Pressing and Infiltration of Metal Matrix Nanocomposites
Abstract
:1. Introduction
2. Experimental Methods
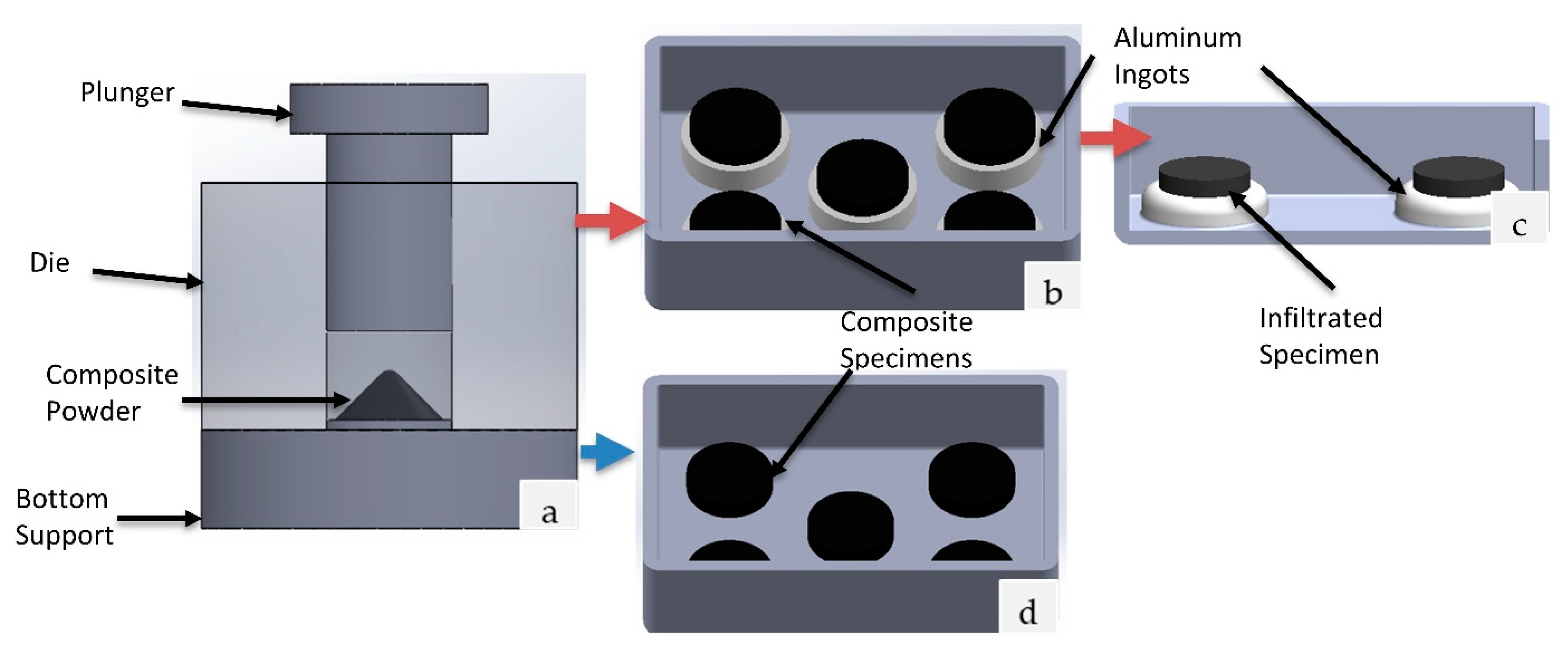
2.1. Specimen Preparation
2.2. Measurement of Density and Porosity
2.3. Characterization of Microstructure and Composition
2.4. Mechanical Testing
3. Results
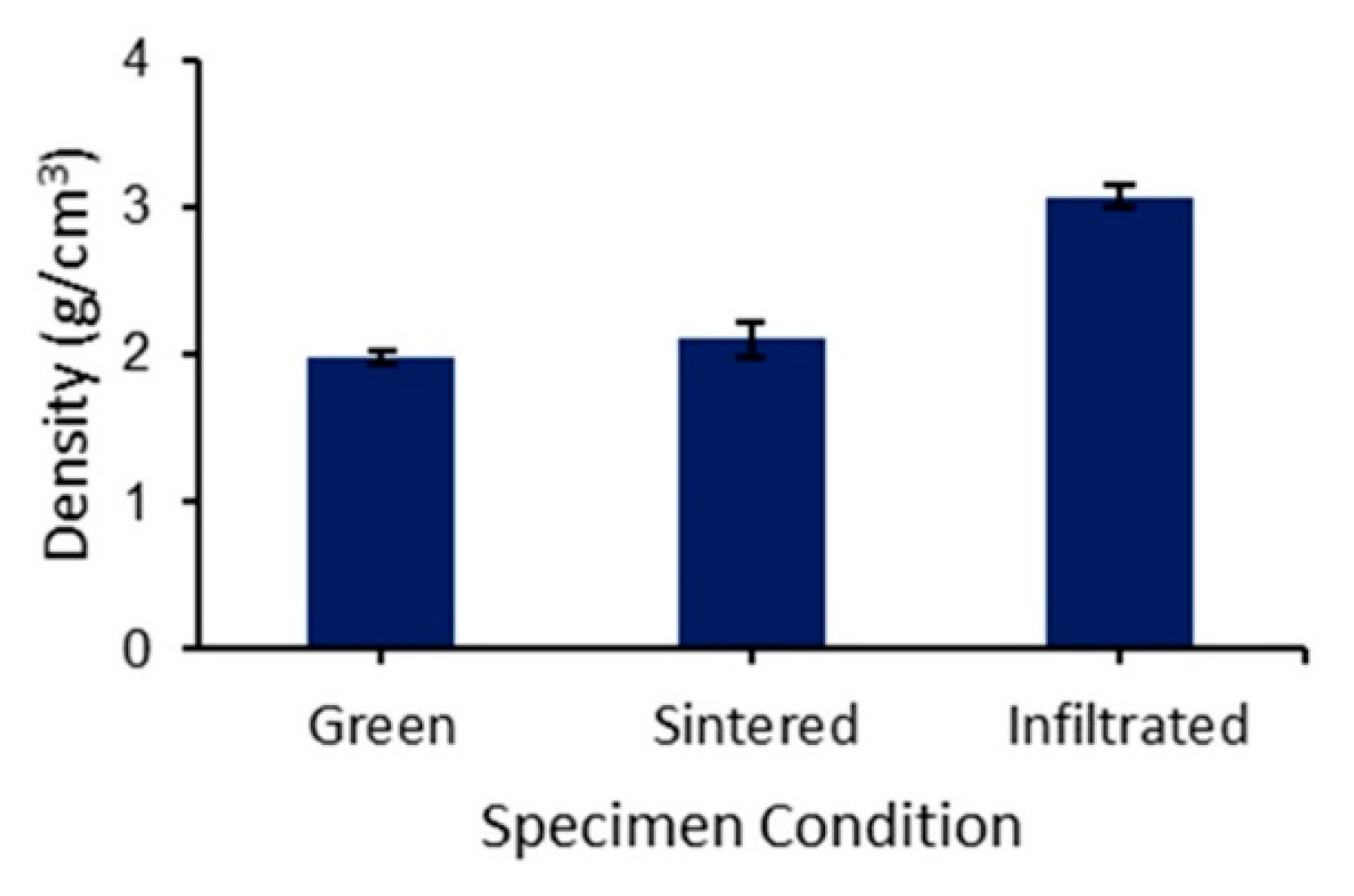
3.1. Density and Porosity
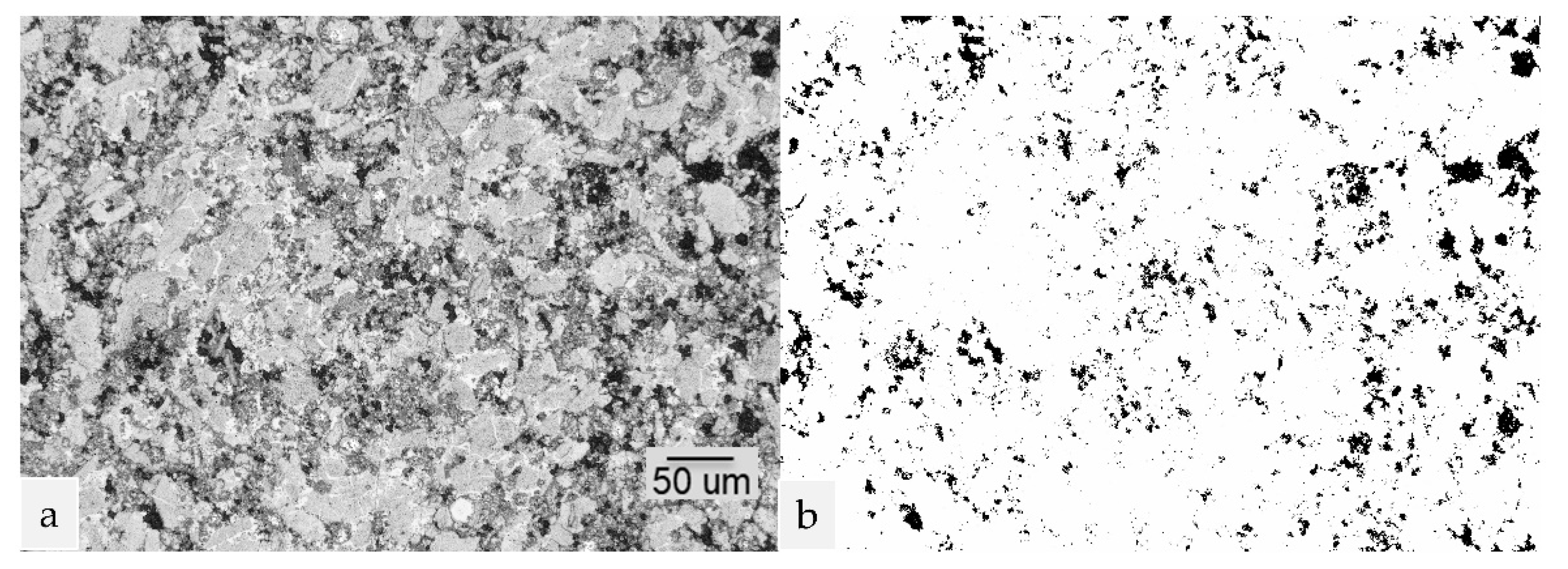
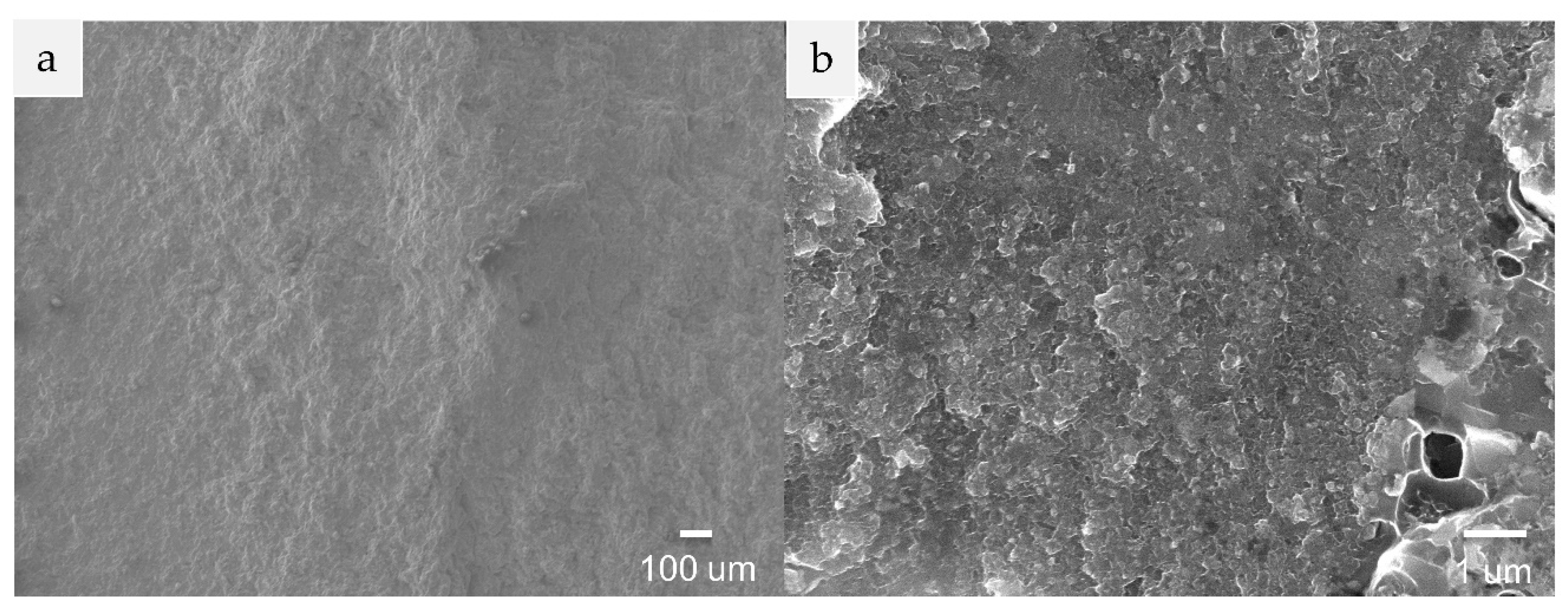
3.2. Microstructure and Composition
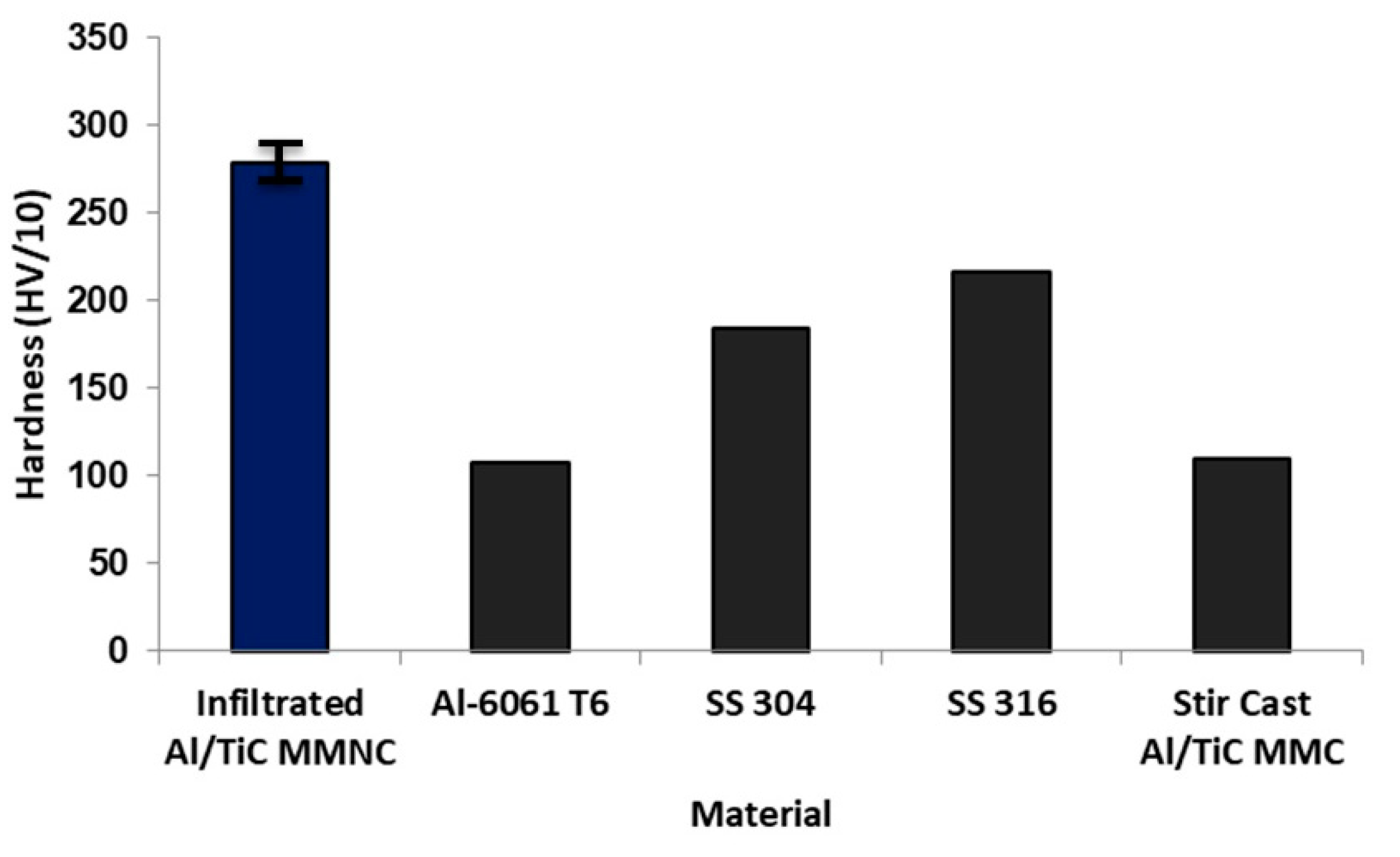
3.3. Hardness
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Samal, P.; Vundavilli, P.R.; Meher, A.; Mahapatra, M.M. Fabrication and Mechanical Properties of Titanium Carbide Reinforced Aluminium Composites. Mater. Today Proc. 2019, 18, 2649–2655. [Google Scholar] [CrossRef]
- Pandey, U.; Purohit, R.; Agarwal, P.; Dhakad, S.; Rana, R. Effect of TiC Particles on the Mechanical Properties of Aluminium Alloy Metal Matrix Composites (MMCs). Mater. Today Proc. 2017, 4, 5452–5460. [Google Scholar] [CrossRef]
- Ravi Kumar, K.; Kiran, K.; Sreebalaji, V.S. Micro Structural Characteristics and Mechanical Behaviour of Aluminium Matrix Composites Reinforced with Titanium Carbide. J. Alloy. Compd. 2017, 723, 795–801. [Google Scholar] [CrossRef]
- Murthy, C.A.H.; Singh, S.K. Influence of TiC Particulate Reinforcement on the Corrosion Behaviour of Al 6061 Metal Matrix Composites. Adv. Mater. Lett. 2015, 6, 633–640. [Google Scholar] [CrossRef]
- Arnold, J.M.; Cramer, C.L.; Elliott, A.M.; Nandwana, P.; Babu, S.S. Microstructure Evolution During Near-Net-Shape Fabrication of NixAly-TiC Cermets Through Binder Jet Additive Manufacturing and Pressureless Melt Infiltration. Int. J. Refract. Met. Hard Mater. 2019, 84, 104985. [Google Scholar] [CrossRef]
- Schaffer, B.G.; Sercombe, T.B.; Lumley, R.N. Liquid Phase Sintering of Aluminium Alloys. Mater. Chem. Phys. 2001, 67, 85–91. [Google Scholar] [CrossRef]
- Awotunde, M.A.; Adegbenjo, A.O.; Obadele, B.A.; Okoro, M.; Shongwe, B.M.; Olubambi, P.A. Influence of Sintering Methods on the Mechanical Properties of Aluminium Nanocomposites Reinforced With Carbonaceous Compounds: A Review. J. Mater. Res. Technol. 2019, 8, 2432–2449. [Google Scholar] [CrossRef]
- Saboori, A.; Novara, C.; Pavese, M.; Badini, C.; Giorgis, F.; Fino, P. An Investigation on the Sinterability and the Compaction Behavior of Aluminum/Graphene Nanoplatelets (GNPs) Prepared by Powder Metallurgy. J. Mater. Eng. Perform. 2017, 26, 993–999. [Google Scholar] [CrossRef]
- Kajikawa, Y.; Nukami, T.; Flemings, M.C. Pressureless Infiltration of Aluminum Metal-Matrix composites. Metall. Mater. Trans. A 1995, 26, 2155–2159. [Google Scholar] [CrossRef]
- Cramer, C.L.; Elliott, A.M.; Kiggans, J.O.; Haberl, B.; Anderson, D.C. Processing of Complex-Shaped Collimators Made via Binder Jet Additive Manufacturing of B4C and Pressureless Melt Infiltration of Al. Mater. Des. 2019, 180, 107956. [Google Scholar] [CrossRef]
- Aguilar, E.A.; León, C.A.; Contreras, A.; López, V.H.; Drew, R.A.L.; Bedolla, E. Wettability and Phase Formation in TiC/Al-Alloys Assemblies. Compos. Part A Appl. Sci. Manuf. 2002, 33, 1425–1428. [Google Scholar] [CrossRef]
- He, B.-L.; Zhu, Y.-F. Microstructure and Properties of TiC/Ni3Al Composites Prepared by Pressureless Melt Infiltration with Porous TiC/Ni3Al Preforms. Mater. Manuf. Process. 2011, 26, 586–591. [Google Scholar] [CrossRef]
- Plucknett, K.P.; Becher, P.F.; Waters, S.B. Flexure Strength of Melt-Infiltration-Processed Titanium Carbide/Nickel Aluminide Composites. J. Am. Ceram. Soc. 1998, 81, 1839–1844. [Google Scholar] [CrossRef]
- Albiter, A.; León, C.; Drew, R.; Bedolla, E. Microstructure and Heat-Treatment Response of Al-2024/TiC Composites. Mater. Sci. Eng. A 2000, 289, 109–115. [Google Scholar] [CrossRef]
- Ma, C.; Chen, L.; Cao, C.; Li, X. Nanoparticle-induced Unusual Melting and Solidification Behaviours of Metals. Nat. Commun. 2017, 8, 14178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saba, F.; Zhang, F.; Liu, S.; Liu, T. Reinforcement Size Dependence of Mechanical Properties and Strengthening Mechanisms in Diamond Reinforced Titanium Metal Matrix Composites. Compos. Part B Eng. 2019, 167, 7–19. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, X.; Zhang, B.; Kondoh, K.; Li, J.; Qian, M. Microstructure, Tensile Properties and Deformation Behaviors of Aluminium Metal Matrix Composites Co-reinforced by Ex-situ Carbon Nanotubes and In-situ Alumina Nanoparticles. Mater. Sci. Eng. A 2020, 795, 139930. [Google Scholar] [CrossRef]
- Tan, Z.; Li, J.; Zhang, Z. Experimental and Numerical Studies on Fabrication of Nanoparticle Reinforced Aluminum Matrix Composites by Friction Stir Additive Manufacturing. J. Mater. Res. Technol. 2021, 12, 1898–1912. [Google Scholar] [CrossRef]
- Singh, M.; Bhandari, D.; Goyal, K. A Review of the Mechanical Performance of Nanoparticles Reinforced Aluminium Matrix Nanocomposites. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Ali, L.F.; Kuppuswamy, N.; Soundararajan, R.; Ramkumar, K.; Sivasankaran, S. Microstructural Evolutions and Mechanical Properties Enhancement of AA 6063 Alloy Reinforced with Tungsten (W) Nanoparticles Processed by Friction Stir Processing. Mater. Charact. 2021, 172, 110903. [Google Scholar] [CrossRef]
- Lin, T.C.; Cao, C.; Sokoluk, M.; Jiang, L.; Wang, X.; Schoenung, J.M.; Lavernia, E.J.; Li, X. Aluminum with Dispersed Nanoparticles by Laser Additive Manufacturing. Nat. Commun. 2019, 10, 4124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO 18754:2020. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Density and Apparent Porosity; ISO: Geneva, Switzerland, 2020. [Google Scholar]
- Albiter, A.; Contreras, A.; Bedolla, E.; Pérez, R. Structural and Chemical Characterization of Precipitates in Al-2024/TiC Composites. Compos. Part A Appl. Sci. Manuf. 2003, 34, 17–24. [Google Scholar] [CrossRef]
- Properties and Selection: Irons, Steels, and High-Performance Alloys; ASM International: Almere, The Netherlands, 1990.
- Properties and Selection: Nonferrous Alloys and Special-Purpose Materials; ASM International: Almere, The Netherlands, 1990.

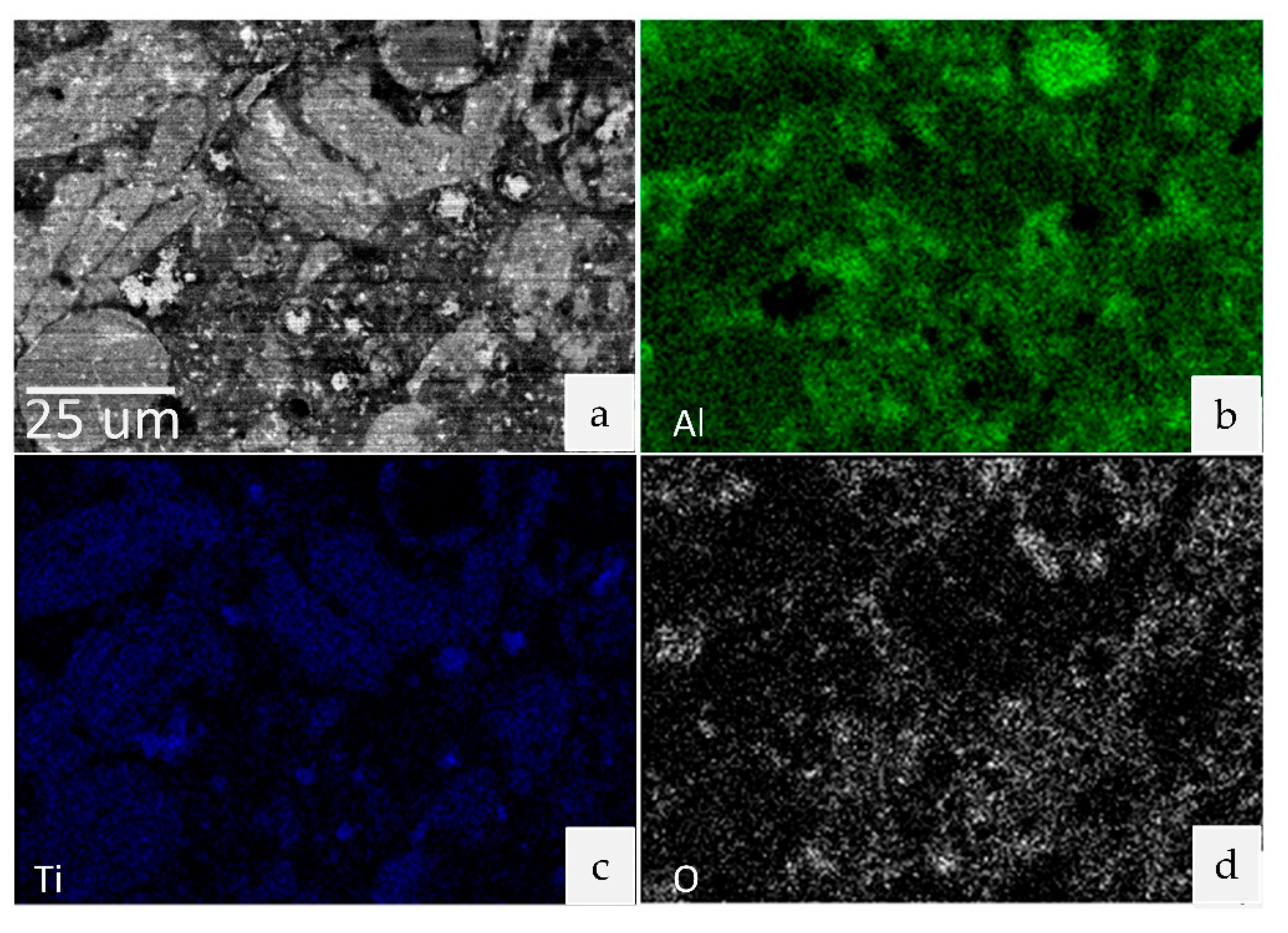
| Specimen | C (wt.%) | Al (wt.%) | Ti (wt.%) | Cu (wt.%) |
|---|---|---|---|---|
| Infiltrated | 23.34 | 50.30 | 24.42 | 1.94 |
| Sintered | 11.79 | 48.05 | 41.61 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porter, Q.; Li, X.; Ma, C. Pressing and Infiltration of Metal Matrix Nanocomposites. J. Manuf. Mater. Process. 2021, 5, 54. https://doi.org/10.3390/jmmp5020054
Porter Q, Li X, Ma C. Pressing and Infiltration of Metal Matrix Nanocomposites. Journal of Manufacturing and Materials Processing. 2021; 5(2):54. https://doi.org/10.3390/jmmp5020054
Chicago/Turabian StylePorter, Quinton, Xiaochun Li, and Chao Ma. 2021. "Pressing and Infiltration of Metal Matrix Nanocomposites" Journal of Manufacturing and Materials Processing 5, no. 2: 54. https://doi.org/10.3390/jmmp5020054
APA StylePorter, Q., Li, X., & Ma, C. (2021). Pressing and Infiltration of Metal Matrix Nanocomposites. Journal of Manufacturing and Materials Processing, 5(2), 54. https://doi.org/10.3390/jmmp5020054