The Detection of Tree of Heaven (Ailanthus altissima) Using Drones and Optical Sensors: Implications for the Management of Invasive Plants and Insects
Abstract
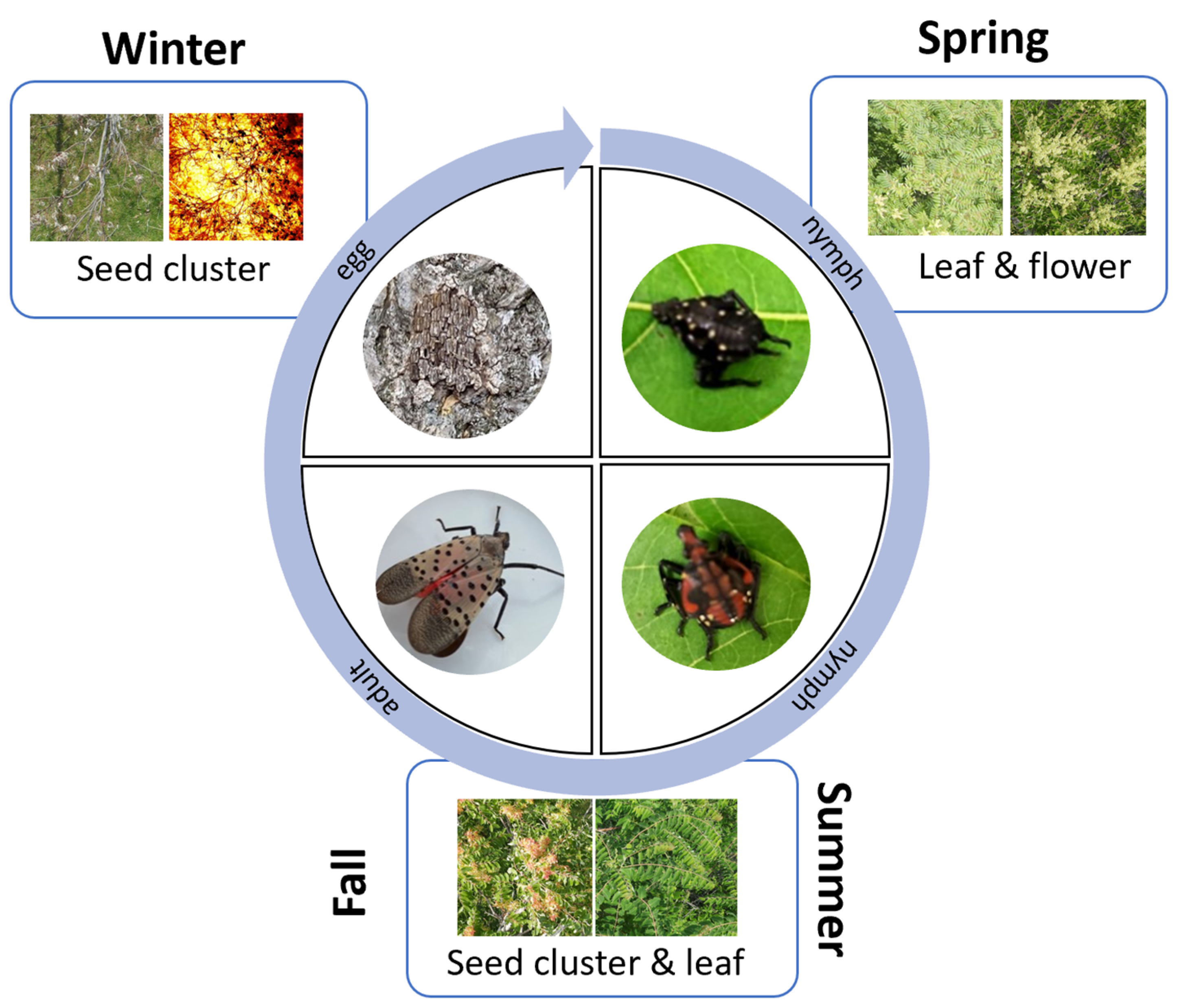
1. Introduction
2. Materials and Methods
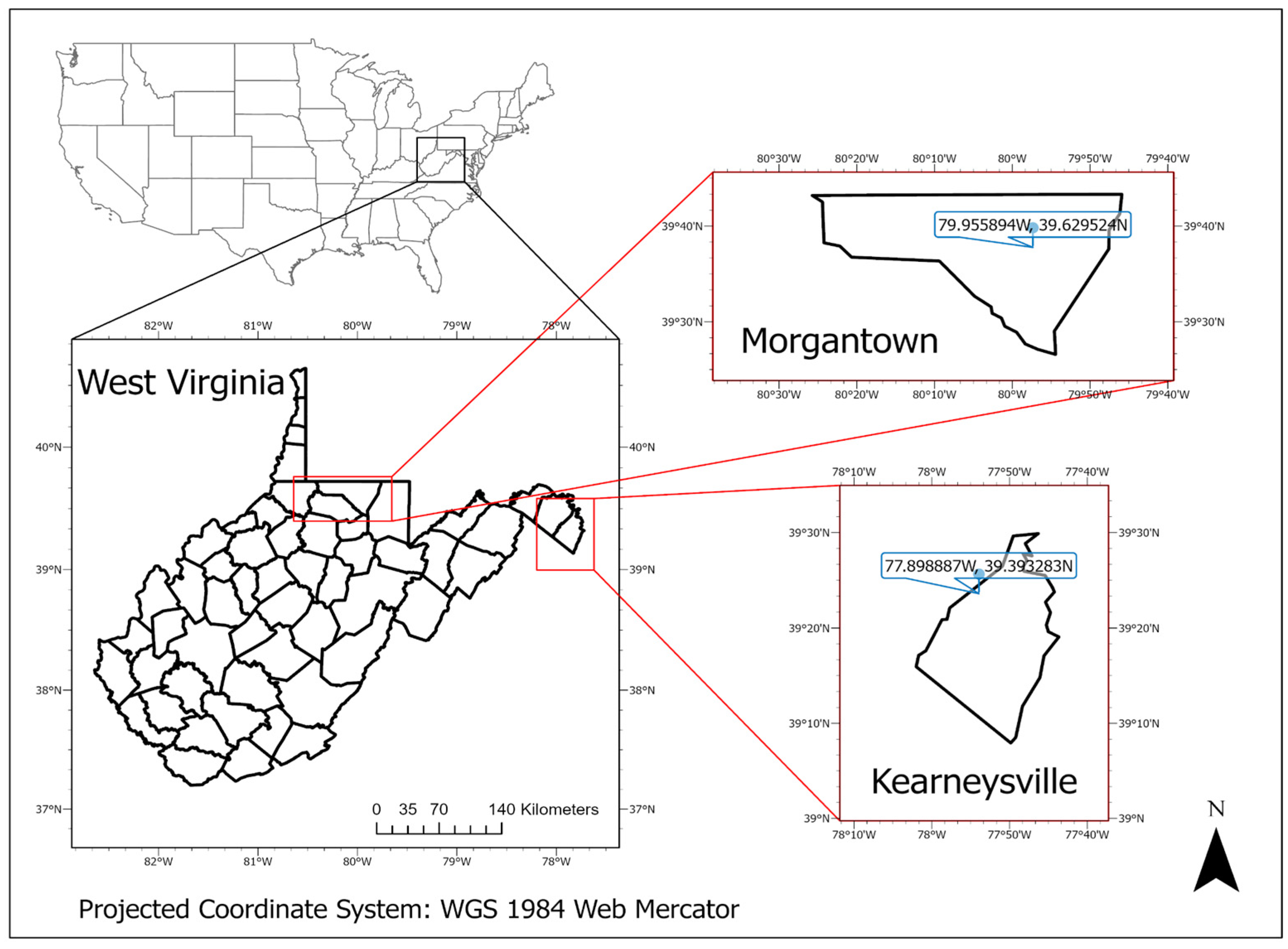
2.1. Study Sites
2.2. Aerial Surveys with Drones
2.2.1. Drones and Optical Sensors
2.2.2. Aerial Survey of A. altissima in Spring
2.2.3. Aerial Survey of A. altissima in Summer and Fall
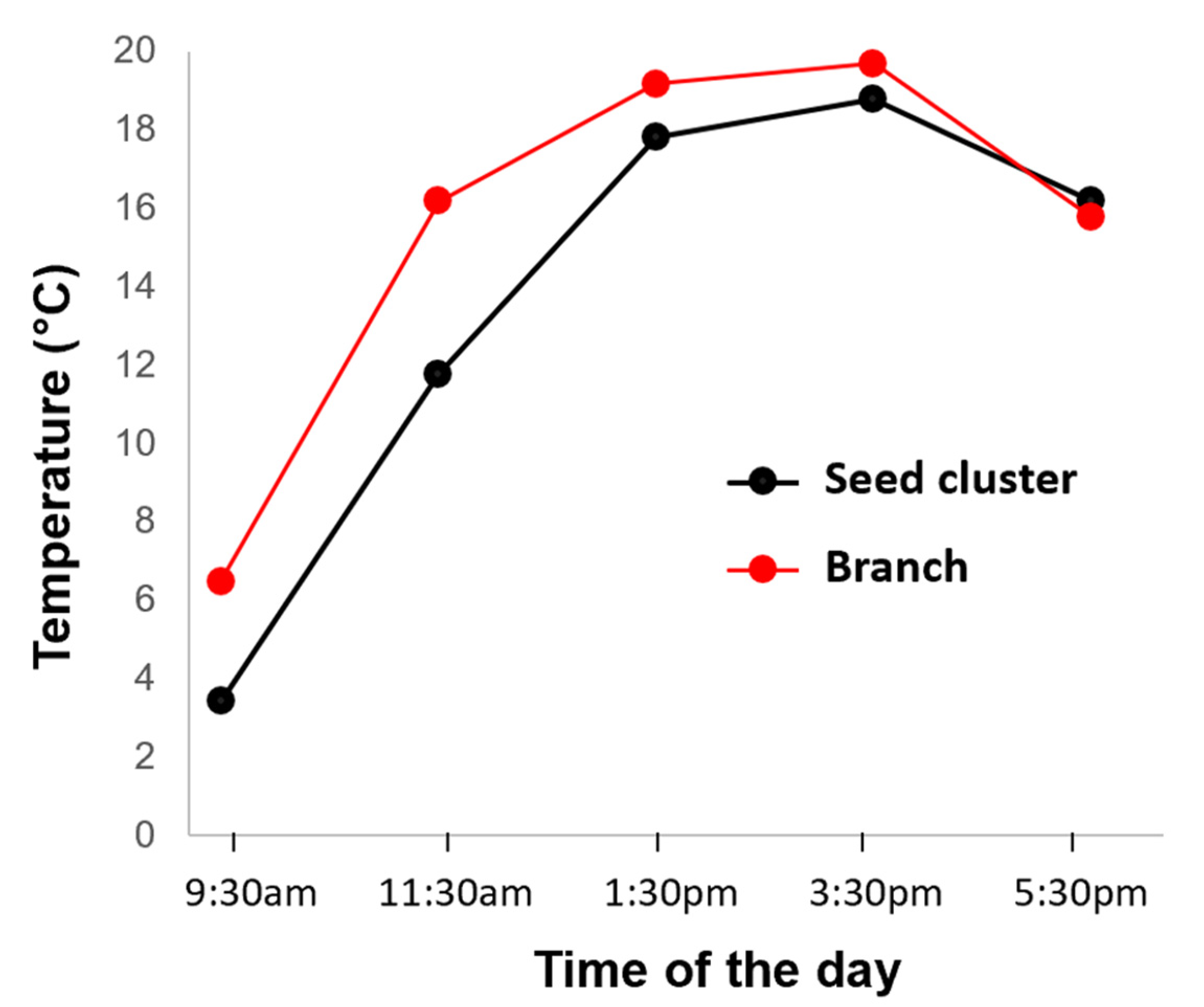
2.2.4. Aerial Survey of A. altissima in Winter
2.3. Data Analysis
3. Results
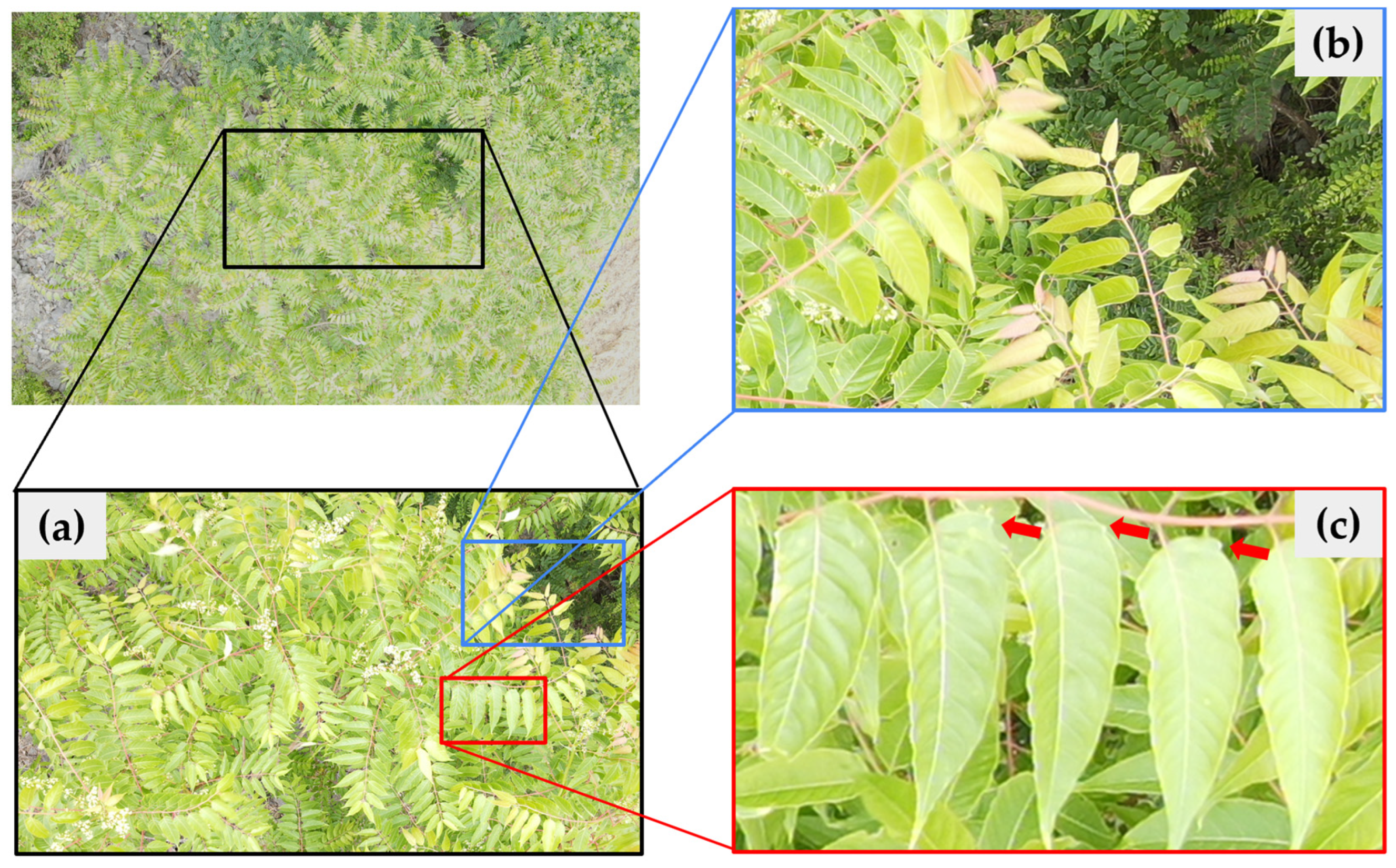
3.1. Detection of A. altissima in Spring
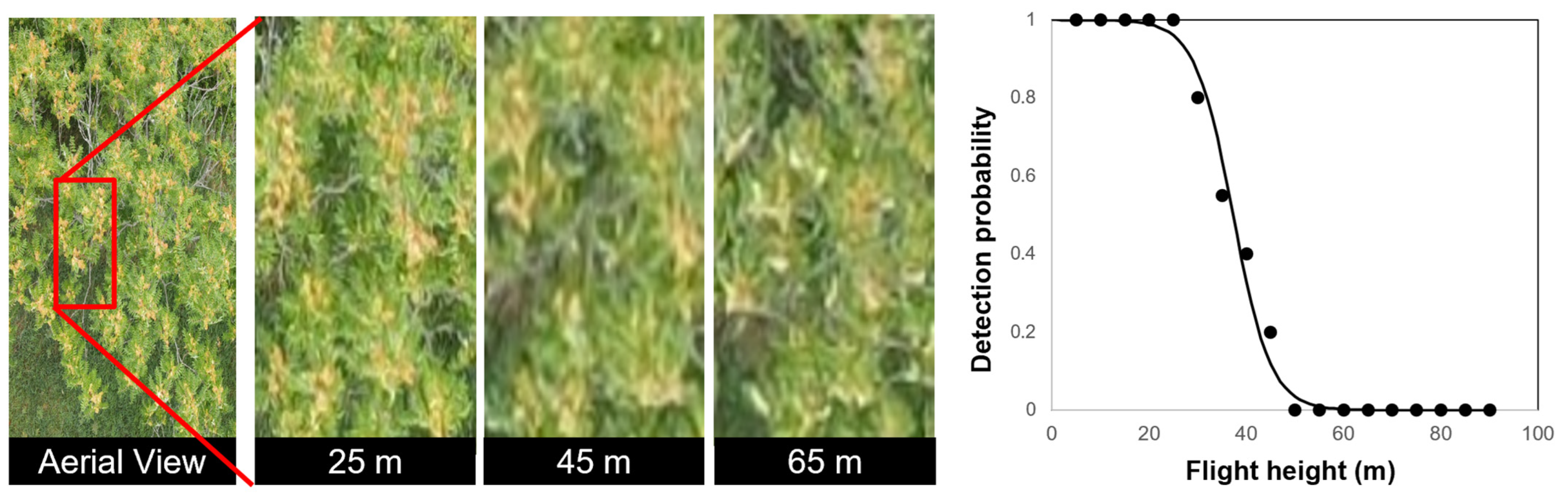
3.2. Detection of A. altissima in Summer and Fall
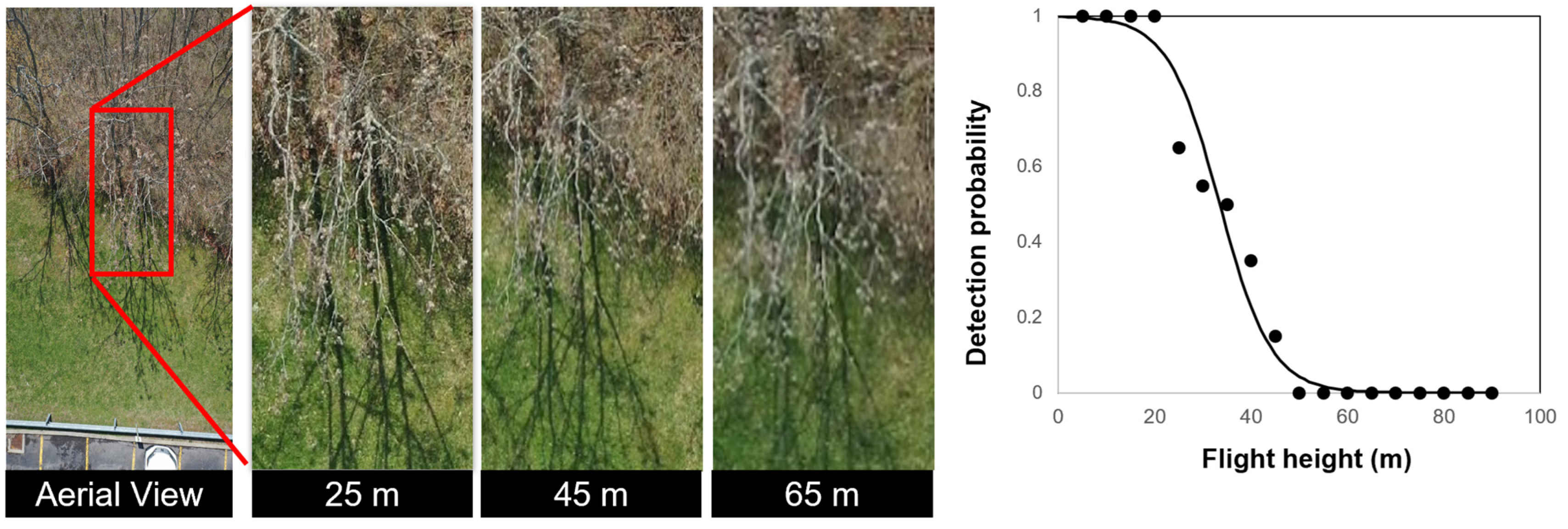
3.3. Detection of A. altissima in Winter
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, S.Y. Ailanthus. Arnoldia 1979, 39, 29–50. [Google Scholar]
- Sladonja, B.; Sušek, M.; Guillermic, J. Review on Invasive Tree of Heaven (Ailanthus Altissima (Mill.) Swingle) Conflicting Values: Assessment of Its Ecosystem Services and Potential Biological Threat. Environ. Manage 2015, 56, 1009–1034. [Google Scholar] [CrossRef] [PubMed]
- Kasson, T.M.; Davis, M.D.; Davis, D.D. The Invasive Ailanthus Altissima in Pennsylvania: A Case Study Elucidating Species Introduction, Migration, Invasion, and Growth Patterns in the Northeastern US. Northeast. Nat. 2013, 20, 1–60. [Google Scholar] [CrossRef]
- EDDMapS. Early Detection & Distribution Mapping System. The University of Georgia—Center for Invasive Species and Ecosystem Health. Available online: http://www.eddmaps.org/ (accessed on 12 September 2023).
- Wickert, K.L.; O’Neal, E.S.; Davis, D.D.; Kasson, M.T. Seed Production, Viability, and Reproductive Limits of the Invasive Ailanthus Altissima (Tree-of-Heaven) within Invaded Environments. Forests 2017, 8, 226. [Google Scholar] [CrossRef]
- Dirr, M.A. Manual of Woody Landscape Plants, 5th ed.; Stipes Publishing LLC: Champaign, IL, USA, 1998; pp. 92–93. [Google Scholar]
- Kowarik, I.; Säumel, I. Biological Flora of Central Europe: Ailanthus Altissima (Mill.) Swingle. Perspect. Plant Ecol. Evol. Syst. 2007, 8, 207–237. [Google Scholar] [CrossRef]
- Ulus, A.; Yilmaz, H.; Akkemik, U.; Yılmaz, O.Y. Assessing Street-level Distribution of Tree of Heaven (Ailanthus altissima) in Istanbul (Turkey). Appl. Ecol. Environ. Res. 2021, 19, 2793–2802. [Google Scholar] [CrossRef]
- Fotiadis, G.; Kyriazopoulos, A.P.; Fraggakis, I. The Behaviour of Ailanthus Altissima Weed and Its Effects on Natural Ecosystems. J. Environ. Biol. 2011, 32, 801–806. Available online: https://pubmed.ncbi.nlm.nih.gov/22471219/ (accessed on 18 September 2023). [PubMed]
- Bory, G.; Clair-Maczulajtys, D. Production, Dissemination and Polymorphism of Seeds in Ailanthus altissima. Revue Génerale de Botanique 1980, 88, 297–311. [Google Scholar]
- Fryer, J.L. Ailanthus altissima. In Fire Effects Information System. U.S.; Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory: Missoula, MT, USA, 2010. Available online: https://www.fs.usda.gov/database/feis/plants/tree/ailalt/all.html (accessed on 18 September 2023).
- Knapp, L.B.; Canham, C.D. Invasion of an Old-Growth Forest in New York by Ailanthus Altissima: Sapling Growth and Recruitment in Canopy Gaps. J. Torrey Bot. Soc. 2000, 127, 307. [Google Scholar] [CrossRef]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the Environmental and Economic Costs Associated with Alien-Invasive Species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Brooks, R.K.; Barney, J.N.; Salom, S.M. The Invasive Tree, Ailanthus Altissima, Impacts Understory Nativity, Not Seedbank Nativity. For. Ecol. Manag. 2021, 489, 119025. [Google Scholar] [CrossRef]
- Forest Service. Field Guide for Managing Tree-of-heaven in the Southwest. United States Department of Agriculture 2014. Available online: https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb5410131.pdf (accessed on 3 September 2023).
- Barringer, L.E.; Donovall, L.R.; Spichiger, S.-E.; Lynch, D.; Henry, D. The First New World Record Of Lycorma Delicatula (Insecta: Hemiptera: Fulgoridae). Entomol. News 2015, 125, 20–23. [Google Scholar] [CrossRef]
- Cook, R.T.; Ward, S.F.; Liebhold, A.M.; Fei, S. Spatial Dynamics of Spotted Lanternfly, Lycorma Delicatula, Invasion of the Northeastern United States. NeoBiota 2021, 70, 23–42. [Google Scholar] [CrossRef]
- Wakie, T.T.; Neven, L.G.; Yee, W.L.; Lu, Z. The Establishment Risk of Lycorma Delicatula (Hemiptera: Fulgoridae) in the United States and Globally. J. Econ. Entomol. 2019, 113, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.; Lee, Y.; Lee, H.-S.; Lee, S.-J.; Lee, J.-H. Tracing the Origin of Korean Invasive Populations of the Spotted Lanternfly, Lycorma Delicatula (Hemiptera: Fulgoridae). Insects 2021, 12, 539. [Google Scholar] [CrossRef] [PubMed]
- Uyi, O.; Keller, J.A.; Swackhamer, E.; Hoover, K. Performance and Host Association of Spotted Lanternfly (Lycorma Delicatula) among Common Woody Ornamentals. Sci. Rep. 2021, 11, 15774. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.M. Perspective: Shedding Light on Spotted Lanternfly Impacts in the USA. Pest. Manag. Sci. 2019, 76, 10–17. [Google Scholar] [CrossRef]
- Dara, S.K.; Barringer, L.; Arthurs, S.P. Lycorma Delicatula (Hemiptera: Fulgoridae): A New Invasive Pest in the United States. J. Integr. Pest. Manag. 2015, 6, 20. [Google Scholar] [CrossRef]
- Urban, J.M.; Leach, H. Biology and Management of the Spotted Lanternfly, Lycorma Delicatula (Hemiptera: Fulgoridae), in the United States. Annu. Rev. Entomol. 2022, 68, 151–167. [Google Scholar] [CrossRef]
- Huang, C.; Asner, G. Applications of Remote Sensing to Alien Invasive Plant Studies. Sensors 2009, 9, 4869–4889. [Google Scholar] [CrossRef]
- Park, Y.-L.; Naharki, K.; Karimzadeh, R.; Seo, B.Y.; Lee, G.S. Rapid Assessment of Insect Pest Outbreak Using Drones: A Case Study with Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) in Soybean Fields. Insects 2023, 14, 555. [Google Scholar] [CrossRef]
- Kattenborn, T.; Lopatin, J.; Förster, M.; Braun, A.C.; Fassnacht, F.E. UAV Data as Alternative to Field Sampling to Map Woody Invasive Species Based on Combined Sentinel-1 and Sentinel-2 Data. Remote Sens. Environ. 2019, 227, 61–73. [Google Scholar] [CrossRef]
- Park, Y.-L.; Cho, J.; Choi, K.-H.; Kim, H.; Kim, J.; Kim, J.; Lee, D.-H.; Park, C.-G.; Cho, Y. Advances, Limitations, and Future Applications of Aerospace and Geospatial Technologies for Apple IPM. Korean J. Appl. Entomol. 2021, 60, 2287–2545. [Google Scholar] [CrossRef]
- Park, Y.-L.; Cho, J.R.; Lee, G.-S.; Seo, B.Y. Detection of Monema Flavescens (Lepidoptera: Limacodidae) Cocoons Using Small Unmanned Aircraft System. J. Econ. Entomol. 2021, 114, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, C.; Casella, F.; Adamo, M.; Lucas, R.; Beierkuhnlein, C.; Blonda, P. Ailanthus Altissima Mapping from Multi-Temporal Very High Resolution Satellite Images. ISPRS J. Photogramm. Remote Sens. 2019, 147, 90–103. [Google Scholar] [CrossRef]
- Burkholder, A.; Warner, T.A.; Culp, M.; Landenberger, R. Seasonal Trends in Separability of Leaf Reflectance Spectra for Ailanthus Altissima and Four Other Tree Species. Photogramm. Eng. Remote Sens. 2011, 77, 793–804. [Google Scholar] [CrossRef]
- Rebbeck, J.; Kloss, A.; Bowden, M.; Coon, C.; Hutchinson, T.F.; Iverson, L.; Guess, G. Aerial Detection of Seed-Bearing Female Ailanthus Altissima: A Cost-Effective Method to Map an Invasive Tree in Forested Landscapes. For. Sci. 2015, 61, 1068–1078. [Google Scholar] [CrossRef]
- Rottenberg, A. A Field Survey of Dioecious Plants in Israel: Sex Ratio in Seven Rare Species. Bot. J. Linn. Soc. 2000, 134, 439–442. [Google Scholar] [CrossRef][Green Version]
- Wiegmann, D.A.; Taneja, N. Analysis of Injuries among Pilots Involved in Fatal General Aviation Airplane. Accidents. Accid. Anal. Prev. 2003, 35, 571–577. [Google Scholar] [CrossRef]
- Watts, A.C.; Perry, J.H.; Smith, S.E.; Burgess, M.A.; Wilkinson, B.E.; Szantoi, Z.; Ifju, P.G.; Percival, H.F. Small Unmanned Aircraft Systems for Low-Altitude Aerial Surveys. J. Wild. Manag. 2010, 74, 1614–1619. [Google Scholar] [CrossRef]
- Dvořák, P.; Müllerová, J.; Bartaloš, T.; Brůna, J. Unmanned Aerial Vehicles for Alien Plant Species Detection and Monitoring. Int. Arch. Photogramm. Remote Sens. Spatial Inf. Sci. 2015, XL-1/W4, 83–90. [Google Scholar] [CrossRef]
- Park, Y.-L.; Krell, R.K.; Carroll, M. Theory, technology, and practice of site-specific insect pest management. J. Asia-Pac. Entomol. 2007, 10, 89–101. [Google Scholar] [CrossRef]
- Rebbeck, J.; Jolliff, J. How Long Do Seeds of the Invasive Tree, Ailanthus Altissima Remain Viable? For. Ecol. Manag. 2018, 429, 175–179. [Google Scholar] [CrossRef]
- Park, Y.-L.; Gururajan, S.; Thistle, H.; Chandran, R.; Reardon, R. Aerial release of Rhinoncomimus latipes (Coleoptera: Curculionidae) to control Persicaria perfoliata (Polygonaceae) using an unmanned aerial system. Pest. Manag. Sci. 2018, 74, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Landenberger, R.E.; Kota, N.L.; McGraw, J.B. Seed Dispersal of the Non-Native Invasive Tree Ailanthus Altissima into Contrasting Environments. Plant Ecol. 2007, 192, 55–70. [Google Scholar] [CrossRef]
- Zhang, C.; Xia, K.; Feng, H.; Yang, Y.; Du, X. Tree Species Classification Using Deep Learning and RGB Optical Images Obtained by an Unmanned Aerial Vehicle. J. For. Res. 2020, 32, 1879–1888. [Google Scholar] [CrossRef]
- Ewane, E.B.; Mohan, M.; Bajaj, S.; Galgamuwa, G.A.P.; Watt, M.S.; Arachchige, P.P.; Hudak, A.T.; Richardson, G.; Ajithkumar, N.; Srinivasan, S.; et al. Climate-Change-Driven Droughts and Tree Mortality: Assessing the Potential of UAV-Derived Early Warning Metrics. Remote Sens. 2023, 15, 2627. [Google Scholar] [CrossRef]
- Federal Aviation Administration. Part 107—Small Unmanned Aircraft Systems. Federal Register. 2016; 81, pp. 42063–42103. Available online: https://www.govinfo.gov/content/pkg/FR-2016-06-28/pdf/2016-15079.pdf (accessed on 5 October 2023).
- Call, L.J.; Nilsen, E.T. Analysis of Spatial Patterns and Spatial Association between the Invasive Tree-of-Heaven (Ailanthus Altissima) and the Native Black Locust (Robinia Pseudoacacia). Am. Midl. Nat. 2003, 150, 1–14. [Google Scholar] [CrossRef]
- Swearingen, J.M.; Pannill, P.D. Factsheet: Tree of Heaven. Plant Conservation Alliance’s Alien Plant Working Group 2009. Available online: https://www.invasive.org/alien/fact/pdf/aial1.pdf (accessed on 2 September 2023).
- Laveaga, E.; Hoover, K.; Acevedo, F.E. Life History Traits of Spotted Lanternfly (Hemiptera: Fulgoridae) When Feeding on Grapevines and Tree of Heaven. Front. Insect Sci. 2023, 3, 1091332. [Google Scholar] [CrossRef]
- Keller, J.A.; Johnson, A.E.; Uyi, O.; Wurzbacher, S.; Long, D.; Hoover, K. Dispersal of Lycorma Delicatula (Hemiptera: Fulgoridae) Nymphs through Contiguous, Deciduous Forest. Environ. Entomol. 2020, 49, 1012–1018. [Google Scholar] [CrossRef]
- Leach, A.; Leach, H. Characterizing the Spatial Distributions of Spotted Lanternfly (Hemiptera: Fulgoridae) in Pennsylvania Vineyards. Sci. Rep. 2020, 10, 20588. [Google Scholar] [CrossRef]
- Oliveira, R.A.; Näsi, R.; Niemeläinen, O.; Nyholm, L.; Alhonoja, K.; Kaivosoja, J.; Jauhiainen, L.; Viljanen, N.; Nezami, S.; Markelin, L.; et al. Machine Learning Estimators for the Quantity and Quality of Grass Swards Used for Silage Production Using Drone-Based Imaging Spectrometry and Photogrammetry. Remote Sens. Environ. 2020, 246, 111830. [Google Scholar] [CrossRef]
- Baker, P.; CNN Underscored. Best Plant Identification Apps for Mobile in 2023, Tested by Our Editors. CNN Underscored. Available online: https://www.cnn.com/cnn-underscored/reviews/best-plant-identification-app (accessed on 12 September 2023).
- Valicharla, S.K.; Li, X.; Greenleaf, J.; Turcotte, R.M.; Hayes, C.J.; Park, Y.-L. Precision Detection and Assessment of Ash Death and Decline Caused by the Emerald Ash Borer Using Drones and Deep Learning. Plants 2023, 12, 798–798. [Google Scholar] [CrossRef]
- Fromm, M.; Schubert, M.; Castilla, G.; Linke, J.; McDermid, G. Automated Detection of Conifer Seedlings in Drone Imagery Using Convolutional Neural Networks. Remote Sens. 2019, 11, 2585. [Google Scholar] [CrossRef]






| Season | Sensors | ||
|---|---|---|---|
| RGB | Thermal | NDVI | |
| Spring | |||
| Leaf | Detectable | Undetectable | Undetectable |
| Inflorescences | Detectable | Undetectable | Undetectable |
| Branching pattern | Detectable | Undetectable | Undetectable |
| Summer and Fall | |||
| Leaf | Detectable | Undetectable | Undetectable |
| Seed clusters | Detectable | Undetectable | Undetectable |
| Branching pattern | Detectable | Undetectable | Undetectable |
| Winter | |||
| Seed clusters | Detectable | Detectable | Undetectable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naharki, K.; Huebner, C.D.; Park, Y.-L. The Detection of Tree of Heaven (Ailanthus altissima) Using Drones and Optical Sensors: Implications for the Management of Invasive Plants and Insects. Drones 2024, 8, 1. https://doi.org/10.3390/drones8010001
Naharki K, Huebner CD, Park Y-L. The Detection of Tree of Heaven (Ailanthus altissima) Using Drones and Optical Sensors: Implications for the Management of Invasive Plants and Insects. Drones. 2024; 8(1):1. https://doi.org/10.3390/drones8010001
Chicago/Turabian StyleNaharki, Kushal, Cynthia D. Huebner, and Yong-Lak Park. 2024. "The Detection of Tree of Heaven (Ailanthus altissima) Using Drones and Optical Sensors: Implications for the Management of Invasive Plants and Insects" Drones 8, no. 1: 1. https://doi.org/10.3390/drones8010001
APA StyleNaharki, K., Huebner, C. D., & Park, Y.-L. (2024). The Detection of Tree of Heaven (Ailanthus altissima) Using Drones and Optical Sensors: Implications for the Management of Invasive Plants and Insects. Drones, 8(1), 1. https://doi.org/10.3390/drones8010001







