Bioprinting of Organ-on-Chip Systems: A Literature Review from a Manufacturing Perspective
Abstract
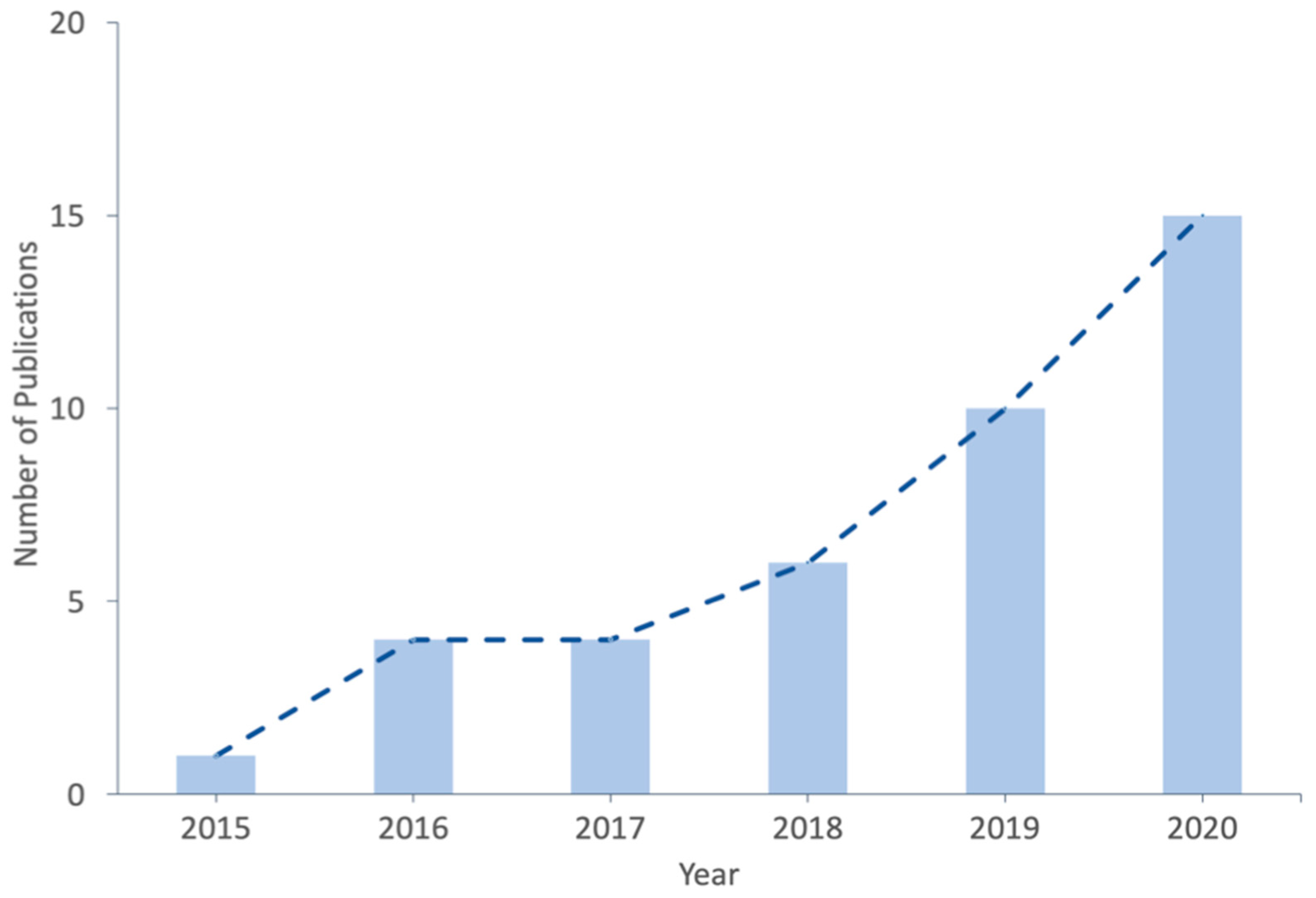
1. Introduction
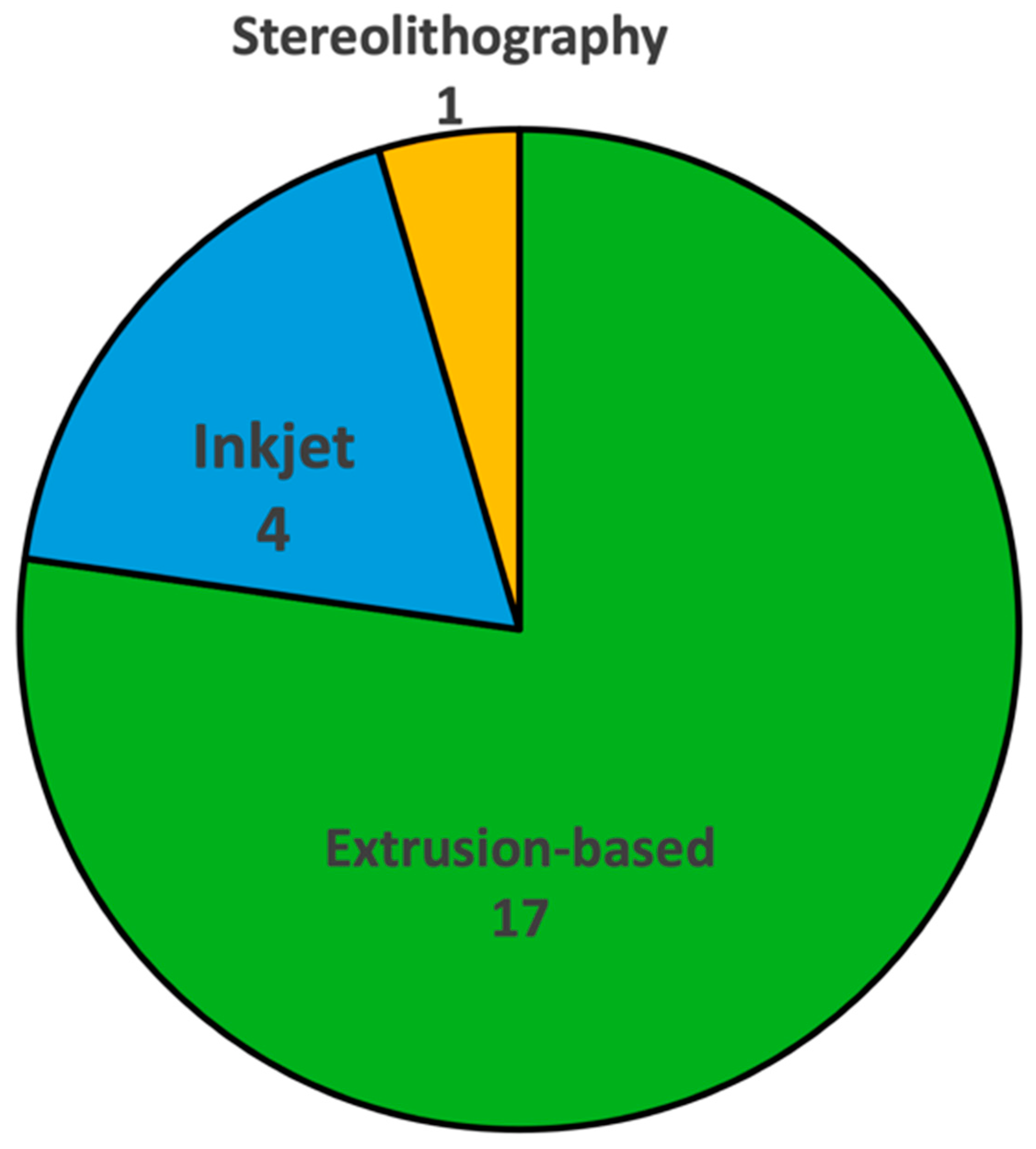
2. Bioprinting Techniques Used to Fabricate Organ-on-Chip Systems
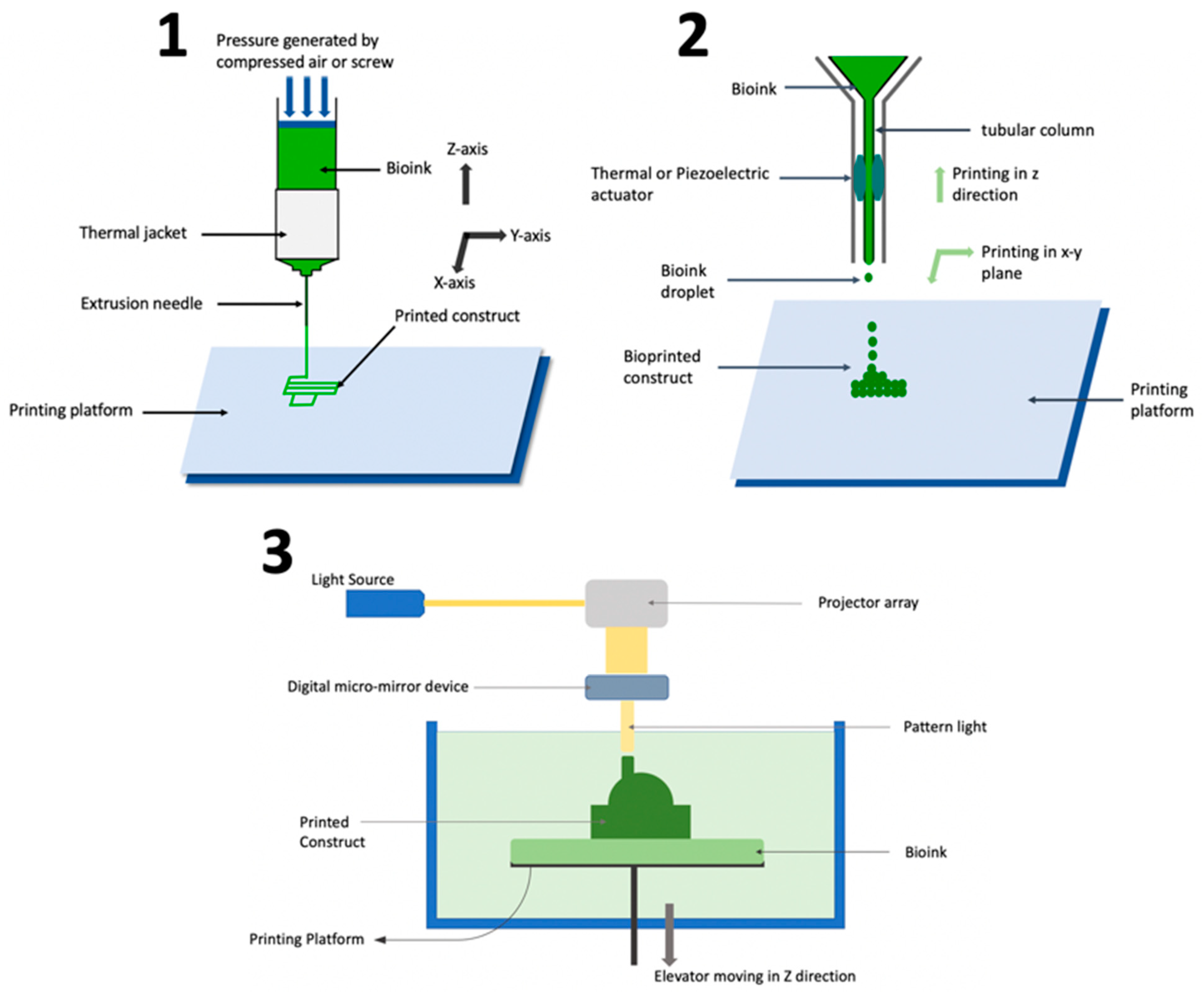
2.1. Extrusion-Based Bioprinting
2.2. Inkjet Bioprinting
2.3. Stereolithography
3. Bioink Used in Bioprinting of Organ-on-Chip Systems
4. Organ-on-Chip Systems Fabricated Using Bioprinting
4.1. Liver-on-Chip
4.2. Kidney-on-Chip
4.3. Heart-on-Chip
4.4. Lung-on-Chip
4.5. Gut-on-Chip
4.6. Bone-on-Chip
4.7. Vessel-on-Chip
4.8. Tumor-on-Chip
5. Challenges and Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morgan, S.; Grootendorst, P.; Lexchin, J.; Cunningham, C.; Greyson, D. The cost of drug development: A systematic review. Health Policy 2011, 100, 4–17. [Google Scholar] [CrossRef]
- Lipsky, M.S.; Sharp, L.K. From idea to market: The drug approval process. J. Am. Board Fam. Pract. 2001, 14, 362–367. [Google Scholar]
- Staff, Reuters. U.S. to develop chip that tests if a drug is toxic. Reuters, 16 September 2011. [Google Scholar]
- Ghaemmaghami, A.M.; Hancock, M.J.; Harrington, H.; Kaji, H.; Khademhosseini, A. Biomimetic tissues on a chip for drug discovery. Drug Discov. Today 2012, 17, 173–181. [Google Scholar] [CrossRef]
- Elçin, Y.M. Organs-on-Chips & 3D—Bioprinting Technologies for Personalized Medicine. Stem Cell Rev. Rep. 2017, 13, 319–320. [Google Scholar]
- Leary, J.F.; Key, J.; Vidi, P.-A.; Cooper, C.L.; Kole, A.; Reece, L.M.; Lelièvre, S.A. Human organ-on-a-chip BioMEMS devices for testing new diagnostic and therapeutic strategies. In Proceedings of the Microfluidics, BioMEMS, and Medical Microsystems XI, International Society for Optics and Photonics, San Francisco, CA, USA, 3–6 February 2013; p. 86150A. [Google Scholar]
- Yang, Q.; Lian, Q.; Xu, F. Perspective: Fabrication of integrated organ-on-a-chip via bioprinting. Biomicrofluidics 2017, 11, 031301. [Google Scholar] [CrossRef]
- Moyer, M.W. Organs-on-a-Chip for Faster Drug Development. In Scientific American; Springer Nature: Basingstoke, UK, 2011. [Google Scholar]
- Avci, H.; Güzel, F.D.; Erol, S.; Akpek, A. Recent advances in organ-on-a-chip technologies and future challenges: A review. Turk. J. Chem. 2018, 42, 587–610. [Google Scholar]
- Xia, Y.; Whitesides, G.M. Soft lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Beebe, D.J.; Mensing, G.A.; Walker, G.M. Physics and applications of microfluidics in biology. Annu. Rev. Biomed. Eng. 2002, 4, 261–286. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.B.; Ng, S.H.; Li, K.H.H.; Yoon, Y.-J. 3D printed microfluidics for biological applications. Lab Chip 2015, 15, 3627–3637. [Google Scholar] [CrossRef] [PubMed]
- Au, A.K.; Lee, W.; Folch, A. Mail-order microfluidics: Evaluation of stereolithography for the production of microfluidic devices. Lab Chip 2014, 14, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.-G.; Lee, H.; Cho, D.-W. 3D printing of organs-on-chips. Bioengineering 2017, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Fetah, K.; Tebon, P.; Goudie, M.J.; Eichenbaum, J.; Ren, L.; Barros, N.; Nasiri, R.; Ahadian, S.; Ashammakhi, N.; Dokmeci, M.R. The emergence of 3D bioprinting in organ-on-chip systems. Prog. Biomed. Eng. 2019, 1, 012001. [Google Scholar] [CrossRef]
- Yu, F.; Choudhury, D. Microfluidic bioprinting for organ-on-a-chip models. Drug Discov. Today 2019, 24, 1248–1257. [Google Scholar] [CrossRef]
- Mittal, R.; Woo, F.W.; Castro, C.S.; Cohen, M.A.; Karanxha, J.; Mittal, J.; Chhibber, T.; Jhaveri, V.M. Organ-on-chip models: Implications in drug discovery and clinical applications. J. Cell. Physiol. 2019, 234, 8352–8380. [Google Scholar] [CrossRef]
- Ning, L.; Chen, X. A brief review of extrusion-based tissue scaffold bio-printing. Biotechnol. J. 2017, 12, 1600671. [Google Scholar] [CrossRef]
- Chen, S.; Jang, T.S.; Pan, H.M.; Jung, H.D.; Sia, M.W.; Xie, S.; Hang, Y.; Chong, S.; Wang, D.; Song, J. 3D freeform printing of nanocomposite hydrogels through in situ precipitation in reactive viscous fluid. Int. J. Bioprint. 2020, 6, 258. [Google Scholar]
- Pati, F.; Jang, J.; Ha, D.-H.; Kim, S.W.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. Part A 2013, 101, 1255–1264. [Google Scholar] [CrossRef]
- Mironov, V.; Boland, T.; Trusk, T.; Forgacs, G.; Markwald, R.R. Organ printing: Computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003, 21, 157–161. [Google Scholar] [CrossRef]
- Tanzeglock, T.; Soos, M.; Stephanopoulos, G.; Morbidelli, M. Induction of mammalian cell death by simple shear and extensional flows. Biotechnol. Bioeng. 2009, 104, 360–370. [Google Scholar] [CrossRef]
- Emmermacher, J.; Spura, D.; Cziommer, J.; Kilian, D.; Wollborn, T.; Fritsching, U.; Steingroewer, J.; Walther, T.; Gelinsky, M.; Lode, A. Engineering considerations on extrusion-based bioprinting: Interactions of material behavior, mechanical forces and cells in the printing needle. Biofabrication 2020, 12, 025022. [Google Scholar] [CrossRef] [PubMed]
- Paxton, N.; Smolan, W.; Böck, T.; Melchels, F.; Groll, J.; Jungst, T. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 2017, 9, 044107. [Google Scholar] [CrossRef] [PubMed]
- Cidonio, G.; Glinka, M.; Dawson, J.I.; Oreffo, R.O.C. The cell in the ink: Improving biofabrication by printing stem cells for skeletal regenerative medicine. Biomaterials 2019, 209, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.G.; Malik, A.N.; Kim, T.K.; Manson, P.N.; Elisseeff, J.H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 2005, 26, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Jakus, A.E.; Rutz, A.L.; Shah, R.N. Advancing the field of 3D biomaterial printing. Biomed. Mater. 2016, 11, 014102. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Guvendiren, M. Recent advances in bioink design for 3D bioprinting of tissues and organs. Front. Bioeng. Biotechnol. 2017, 5, 23. [Google Scholar] [CrossRef]
- Mobaraki, M.; Ghaffari, M.; Yazdanpanah, A.; Luo, Y.; Mills, D. Bioinks and bioprinting: A focused review. Bioprinting 2020, 18, e00080. [Google Scholar] [CrossRef]
- Sears, N.A.; Seshadri, D.R.; Dhavalikar, P.S.; Cosgriff-Hernandez, E. A review of three-dimensional printing in tissue engineering. Tissue Eng. Part B Rev. 2016, 22, 298–310. [Google Scholar] [CrossRef]
- Kador, K.E.; Grogan, S.P.; Dorthé, E.W.; Venugopalan, P.; Malek, M.F.; Goldberg, J.L.; D’lima, D.D. Control of retinal ganglion cell positioning and neurite growth: Combining 3D printing with radial electrospun scaffolds. Tissue Eng. Part A 2016, 22, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.; Yu, H.; Ahmadi, A.; Cheung, K.C. Investigation of the hydrodynamic response of cells in drop on demand piezoelectric inkjet nozzles. Biofabrication 2016, 8, 015008. [Google Scholar] [CrossRef]
- Christensen, K.; Xu, C.; Chai, W.; Zhang, Z.; Fu, J.; Huang, Y. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol. Bioeng. 2015, 112, 1047–1055. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef]
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent US4575330A, 11 March 1986. [Google Scholar]
- Wilson, W.C., Jr.; Boland, T. Cell and organ printing 1: Protein and cell printers. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 272, 491–496. [Google Scholar] [CrossRef]
- Calvert, P. Inkjet printing for materials and devices. Chem. Mater. 2001, 13, 3299–3305. [Google Scholar] [CrossRef]
- Raman, R.; Bashir, R. Stereolithographic 3D bioprinting for biomedical applications. In Essentials of 3D Biofabrication and Translation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 89–121. [Google Scholar]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.-C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Guvendiren, M.; Lu, H.D.; Burdick, J.A. Shear-thinning hydrogels for biomedical applications. Soft Matter 2012, 8, 260–272. [Google Scholar] [CrossRef]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef] [PubMed]
- Gruene, M.; Unger, C.; Koch, L.; Deiwick, A.; Chichkov, B. Dispensing pico to nanolitre of a natural hydrogel by laser-assisted bioprinting. Biomed. Eng. Online 2011, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl. Mater. Today 2020, 18, 100479. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.S.; Galarraga, J.H.; Cui, X.; Lindberg, G.C.; Burdick, J.A.; Woodfield, T.B. Fundamentals and applications of photo-cross-linking in bioprinting. Chem. Rev. 2020, 120, 10662–10694. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Ashammakhi, N.; Wu, X.Y.; Khademhosseini, A. Crosslinking strategies for 3D bioprinting of polymeric hydrogels. Small 2020, 16, 2002931. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Liu, Z.; Lin, Z.; Qiu, J.; Liu, Y.; Liu, A.; Wang, Y.; Xiang, M.; Chen, B.; Fu, J. 3D bioprinting of vessel-like structures with multilevel fluidic channels. ACS Biomater. Sci. Eng. 2017, 3, 399–408. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.-R.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell’Erba, V. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016, 110, 45–59. [Google Scholar] [CrossRef]
- Grix, T.; Ruppelt, A.; Thomas, A.; Amler, A.-K.; Noichl, B.P.; Lauster, R.; Kloke, L. Bioprinting perfusion-enabled liver equivalents for advanced organ-on-a-chip applications. Genes 2018, 9, 176. [Google Scholar] [CrossRef]
- Homan, K.A.; Kolesky, D.B.; Skylar-Scott, M.A.; Herrmann, J.; Obuobi, H.; Moisan, A.; Lewis, J.A. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci. Rep. 2016, 6, 34845. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef]
- Lee, V.K.; Kim, D.Y.; Ngo, H.; Lee, Y.; Seo, L.; Yoo, S.-S.; Vincent, P.A.; Dai, G. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials 2014, 35, 8092–8102. [Google Scholar] [CrossRef]
- Cheng, F.; Cao, X.; Li, H.; Liu, T.; Xie, X.; Huang, D.; Maharjan, S.; Bei, H.P.; Gómez, A.; Li, J. Generation of cost-effective paper-based tissue models through matrix-assisted sacrificial 3D printing. Nano Lett. 2019, 19, 3603–3611. [Google Scholar] [CrossRef]
- Schöneberg, J.; De Lorenzi, F.; Theek, B.; Blaeser, A.; Rommel, D.; Kuehne, A.J.; Kießling, F.; Fischer, H. Engineering biofunctional in vitro vessel models using a multilayer bioprinting technique. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Kim, W.; Kim, G. Intestinal villi model with blood capillaries fabricated using collagen-based bioink and dual-cell-printing process. ACS Appl. Mater. Interfaces 2018, 10, 41185–41196. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Ryu, H.; Lee, B.; Ha, D.-H.; Ahn, M.; Kim, S.; Kim, J.Y.; Jeon, N.L.; Cho, D.-W. Development of a functional airway-on-a-chip by 3D cell printing. Biofabrication 2018, 11, 015002. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Cho, D.-W. One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab Chip 2016, 16, 2618–2625. [Google Scholar] [CrossRef] [PubMed]
- Abudupataer, M.; Chen, N.; Yan, S.; Alam, F.; Shi, Y.; Wang, L.; Lai, H.; Li, J.; Zhu, K.; Wang, C. Bioprinting a 3D vascular construct for engineering a vessel-on-a-chip. Biomed. Microdevices 2020, 22, 10. [Google Scholar] [CrossRef]
- Bhise, N.S.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Zhang, Y.S.; Shin, S.R.; Calzone, G. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 2016, 8, 014101. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ashfaq, R.; Cheng, F.; Maharjan, S.; Li, J.; Ying, G.; Hassan, S.; Xiao, H.; Yue, K.; Zhang, Y.S. A tumor-on-a-chip system with bioprinted blood and lymphatic vessel pair. Adv. Funct. Mater. 2019, 29, 1807173. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Davoudi, F.; Walch, P.; Manbachi, A.; Luo, X.; Dell’Erba, V.; Miri, A.K.; Albadawi, H.; Arneri, A.; Li, X. Bioprinted thrombosis-on-a-chip. Lab Chip 2016, 16, 4097–4105. [Google Scholar] [CrossRef]
- Snyder, J.; Hamid, Q.; Wang, C.; Chang, R.; Emami, K.; Wu, H.; Sun, W. Bioprinting cell-laden matrigel for radioprotection study of liver by pro-drug conversion in a dual-tissue microfluidic chip. Biofabrication 2011, 3, 034112. [Google Scholar] [CrossRef]
- Lind, J.U.; Busbee, T.A.; Valentine, A.D.; Pasqualini, F.S.; Yuan, H.; Yadid, M.; Park, S.-J.; Kotikian, A.; Nesmith, A.P.; Campbell, P.H. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat. Mater. 2017, 16, 303–308. [Google Scholar] [CrossRef]
- Lee, J.-H.; Gu, Y.; Wang, H.; Lee, W.Y. Microfluidic 3D bone tissue model for high-throughput evaluation of wound-healing and infection-preventing biomaterials. Biomaterials 2012, 33, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.-G.; Jeong, Y.H.; Kim, Y.; Choi, Y.-J.; Moon, H.E.; Park, S.H.; Kang, K.S.; Bae, M.; Jang, J.; Youn, H. A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat. Biomed. Eng. 2019, 3, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Yang, S.; Liu, T.; Du, Z.; Xu, Y.; Li, B.; Sun, W. A novel controllable cell array printing technique on microfluidic chips. IEEE Trans. Biomed. Eng. 2019, 66, 2512–2520. [Google Scholar] [CrossRef]
- Hamid, Q.; Wang, C.; Zhao, Y.; Snyder, J.; Sun, W. A three-dimensional cell-laden microfluidic chip for in vitro drug metabolism detection. Biofabrication 2014, 6, 025008. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Q.; Wang, C.; Snyder, J.; Williams, S.; Liu, Y.; Sun, W. Maskless fabrication of cell-laden microfluidic chips with localized surface functionalization for the co-culture of cancer cells. Biofabrication 2015, 7, 015012. [Google Scholar] [CrossRef] [PubMed]
- Khetani, S.R.; Bhatia, S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008, 26, 120–126. [Google Scholar] [CrossRef]
- Xu, J.J.; Henstock, P.V.; Dunn, M.C.; Smith, A.R.; Chabot, J.R.; de Graaf, D. Cellular imaging predictions of clinical drug-induced liver injury. Toxicol. Sci. 2008, 105, 97–105. [Google Scholar] [CrossRef]
- Naughton, C.A. Drug-induced nephrotoxicity. Am. Fam. Physician 2008, 78, 743–750. [Google Scholar]
- Boschetto, P.; Quintavalle, S.; Miotto, D.; Cascio, N.L.; Zeni, E.; Mapp, C.E. Chronic obstructive pulmonary disease (COPD) and occupational exposures. J. Occup. Med. Toxicol. 2006, 1, 11. [Google Scholar] [CrossRef][Green Version]
- McKim, J.; James, M. Building a tiered approach to in vitro predictive toxicity screening: A focus on assays with in vivo relevance. Comb. Chem. High Throughput Screen. 2010, 13, 188–206. [Google Scholar] [CrossRef] [PubMed]
- Panek, M.; Grabacka, M.; Pierzchalska, M. The formation of intestinal organoids in a hanging drop culture. Cytotechnology 2018, 70, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Brown, C. Staying strong. Nature 2017, 550, S15–S17. [Google Scholar] [CrossRef]
- Zhang, B.; Radisic, M. Organ-on-a-chip devices advance to market. Lab Chip 2017, 17, 2395–2420. [Google Scholar] [CrossRef] [PubMed]
- Bertassoni, L.E.; Cecconi, M.; Manoharan, V.; Nikkhah, M.; Hjortnaes, J.; Cristino, A.L.; Barabaschi, G.; Demarchi, D.; Dokmeci, M.R.; Yang, Y. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 2014, 14, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.; van Bernum, R.; El-Siblani, A. Preclinical trials using the 3D-Bioplotter for Tissue Engineering. In Proceedings of the Tissue Engineering and Regenerative Medicine International Society 2010, Galway, Ireland, 13–17 June 2010. [Google Scholar]
- Becker, H.; Locascio, L.E. Polymer microfluidic devices. Talanta 2002, 56, 267–287. [Google Scholar] [CrossRef]
- Gong, H.; Beauchamp, M.; Perry, S.; Woolley, A.T.; Nordin, G.P. Optical approach to resin formulation for 3D printed microfluidics. RSC Adv. 2015, 5, 106621–106632. [Google Scholar] [CrossRef]
- Kratz, S.R.A.; Höll, G.; Schuller, P.; Ertl, P.; Rothbauer, M. Latest trends in biosensing for microphysiological organs-on-a-chip and body-on-a-chip systems. Biosensors 2019, 9, 110. [Google Scholar] [CrossRef]
- Rothbauer, M.; Ertl, P. Emerging Biosensor Trends in Organ-on-a-Chip. In Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
| Hydrogel as Bioink Constituent | Bioprinting Technique | Crosslinking Mechanism | Organ-on-Chip System | Reference |
|---|---|---|---|---|
| Alginate | Extrusion | Physical | Vessel, heart | [52,53] |
| Gelatin | Extrusion, stereolithography | Chemical | Vessel, liver, kidney | [54,55,56,57] |
| Cellulose | Extrusion | Chemical | Tumor | [58] |
| Fibrin | Extrusion, inkjet | Physical | Vessel, kidney | [55,56,59] |
| Collagen | Extrusion, inkjet | Chemical | Vessel, gut, lung | [57,59,60,61] |
| Poly (ethylene glycol) (PEG) | Stereolithography | Photo | Liver | [54] |
| Poly (ε-caprolactone) (PCL) | Extrusion | Photo | Liver | [62] |
| Gelatin methacryloyl (GelMA) | Extrusion, inkjet | Photo | Vessel, heart, liver, tumor | [53,63,64,65,66] |
| Pluronic | Extrusion | Photo | Kidney | [55] |
| Organ-on-Chip System | Bioprinting Technique | Bioinks Used | Reference |
|---|---|---|---|
| Liver | Extrusion, inkjet, stereolithography | Gelatin, PCL, PEG, GelMA | [54,62,64,67] |
| Kidney | Extrusion | Fibrin, Pluronic | [55] |
| Heart | Extrusion | Alginate | [53,68] |
| Lung | Extrusion | Collagen | [61] |
| Gut | Extrusion | Collagen | [60] |
| Bone | Inkjet | PLGA | [69] |
| Vessel | Extrusion, inkjet | Alginate, gelatin, fibrin, collagen, GelMA | [52,56,57,59,63,66] |
| Tumor | Extrusion | Cellulose | [58,65,70,71,72,73] |
| Organ-on-Chip System | Major Result | Reference |
|---|---|---|
| Liver | Models drug toxicity | [54,62,64,67] |
| Kidney | Models drug toxicity | [55] |
| Heart | Models drug toxicity, mimics heartbeat | [53,68] |
| Lung | Mimics disease response | [61] |
| Gut | Capable of forming tissue with multiple cell types | [60] |
| Bone | Disease modeling | [69] |
| Vessel | Mimics blood flow, disease modeling | [52,56,57,59,63,66] |
| Tumor | Disease modeling, drug testing | [58,65,70,71,72,73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakare, K.; Jerpseth, L.; Pei, Z.; Elwany, A.; Quek, F.; Qin, H. Bioprinting of Organ-on-Chip Systems: A Literature Review from a Manufacturing Perspective. J. Manuf. Mater. Process. 2021, 5, 91. https://doi.org/10.3390/jmmp5030091
Thakare K, Jerpseth L, Pei Z, Elwany A, Quek F, Qin H. Bioprinting of Organ-on-Chip Systems: A Literature Review from a Manufacturing Perspective. Journal of Manufacturing and Materials Processing. 2021; 5(3):91. https://doi.org/10.3390/jmmp5030091
Chicago/Turabian StyleThakare, Ketan, Laura Jerpseth, Zhijian Pei, Alaa Elwany, Francis Quek, and Hongmin Qin. 2021. "Bioprinting of Organ-on-Chip Systems: A Literature Review from a Manufacturing Perspective" Journal of Manufacturing and Materials Processing 5, no. 3: 91. https://doi.org/10.3390/jmmp5030091
APA StyleThakare, K., Jerpseth, L., Pei, Z., Elwany, A., Quek, F., & Qin, H. (2021). Bioprinting of Organ-on-Chip Systems: A Literature Review from a Manufacturing Perspective. Journal of Manufacturing and Materials Processing, 5(3), 91. https://doi.org/10.3390/jmmp5030091







