Canopy Composition and Spatial Configuration Influences Beta Diversity in Temperate Regrowth Forests of Southeastern Australia
Abstract
1. Introduction
2. Materials and Methods
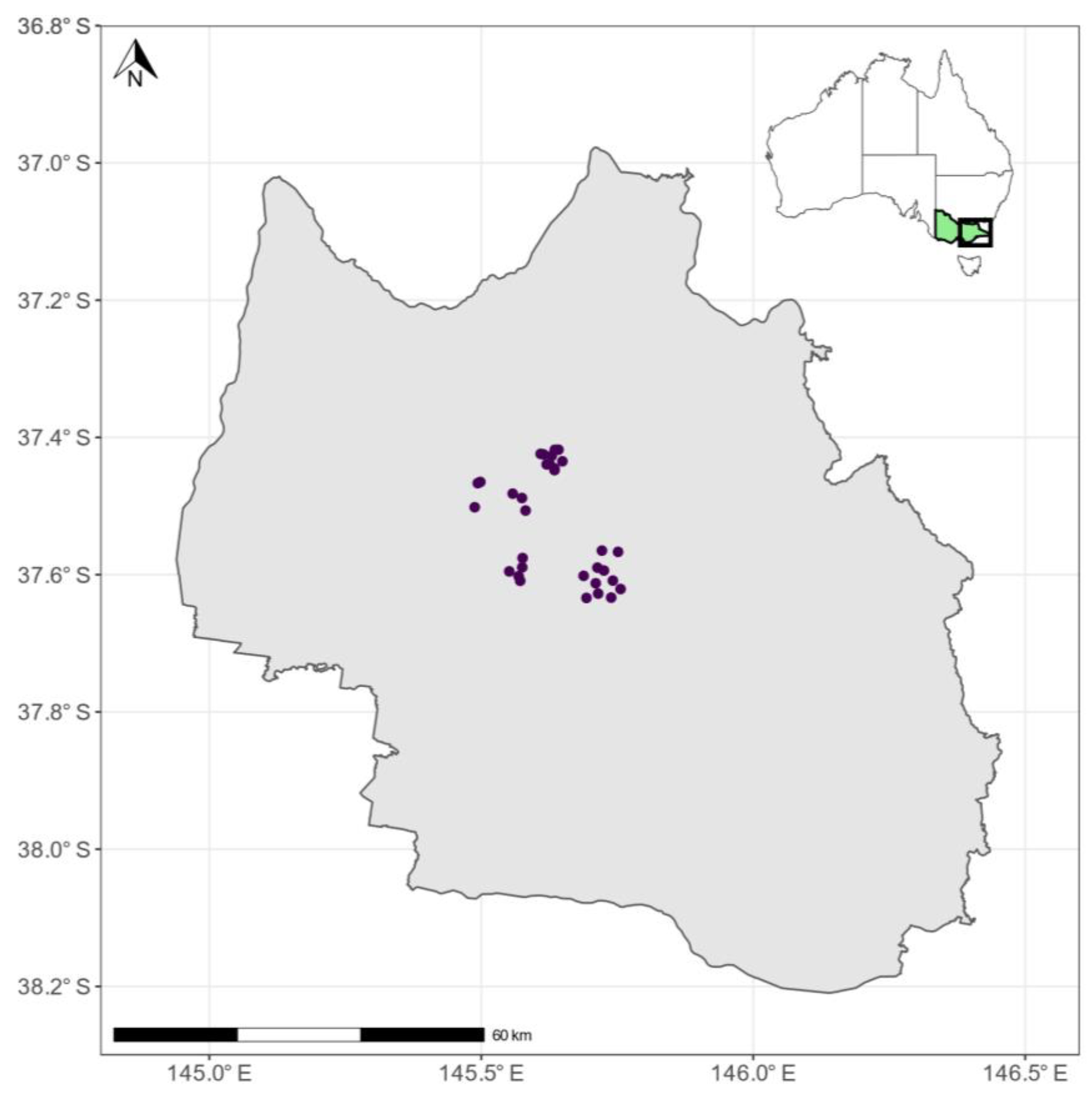
2.1. Study Area
2.2. Field Observation Data
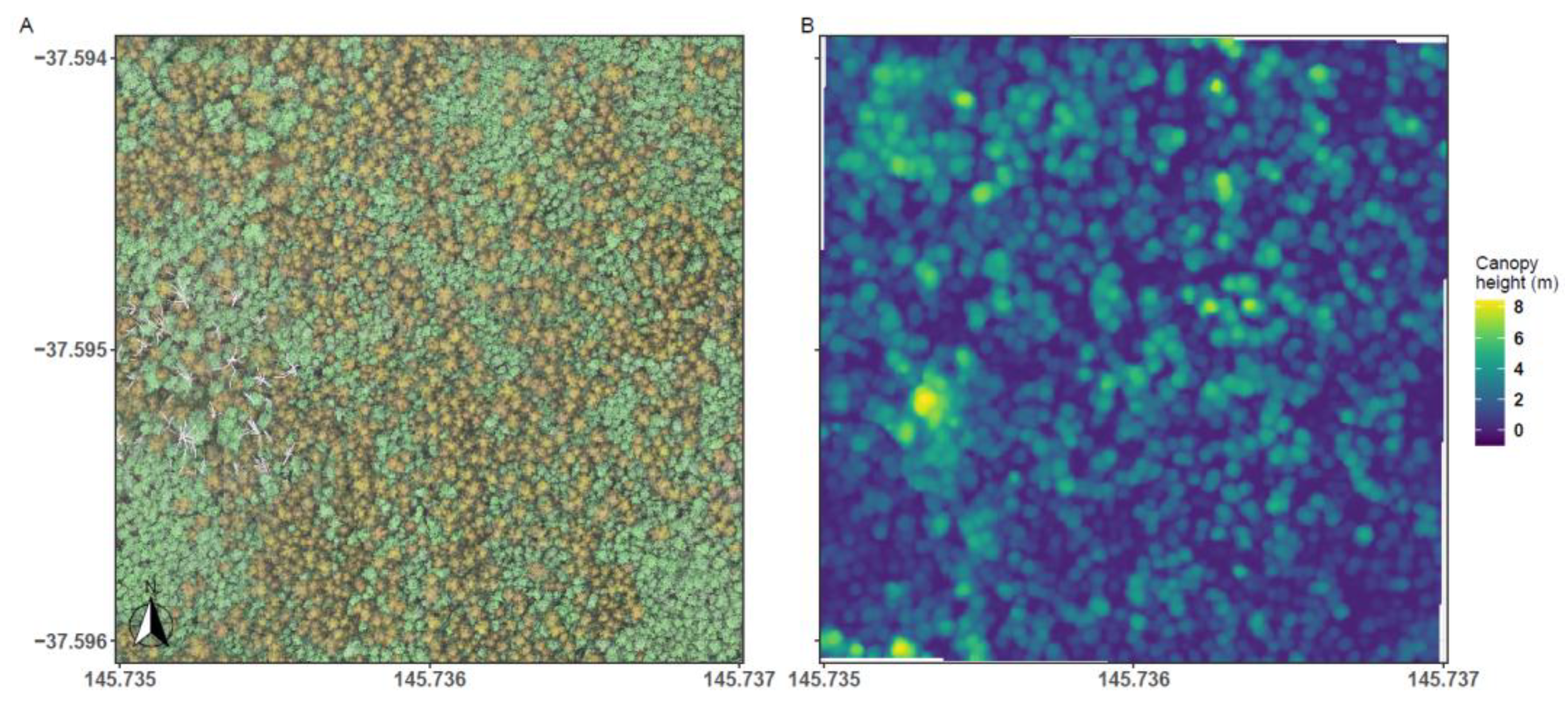
2.3. UAV Imagery
2.4. Imagery Processing
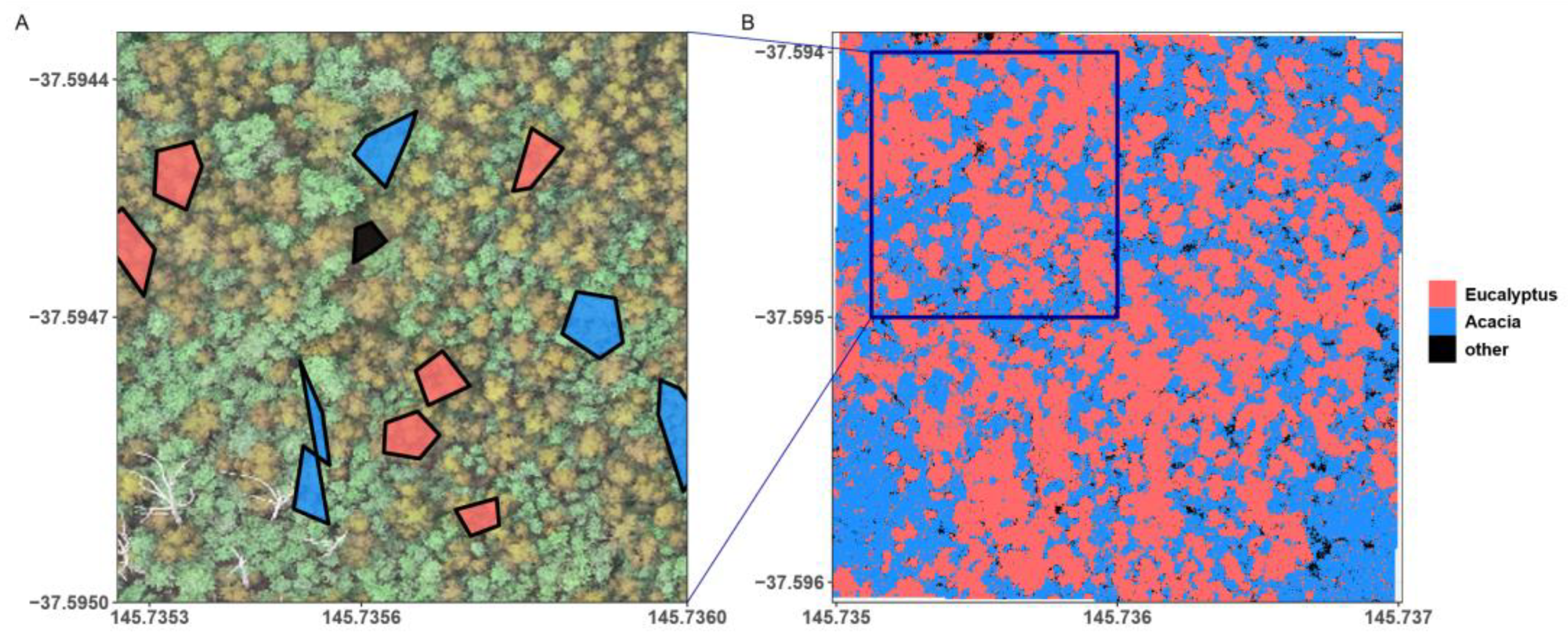
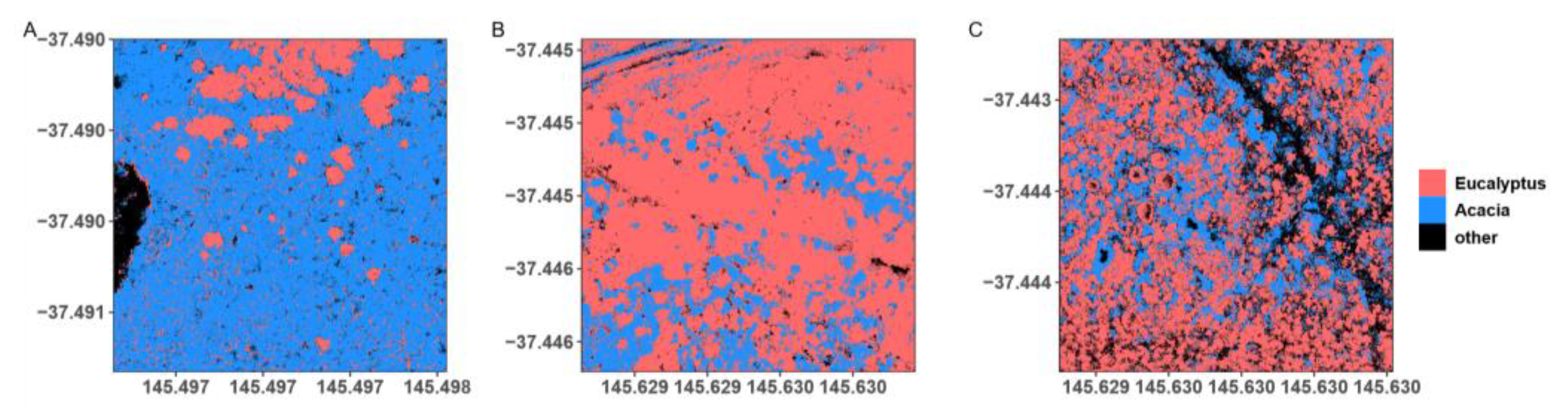
2.5. Forest Cover Classification and Spatial Metrics
2.6. Relationship between Field and Imagery Data
3. Results
3.1. Observed Species Diversity
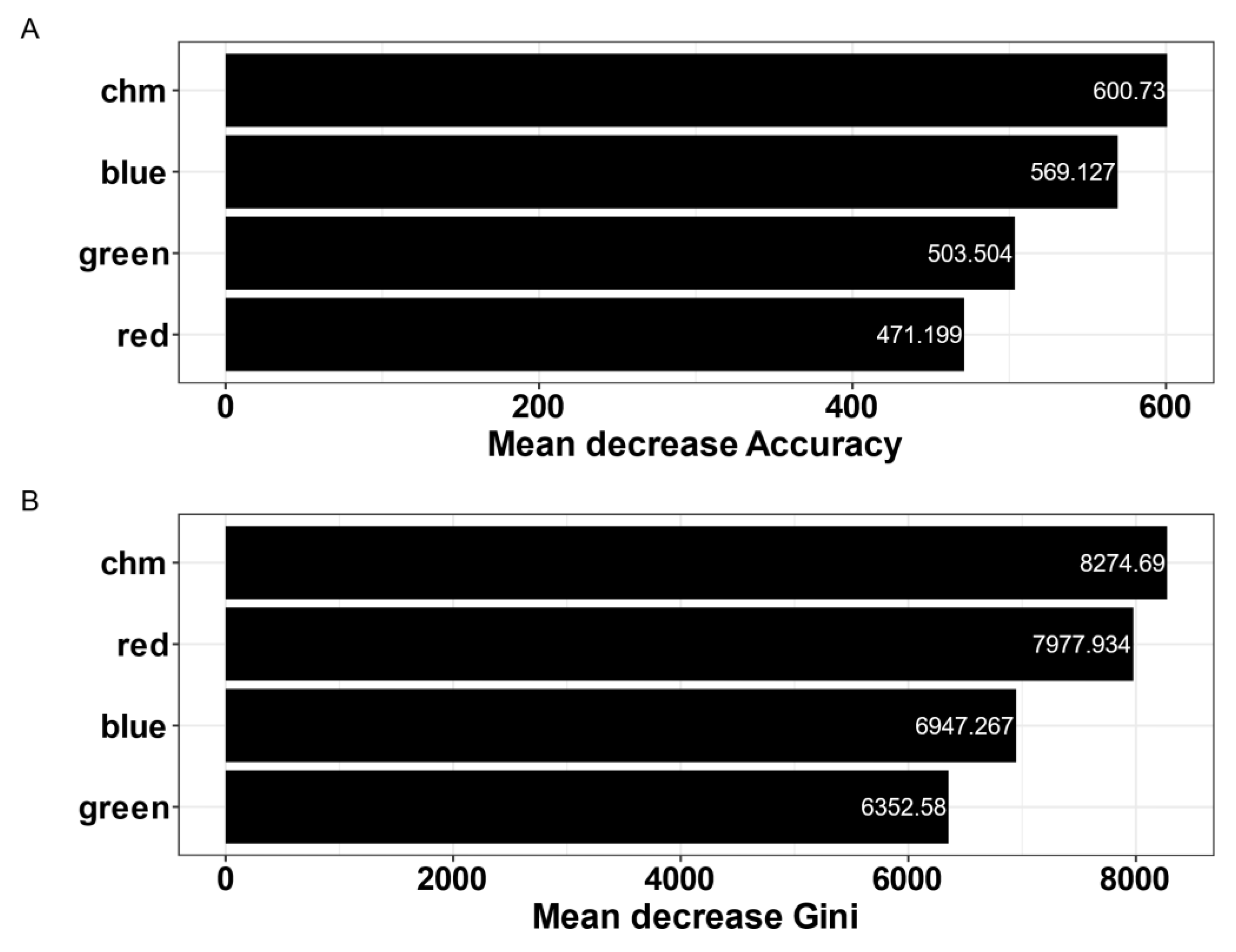
3.2. Supervised Classification: Variable Importance and Model Performance
3.3. Spatial Configuration and Arrangement
3.4. Influence of Remotely Sensed Forest Structure on Beta Diversity
4. Discussion
4.1. Spatial Aggregation of Acacia
4.2. Implications for Forest Management and Future Directions
4.3. Limitations, Implications, and Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bowman, D.M.; Kolden, C.A.; Abatzoglou, J.T.; Johnston, F.H.; van der Werf, G.R.; Flannigan, M. Vegetation fires in the Anthropocene. Nat. Rev. Earth Environ. 2020, 1, 500–515. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Allen, C.D.; Franklin, J.F.; Frelich, L.E.; Harvey, B.J.; Higuera, P.E.; Mack, M.C.; Meentemeyer, R.K.; Metz, M.R.; Perry, G.L. Changing disturbance regimes, ecological memory, and forest resilience. Front. Ecol. Environ. 2016, 14, 369–378. [Google Scholar] [CrossRef]
- Bratton, S.P. Resource division in an understory herb community: Responses to temporal and microtopographic gradients. Am. Nat. 1976, 110, 679–693. [Google Scholar] [CrossRef]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of tree species on understory vegetation diversity and mechanisms involved—A critical review for temperate and boreal forests. For. Ecol. Manag. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Cole, D.; Rapp, M. Elemental cycling in forest ecosystems. Dyn. Prop. For. Ecosyst. 1981, 23, 341–409. [Google Scholar]
- Wilson, S.D.; Tilman, D. Plant competition and resource availability in response to disturbance and fertilization. Ecology 1993, 74, 599–611. [Google Scholar] [CrossRef]
- Eilts, J.A.; Mittelbach, G.G.; Reynolds, H.L.; Gross, K.L. Resource heterogeneity, soil fertility, and species diversity: Effects of clonal species on plant communities. Am. Nat. 2011, 177, 574–588. [Google Scholar] [CrossRef]
- Chen, H.Y.; Légaré, S.; Bergeron, Y. Variation of the understory composition and diversity along a gradient of productivity in Populus tremuloides stands of northern British Columbia, Canada. Can. J. Bot. 2004, 82, 1314–1323. [Google Scholar] [CrossRef]
- Bartemucci, P.; Messier, C.; Canham, C.D. Overstory influences on light attenuation patterns and understory plant community diversity and composition in southern boreal forests of Quebec. Can. J. For. Res. 2006, 36, 2065–2079. [Google Scholar] [CrossRef]
- Bartels, S.F.; Chen, H.Y. Interactions between overstorey and understorey vegetation along an overstorey compositional gradient. J. Veg. Sci. 2013, 24, 543–552. [Google Scholar] [CrossRef]
- Bartels, S.F.; Chen, H.Y. Is understory plant species diversity driven by resource quantity or resource heterogeneity? Ecology 2010, 91, 1931–1938. [Google Scholar] [CrossRef]
- Getzin, S.; Wiegand, K.; Schöning, I. Assessing biodiversity in forests using very high-resolution images and unmanned aerial vehicles. Methods Ecol. Evol. 2012, 3, 397–404. [Google Scholar] [CrossRef]
- Seidl, R.; Rammer, W.; Spies, T.A. Disturbance legacies increase the resilience of forest ecosystem structure, composition, and functioning. Ecol. Appl. 2014, 24, 2063–2077. [Google Scholar] [CrossRef]
- Ashton, D. The development of even-aged stands of Eucalyptus regnans F. Muell. in central Victoria. Aust. J. Bot. 1976, 24, 397–414. [Google Scholar] [CrossRef]
- Ashton, D.; Martin, D.G. Regeneration in a pole-stage forest of Eucalyptus regnans subjected to different fire intensities in 1982. Aust. J. Bot. 1996, 44, 393–410. [Google Scholar] [CrossRef]
- Singh, A. Environmental Filtering Shapes Plant Turnover and Species Occurrence in Post-Logging Regrowth Forest in Southeastern Australia; The University of Melbourne: Melbourne, Australia, 2021. [Google Scholar]
- Blair, D.P.; McBurney, L.M.; Blanchard, W.; Banks, S.C.; Lindenmayer, D.B. Disturbance gradient shows logging affects plant functional groups more than fire. Ecol. Appl. 2016, 26, 2280–2301. [Google Scholar] [CrossRef]
- Bowd, E.J.; Lindenmayer, D.B.; Banks, S.C.; Blair, D.P. Logging and fire regimes alter plant communities. Ecol. Appl. 2018, 28, 826–841. [Google Scholar] [CrossRef]
- Trouvé, R.; Nitschke, C.R.; Andrieux, L.; Willersdorf, T.; Robinson, A.P.; Baker, P.J. Competition drives the decline of a dominant midstorey tree species. Habitat implications for an endangered marsupial. For. Ecol. Manag. 2019, 447, 26–34. [Google Scholar] [CrossRef]
- Kasel, S.; Bennett, L.T.; Aponte, C.; Fedrigo, M.; Nitschke, C.R. Environmental heterogeneity promotes floristic turnover in temperate forests of south-eastern Australia more than dispersal limitation and disturbance. Landsc. Ecol. 2017, 32, 1613–1629. [Google Scholar] [CrossRef]
- Jones, H.G.; Vaughan, R.A. Remote Sensing of Vegetation: Principles, Techniques, and Applications; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Anderson, K.; Gaston, K.J. Lightweight unmanned aerial vehicles will revolutionize spatial ecology. Front. Ecol. Environ. 2013, 11, 138–146. [Google Scholar] [CrossRef]
- Heaphy, M.; Watt, M.S.; Dash, J.P.; Pearse, G.D. UAVs for data collection-plugging the gap. N. Z. J. For. 2017, 62, 23–30. [Google Scholar]
- Jayathunga, S.; Owari, T.; Tsuyuki, S.; Hirata, Y. Potential of UAV photogrammetry for characterization of forest canopy structure in uneven-aged mixed conifer-broadleaf forests. Int. J. Remote Sens. 2020, 41, 53–73. [Google Scholar] [CrossRef]
- Swinfield, T.; Lindsell, J.A.; Williams, J.V.; Harrison, R.D.; Agustiono; Habibi; Gemita, E.; Schönlieb, C.B.; Coomes, D.A. Accurate Measurement of Tropical Forest Canopy Heights and Aboveground Carbon Using Structure From Motion. Remote Sens. 2019, 11, 928. [Google Scholar] [CrossRef]
- Nevalainen, O.; Honkavaara, E.; Tuominen, S.; Viljanen, N.; Hakala, T.; Yu, X.; Hyyppä, J.; Saari, H.; Pölönen, I.; Imai, N.; et al. Individual Tree Detection and Classification with UAV-Based Photogrammetric Point Clouds and Hyperspectral Imaging. Remote Sens. 2017, 9, 185. [Google Scholar] [CrossRef]
- Xie, Y.; Sha, Z.; Yu, M. Remote sensing imagery in vegetation mapping: A review. J. Plant Ecol. 2008, 1, 9–23. [Google Scholar] [CrossRef]
- Dash, J.P.; Watt, M.S.; Pearse, G.D.; Heaphy, M.; Dungey, H.S. Assessing very high resolution UAV imagery for monitoring forest health during a simulated disease outbreak. ISPRS J. Photogramm. Remote Sens. 2017, 131, 1–14. [Google Scholar] [CrossRef]
- Mohan, M.; Silva, C.A.; Klauberg, C.; Jat, P.; Catts, G.; Cardil, A.; Hudak, A.T.; Dia, M. Individual tree detection from unmanned aerial vehicle (UAV) derived canopy height model in an open canopy mixed conifer forest. Forests 2017, 8, 340. [Google Scholar] [CrossRef]
- Ma, L.; Li, M.; Ma, X.; Cheng, L.; Du, P.; Liu, Y. A review of supervised object-based land-cover image classification. ISPRS J. Photogramm. Remote Sens. 2017, 130, 277–293. [Google Scholar] [CrossRef]
- He, H.S.; DeZonia, B.E.; Mladenoff, D.J. An aggregation index (AI) to quantify spatial patterns of landscapes. Landsc. Ecol. 2000, 15, 591–601. [Google Scholar] [CrossRef]
- Wagner, B.; Baker, P.J.; Nitschke, C.R. The influence of spatial patterns in foraging habitat on the abundance and home range size of a vulnerable arboreal marsupial in southeast Australia. Conserv. Sci. Pract. 2021, 3, e566. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Hesselbarth, M.H.K.; Sciaini, M.; With, K.A.; Wiegand, K.; Nowosad, J. landscapemetrics: An open-source R tool to calculate landscape metrics. Ecography 2019, 42, 1648–1657. [Google Scholar] [CrossRef]
- Araujo, R.F.; Chambers, J.Q.; Celes, C.H.S.; Muller-Landau, H.C.; Santos, A.; Emmert, F.; Ribeiro, G.; Gimenez, B.O.; Lima, A.J.N.; Campos, M.A.A.; et al. Integrating high resolution drone imagery and forest inventory to distinguish canopy and understory trees and quantify their contributions to forest structure and dynamics. PLoS ONE 2020, 15, e0243079. [Google Scholar] [CrossRef]
- Egerer, M.; Wagner, B.; Lin, B.B.; Kendal, D.; Zhu, K. New methods of spatial analysis in urban gardens inform future vegetation surveying. Landsc. Ecol. 2020, 35, 761–778. [Google Scholar] [CrossRef]
- Feng, Q.L.; Liu, J.T.; Gong, J.H. UAV Remote Sensing for Urban Vegetation Mapping Using Random Forest and Texture Analysis. Remote Sens. 2015, 7, 1074–1094. [Google Scholar] [CrossRef]
- Wagner, B.; Baker, P.J.; Moore, B.D.; Nitschke, C.R. Mapping canopy nitrogen-scapes to assess foraging habitat for a vulnerable arboreal folivore in mixed-species Eucalyptus forests. Ecol. Evol. 2021, 11, 18401–18421. [Google Scholar] [CrossRef]
- Olsoy, P.J.; Forbey, J.S.; Shipley, L.A.; Rachlow, J.L.; Robb, B.C.; Nobler, J.D.; Thornton, D.H. Mapping foodscapes and sagebrush morphotypes with unmanned aerial systems for multiple herbivores. Landsc. Ecol. 2020, 35, 921–936. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E.; Knapp, D.E.; Tupayachi, R.; Anderson, C.B.; Sinca, F.; Vaughn, N.R.; Llactayo, W. Airborne laser-guided imaging spectroscopy to map forest trait diversity and guide conservation. Science 2017, 355, 385–389. [Google Scholar] [CrossRef]
- Baldeck, C.A.; Asner, G.P.; Martin, R.E.; Anderson, C.B.; Knapp, D.E.; Kellner, J.R.; Wright, S.J. Operational Tree Species Mapping in a Diverse Tropical Forest with Airborne Imaging Spectroscopy. PLoS ONE 2015, 10, e0118403. [Google Scholar] [CrossRef]
- Attiwill, P.M. The disturbance of forest ecosystems: The ecological basis for conservative management. For. Ecol. Manag. 1994, 63, 247–300. [Google Scholar] [CrossRef]
- Kasel, S.; Nitschke, C.R.; Baker, S.C.; Pryde, E.C. Concurrent assessment of functional types in extant vegetation and soil seed banks informs environmental constraints and mechanisms of plant community turnover in temperate forests of south-eastern Australia. For. Ecol. Manag. 2022, 519, 120321. [Google Scholar] [CrossRef]
- Vickers, H.; Kasel, S.; Duff, T.; Nitschke, C. Recruitment and growth dynamics of a temperate forest understorey species following wildfire in southeast Australia. Dendrochronologia 2021, 67, 125829. [Google Scholar] [CrossRef]
- Stewart, S.B.; Fedrigo, M.; Roxburgh, S.H.; Kasel, S.; Nitschke, C. Climate Victoria: Precipitation (9 Second, Approx. 250 m); v1; CSIRO, 1 ed.; The University of Melbourne: Melbourne, Australia, 2020. [Google Scholar] [CrossRef]
- Stewart, S.B.; Nitschke, C.R. Improving temperature interpolation using MODIS LST and local topography: A comparison of methods in south east Australia. Int. J. Climatol. 2017, 37, 3098–3110. [Google Scholar] [CrossRef]
- Lutze, M.T.; Campbell, R.G.; Fagg, P.C. Development of silviculture in the native State forests of Victoria. Aust. For. 1999, 62, 236–244. [Google Scholar] [CrossRef]
- Anderson, M.J.; Crist, T.O.; Chase, J.M.; Vellend, M.; Inouye, B.D.; Freestone, A.L.; Sanders, N.J.; Cornell, H.V.; Comita, L.S.; Davies, K.F.; et al. Navigating the multiple meanings of β diversity: A roadmap for the practicing ecologist. Ecol. Lett. 2011, 14, 19–28. [Google Scholar] [CrossRef]
- Socolar, J.B.; Gilroy, J.J.; Kunin, W.E.; Edwards, D.P. How Should Beta-Diversity Inform Biodiversity Conservation? Trends Ecol. Evol. 2016, 31, 67–80. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, R Package Version 2.2-0; R Foundation for Statistical Computing: Vienna, Austria, 2020.
- CASA. Flying in Public Spaces. Available online: https://www.casa.gov.au/drones/rules/public-spaces (accessed on 10 January 2020).
- Dandois, J.P.; Olano, M.; Ellis, E.C. Optimal altitude, overlap, and weather conditions for computer vision UAV estimates of forest structure. Remote Sens. 2015, 7, 13895–13920. [Google Scholar] [CrossRef]
- Roussel, J.-R.; Auty, D. lidR: Airborne LiDAR Data Manipulation and Visualization for Forestry Applications; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Pebesma, E. Simple features for R: Standardized support for spatial vector data. R J. 2018, 10, 439–446. [Google Scholar] [CrossRef]
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling, 3.6-14; R Foundation for Statistical Computing: Vienna, Austria, 2019.
- R Core Development Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Khosravipour, A.; Skidmore, A.K.; Isenburg, M.; Wang, T.J.; Hussin, Y.A. Generating Pit-free Canopy Height Models from Airborne Lidar. Photogramm. Eng. Remote Sens. 2014, 80, 863–872. [Google Scholar] [CrossRef]
- Wing, J.; Kuhn, M.; Weston, S.; Williams, A.; Keefer, C.; Engelhardt, A.; Cooper, T.; Mayer, Z.; Kenkel, B.; R Core Development Team; et al. Caret: Classification and Regression Training, 6.0-93; R Foundation for Statistical Computing: Vienna, Austria, 2019.
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Gislason, P.O.; Benediktsson, J.A.; Sveinsson, J.R. Random forests for land cover classification. Pattern Recognit. Lett. 2006, 27, 294–300. [Google Scholar] [CrossRef]
- Pal, M. Random forest classifier for remote sensing classification. Int. J. Remote Sens. 2005, 26, 217–222. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Wang, T.; Hamann, A.; Yanchuk, A.; O’Neill, G.A.; Aitken, S.N. Use of response functions in selecting lodgepole pine populations for future climates. Glob. Change Biol. 2006, 12, 2404–2416. [Google Scholar] [CrossRef]
- Paudel, S.K.; Waeber, P.O.; Simard, S.W.; Innes, J.L.; Nitschke, C.R. Multiple factors influence plant richness and diversity in the cold and dry boreal forest of southwest Yukon, Canada. Plant Ecol. 2016, 217, 505–519. [Google Scholar] [CrossRef]
- Stewart, S.B.; Nitschke, C. Climate Victoria: Maximum Temperature (3DS; 9 Second, Approx. 250 m); v2; CSIRO; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar] [CrossRef]
- Stewart, S.B.; Nitschke, C. Climate Victoria: Minimum Temperature (3DS-TM; 9 Second, Approx. 250 m); v2; CSIRO; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar] [CrossRef]
- Fedrigo, M.; Stewart, S.B.; Roxburgh, S.H.; Kasel, S.; Bennett, L.T.; Vickers, H.; Nitschke, C.R. Predictive Ecosystem Mapping of South-Eastern Australian Temperate Forests Using Lidar-Derived Structural Profiles and Species Distribution Models. Remote Sens. 2019, 11, 93. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference, R package version 1.0.0; R Foundation for Statistical Computing: Vienna, Austria, 2009. Available online: http://r-forge.r-project.org/projects/mumin/2009 (accessed on 23 November 2019).
- Singh, A.; Baker, P.J.; Kasel, S.; Trouvé, R.; Stewart, S.B.; Nitschke, C.R. The role of climatic variability on Eucalyptus regeneration in southeastern Australia. Glob. Ecol. Conserv. 2021, 32, e01929. [Google Scholar] [CrossRef]
- Trouvé, R.; Sherriff, R.M.; Holt, L.M.; Baker, P.J. Differing regeneration patterns after catastrophic fire and clearfelling: Implications for future stand dynamics and forest management. For. Ecol. Manag. 2021, 498, 119555. [Google Scholar] [CrossRef]
- Bowman, D.M.; Murphy, B.P.; Neyland, D.L.; Williamson, G.J.; Prior, L.D. Abrupt fire regime change may cause landscape-wide loss of mature obligate seeder forests. Glob. Change Biol. 2014, 20, 1008–1015. [Google Scholar] [CrossRef]
- Doherty, M.D.; Lavorel, S.; Colloff, M.J.; Williams, K.J.; Williams, R.J. Moving from autonomous to planned adaptation in the montane forests of southeastern Australia under changing fire regimes. Austral Ecol. 2017, 42, 309–316. [Google Scholar] [CrossRef]
- Lutze, M.; Ades, P.; Campbell, R. Review of measures of site occupancy by regeneration. Aust. For. 2004, 67, 164–171. [Google Scholar] [CrossRef]
- May, B. Silver Wattle (Acacia dealbata): Its Role in the Ecology of the Mountain Ash Forest and the Effect of Alternative Silvicultural Systems on Its Regeneration. Ph.D. Thesis, Department of Botany, The University of Melbourne, Melbourne, Australia, 1999. [Google Scholar]
- Bowd, E.J.; McBurney, L.; Blair, D.P.; Lindenmayer, D.B. Temporal patterns of forest seedling emergence across different disturbance histories. Ecol. Evol. 2021, 11, 9254–9292. [Google Scholar] [CrossRef] [PubMed]
- Bauhus, J.; Van Winden, A.P.; Nicotra, A.B. Aboveground interactions and productivity in mixed-species plantations of Acacia mearnsii and Eucalyptus globulus. Can. J. For. Res. 2004, 34, 686–694. [Google Scholar] [CrossRef]
- Bowd, E.J.; McBurney, L.; Lindenmayer, D.B. The characteristics of regeneration failure and their potential to shift wet temperate forests into alternate stable states. For. Ecol. Manag. 2023, 529, 120673. [Google Scholar] [CrossRef]
- DAWE. Victorian Regional Forest Agreements Major Event Review of the 2019–20 Bushfires; Australian Government—Department of Agriculture, Water and the Environment: Melbourne, Australia, 2021.
- Goodbody, T.R.; Coops, N.C.; Marshall, P.L.; Tompalski, P.; Crawford, P. Unmanned aerial systems for precision forest inventory purposes: A review and case study. For. Chron. 2017, 93, 71–81. [Google Scholar] [CrossRef]
- Adão, T.; Hruška, J.; Pádua, L.; Bessa, J.; Peres, E.; Morais, R.; Sousa, J.J. Hyperspectral imaging: A review on UAV-based sensors, data processing and applications for agriculture and forestry. Remote Sens. 2017, 9, 1110. [Google Scholar] [CrossRef]
- Krůček, M.; Král, K.; Cushman, K.C.; Missarov, A.; Kellner, J.R. Supervised Segmentation of Ultra-High-Density Drone Lidar for Large-Area Mapping of Individual Trees. Remote Sens. 2020, 12, 3260. [Google Scholar] [CrossRef]
- Popescu, S.C.; Wynne, R.H. Seeing the trees in the forest: Using lidar and multispectral data fusion with local filtering and variable window size for estimating tree height. Photogramm. Eng. Remote Sens. 2004, 70, 589–604. [Google Scholar] [CrossRef]
- Hakkenberg, C.R.; Peet, R.K.; Urban, D.L.; Song, C. Modeling plant composition as community-continua in a forest landscape with LiDAR and hyperspectral remote sensing. Ecol. Appl. 2017, 28, 177–190. [Google Scholar] [CrossRef]
- Bunting, P.; Lucas, R. The delineation of tree crowns in Australian mixed species forests using hyperspectral Compact Airborne Spectrographic Imager (CASI) data. Remote Sens Environ. 2006, 101, 230–248. [Google Scholar] [CrossRef]
- Baker, P.J.; Nitschke, C.R.; Trouvé, R.; Robinson, A.P. Forest Stand Dynamics Drive a Conservation Conundrum for the Critically Endangered Leadbeater’s Possum. In Forests as Complex Social and Ecological Systems: A Festschrift for Chadwick D. Oliver; Baker, P.J., Larsen, D.R., Saxena, A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 93–113. [Google Scholar]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics: Updated Edition; John Wiley and Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
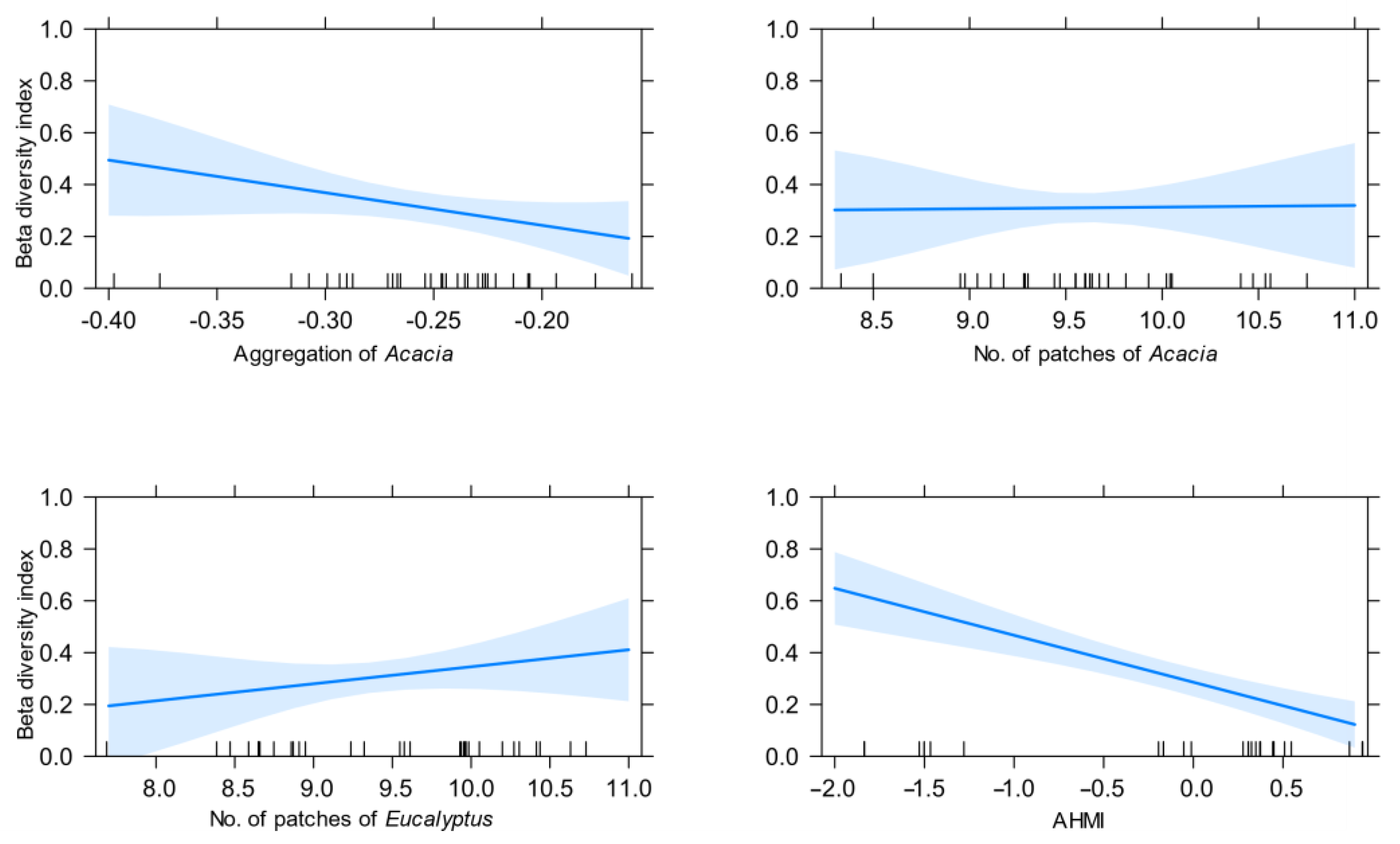
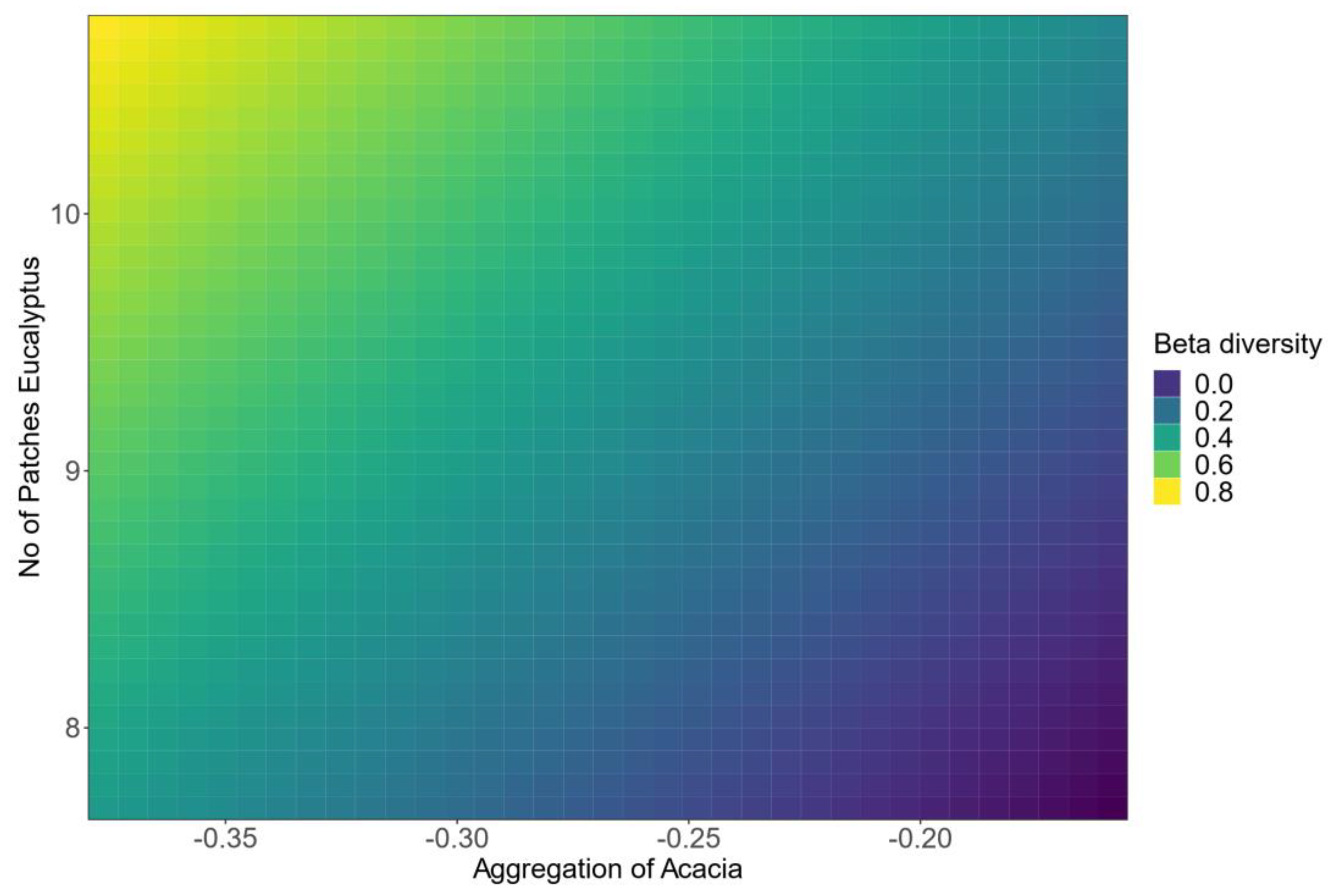
| Coefficients | Estimate ± SE | Significance Level (p-Value) |
|---|---|---|
| Intercept | −0.48 ± 0.48 | <0.1 |
| Aggregation of Acacia | −2.34 ± 0.46 | <0.00001 |
| Number of Acacia patches | −0.12 ± 0.07 | >0.05 |
| Number of Eucalyptus patches | 0.14 ± 0.06 | ≤0.05 |
| AHMI | −0.19 ± 0.03 | <0.00001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Wagner, B.; Kasel, S.; Baker, P.J.; Nitschke, C.R. Canopy Composition and Spatial Configuration Influences Beta Diversity in Temperate Regrowth Forests of Southeastern Australia. Drones 2023, 7, 155. https://doi.org/10.3390/drones7030155
Singh A, Wagner B, Kasel S, Baker PJ, Nitschke CR. Canopy Composition and Spatial Configuration Influences Beta Diversity in Temperate Regrowth Forests of Southeastern Australia. Drones. 2023; 7(3):155. https://doi.org/10.3390/drones7030155
Chicago/Turabian StyleSingh, Anu, Benjamin Wagner, Sabine Kasel, Patrick J. Baker, and Craig R. Nitschke. 2023. "Canopy Composition and Spatial Configuration Influences Beta Diversity in Temperate Regrowth Forests of Southeastern Australia" Drones 7, no. 3: 155. https://doi.org/10.3390/drones7030155
APA StyleSingh, A., Wagner, B., Kasel, S., Baker, P. J., & Nitschke, C. R. (2023). Canopy Composition and Spatial Configuration Influences Beta Diversity in Temperate Regrowth Forests of Southeastern Australia. Drones, 7(3), 155. https://doi.org/10.3390/drones7030155








