Electropolishing of Laser Powder Bed-Fused IN625 Components in an Ionic Electrolyte
Abstract
:1. Introduction
2. Experimental Setup
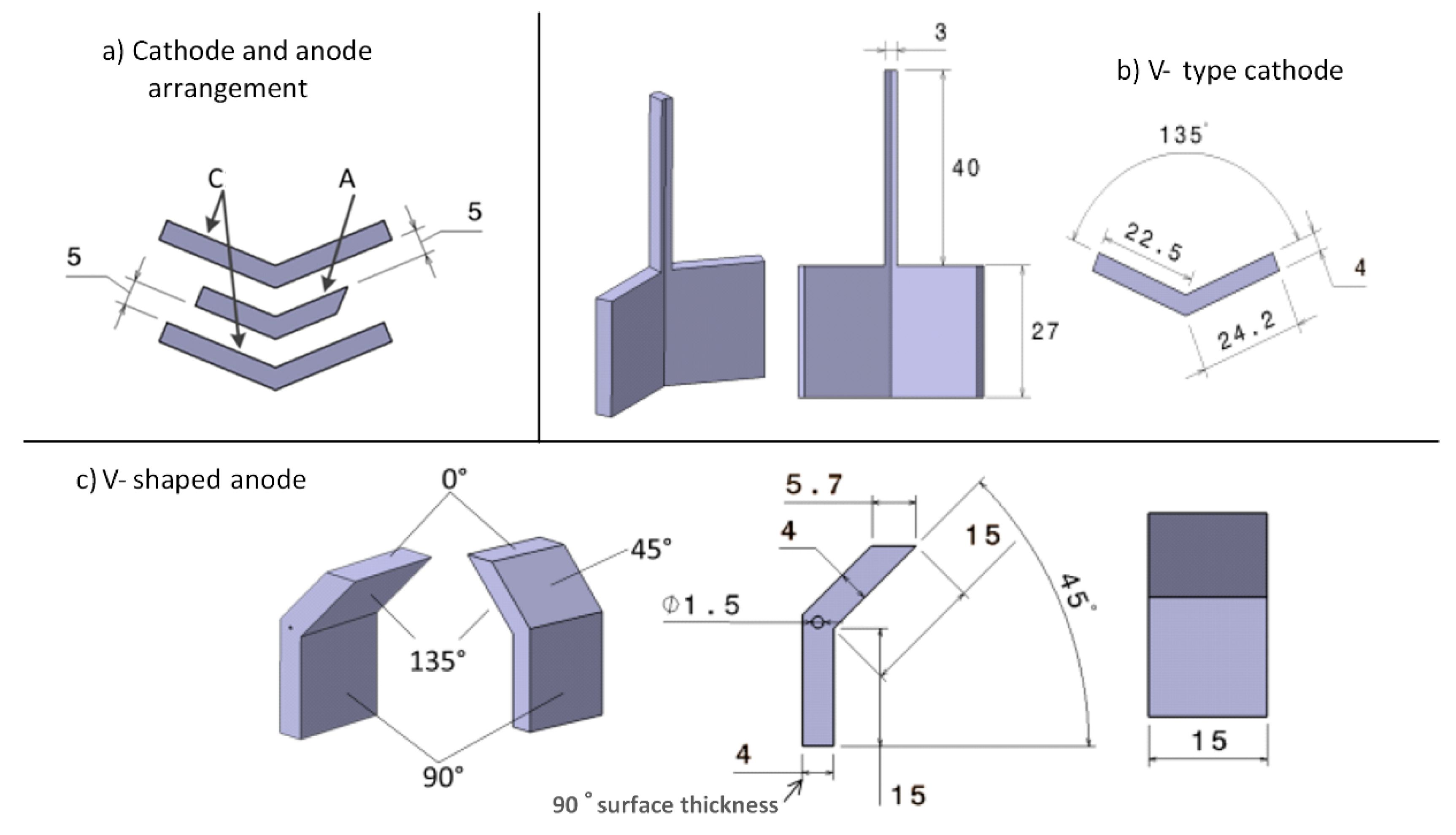
2.1. Materials EP Cell Setup and Design of Electrodes
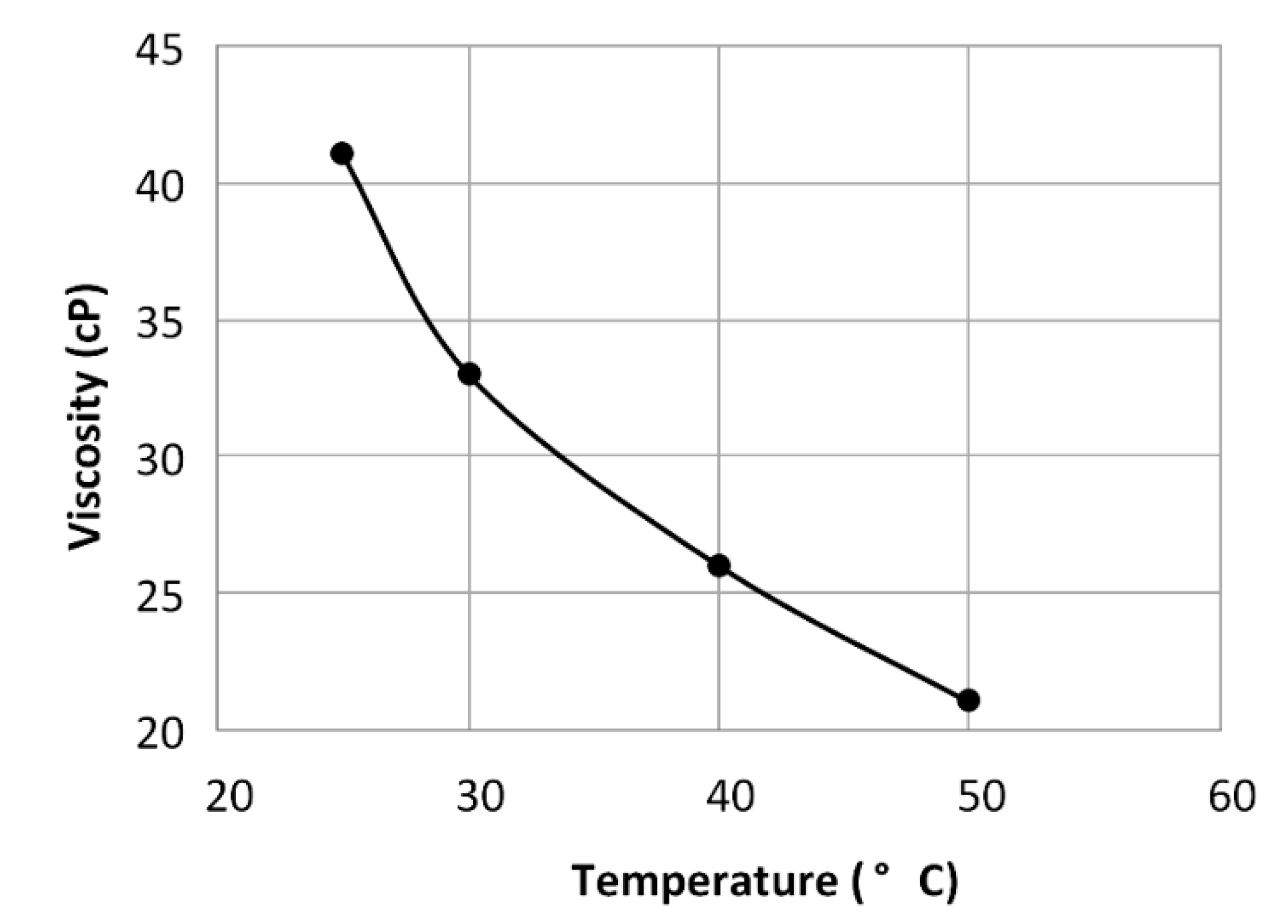
2.2. Green Electrolyte Preparation and Measurements of the Viscosity-Temperature Dependence
3. Experimental Procedure
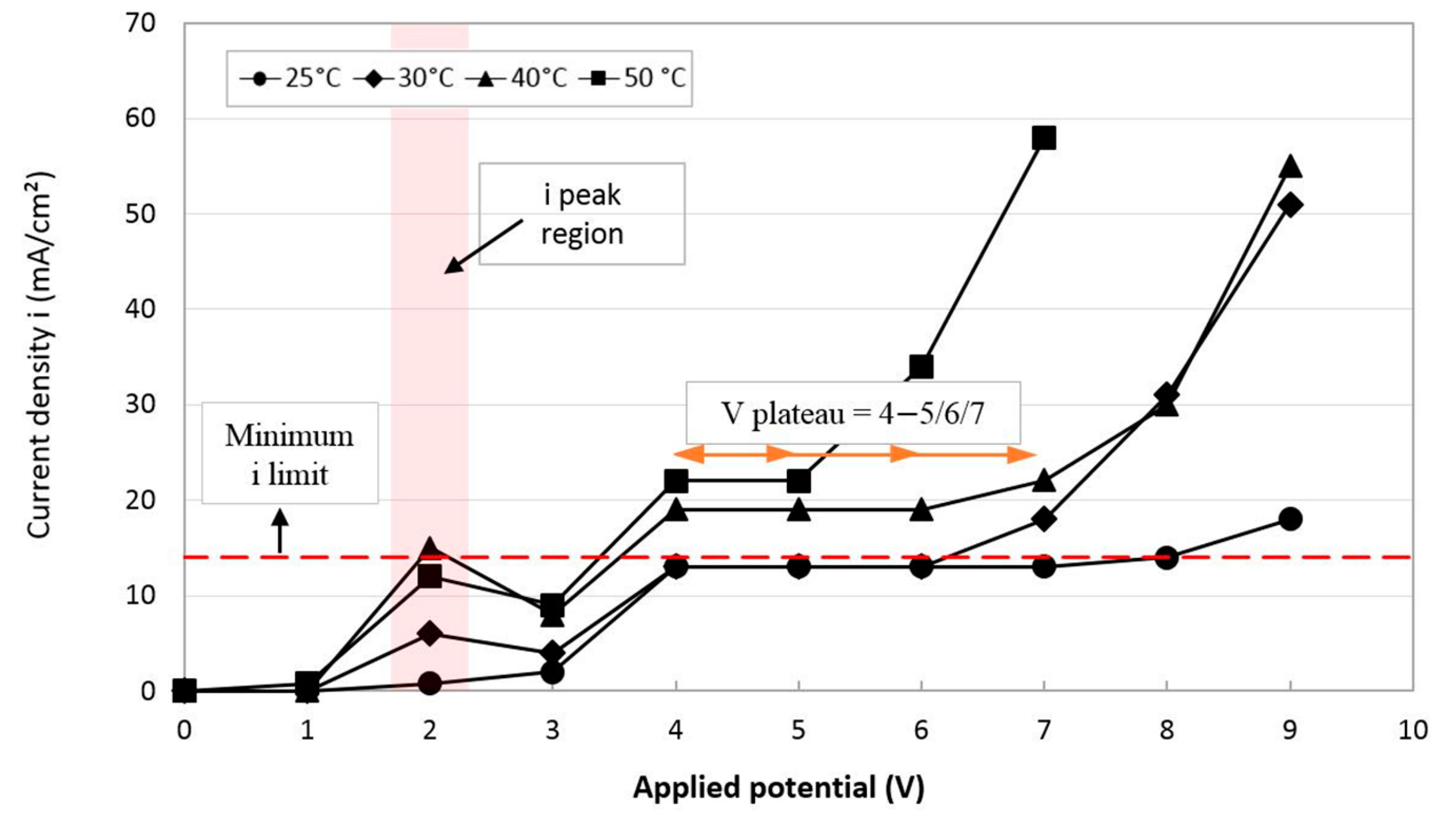
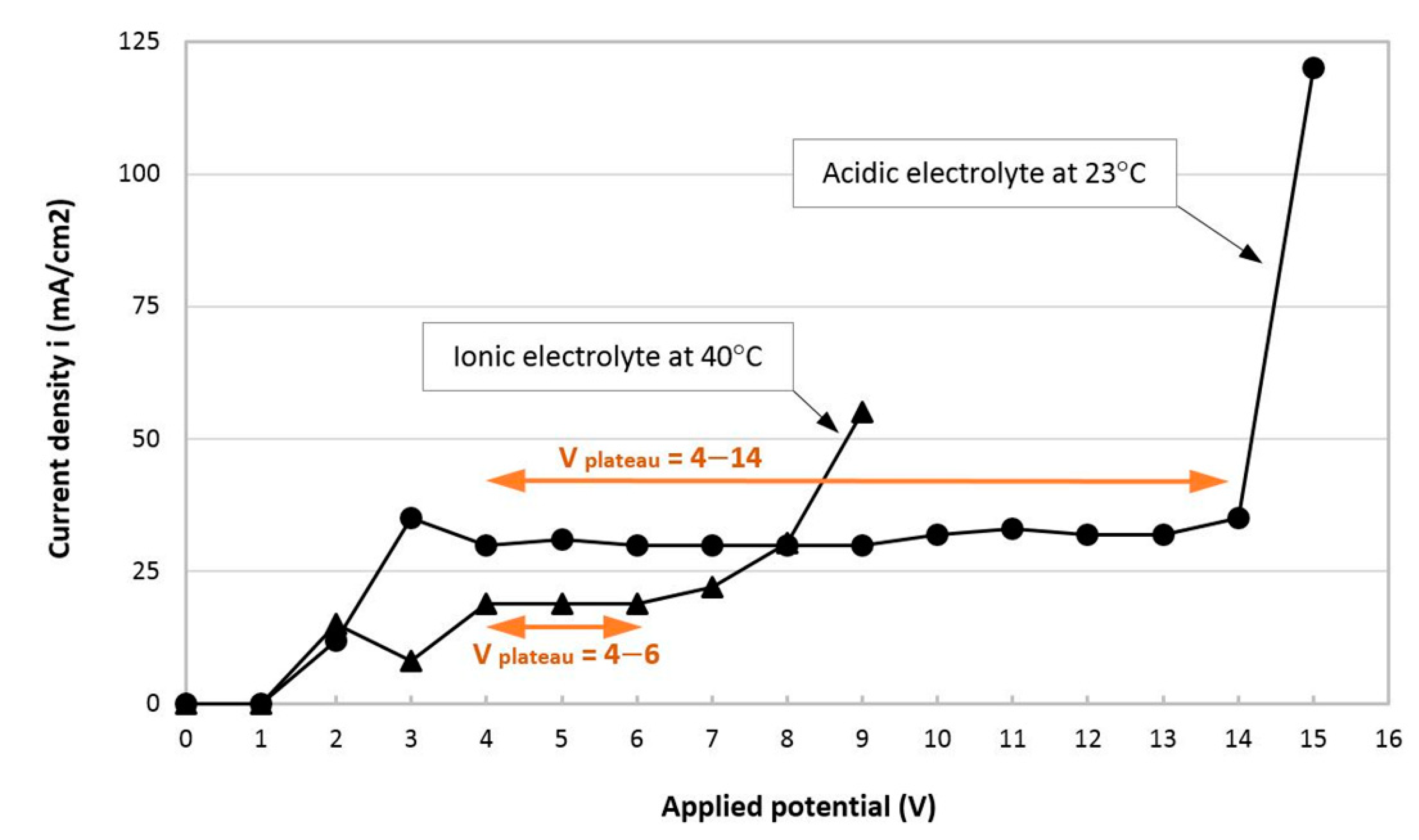
3.1. i-Va Measurements and Optimization of the EP Working Conditions
- V plateau range: As the temperature increases from 25 to 50 °C, a stable part of the i plateau, or the polishing region, shortens from 4–7 V to 4–5 V. The characteristic constant current density of the V plateau is controlled by the mass transport phenomenon and is independent of the applied voltage.
- Current density: As the temperature increases from 25 to 50 °C, the current density corresponding to the i plateau increases from 13 to 22 mA/cm2. This is attributable to a decrease in viscosity due to the increasing temperature, which leads to a higher current efficiency and current density of the electrolyte, as discussed in Section 2.2.
- Minimum allowable current density: According to the results of preliminary testing carried out at temperatures of 25 and 30 °C in the applied potential range of 4, 5 and 6 V (i ≈ 14 mA/cm2), no surface roughness improvements were observed. The reason could be that in these cases, anode surface dissolution led to etching instead of surface polishing. To explain, electropolishing is able to dissolve asperities (peaks) of the surface layer much faster than the surface of the micro-valleys, resulting in overall improvement of the surface roughness. In contrast, electroetching does not have such a selective capacity, ending up with no change or more roughness [2]. Therefore, the current density of i ≈ 14 mA/cm2 was defined as a minimum allowable current density for the selection of adequate EP conditions.
- i peak region: On the i-V graphs in Figure 3, an i peak was observed at 2 V, and the higher the electrolyte temperature, the greater the peak. The nature of the current density peak before the Vplateau region depends on the EP mechanism for the electrolyte system. In an ionic electrolyte with the salt film mechanism, the i peak is related to the super-saturation of the forming salt on the surface. In an acidic electrolyte with the passivation mechanism, the i peak is the characteristic passivation response [18].
- Negative i-Va relationship after i peak region: There exists a window of negative current density-voltage relationship after the i peak, whereas there is no such behavior for EP in acidic electrolytes. This can also be explained by the salt precipitation mechanism in the ionic electrolyte. During EP, the salt film is formed at the bottom of surface pits. As the temperature rises, the i peak value increases, which is an indication of the super-saturation of the salt forming with the dissolved metal ions. There could be easier ion transport through the salt layer because of the higher electrical field across this layer. The concentration gradient of the metal ions across the salt layer decreases after passing the voltage of the i peak region, resulting in a drop in the current density value [19]. Afterwards, the concentration gradient of metal ions increases to the V-plateau current density level and becomes saturated and constant through the plateau region. The transition from the electrical current peak corresponding to the super-saturation of the salt layer to the current density of the V plateau (equilibrium state) is discussed in detail in Reference [20].
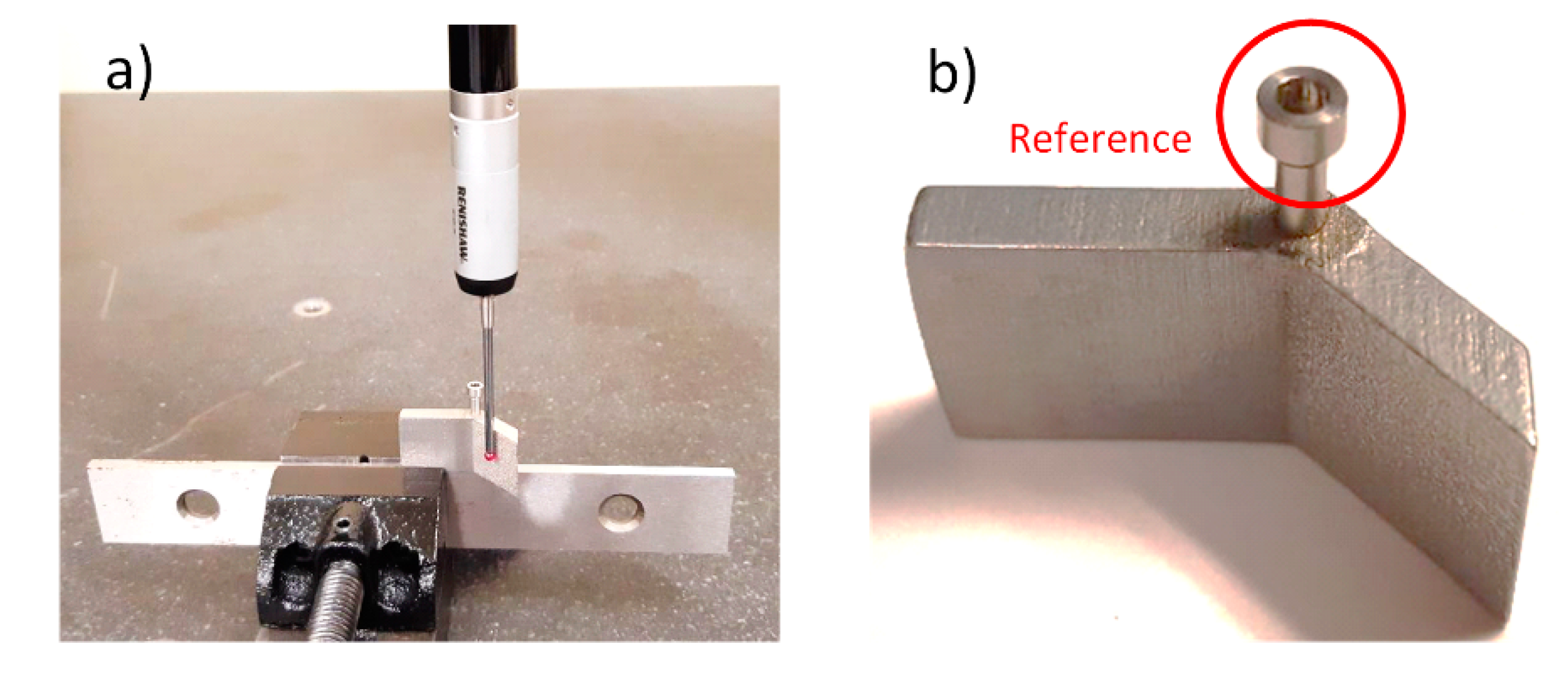
3.2. Roughness and Material Removal Measurements
4. Results
4.1. Roughness Measurements
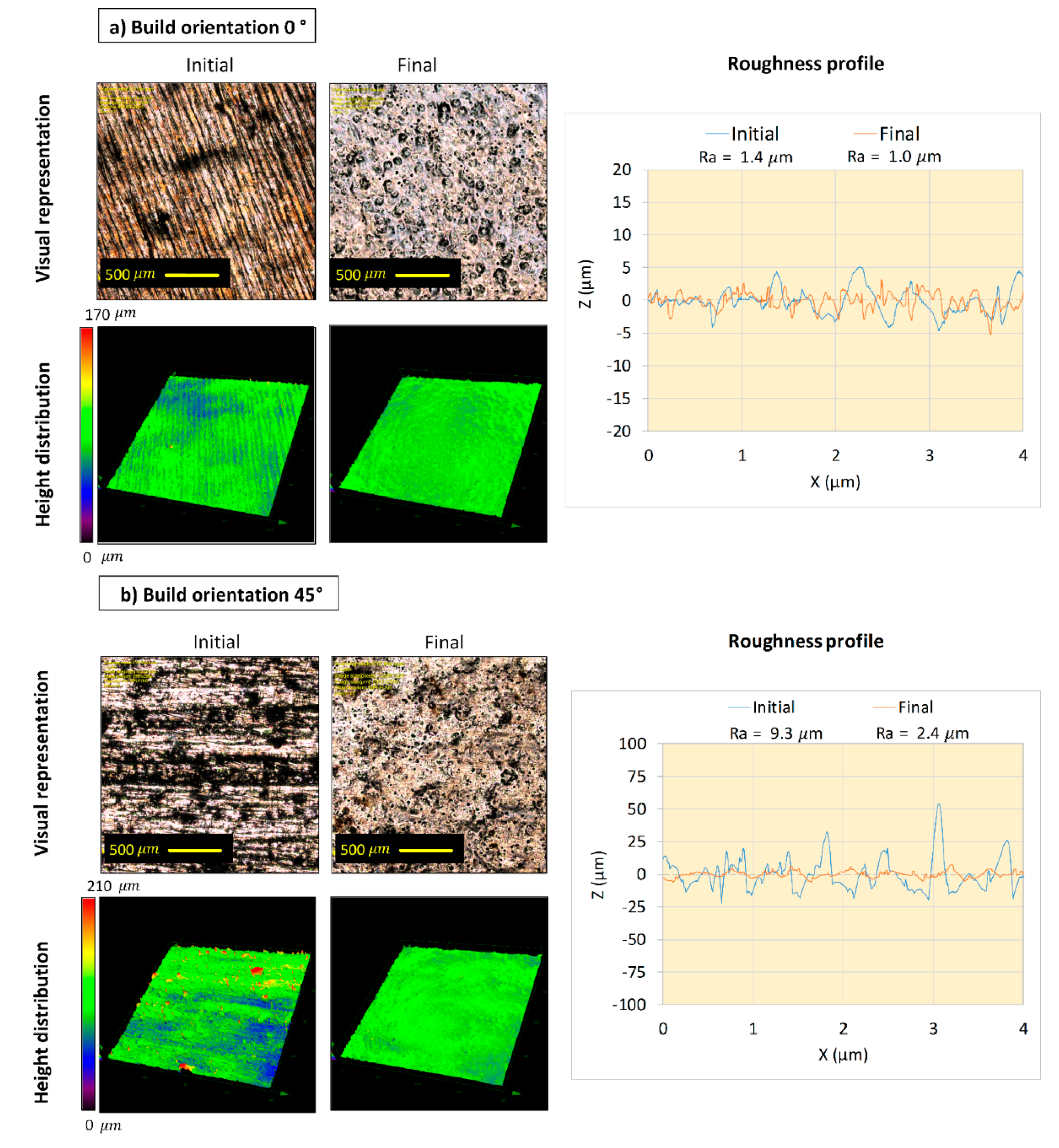
4.2. Surface Topography Characterization and Material Removal Measurements
5. Discussion
5.1. EP Optimization, Polishing Mechanism and Viscosity of 1 Choline Chloride:2 Ethylene Glycol (1ChCl:2EG) Electrolyte
5.1.1. EP Optimization and Polishing Mechanisms
5.1.2. Viscosity
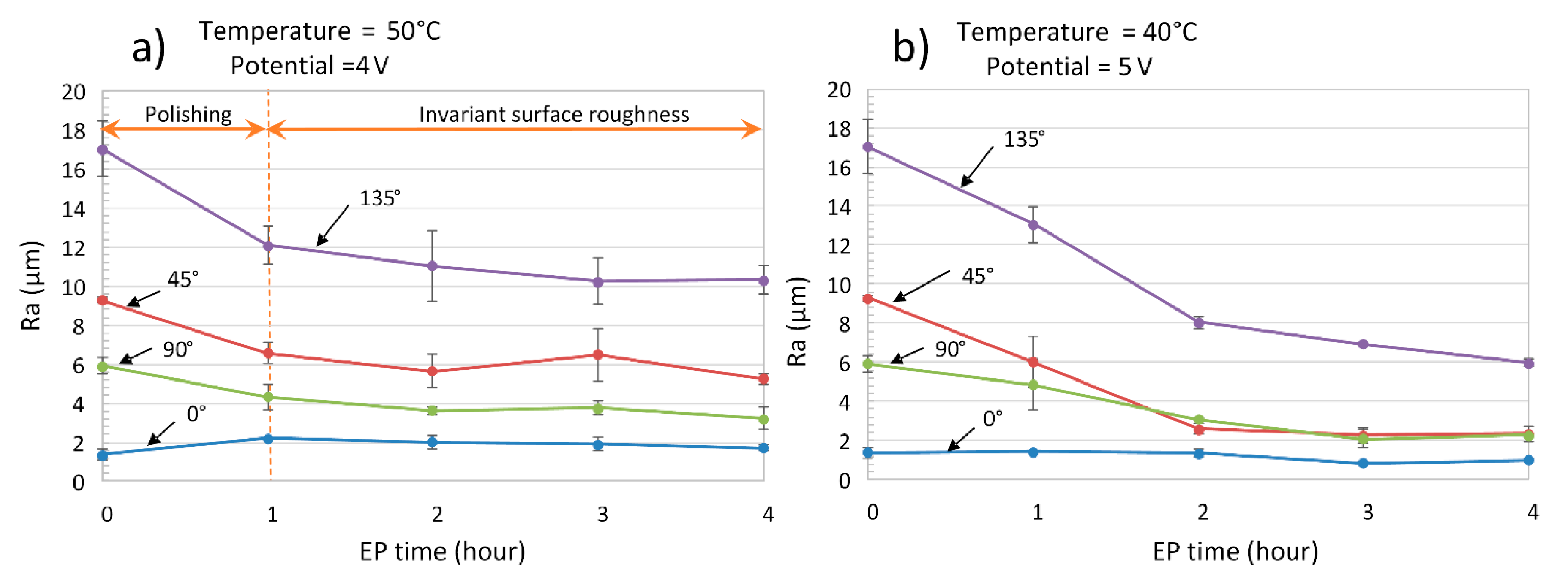
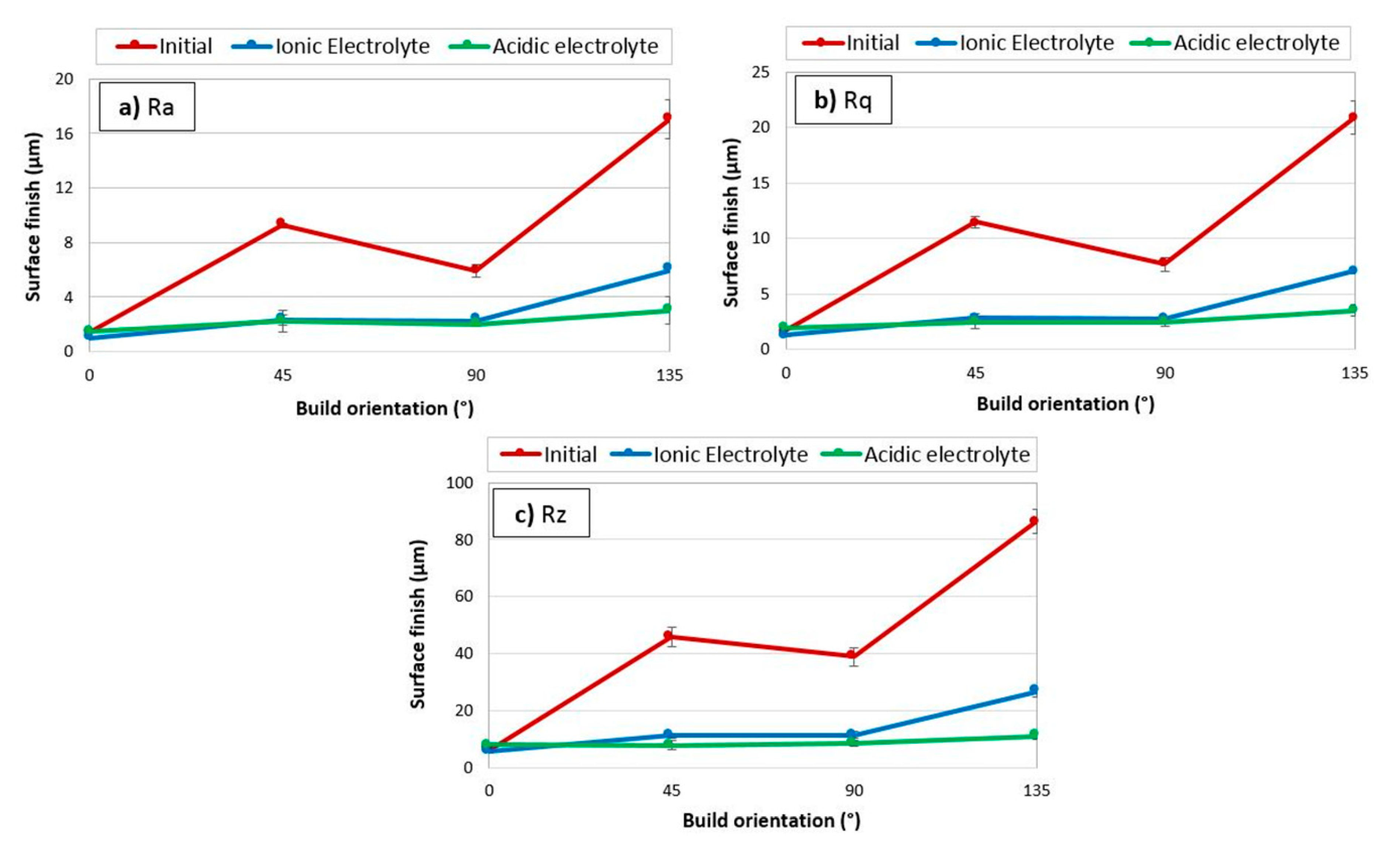
5.2. Roughness Evolution Time in Ionic Electrolyte as Compared to Acidic Electrolyte
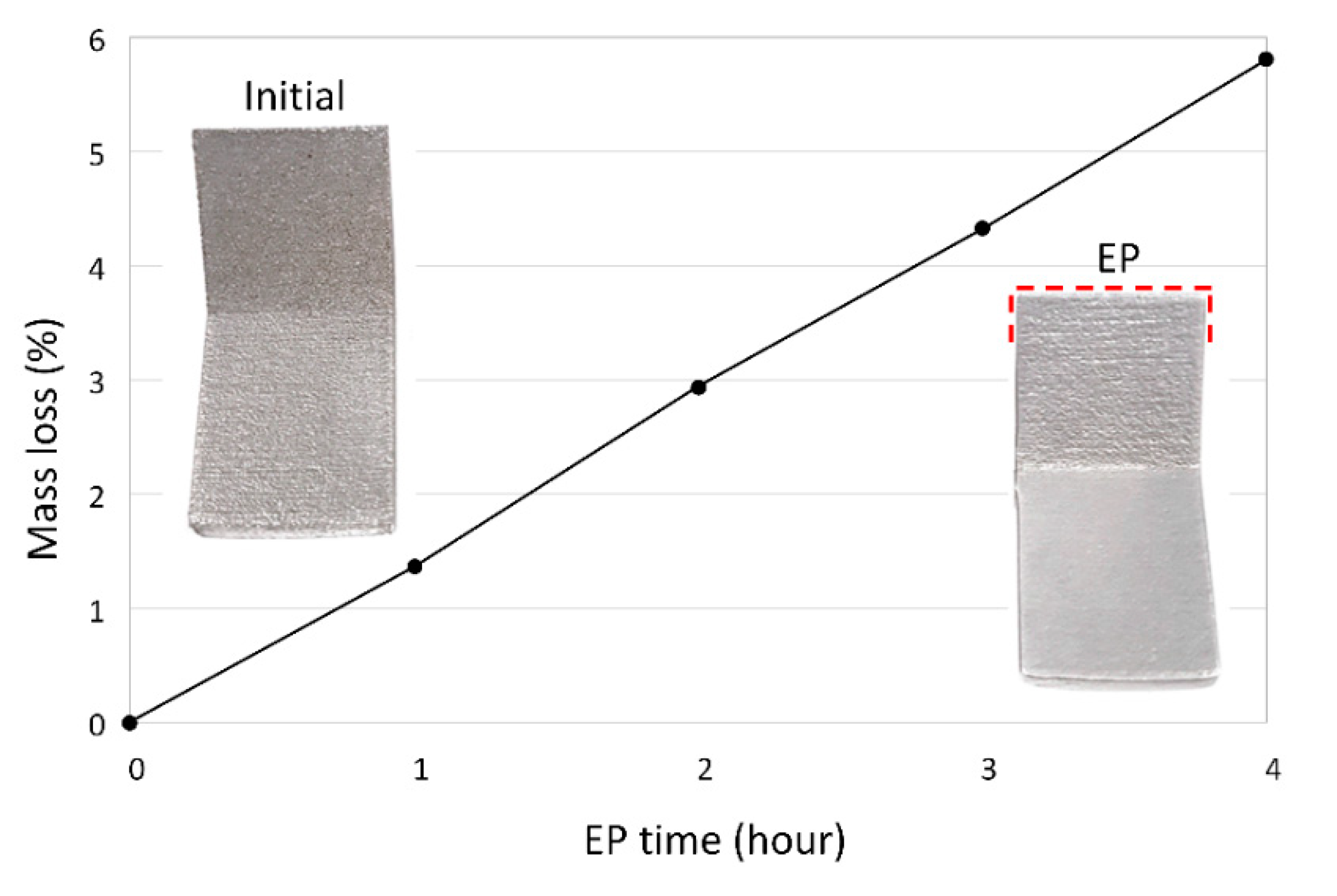
5.3. Mass Loss and Current Efficiency
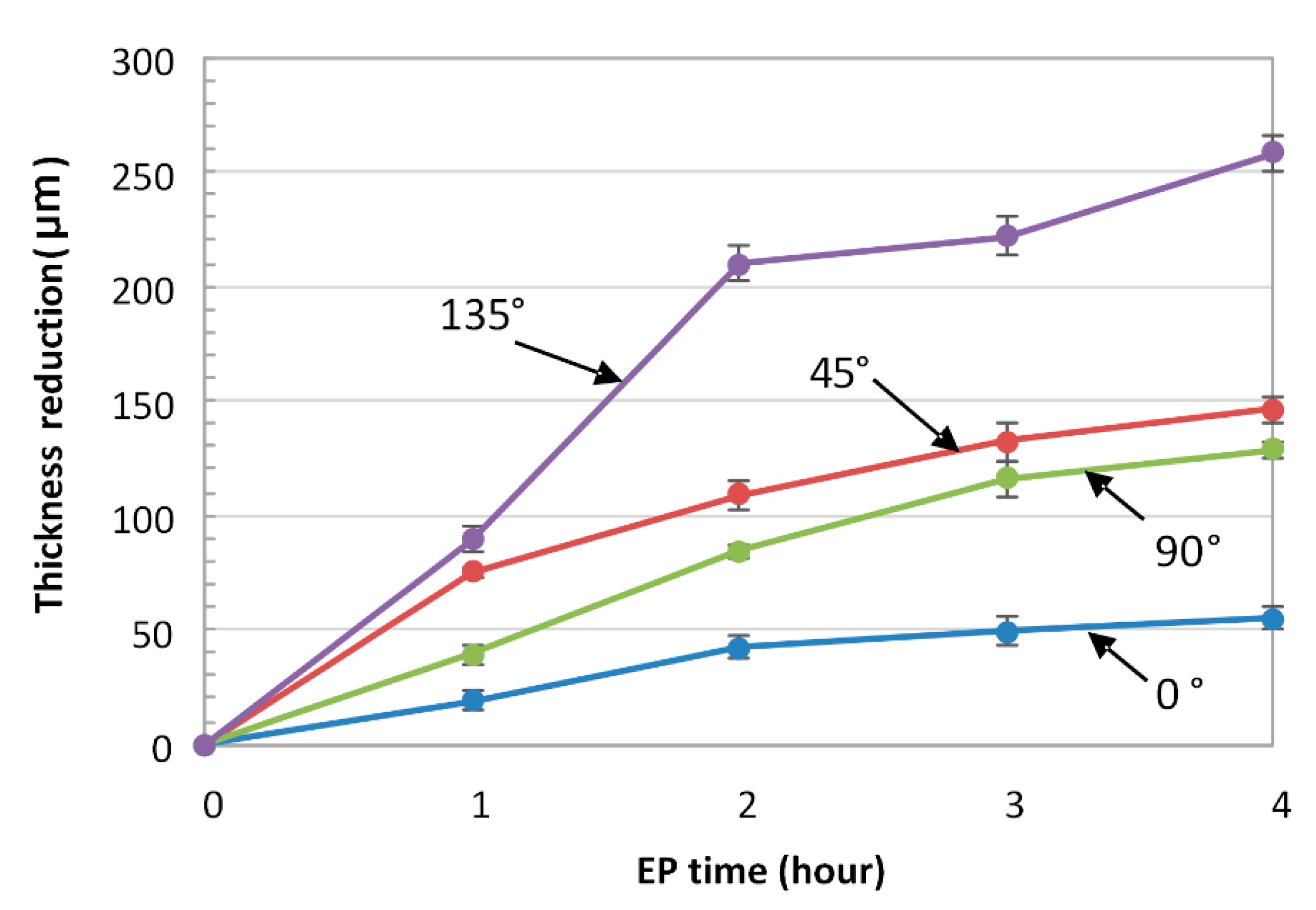
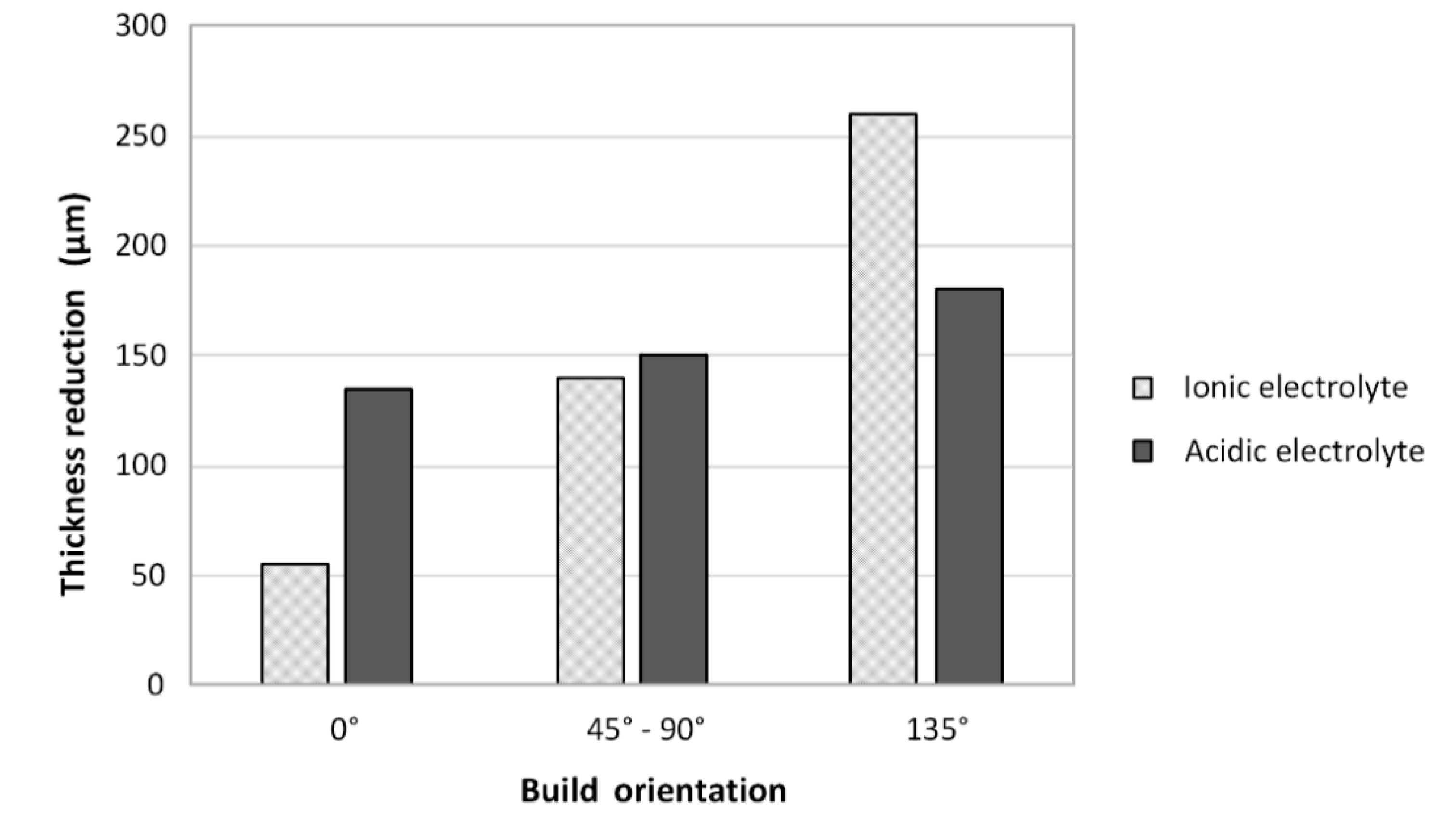
5.4. Thickness Loss and the Determination of EP Allowances (Dimensions of an as-Built Part before EP)
Future Work
6. Conclusions
- For the first time, the EP of a laser powder bed fusion-processed IN625 nickel-based superalloy was successfully conducted in an ionic liquid (1ChCl:2EG).
- Narrower V-plateaus at lower applied potentials and current densities were obtained for the ionic electrolyte in the 25 to 50 °C temperature range, as compared to the acidic electrolyte at room temperature. As the temperature of the ionic electrolyte increased, the V-plateau range became narrower and the current density increased.
- No passive layer was created on the anode surface and no aggressive gassing on the cathodes was observed in the ionic electrolyte. On the anode surface, oxide layer removal was followed by the salt EP precipitation mechanism, allowing a complete electrochemical control of the polishing process.
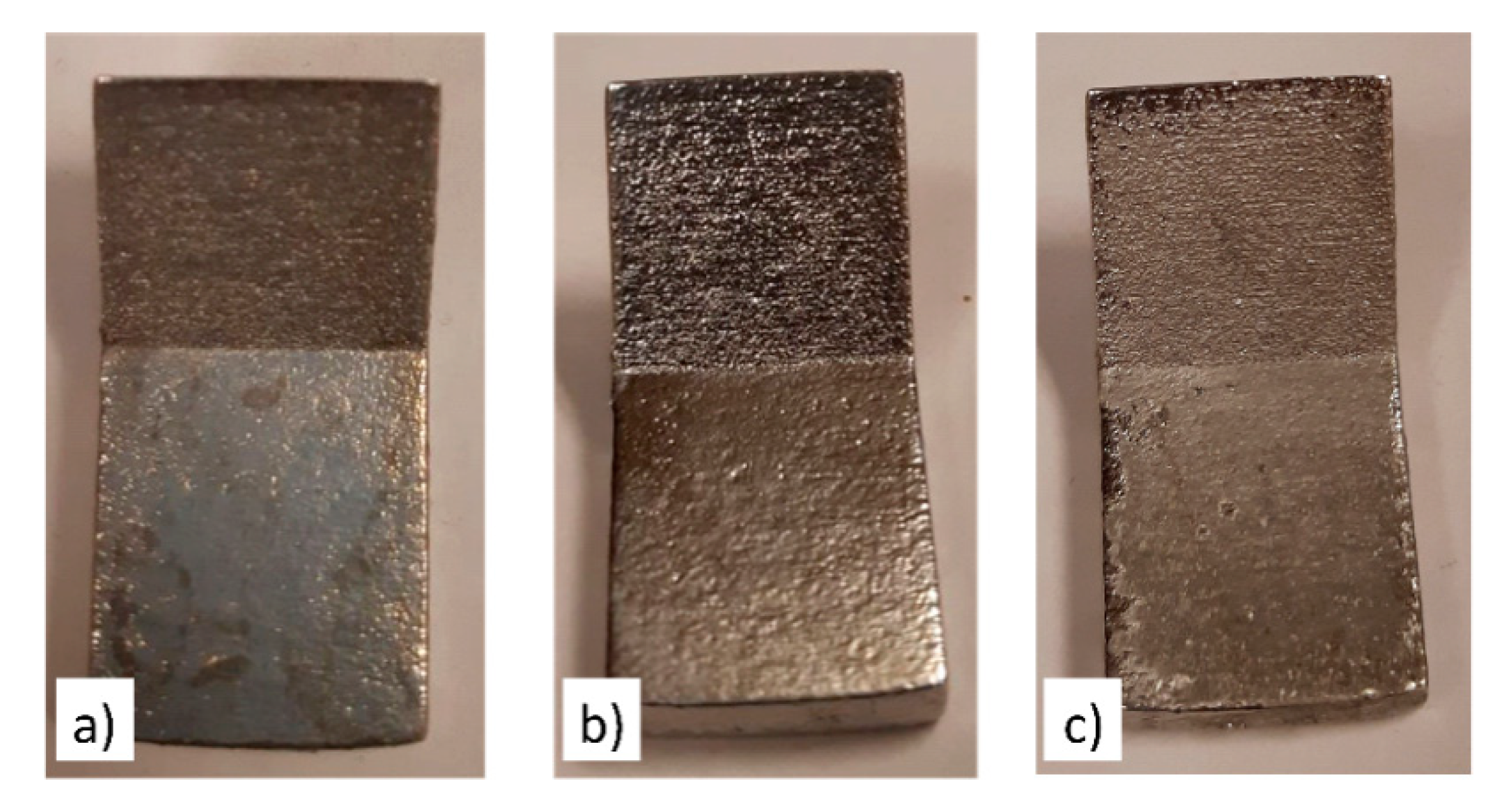
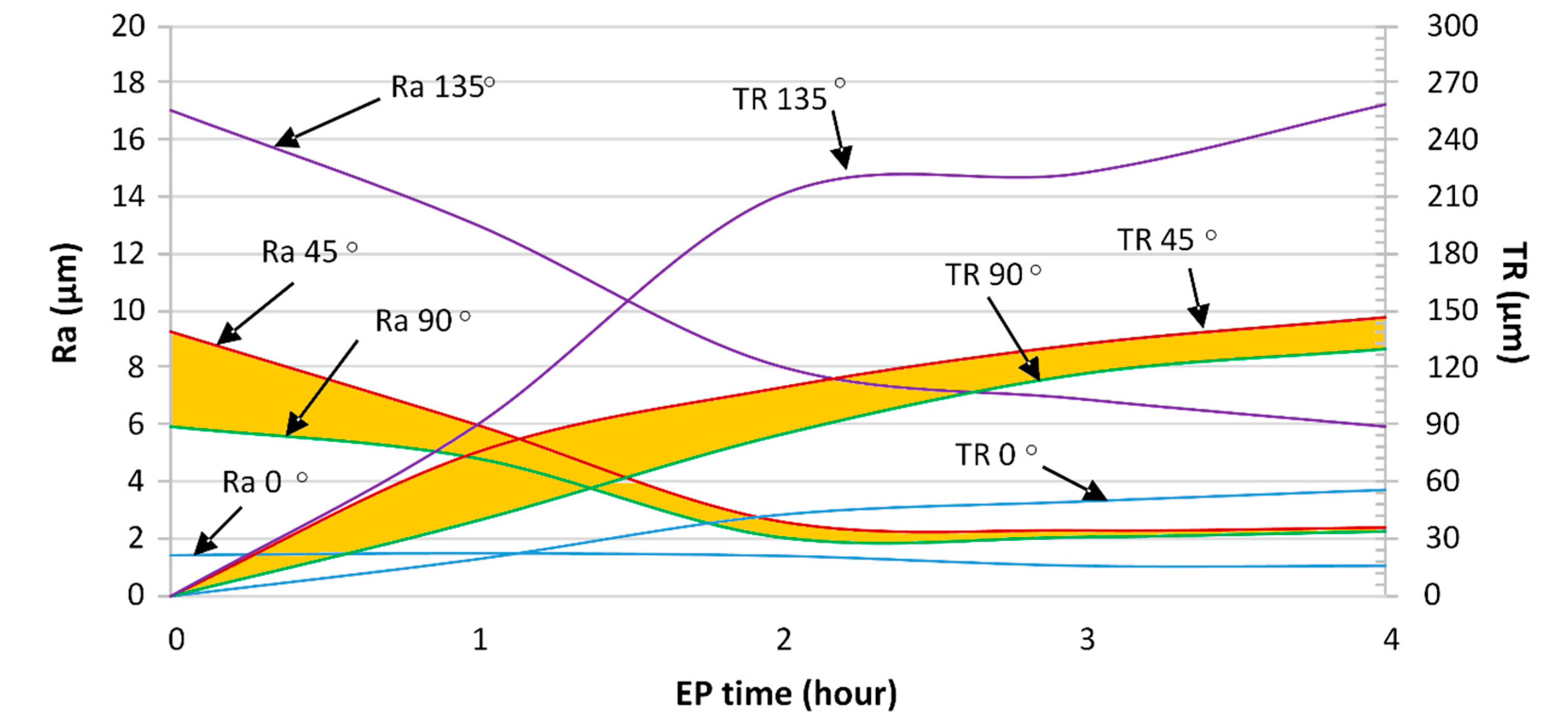
- A continuous roughness reduction over time was observed for EP in the 1ChCl:2EG solution by regulating the electrolyte temperature at 40 °C with a viscosity of 26 cP. Uniform distribution of peaks and valleys and a homogeneous dissolution were obtained by EP in the ionic electrolyte, eliminating the edge rounding effect.
- After 4 h of EP in both the ionic and acidic electrolytes, the Ra roughness on all surfaces reached Ra 6.3 µm (ISO N9 grade number for roughness). By comparing the ionic and acidic electrolytes, after 4 h of EP, the same surface roughness reductions of 75% and 65% were obtained for the 45°- and 90°-oriented surfaces, respectively. While the ionic liquid showed a 30% roughness reduction with some pitting on the surface for the 0°-oriented surface, the acidic electrolyte had an unstable roughness evolution profile over time on this surface.
- EP of IN625 in the ionic electrolyte had a current efficiency of 90%, whereas this value was only 46% for the acidic electrolyte.
- For the ionic electrolyte, the higher the initial surface roughness, the higher the thickness reduction during EP. The ionic electrolyte polishes the surface selectively, avoiding unnecessary material removal from the already smooth surfaces. In the acidic electrolyte however, the surface orientation, and thus the initial surface roughness, does not affect the amount of material dissolved during EP.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ASM. Chemical and Electrolytic Polishing—Metallography and Microstructures—Vol. 9: ASM Handbook; Vander, V., George, F., Eds.; ASM Intermnational: Novelty, OH, USA, 2004; pp. 281–293. ISBN 978-0-87170-706-2. [Google Scholar]
- Huang, C.A.; Chen, Y.C.; Chang, J.H. The Electrochemical Polishing Behavior of the Inconel 718 Alloy in Perchloric–Acetic Mixed Acids. Corros. Sci. 2008, 50, 480–489. [Google Scholar] [CrossRef]
- Urlea, V.; Brailovski, V. Electropolishing and Electropolishing-Related Allowances for In625 Alloy Components Fabricated by Laser Powder-Bed Fusion. Int. J. Adv. Manuf. Technol. 2017, 92, 4487–4499. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Swain, B.G.; Wheeler, D.A. Electropolishing of Stainless Steel in an Ionic Liquid. Trans. IMF 2005, 83, 51–53. [Google Scholar] [CrossRef]
- Zhao, Y.; VanderNoot, T.J. Electrodeposition of Aluminium from Room Temperature Alcl3-Tmpac Molten Salts. Electrochim. Acta 1997, 42, 1639–1643. [Google Scholar] [CrossRef]
- Lebedeva, O.; Jungurova, G.; Kultin, D.; Kustov, L.; Zakharov, A.; Kalmikov, K.; Chernikovab, E.; Krasovskiyb, V. Ionic Liquids Based on the Imidazolium Cation in Platinum and Titanium Electropolishing. Green Chemistr. 2011, 13, 1004–1008. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M. Ionic Liquids as Green Solvents: Progress and Prospects; Mohammad, A., Inamuddin, Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 1–32. ISBN 978-94-007-2890-5. [Google Scholar]
- Eozénou, F.; Antoine, C.; Aspart, A.; Berry, S.; Denis, J.F.; Brahim, M. Efficiency of Electropolishing Vs Bath Composition and Aging: First Results. In Proceedings of the CARE Conference-05-025-SRF, Contribution to the SRF, Ithaca, NY, USA, 10–15 July 2017. [Google Scholar]
- Abbott, A.P.; Capper, G.; McKenzie, K.J.; Ryder, K.S. Voltammetric and Impedance Studies of the Electropolishing of Type 316 Stainless Steel in a Choline Chloride Based Ionic Liquid. Electrochim. Acta 2006, 51, 4420–4425. [Google Scholar] [CrossRef]
- Hryniewicz, T. Electropolishing Processes for Better Implants’ Performance. Biomed. J. Sci. Tech. Res. 2018, 5, 1–5. [Google Scholar] [CrossRef]
- Rokosz, K. High-Current-Density Electropolishing (HDEP) of AISI 316L (EN 1.4404) Stainless Steel. Teh. Vjesn. 2015, 22, 415–424. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Raaen, S.; Chapon, P.; Prima, F. Development of Copper-Enriched Porous Coatings on Ternary Ti-Nb-Zr Alloy by Plasma Electrolytic Oxidation. Int. J. Adv. Manuf. Tech. 2017, 89, 2953–2965. [Google Scholar] [CrossRef]
- Hryniewicz, T.; Rokicki, R.; Rokosz, K. Magnetoelectropolishing for Metal Surface Modification. Trans. IMF 2007, 85, 325–332. [Google Scholar] [CrossRef]
- Rotty, C.; Doche, M.L.; Mandroyan, A.; Hihn, J.Y. Electropolishing Behavior of Additive Layer Manufacturing 316l Stainless Steel in Deep Eutectic Solvents. ECS Trans. 2017, 77, 1199–1207. [Google Scholar] [CrossRef]
- Abbott, A.P.; Ryder, K.S.; König, U. Electrofinishing of Metals Using Eutectic Based Ionic Liquids. Trans. IMF 2008, 86, 196–204. [Google Scholar] [CrossRef]
- Urlea, V.; Brailovski, V. Electropolishing and Electropolishing-Related Allowances for Powder Bed Selectively Laser-Melted Ti-6Al-4V Alloy Components. J. Mater. Process. Technol. 2017, 242, 1–11. [Google Scholar] [CrossRef]
- Lee, E.S. Machining Characteristics of the Electropolishing of Stainless Steel (STS316L). Int. J. Adv. Manuf. Technol. 2000, 16, 591–599. [Google Scholar] [CrossRef]
- Chandra, A.; Sumption, M.; Frankel, G.S. On the Mechanism of Niobium Electropolishing. J. Electrochem. Soc. 2012, 159, C485–C491. [Google Scholar] [CrossRef]
- Yang, G.; Wang, B.; Tawfiq, K.; Wei, H.; Zhou, S.; Chen, G. Electropolishing of Surfaces: Theory and Applications. Surf. Eng. 2017, 33, 149–166. [Google Scholar] [CrossRef]
- Frankel, G. MSE 735 Kinetics II; Fontana Corrosion Center: Columbus, OH, USA, 2010. [Google Scholar]
- Leszek, Z.; Kurowska, E.; Sulka, G.D.; Senyk, I.; Jaskula, M. The Effect of Anode Surface Area on Nanoporous Oxide Formation During Anodizing of Low Purity Aluminum (Aa1050 Alloy). J. Solid State Electrochem. 2014, 18, 361–368. [Google Scholar] [CrossRef]
- Wu, A.T.; Mammosser, J.; Phillips, L.; Delayen, J.; Reece, C.; Wilkerson, A.; Smith, D.; Ike, R. Smooth Nb Surfaces Fabricated by Buffered Electropolishing. Appl. Surf. Sci. 2007, 253, 3041–3052. [Google Scholar] [CrossRef]
- Alrbaey, K.; Wimpenny, D.I.; Al-Barzinjy, A.A.; Moroz, A. Electropolishing of Re-Melted Slm Stainless Steel 316l Parts Using Deep Eutectic Solvents: 3 × 3 Full Factorial Design. J. Mater. Eng. Perform. 2016, 25, 2836–2846. [Google Scholar] [CrossRef]
- Nandi, B.K.; Patel, S. Effects of Operational Parameters on the Removal of Brilliant Green Dye from Aqueous Solutions by Electrocoagulation. Arab. J. Chem. 2017, 10, S2961–S2968. [Google Scholar] [CrossRef]
- Yun, J.Y.; Park, D.; Wang, J.P. A Study on the Oxidation Behavior of Nickel Alloys at Elevated Temperatures. In IOP Conference Series: Materials Science and Engineering, Proceedings of the 2nd International Conference on Mining, Material and Metallurgical Engineering, Bangkok, Thailand, 17–19 March 2017; IOP Publishing: Bristol, UK, 2017; Volume 191, p. 012039. [Google Scholar] [CrossRef]
- Yang, L.; O’Neil, C.; Wu, Y. The Use of Electropolishing Surface Treatment on In718 Parts Fabricated by Laser Powder Bed Fusion Process. In Proceedings of the Solid Freeform Fabrication 2017: Proceedings of the 28th Annual International, Austin, TX, USA, 7–9 August 2017. [Google Scholar]
- Tachikawa, N.; Park, J.; Yoshida, K.; Tamura, T.; Dokko, K.; Watanabe, M. Limiting Current Density in Ionic Liquid Electrolyte for Lithium Batteries. Electroch 2010, 5, 349–352. [Google Scholar] [CrossRef]
- Khorsand, S.; Sheikhi, A.; Raeissi, K.; Golozar, M. Hot Corrosion Behavior of Inconel 625 Superalloy in Eutectic Molten Nitrate Salts. Oxid. Met. 2018, 90, 169–186. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadian, N.; Turenne, S.; Brailovski, V. Electropolishing of Laser Powder Bed-Fused IN625 Components in an Ionic Electrolyte. J. Manuf. Mater. Process. 2019, 3, 86. https://doi.org/10.3390/jmmp3040086
Mohammadian N, Turenne S, Brailovski V. Electropolishing of Laser Powder Bed-Fused IN625 Components in an Ionic Electrolyte. Journal of Manufacturing and Materials Processing. 2019; 3(4):86. https://doi.org/10.3390/jmmp3040086
Chicago/Turabian StyleMohammadian, Neda, Sylvain Turenne, and Vladimir Brailovski. 2019. "Electropolishing of Laser Powder Bed-Fused IN625 Components in an Ionic Electrolyte" Journal of Manufacturing and Materials Processing 3, no. 4: 86. https://doi.org/10.3390/jmmp3040086
APA StyleMohammadian, N., Turenne, S., & Brailovski, V. (2019). Electropolishing of Laser Powder Bed-Fused IN625 Components in an Ionic Electrolyte. Journal of Manufacturing and Materials Processing, 3(4), 86. https://doi.org/10.3390/jmmp3040086



