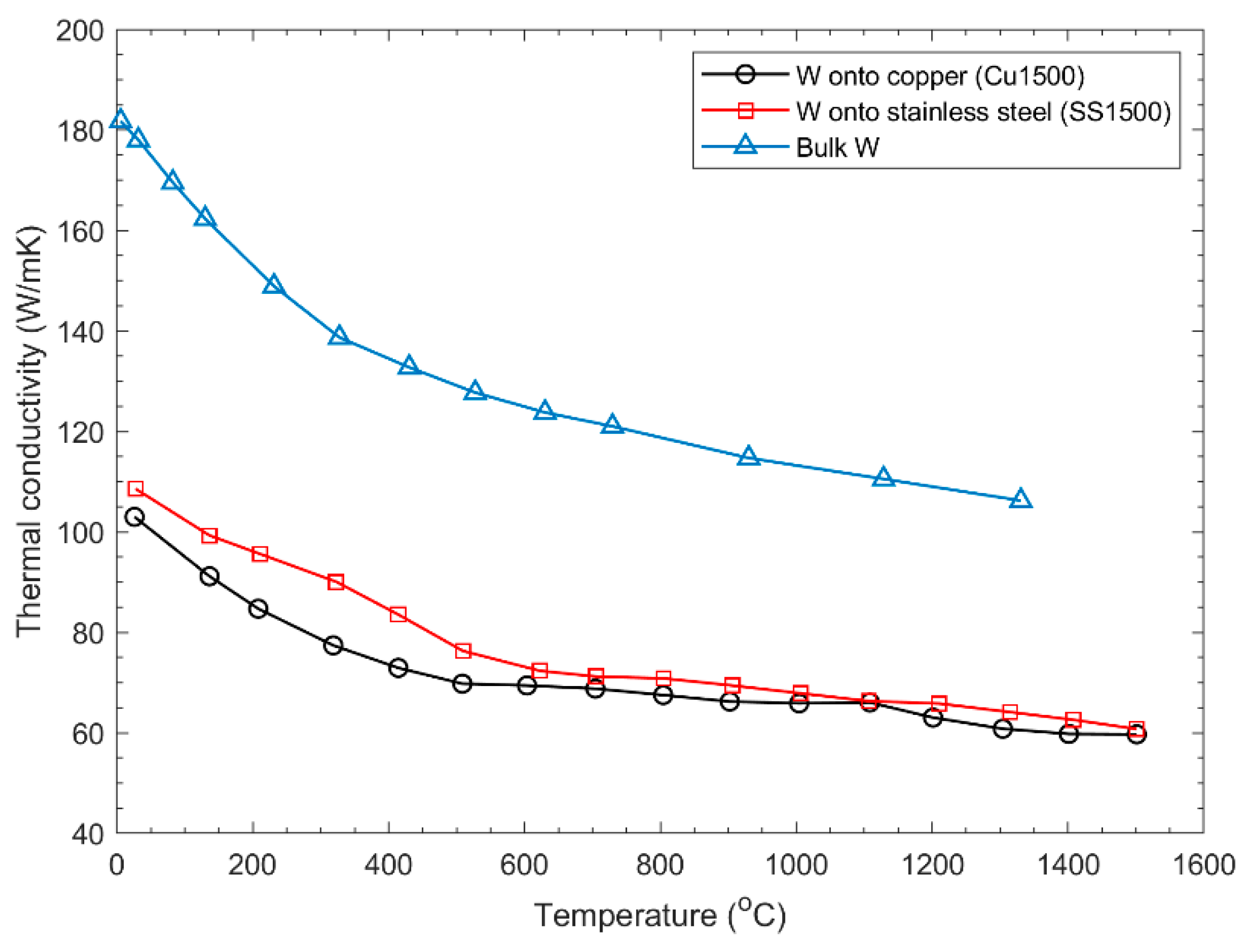
5. Coating Technologies for Tungsten Thin Films
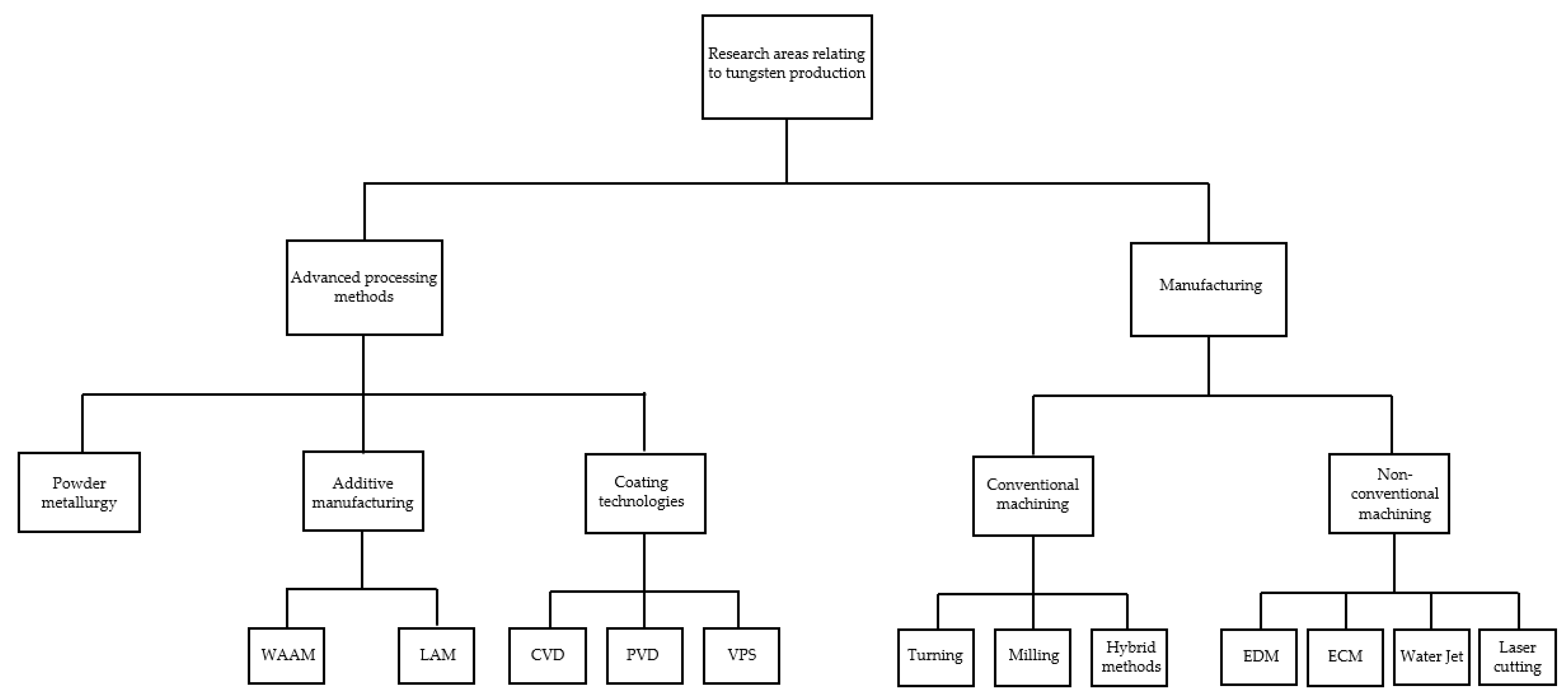
6. Machining of Tungsten
6.1. Non-Conventional Machining of Tungsten
6.1.1. Electro Discharge Machining (EDM) of Tungsten
6.1.2. Electro Chemical Machining (ECM) of Tungsten
6.1.3. Other Non-Conventional Techniques
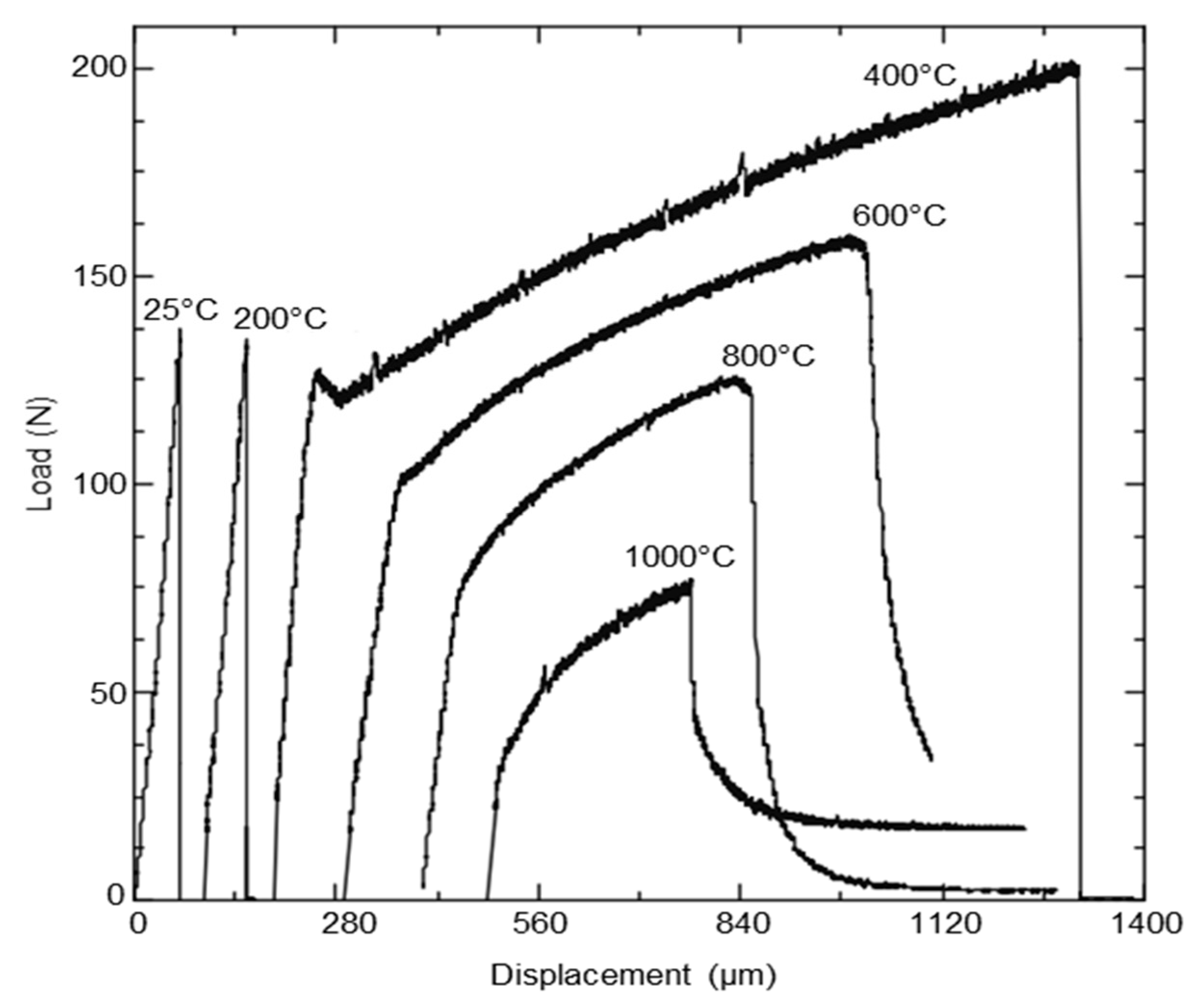
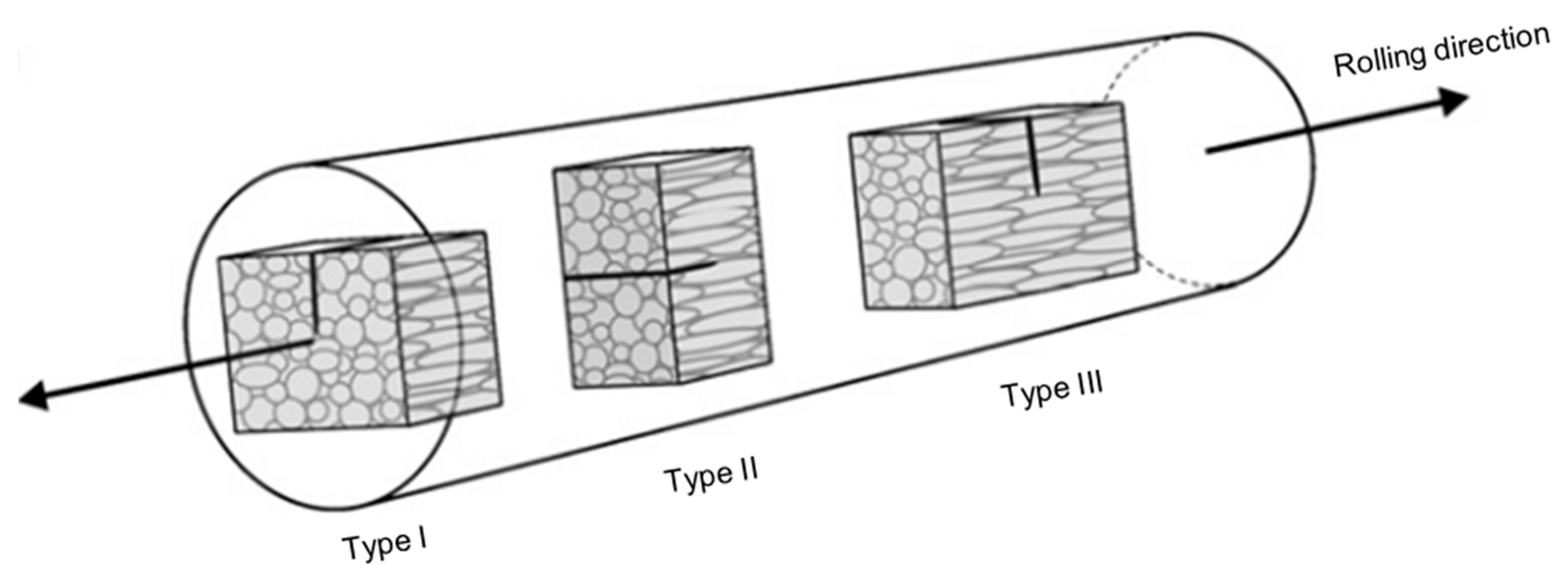
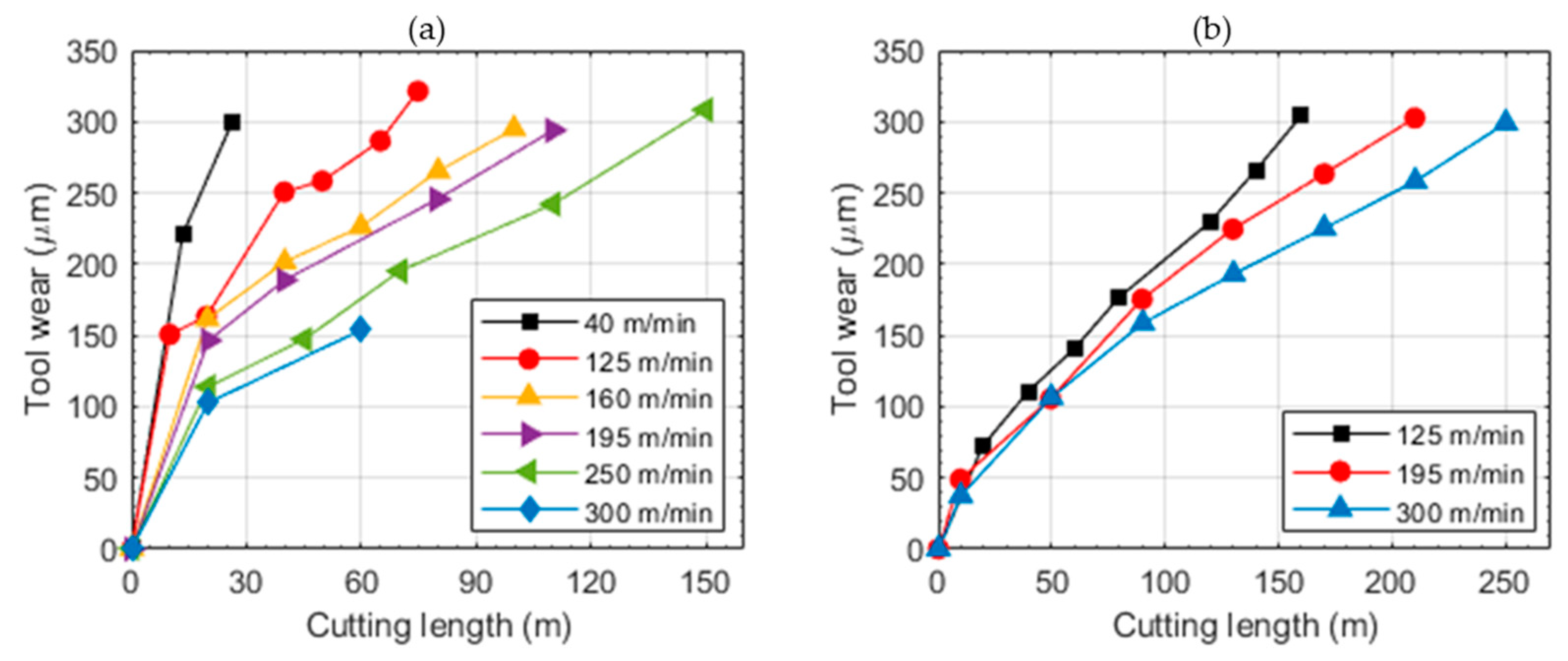
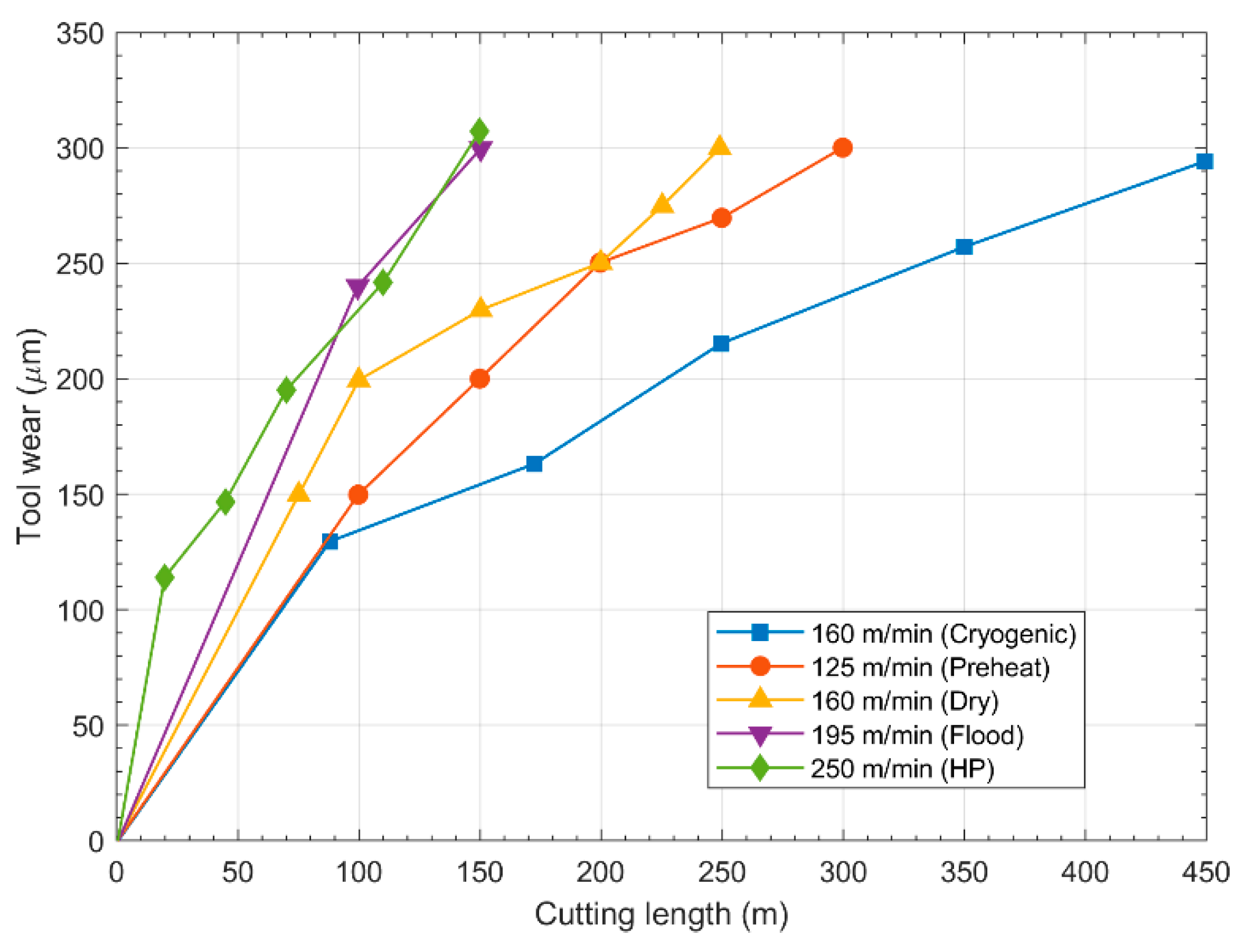
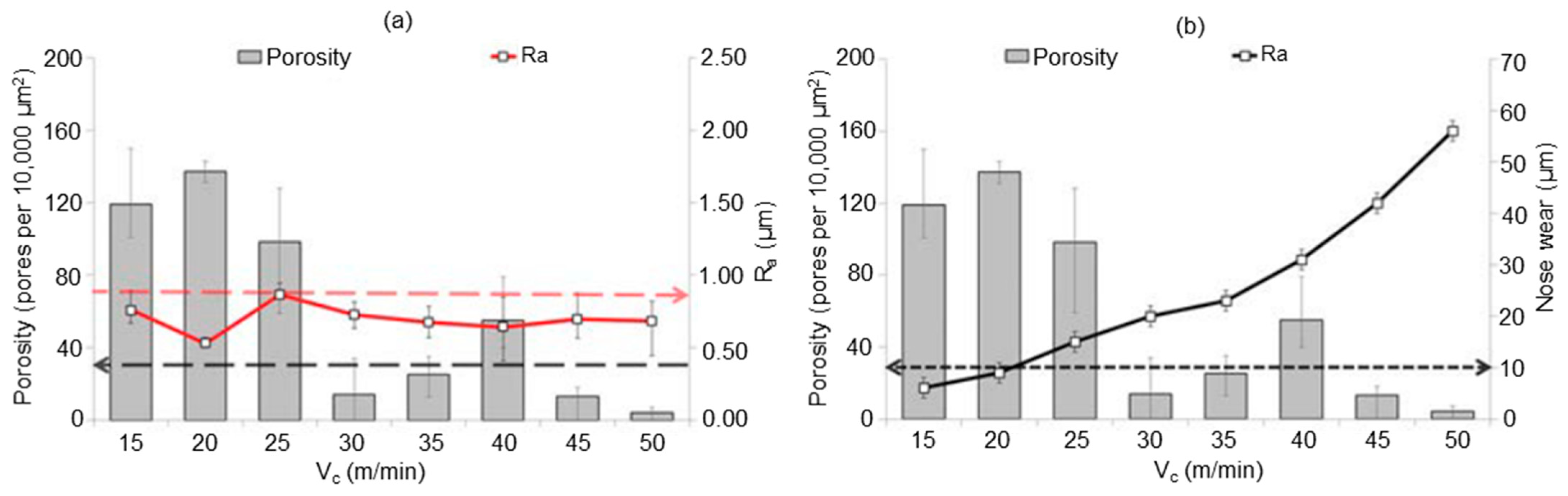
6.2. Conventional Machining of Tungsten
- High DBTT;
- High hardness and strength;
- High strain hardening tendency;
- High shear strength;
- Increase in recrystallisation tendency with increase in level of deformation.
7. Discussion and Future Research Direction
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Energy Agency (IEA). World Energy Outlook 2019. 2019. Available online: https://www.iea.org/reports/world-energy-outlook-2019 (accessed on 12 July 2020).
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Threlfall, R.; King, A.; Bartels, W.; Shulman, J.; Hayes, M. Towards Net Zero: How the World’s Largest Companies Report on Climate Risk and Net Zero Transition; KPMG: Amstelveen, The Netherlands, 2020. [Google Scholar]
- United Nations Environment Programme. Emissions Gap Report 2020; United Nations Environment Programme: Nairobi, Kenya, 2020. [Google Scholar]
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, R. Predicting the performance of tungsten in a fusion environment: A literature review. Mater. Sci. Technol. 2017, 33, 388–399. [Google Scholar] [CrossRef]
- Ward, D.; Cook, I.; Lechon, Y.; Saez, R. The economic viability of fusion power. Fusion Eng. Des. 2005, 75-79, 1221–1227. [Google Scholar] [CrossRef]
- Ueda, Y.; Coenen, J.; De Temmerman, G.; Doerner, R.; Linke, J.; Philipps, V.; Tsitrone, E. Research status and issues of tungsten plasma facing materials for ITER and beyond. Fusion Eng. Des. 2014, 89, 901–906. [Google Scholar] [CrossRef]
- Barabash, V.; Peacock, A.; Fabritsiev, S.; Kalinin, G.; Zinkle, S.; Rowcliffe, A.; Rensman, J.-W.; Tavassoli, A.; Marmy, P.; Karditsas, P.; et al. Materials challenges for ITER—Current status and future activities. J. Nucl. Mater. 2007, 367–370, 21–32. [Google Scholar] [CrossRef]
- Lassner, E.; Schubert, W.D. Properties, Chemistry, Technology of the Element, Alloys, and Chemical Compounds; Vienna University of Technology: Vienna, Austria, 2005. [Google Scholar]
- Bailar, J.C.; Trotman-Dickenson, A.F. (Eds.) Comprehensive Inorganic Chemistry, 4th ed.; Pergamon Press: Oxford, UK, 1973. [Google Scholar]
- Holden, N.E. Element 74, the Wolfram versus Tungsten Controversy. In Proceedings of the IUPAC Inorganic Chemistry Division Committee Meeting, Helsinki, Finland, 11–12 August 2008; Brookhaven National Lab.: Upton, NY, USA, 2008. [Google Scholar]
- Lennon, A.; Ramesh, K. The thermoviscoplastic response of polycrystalline tungsten in compression. Mater. Sci. Eng. A 2000, 276, 9–21. [Google Scholar] [CrossRef]
- Snead, L.L.; Hoelzer, D.T.; Rieth, M.; Nemith, A.A. Refractory Alloys: Vanadium, Niobium, Molybdenum, Tungsten. In Structural Alloys for Nuclear Energy Applications; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 585–640. [Google Scholar]
- Barabash, V. Historical Baseline Document: Appendix A, Materials Design Limit Data; ITER Organization: Saint-Paul-lez-Durance, France, 2012. [Google Scholar]
- Tungsten, W. Available online: http://www.matweb.com/search/datasheet_print.aspx?matguid=41e0851d2f3c417ba69ea0188fa570e3 (accessed on 24 March 2021).
- Li, H.; Wurster, S.; Motz, C.; Romaner, L.; Draxl, C.; Pippan, R. Dislocation-core symmetry and slip planes in tungsten alloys: Ab initio calculations and microcantilever bending experiments. Acta Mater. 2012, 60, 748–758. [Google Scholar] [CrossRef]
- Callister, W.D. Dislocation and Strengthening Mechanisms. In Materials Science and Engineering: An Introduction, 7th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2006; pp. 179–180. [Google Scholar]
- Liu, A. Lattice Structure and Deformation Mechanisms in Metallic Single Crystals. In Mechanics and Mechanisms of Fracture: An Introduction; ASM International: Almere, The Netherlands, 2005; pp. 357–372. [Google Scholar]
- Hirsch, P.B. First suggestion about screws. In Proceedings of the 5th International Conference on Crystallography, Cambridge, UK, 15–24 August 1960; pp. 139–145. [Google Scholar]
- Gröger, R.; Bailey, A.G.; Vitek, V. Multiscale modeling of plastic deformation of molybdenum and tungsten: I. Atomistic studies of the core structure and glide of 1/2<1 1 1> screw dislocations at 0 K. Acta Mater. 2008, 56, 5401–5411. [Google Scholar] [CrossRef]
- Cheng, G.; Xu, W.; Jian, W.; Yuan, H.; Tsai, M.-H.; Zhu, Y.; Zhang, Y.; Millett, P. Dislocations with edge components in nanocrystalline bcc Mo. J. Mater. Res. 2013, 28, 1820–1826. [Google Scholar] [CrossRef]
- Setyawan, W.; Kurtz, R.J. Effects of transition metals on the grain boundary cohesion in tungsten. Scr. Mater. 2012, 66, 558–561. [Google Scholar] [CrossRef]
- Gludovatz, B.; Wurster, S.; Weingärtner, T.; Hoffmann, A.; Pippan, R. Influence of impurities on the fracture behaviour of tungsten. Philos. Mag. 2011, 91, 3006–3020. [Google Scholar] [CrossRef]
- Joshi, a.; Stein, D.F. Intergranular brittleness studies in Tungsten Using Auger Spectroscopy. Metall. Trans. 1970, 1, 2543–2546. [Google Scholar]
- Funkenbusch, A.W.; Bacon, F.; Lee, D. The influence of microstructure on fracture of drawn tungsten wire. Met. Mater. Trans. A 1979, 10, 1085–1091. [Google Scholar] [CrossRef]
- Schwartzberg, F.R.; Ogden, H.R.; Jaffee, R.I. Ductile-Brittle Transition in the Refractory Metals; Defense Metals Information Center, Battelle Memorial Institute: Columbus, OH, USA, 1959. [Google Scholar]
- Aguirre, M.V.; Martín, A.; Pastor, J.Y.; Llorca, J.; Monge, M.A.; Pareja, R. Mechanical behavior of W-Y2O3 and W-Ti Alloys from 25 °C to 1000 °C. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2009, 40, 2283–2290. [Google Scholar] [CrossRef]
- Faleschini, M.; Kreuzer, H.; Kiener, D.; Pippan, R. Fracture toughness investigations of tungsten alloys and SPD tungsten alloys. J. Nucl. Mater. 2007, 367–370, 800–805. [Google Scholar] [CrossRef]
- Riedle, J. Bruchwiderstand in Wolfram-Einkristallen: Einfluß der Kristallographischen Orientierung, der Temperatur und der Lastrate; VDI-Verlag: Düsseldorf, Germany, 1995. [Google Scholar]
- Yih, S.W.H.; Wang, C.T. Tungsten: Sources, Metallurgy, Properties, and Applications; Plenum Press: New York, NY, USA, 1979; p. 358. [Google Scholar]
- Norajitra, P.; Boccaccini, L.; Diegele, E.; Filatov, V.; Gervash, A.; Giniyatulin, R.; Gordeev, S.; Heinzel, V.; Janeschitz, G.; Konys, J.; et al. Development of a helium-cooled divertor concept: Design-related requirements on materials and fabrication technology. J. Nucl. Mater. 2004, 329–333, 1594–1598. [Google Scholar] [CrossRef]
- Ren, C.; Fang, Z.; Koopman, M.; Butler, B.; Paramore, J.; Middlemas, S. Methods for improving ductility of tungsten—A review. Int. J. Refract. Met. Hard Mater. 2018, 75, 170–183. [Google Scholar] [CrossRef]
- Shen, T.; Dai, Y.; Lee, Y. Microstructure and tensile properties of tungsten at elevated temperatures. J. Nucl. Mater. 2016, 468, 348–354. [Google Scholar] [CrossRef]
- Rupp, D.; Mönig, R.; Gruber, P.; Weygand, S. Fracture toughness and microstructural characterization of polycrystalline rolled tungsten. Int. J. Refract. Met. Hard Mater. 2010, 28, 669–673. [Google Scholar] [CrossRef]
- Reiser, J.; Hoffmann, J.; Jäntsch, U.; Klimenkov, M.; Bonk, S.; Bonnekoh, C.; Rieth, M.; Hoffmann, A.; Mrotzek, T. Ductilisation of tungsten (W): On the shift of the brittle-to-ductile transition (BDT) to lower temperatures through cold rolling. Int. J. Refract. Met. Hard Mater. 2016, 54, 351–369. [Google Scholar] [CrossRef]
- Wei, Q.; Kecskes, L. Effect of low-temperature rolling on the tensile behavior of commercially pure tungsten. Mater. Sci. Eng. A 2008, 491, 62–69. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Q.; Lang, S.; Xia, M.; Ge, C. Texture evolution and basic thermal–mechanical properties of pure tungsten under various rolling reductions. J. Nucl. Mater. 2016, 468, 339–347. [Google Scholar] [CrossRef]
- Hall, E.O. The Deformation and Ageing of Mild Steel: III Discussion of Results. Proc. Phys. Soc. Sect. B 1951, 64, 747. [Google Scholar] [CrossRef]
- Petch, N.J. The cleavage strength of polycrystals. J. Iron Steel Inst. 1953, 174, 25–28. [Google Scholar]
- Vashi, U.K.; Armstrong, R.W.; Zima, G.E. The hardness and grain size of consolidated fine tungsten powder. Met. Mater. Trans. A 1970, 1, 1769–1771. [Google Scholar] [CrossRef]
- Reiser, J.; Rieth, M.; Dafferner, B.; Hoffmann, A.; Yi, X.; Armstrong, D.E. Tungsten foil laminate for structural divertor applications—Analyses and characterisation of tungsten foil. J. Nucl. Mater. 2012, 424, 197–203. [Google Scholar] [CrossRef]
- Terentyev, D.; Xiao, X.; Dubinko, A.; Bakaeva, A.; Duan, H. Dislocation-mediated strain hardening in tungsten: Thermo-mechanical plasticity theory and experimental validation. J. Mech. Phys. Solids 2015, 85, 1–15. [Google Scholar] [CrossRef]
- Romaner, L.; Draxl, C.; Pippan, R. Effect of Rhenium on the Dislocation Core Structure in Tungsten. Phys. Rev. Lett. 2010, 104, 195503. [Google Scholar] [CrossRef] [PubMed]
- Stephens, J.R. Dislocation structures in single-crystal tungsten and tungsten alloys. Met. Mater. Trans. A 1970, 1, 1293–1301. [Google Scholar] [CrossRef]
- Geach, G.A.; Hughes, J.E. The alloys of rhenium with molybdenum or with tungsten and having good high temperature properties. In Plansee Proceedings; Pergamon Press: London, UK, 1955; pp. 245–253. [Google Scholar]
- Mutoh, Y.; Ichikawa, K.; Nagata, K.; Takeuchi, M. Effect of rhenium addition on fracture toughness of tungsten at elevated temperatures. J. Mater. Sci. 1995, 30, 770–775. [Google Scholar] [CrossRef]
- Wurster, S.; Gludovatz, B.; Pippan, R. High temperature fracture experiments on tungsten–rhenium alloys. Int. J. Refract. Met. Hard Mater. 2010, 28, 692–697. [Google Scholar] [CrossRef]
- Watanabe, S.; Nogami, S.; Reiser, J.; Rieth, M.; Sickinger, S.; Baumgärtner, S.; Miyazawa, T.; Hasegawa, A. Tensile and impact properties of tungsten-rhenium alloy for plasma-facing components in fusion reactor. Fusion Eng. Des. 2019, 148, 111323. [Google Scholar] [CrossRef]
- Klopp, W.D.; Witzke, W.R.; Raffo, P.L. Mechanical Dilute Properties of and Tungsten-Rhenium; National Aeronautics and Space Administration: Washington, DC, USA, September 1966. [Google Scholar]
- Wurster, S.; Baluc, N.; Battabyal, M.; Crosby, T.; Du, J.; García-Rosales, C.; Hasegawa, A.; Hoffmann, A.; Kimura, A.; Kurishita, H.; et al. Recent progress in R&D on tungsten alloys for divertor structural and plasma facing materials. J. Nucl. Mater. 2013, 442, S181–S189. [Google Scholar] [CrossRef]
- El-Guebaly, L.; Kurtz, R.; Rieth, M.; Kurishita, H.; Robinson, A.; Team, A. W-Based Alloys for Advanced Divertor Designs: Options and Environmental Impact of State-of-the-Art Alloys. Fusion Sci. Technol. 2011, 60, 185–189. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Ren, C.; Simmons, M.; Sun, P. The effect of Ni doping on the mechanical behavior of tungsten. Int. J. Refract. Met. Hard Mater. 2020, 92, 105281. [Google Scholar] [CrossRef]
- Ren, C.; Fang, Z.Z.; Koopman, M.; Zhang, H. The Effects of Molybdenum Additions on the Sintering and Mechanical Behavior of Ultrafine-Grained Tungsten. JOM 2018, 70, 2567–2573. [Google Scholar] [CrossRef]
- Patra, A.; Saxena, R.; Karak, S. Combined effect of Ni and nano-Y2O3 addition on microstructure, mechanical and high temperature behavior of mechanically alloyed W-Mo. Int. J. Refract. Met. Hard Mater. 2016, 60, 131–146. [Google Scholar] [CrossRef]
- Wurster, S.; Gludovatz, B.; Hoffmann, A.; Pippan, R. Fracture behaviour of tungsten–vanadium and tungsten–tantalum alloys and composites. J. Nucl. Mater. 2011, 413, 166–176. [Google Scholar] [CrossRef]
- Rieth, M.; Reister, J.; Dafferner, B.; Baumgärtner, S. The Impact of Refractory Material Properties on the Helium Cooled Divertor Design. Fusion Sci. Technol. 2012, 61, 381–384. [Google Scholar] [CrossRef]
- Bose, A.; Schuh, C.A.; Tobia, J.C.; Tuncer, N.; Mykulowycz, N.M.; Preston, A.; Barbati, A.C.; Kernan, B.; Gibson, M.A.; Krause, D.; et al. Traditional and additive manufacturing of a new Tungsten heavy alloy alternative. Int. J. Refract. Met. Hard Mater. 2018, 73, 22–28. [Google Scholar] [CrossRef]
- Ye, H.; Liu, X.Y.; Hong, H. Fabrication of metal matrix composites by metal injection molding—A review. J. Mater. Process. Technol. 2008, 200, 12–24. [Google Scholar] [CrossRef]
- Blagoeva, D.T.; Opschoor, J.; Pintsuk, G.; Sarbu, C. Development and Qualification of Tungsten and Tungsten Alloys for Fusion. Fusion Sci. Technol. 2013, 64, 203–210. [Google Scholar] [CrossRef]
- Piotter, V.; Zeep, B.; Norajitra, P.; Ruprecht, R.; von der Weth, A.; Hausselt, J. Development of a powder metallurgy process for tungsten components. Fusion Eng. Des. 2008, 83, 1517–1520. [Google Scholar] [CrossRef]
- Zeep, B.; Norajitra, P.; Piotter, V.; Boehm, J.; Ruprecht, R.; Hausselt, J. Net shaping of tungsten components by micro powder injection moulding. Fusion Eng. Des. 2007, 82, 2660–2665. [Google Scholar] [CrossRef]
- Antusch, S.; Norajitra, P.; Piotter, V.; Ritzhaupt-Kleissl, H.-J.; Spatafora, L. Powder Injection Molding—An innovative manufacturing method for He-cooled DEMO divertor components. Fusion Eng. Des. 2011, 86, 1575–1578. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying and Milling; Cambridge International Science Publishing, Limited: Cambridge, UK, 2004; Volume 46, pp. 1–184. [Google Scholar] [CrossRef]
- Oghbaei, M.; Mirzaee, O. Microwave versus conventional sintering: A review of fundamentals, advantages and applications. J. Alloy. Compd. 2010, 494, 175–189. [Google Scholar] [CrossRef]
- Li, Y.; Hu, K.; Li, X.; Ai, X.; Qu, S. Fine-grained 93W–5.6Ni–1.4Fe heavy alloys with enhanced performance prepared by spark plasma sintering. Mater. Sci. Eng. A 2013, 573, 245–252. [Google Scholar] [CrossRef]
- Caldwell, S.G.; Bose, A.; Dowding, R.J. (Eds.) Tungsten & Tungsten Alloys-1992. In Proceedings of the First International Conference on Tungsten and Tungsten Alloys, Princeton, NJ, USA, 1 January 1993; pp. 89–96. [Google Scholar]
- Şahin, Y. Recent Progress in Processing of Tungsten Heavy Alloys. J. Powder Technol. 2014, 2014, 1–22. [Google Scholar] [CrossRef]
- Edstrom, C.M.; Phillips, A.G.; Johnson, L.D.; Corle, R.R. Literature on Fabrication of Tungtsen for Application in Pyrochemical Processing of Spent Nuclear Fuels; Technical Report No. RFP-2864; Rockwell International Corp.: Golden, CO, USA, 1980. [Google Scholar]
- Marinelli, G.; Martina, F.; Lewtas, H.; Hancock, D.; Mehraban, S.; Lavery, N.P.; Ganguly, S.; Williams, S. Microstructure and thermal properties of unalloyed tungsten deposited by Wire + Arc Additive Manufacture. J. Nucl. Mater. 2019, 522, 45–53. [Google Scholar] [CrossRef]
- Marinelli, G.; Martina, F.; Ganguly, S.; Williams, S. Development of Wire + Arc additive manufacture for the production of large-scale unalloyed tungsten components. Int. J. Refract. Met. Hard Mater. 2019, 82, 329–335. [Google Scholar] [CrossRef]
- Lv, Y.; Lian, Y.; Song, J.; Yu, Y.; Liu, X.; Zhuang, Z. WITHDRAWN: The thermal properties of high purity and fully dense tungsten produced by chemical vapor deposition. J. Nucl. Mater. 2014, 457, 317–323. [Google Scholar] [CrossRef]
- Niu, Y.; Zheng, X.; Ji, H.; Qi, L.; Ding, C.; Chen, J.; Luo, G. Microstructure and thermal property of tungsten coatings prepared by vacuum plasma spraying technology. Fusion Eng. Des. 2010, 85, 1521–1526. [Google Scholar] [CrossRef]
- Wang, D.; Yu, C.; Zhou, X.; Ma, J.; Liu, W.; Shen, Z. Dense Pure Tungsten Fabricated by Selective Laser Melting. Appl. Sci. 2017, 7, 430. [Google Scholar] [CrossRef]
- Braun, J.; Kaserer, L.; Stajkovic, J.; Leitz, K.-H.; Tabernig, B.; Singer, P.; Leibenguth, P.; Gspan, C.; Kestler, H.; Leichtfried, G. Molybdenum and tungsten manufactured by selective laser melting: Analysis of defect structure and solidification mechanisms. Int. J. Refract. Met. Hard Mater. 2019, 84, 104999. [Google Scholar] [CrossRef]
- Iveković, A.; Omidvari, N.; Vrancken, B.; Lietaert, K.; Thijs, L.; Vanmeensel, K.; Vleugels, J.; Kruth, J.-P. Selective laser melting of tungsten and tungsten alloys. Int. J. Refract. Met. Hard Mater. 2018, 72, 27–32. [Google Scholar] [CrossRef]
- Nie, B.; Yang, L.; Huang, H.; Bai, S.; Wan, P.; Liu, J. Femtosecond laser additive manufacturing of iron and tungsten parts. Appl. Phys. A 2015, 119, 1075–1080. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Zhang, D.; Shen, Z.; Liu, W. Balling phenomena in selective laser melted tungsten. J. Mater. Process. Technol. 2015, 222, 33–42. [Google Scholar] [CrossRef]
- Guan, K.; Wang, Z.; Gao, M.; Li, X.; Zeng, X. Effects of processing parameters on tensile properties of selective laser melted 304 stainless steel. Mater. Des. 2013, 50, 581–586. [Google Scholar] [CrossRef]
- Wang, M.; Li, R.; Yuan, T.; Chen, C.; Zhang, M.; Weng, Q.; Yuan, J. Selective laser melting of W-Ni-Cu composite powder: Densification, microstructure evolution and nano-crystalline formation. Int. J. Refract. Met. Hard Mater. 2018, 70, 9–18. [Google Scholar] [CrossRef]
- Wang, X.; Wraith, M.; Burke, S.; Rathbun, H.; DeVlugt, K. Densification of W-Ni-Fe powders using laser sintering. Int. J. Refract. Met. Hard Mater. 2016, 56, 145–150. [Google Scholar] [CrossRef]
- Field, A.C.; Carter, L.N.; Adkins, N.J.E.; Attallah, M.M.; Gorley, M.J.; Strangwood, M. The Effect of Powder Characteristics on Build Quality of High-Purity Tungsten Produced via Laser Powder Bed Fusion (LPBF). Met. Mater. Trans. A 2020, 51, 1367–1378. [Google Scholar] [CrossRef]
- Zhang, D.; Cai, Q.; Liu, J. Formation of Nanocrystalline Tungsten by Selective Laser Melting of Tungsten Powder. Mater. Manuf. Process. 2012, 27, 1267–1270. [Google Scholar] [CrossRef]
- Dorow-Gerspach, D.; Kirchner, A.; Loewenhoff, T.; Pintsuk, G.; Weißgärber, T.; Wirtz, M. Additive manufacturing of high density pure tungsten by electron beam melting. Nucl. Mater. Energy 2021, 28, 101046. [Google Scholar] [CrossRef]
- Neiser, R.A.; Smolik, G.R.; Hollis, K.J.; Watson, R.D. Evaluation of plasma-sprayed tungsten for fusion reactors. J. Therm. Spray Technol. 1993, 2, 393–399. [Google Scholar] [CrossRef]
- Sato, K.; Nakamura, K.; Suzuki, S.; Araki, M.; Dairaku, M.; Yokoyama, K.; Akiba, M. High Heat Flux Test of CVD-Tungsten Coated Cu Heat Sink Divertor Mock-Up. Fusion Technol. 1996, 30, 769–773. [Google Scholar] [CrossRef]
- Nakamura, K.; Suzuki, S.; Satoh, K.; Araki, M.; Yokoyama, K.; Dairaku, M.; Akiba, M. Erosion of CFCs and W at high temperature under high heat loads. J. Nucl. Mater. 1994, 212–215, 1201–1205. [Google Scholar] [CrossRef]
- Smid, I.; Akiba, M.; Vieider, G.; Plöchl, L. Development of tungsten armor and bonding to copper for plasma-interactive components. J. Nucl. Mater. 1998, 258–263, 160–172. [Google Scholar] [CrossRef]
- Salhi, Z.; Klein, D.; Gougeon, P.; Coddet, C. Development of coating by thermal plasma spraying under very low-pressure condition. Vacuum 2005, 77, 145–150. [Google Scholar] [CrossRef]
- Matejicek, J.; Chráska, P.; Linke, J. Thermal Spray Coatings for Fusion Applications—Review. J. Therm. Spray Technol. 2007, 16, 64–83. [Google Scholar] [CrossRef]
- Murphy, J.; Giannattasio, A.; Yao, Z.; Hetherington, C.; Nellist, P.; Roberts, S. The mechanical properties of tungsten grown by chemical vapour deposition. J. Nucl. Mater. 2009, 386–388, 583–586. [Google Scholar] [CrossRef]
- Giannattasio, A.; Roberts, S. Strain-rate dependence of the brittle-to-ductile transition temperature in tungsten. Philos. Mag. 2007, 87, 2589–2598. [Google Scholar] [CrossRef]
- Lassila, D.H.; Connor, A. Tungsten and Tungsten Alloys—Recent Advances; The Minerals, Metals and Materials Society: Warrendale, PA, USA, 1991; pp. 79–85. [Google Scholar]
- Rieth, M.; Dudarev, S.; de Vicente, S.G.; Aktaa, J.; Ahlgren, T.; Antusch, S.; Armstrong, D.; Balden, M.; Baluc, N.; Barthe, M.-F.; et al. Recent progress in research on tungsten materials for nuclear fusion applications in Europe. J. Nucl. Mater. 2013, 432, 482–500. [Google Scholar] [CrossRef]
- Krauss, W.; Holstein, N.; Konys, J.; Mazul, I. Investigation of the impact of fabrication methods on the microstructure features of W-components of a He-cooled divertor. Fusion Eng. Des. 2005, 81, 259–264. [Google Scholar] [CrossRef]
- Holstein, N.; Krauss, W.; Konys, J. Development of novel tungsten processing technologies for electro-chemical machining (ECM) of plasma facing components. Fusion Eng. Des. 2011, 86, 1611–1615. [Google Scholar] [CrossRef]
- Ho, K.; Newman, S. State of the art electrical discharge machining (EDM). Int. J. Mach. Tools Manuf. 2003, 43, 1287–1300. [Google Scholar] [CrossRef]
- Sundaram, M.; Rajurkar, K. Electrical and Electrochemical Processes. In Intelligent Energy Field Manufacturing; CRC Press: Boca Raton, FL, USA, 2010; pp. 173–212. [Google Scholar]
- Wang, Y.; Wang, L.; Zhang, X.; Mou, N.; Yao, D. Cutting of tungsten plate for fusion device via pre-mixed abrasive water jet. Fusion Eng. Des. 2020, 159, 111790. [Google Scholar] [CrossRef]
- Šniaukas, R.; Račiukaitis, G. Laser Micro-Cutting of Thick Tungsten Sheets. In Proceedings of the Lasers in Manufacturing Conference 2015, Munich, Germany, 22–25 June 2015. [Google Scholar]
- Begic-Hajdarevic, D.; Vucijak, B.; Pasic, M.; Bijelonja, I. Analysis of the influence of cutting parameters on surface roughness in laser cutting of tungsten alloy using control charts. Teh. Vjesn.-Tech. Gaz. 2017, 24, 339–344. [Google Scholar] [CrossRef][Green Version]
- Uebel, M.; Bliedtner, J. Laser Precision Cutting of High-melting Metal Foils. Procedia Eng. 2014, 69, 99–103. [Google Scholar] [CrossRef]
- Spiro, A.; Lowe, M.; Pasmanik, G. Drilling rate of five metals with picosecond laser pulses at 355, 532, and 1064 nm. Appl. Phys. A 2012, 107, 801–808. [Google Scholar] [CrossRef]
- Begic-Hajdarevic, D.; Bijelonja, I. Experimental and Numerical Investigation of Temperature Distribution and Hole Geometry during Laser Drilling Process. Procedia Eng. 2015, 100, 384–393. [Google Scholar] [CrossRef]
- König, W.; Dauw, D.; Levy, G.; Panten, U. EDM-Future Steps towards the Machining of Ceramics. CIRP Ann. 1988, 37, 623–631. [Google Scholar] [CrossRef]
- Masuzawa, T. State of the Art of Micromachining. CIRP Ann. 2000, 49, 473–488. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, F.; Sundaram, M.M.; Rajurkar, K.P. Study on the surface integrity of machined tool in micro EDM. In Proceedings of the 16th International Symposium on Electromachining, Shanghai, China, 19–23 April 2010; pp. 685–689. [Google Scholar]
- Krauss, W.; Holstein, N.; Konys, J. Development and fabrication aspects regarding tungsten components for a He-cooled divertor. Fusion Eng. Des. 2005, 75–79, 775–778. [Google Scholar] [CrossRef]
- Han, W.; Kunieda, M. A novel method to switch machining mode between Micro-ECM and Micro-EDM using oxide film on surface of tungsten electrode. Precis. Eng. 2019, 56, 455–465. [Google Scholar] [CrossRef]
- Rajurkar, K.; Zhu, D.; McGeough, J.; Kozak, J.; De Silva, A. New Developments in Electro-Chemical Machining. CIRP Ann. 1999, 48, 567–579. [Google Scholar] [CrossRef]
- McGeough, J.A. Principles of Electrochemical Machining; Chapman & Hall: London, UK, 1974. [Google Scholar]
- Krauss, W.; Holstein, N.; Konys, J. Strategies in electro-chemical machining of tungsten for divertor application. Fusion Eng. Des. 2007, 82, 1799–1805. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Chen, X.; Li, W.; Liu, G. Investigation of the electrochemical dissolution behavior of tungsten during electrochemical machining. Int. J. Adv. Manuf. Technol. 2018, 97, 3575–3582. [Google Scholar] [CrossRef]
- Fan, Z.-W.; Hourng, L.-W.; Wang, C.-Y. Fabrication of tungsten microelectrodes using pulsed electrochemical machining. Precis. Eng. 2010, 34, 489–496. [Google Scholar] [CrossRef]
- Liu, Z.; Zeng, Y.; Zhang, W. Fabrication of metal microtool applying wire electrochemical machining. Adv. Mech. Eng. 2014, 6, 382105. [Google Scholar] [CrossRef]
- He, H.; Zeng, Y.; Qu, N. An investigation into wire electrochemical micro machining of pure tungsten. Precis. Eng. 2016, 45, 285–291. [Google Scholar] [CrossRef]
- Lauer, B.; Jäggi, B.; Neuenschwander, B. Influence of the Pulse Duration onto the Material Removal Rate and Machining Quality for Different Types of Steel. Phys. Procedia 2014, 56, 963–972. [Google Scholar] [CrossRef]
- Klancnik, S.; Begic-Hajdarevic, D.; Paulic, M.; Ficko, M.; Cekic, A.; Husic, M.C. Prediction of Laser Cut Quality for Tungsten Alloy Using the Neural Network Method. Stroj. Vestn. J. Mech. Eng. 2015, 61, 714–720. [Google Scholar] [CrossRef]
- Shokrani, A.; Dhokia, V.; Newman, S. Environmentally conscious machining of difficult-to-machine materials with regard to cutting fluids. Int. J. Mach. Tools Manuf. 2012, 57, 83–101. [Google Scholar] [CrossRef]
- Zhong, L.; Li, L.; Wu, X.; He, N. Micro cutting of pure tungsten using self-developed polycrystalline diamond slotting tools. Int. J. Adv. Manuf. Technol. 2016, 89, 2435–2445. [Google Scholar] [CrossRef]
- Legutko, S.; Winiarski, P.; Chwalczuk, T.; Marcincinova-Novakova, L.; Zak, K. Tool life of ceramic wedges during precise turning of tungsten. In Proceedings of the MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2017; pp. 1–9. [Google Scholar]
- Olsson, M.; Bushlya, V.; Lenrick, F.; Ståhl, J.-E. Evaluation of tool wear mechanisms and tool performance in machining single-phase tungsten. Int. J. Refract. Met. Hard Mater. 2021, 94, 105379. [Google Scholar] [CrossRef]
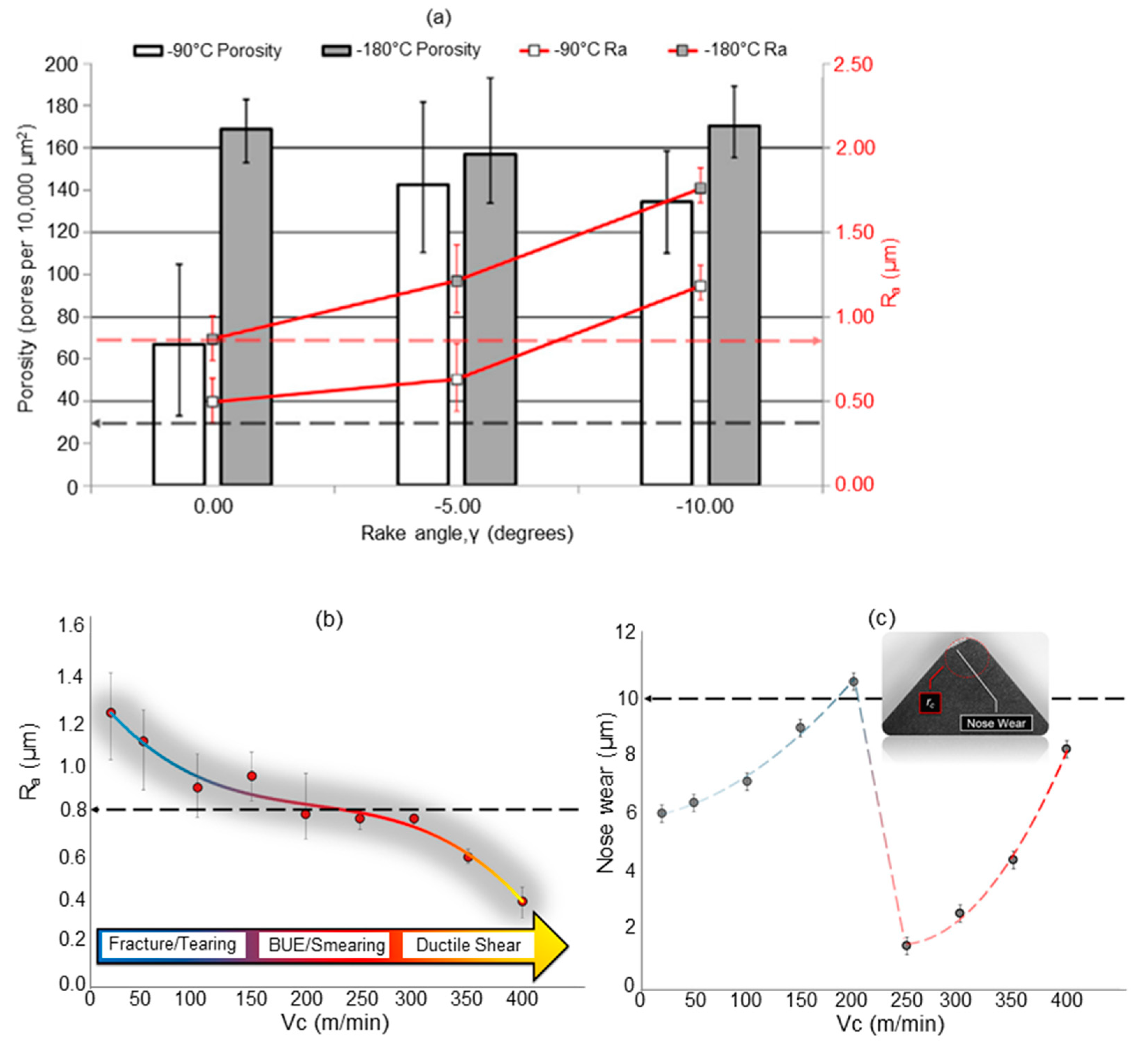
- Olsson, M.; Akujärvi, V.; Ståhl, J.E.; Bushlya, V. Cryogenic and hybrid induction-assisted machining strategies as alternatives for conventional machining of refractory tungsten and niobium. Int. J. Refract. Met. Hard Mater. 2021, 97, 105520. [Google Scholar] [CrossRef]
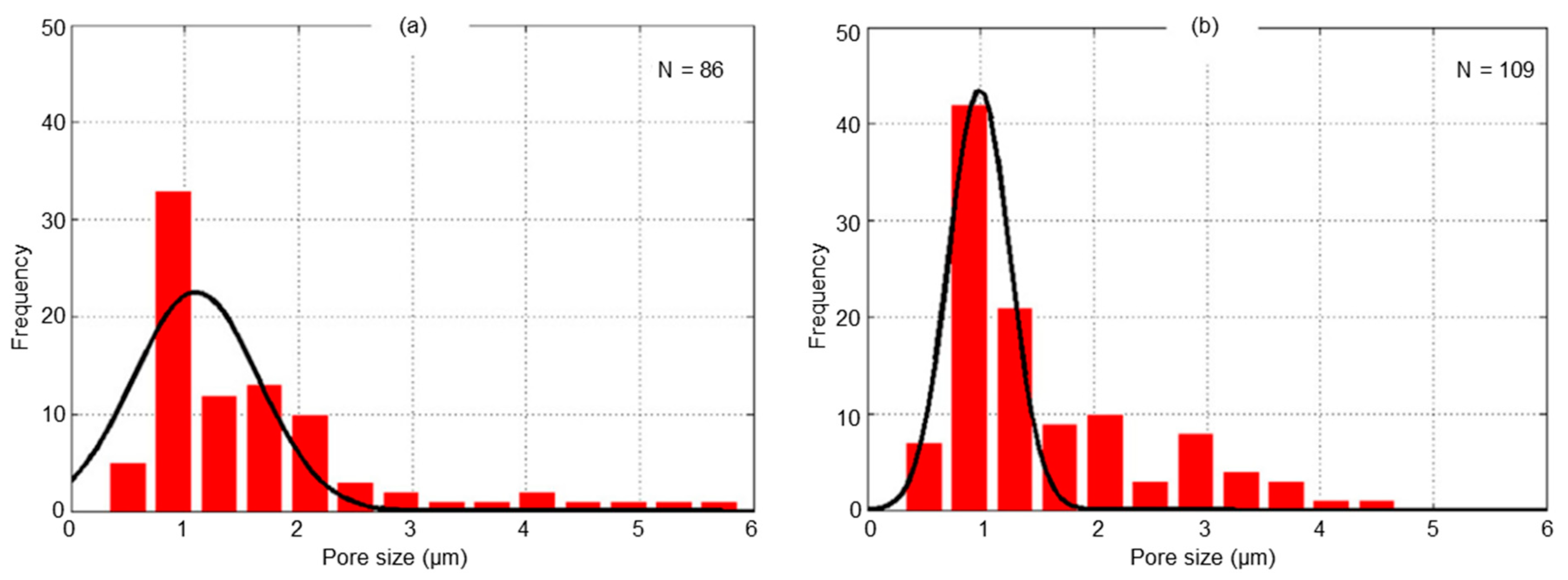
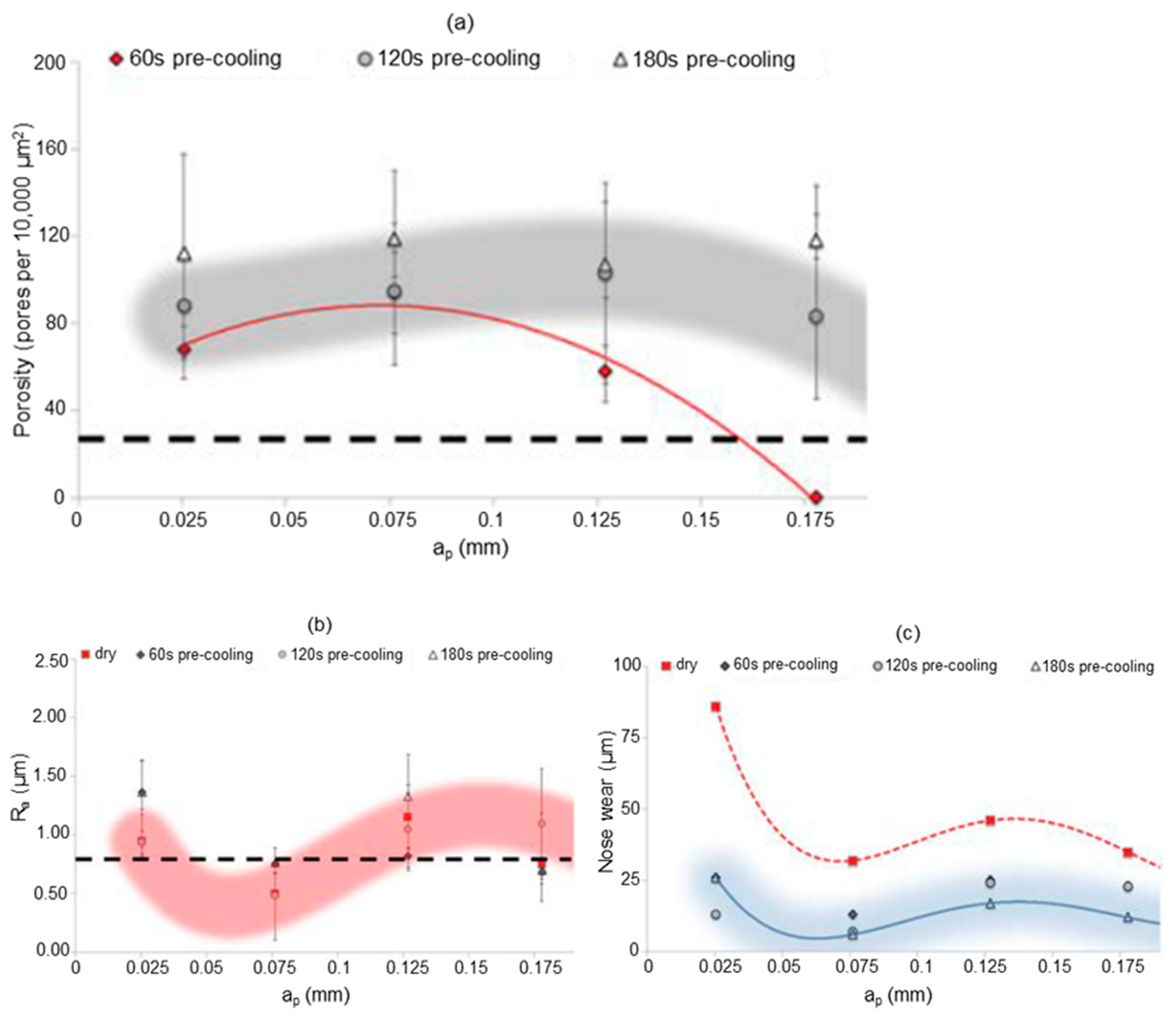
- Pusavec, F. Porous tungsten machining under cryogenic conditions. Int. J. Refract. Met. Hard Mater. 2012, 35, 84–89. [Google Scholar] [CrossRef]
- Das, J.; Chakraborty, A.; Bagchi, T.; Sarma, B. Improvement of machinability of tungsten by copper infiltration technique. Int. J. Refract. Met. Hard Mater. 2008, 26, 530–539. [Google Scholar] [CrossRef]
- Tarter, J.O.; Effgen, M.; Pušavec, F.; Jawahir, I. Cryogenic machining of porous tungsten for dispenser cathode applications. In Proceedings of the 2008 IEEE International Vacuum Electronics Conference, Monterey, CA, USA, 22–24 April 2008; IEEE: Piscataway, NJ, USA, 2008; pp. 293–294. [Google Scholar]
- Nee, A.; Song, B.; Ong, S. Re-engineering Manufacturing for Sustainability. In Proceedings of the 20th CIRP International Conference on Life Cycle Engineering, Singapore, 17–19 April 2013. [Google Scholar]
- Schoop, J.; Ambrosy, F.; Zanger, F.; Schulze, V.; Balk, T.; Jawahir, I. Cryogenic machining of porous tungsten for enhanced surface integrity. J. Mater. Process. Technol. 2016, 229, 614–621. [Google Scholar] [CrossRef]
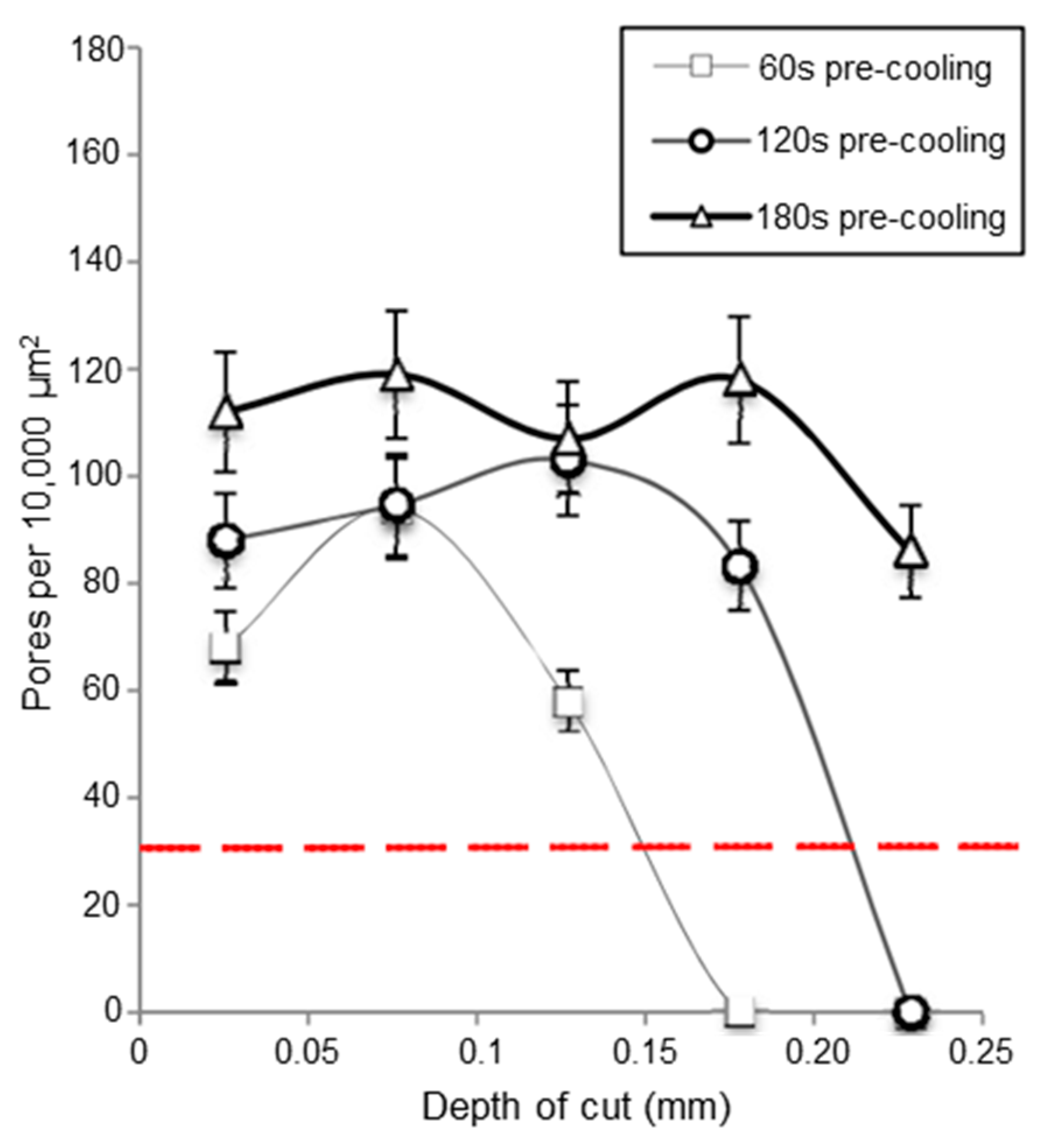
- Schoop, J.; Effgen, M.; Balk, T.; Jawahir, I. The Effects of Depth of Cut and Pre-cooling on Surface Porosity in Cryogenic Machining of Porous Tungsten. Procedia CIRP 2013, 8, 357–362. [Google Scholar] [CrossRef]
- Schoop, J.; Ambrosy, F.; Zanger, F.; Schulze, V.; Jawahir, I.S.; Balk, T.J. Increased Surface Integrity in Porous Tungsten from Cryogenic Machining with Cermet Cutting Tool. Mater. Manuf. Process. 2016, 31, 823–831. [Google Scholar] [CrossRef]
- Campbell, F.C. Refractory Metals and Alloys. In Metals Handbook Desk Edition; ASM International: Almere, The Netherlands, 1998; pp. 629–633. [Google Scholar]
- Gibson, J.S.-L.; Roberts, S.; Armstrong, D. High temperature indentation of helium-implanted tungsten. Mater. Sci. Eng. A 2015, 625, 380–384. [Google Scholar] [CrossRef]
- Pisarenko, G.S.; Borisenko, V.A.; Kashtalyan, Y.A. The effect of temperature on the hardness and modulus of elasticity of tungsten and molybdenum (20–2700 °C). Powder Metall. Met. Ceram. 1964, 1, 371–374. [Google Scholar] [CrossRef]
- Sun, S.; Brandt, M.; Dargusch, M. Thermally enhanced machining of hard-to-machine materials—A review. Int. J. Mach. Tools Manuf. 2010, 50, 663–680. [Google Scholar] [CrossRef]
- Gulbransen, E.A.; Wysong, W.S. Thin Oxide Films on Tungsten. Met. Technol. 1947, 14, 611–627. [Google Scholar]
- Cifuentes, S.C.; Monge, M.A.; Pérez, P. On the oxidation mechanism of pure tungsten in the temperature range 600–800 °C. Corros. Sci. 2012, 57, 114–121. [Google Scholar] [CrossRef]

| Property | Title 2 |
|---|---|
| Density, ρ (kg/m3) 20 °C ≤ T ≤ 1200 °C | (19.3027 − 2.3786 × 10−4 T − 2.2448 × 10−8 T2) × 1000 |
| Hardness [16] | Brinell (294) |
| Knoop (318) | |
| Rockwell A (66) | |
| Rockwell C (31) | |
| Vickers (310) | |
| Modulus of Elasticity, E (GPa) 20 °C ≤ T ≤ 800 °C | 397.903 − 2.3066 × 10−3 T − 2.7162 × 10−5 T2 |
| Average tensile strength, Su (MPa) at 20 °C | 1432 (stress-relieved) 380 (recrystallized) |
| Average yield strength, Sy (MPa) at 20 °C and 0.2% offset | 1360 (stress-relieved) 94 (recrystallized) |
| Average uniform elongation, εu (%) for stress-relieved conditions 300 °C ≤ T ≤ 700 °C | −0.1713 + 9.39 × 10−3 T − 1.11 × 10−5 T2 |
| Average total elongation, εt (%) 500 °C ≤ T ≤ 2500 °C (annealed) 800 °C ≤ T ≤ 2500 °C (stress-relieved) | 20.80 + 5.30 × 10−2 T − 2.18 × 10−5 T2 (annealed) 2.35 − 3.05 × 10−2 T + 4.99 × 10−5 T2 − 1.44 × 10−8 T3 (stress-relieved) |
| Average true strain at rupture, εtr = 500 °C ≤ T ≤ 2500 °C | Average reduction in area, %RA = −4.88 + 0.1931 T − 1.13 × 10−5 T2 + 1.74 × 10−8 T3 (annealed) |
| Poisson’s ratio | 0.29 |
| Specific heat capacity, Cp (J/KgK) 20 °C ≤ T ≤ 1000 °C | 128.308 + 3.2797 × 10−2 T − 3.4097 × 10−6 T2 |
| Thermal conductivity, λ (W/mK) 20 °C ≤ T ≤ 1000 °C | 174.9274 − 0.1067 T + 5.0067 × 10−5 T2 − 7.8349 × 10−9 T3 |
| Melting point (°C) [14] | 3423 |
| Boiling point (°C) [16] | 5900 |
| Coefficient of linear thermal expansion, α (10−6K−1) 20 °C ≤ T ≤ 1200 °C | 3.9225 + 5.8352 × 10−4 T + 5.7054 × 10−11 T2 − 2.0463 × 10−14 T3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omole, S.; Lunt, A.; Kirk, S.; Shokrani, A. Advanced Processing and Machining of Tungsten and Its Alloys. J. Manuf. Mater. Process. 2022, 6, 15. https://doi.org/10.3390/jmmp6010015
Omole S, Lunt A, Kirk S, Shokrani A. Advanced Processing and Machining of Tungsten and Its Alloys. Journal of Manufacturing and Materials Processing. 2022; 6(1):15. https://doi.org/10.3390/jmmp6010015
Chicago/Turabian StyleOmole, Samuel, Alexander Lunt, Simon Kirk, and Alborz Shokrani. 2022. "Advanced Processing and Machining of Tungsten and Its Alloys" Journal of Manufacturing and Materials Processing 6, no. 1: 15. https://doi.org/10.3390/jmmp6010015
APA StyleOmole, S., Lunt, A., Kirk, S., & Shokrani, A. (2022). Advanced Processing and Machining of Tungsten and Its Alloys. Journal of Manufacturing and Materials Processing, 6(1), 15. https://doi.org/10.3390/jmmp6010015








