Unmanned Aerial Vehicles (UAVs) in Marine Mammal Research: A Review of Current Applications and Challenges
Abstract
:1. Introduction
2. Methodology
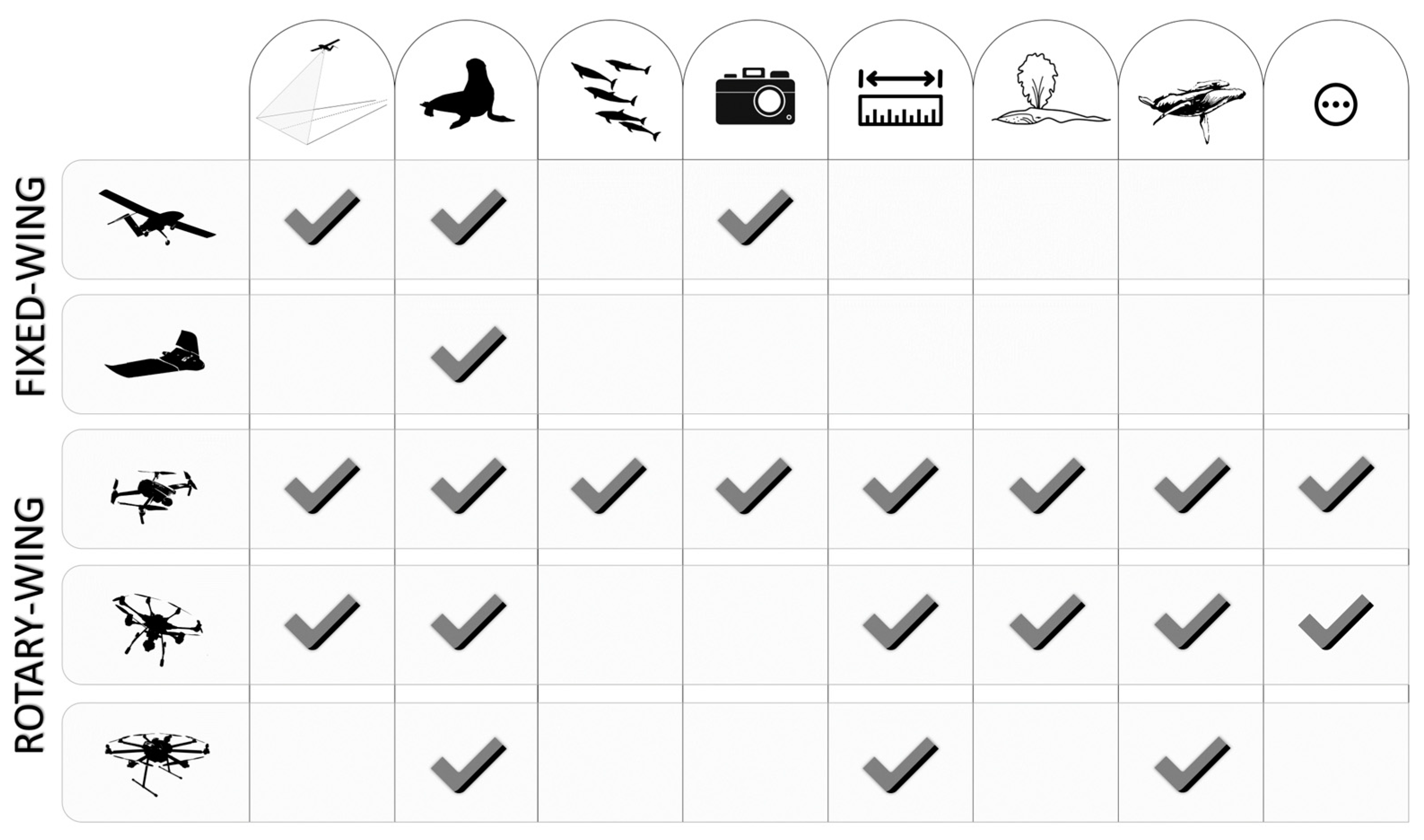
3. Main Uses of UAVs for the Study of Marine Mammals
3.1. Abundance and Distribution Monitoring
3.1.1. Line-Transect Surveys
3.1.2. Pinniped Aggregation Census
3.1.3. Group Size
3.2. Photo-ID
3.3. Photogrammetry
3.4. Blow Sample Collection
3.5. Behavioural Studies
3.6. Other Approaches
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Brooke, S.; Graham, D.; Jacobs, T.; Littnan, C.; Manuel, M.; O’Conner, R. Testing marine conservation applications of unmanned aerial systems (UAS) in a remote marine protected area. J. Unmanned Veh. Sys. 2015, 3, 237–251. [Google Scholar] [CrossRef]
- Koski, W.R.; Gamage, G.; Davis, A.R.; Mathews, T.; LeBlanc, B.; Ferguson, S.H. Evaluation of UAS for photographic re-identification of bowhead whales, Balaena mysticetus. J. Unmanned Veh. Syst. 2015, 3, 22–29. [Google Scholar] [CrossRef]
- Zmarz, A.; Rodzewicz, M.; Dąbski, M.; Karsznia, I.; Korczak-Abshire, M.; Chwedorzewska, K.J. Application of UAV BVLOS remote sensing data for multi-faceted analysis of Antarctic ecosystem. Remote Sens. Environ. 2018, 217, 375–388. [Google Scholar] [CrossRef]
- Korczak-Abshire, M.; Zmarz, A.; Rodzewicz, M.; Kycko, M.; Karsznia, I.; Chwedorzewska, K.J. Study of fauna population changes on Penguin Island and Turret Point Oasis (King George Island, Antarctica) using an unmanned aerial vehicle. Polar Biol. 2019, 42, 217–224. [Google Scholar] [CrossRef]
- Ferguson, M.C.; Angliss, R.P.; Hall, P.; Helker, V.; Kennedy, A.; Sformo, T. Comparing manned to unmanned aerial surveys for cetacean monitoring in the arctic: Methods and operational results. J. Unmanned Veh. Syst. 2018, 6, 109–127. [Google Scholar] [CrossRef]
- Angliss, R.P.; Ferguson, M.C.; Kennedy, A.; Lynch, B.; Willoughby, A.; Helker, V.; Brower, A.A.; Clarke, J.T. Performance of manned and unmanned aerial surveys to collect visual data and imagery for estimating Arctic cetacean density and associated uncertainty. J. Unmanned Veh. Syst. 2018, 6, 128–154. [Google Scholar] [CrossRef]
- Hodgson, A.; Peel, D.; Kelly, N. Unmanned aerial vehicles for surveying marine fauna: Assessing detection probability. Ecol. Appl. 2017, 27, 1253–1267. [Google Scholar] [CrossRef]
- Hodgson, A.; Kelly, N.; Peel, D. Unmanned aerial vehicles (UAVs) for surveying marine fauna: A dugong case study. PLoS ONE 2013, 8, e79556. [Google Scholar] [CrossRef]
- Moreland, E.E.; Cameron, M.F.; Angliss, R.P.; Boveng, P.L. Evaluation of a ship-based unoccupied aircraft system (UAS) for surveys of spotted and ribbon seals in the Bering Sea pack ice. J. Unmanned Veh. Syst. 2015, 3, 114–122. [Google Scholar] [CrossRef]
- Koski, W.R.; Allen, T.; Ireland, D.; Buck, G.; Smith, P.R.; Macrender, A.M.; Halick, M.A.; Rushing, C.; Sliwa, D.J.; McDonald, T.L. Evaluation of an unmanned airborne system for monitoring marine mammals. Aquat. Mamm. 2009, 35, 347–357. [Google Scholar] [CrossRef]
- Aniceto, A.S.; Biuw, M.; Lindstrøm, U.; Solbø, S.A.; Broms, F.; Carroll, J. Monitoring marine mammals using unmanned aerial vehicles: Quantifying detection certainty. Ecosphere 2018, 9, e02122. [Google Scholar] [CrossRef]
- Barnas, A.F.; Felege, C.J.; Rockwell, R.F.; Ellis-Felege, S.N. A pilot(less) study on the use of an unmanned aircraft system for studying polar bears (Ursus maritimus). Polar Biol. 2018, 41, 1055–1062. [Google Scholar] [CrossRef]
- Sweeney, K.L.; Helker, V.T.; Perryman, W.L.; LeRoi, D.J.; Fritz, L.W.; Gelatt, T.S.; Angliss, R.P. Flying beneath the clouds at the edge of the world: Using a hexacopter to supplement abundance surveys of Steller sea lions (Eumetopias jubatus) in Alaska. J. Unmanned Veh. Syst. 2015, 4, 70–81. [Google Scholar] [CrossRef]
- Johnston, D.W.; Dale, J.; Murray, K.T.; Josephson, E.; Newton, E.; Wood, S. Comparing occupied and unoccupied aircraft surveys of wildlife populations: Assessing the gray seal (Halichoerus grypus) breeding colony on Muskeget Island, USA. J. Unmanned Veh. Syst. 2017, 5, 178–191. [Google Scholar] [CrossRef]
- Seymour, A.C.; Dale, J.; Hammill, M.; Halpin, P.N.; Johnston, D.W. Automated detection and enumeration of marine wildlife using unmanned aircraft systems (UAS) and thermal imagery. Sci. Rep. 2017, 7, 45127. [Google Scholar] [CrossRef]
- Arona, L.; Dale, J.; Heaslip, S.G.; Hammill, M.O.; Johnston, D.W. Assessing the disturbance potential of small unoccupied aircraft systems (UAS) on gray seals (Halichoerus grypus) at breeding colonies in Nova Scotia, Canada. PeerJ 2018, 6, e4467. [Google Scholar] [CrossRef]
- Larsen, G.D.; Seymour, A.C.; Richmond, E.L.; Divine, L.M.; Moreland, E.E.; Newton, E.; London, J.M.; Johnston, D.W. Drones reveal spatial patterning of sympatric Alaskan pinniped species and drivers of their local distributions. Drone Syst. Appl. 2022, 10, 235–255. [Google Scholar] [CrossRef]
- Oliveira-da-Costa, M.; Marmontel, M.; Da-Rosa, D.S.X.; Coelho, A.; Wich, S.; Mosquera-Guerra, F.; Trujillo, F. Effectiveness of unmanned aerial vehicles to detect Amazon dolphins. Oryx 2020, 54, 696–698. [Google Scholar] [CrossRef]
- Vergara, V.; Mikus, M.A. Contact call diversity in natural beluga entrapments in an Arctic estuary: Preliminary evidence of vocal signatures in wild belugas. Mar. Mamm. Sci. 2019, 35, 434–465. [Google Scholar] [CrossRef]
- Ryan, K.P.; Ferguson, S.H.; Koski, W.R.; Young, B.G.; Roth, J.D.; Watt, C.A. Use of drones for the creation and development of a photographic identification catalogue for an endangered whale population. Arct. Sci. 2022, 8, 1191–1201. [Google Scholar] [CrossRef]
- Dickson, L.C.D.; Negus, S.R.B.; Eizaguirre, C.; Katselidis, K.A.; Schofield, G. Aerial drone surveys reveal the efficacy of a protected area network for marine megafauna and the value of sea turtles as umbrella species. Drones 2022, 6, 291. [Google Scholar] [CrossRef]
- Landeo-Yauri, S.S.; Ramos, E.A.; Castelblanco-Martínez, D.N.; Niño-Torres, C.A.; Searle, L. Using small drones to photo-identify Antillean manatees: A novel method for monitoring an endangered marine mammal in the Caribbean Sea. Endang. Species Res. 2020, 41, 79–90. [Google Scholar] [CrossRef]
- Burnett, J.D.; Lemos, L.; Barlow, D.; Wing, M.G.; Chandler, T.; Torres, L.G. Estimating morphometric attributes of baleen whales with photogrammetry from small UASs: A case study with blue and gray whales. Mar. Mamm. Sci. 2019, 35, 108–139. [Google Scholar] [CrossRef]
- Gough, W.T.; Smith, H.J.; Savoca, M.S.; Czapanskiy, M.F.; Fish, F.E.; Potvin, J.; Bierlich, K.C.; Cade, D.E.; Di Clemente, J.; Kennedy, J.; et al. Scaling of oscillatory kinematics and Froude efficiency in baleen whales. J. Exp. Biol. 2021, 224, jeb237586. [Google Scholar] [CrossRef]
- Gough, W.T.; Segre, P.S.; Bierlich, K.C.; Cade, D.E.; Potvin, J.; Fish, F.E.; Dale, J.; Di Clemente, J.; Friedlaender, A.S.; Johnston, D.W.; et al. Scaling of swimming performance in baleen whales. J. Exp. Biol. 2019, 222, jeb204172. [Google Scholar] [CrossRef]
- Soledade Lemos, L.; Burnett, J.D.; Chandler, T.E.; Sumich, J.L.; Torres, L.G. Intra- and inter-annual variation in gray whale body condition on a foraging ground. Ecosphere 2020, 11, e03094. [Google Scholar] [CrossRef]
- Torres, L.G.; Bird, C.N.; Rodríguez-González, F.; Christiansen, F.; Bejder, L.; Lemos, L.; Urban, R.J.; Swartz, S.; Willoughby, A.; Hewitt, J.; et al. Range-wide comparison of gray whale body condition reveals contrasting sub-population health characteristics and vulnerability to environmental change. Front. Mar. Sci. 2022, 9, 867258. [Google Scholar] [CrossRef]
- Werth, A.J.; Kosma, M.M.; Chenoweth, E.M.; Straley, J.M. New views of humpback whale flow dynamics and oral morphology during prey engulfment. Mar. Mamm. Sci. 2019, 35, 1556–1578. [Google Scholar] [CrossRef]
- Nielsen, M.L.K.; Sprogis, K.R.; Bejder, L.; Madsen, P.T.; Christiansen, F. Behavioural development in southern right whale calves. Mar. Ecol. Prog. Ser. 2019, 629, 219–234. [Google Scholar] [CrossRef]
- Azizeh, T.R.; Sprogis, K.R.; Soley, R.; Nielsen, M.L.K.; Uhart, M.M.; Sironi, M.; Marón, C.F.; Bejder, L.; Madsen, P.T.; Christiansen, F. Acute and chronic behavioral effects of kelp gull micropredation on southern right whale mother-calf pairs off Península Valdés, Argentina. Mar. Ecol. Prog. Ser. 2021, 668, 133–148. [Google Scholar] [CrossRef]
- Torres, L.G.; Nieukirk, S.L.; Lemos, L.; Chandler, T.E. Drone up! Quantifying whale behavior from a new perspective improves observational capacity. Front. Mar. Sci. 2018, 5, 319. [Google Scholar] [CrossRef]
- Izadi, S.; Aguilar de Soto, N.; Constantine, R.; Johnson, M. Feeding tactics of resident Bryde’s whales in New Zealand. Mar. Mamm. Sci. 2022, 38, 1104–1117. [Google Scholar] [CrossRef]
- Jagielski, P.M.; Dey, C.J.; Gilchrist, H.G.; Richardson, E.S.; Semeniuk, C.A.D. Polar bear foraging on common eider eggs: Estimating the energetic consequences of a climate-mediated behavioural shift. Anim. Behav. 2021, 171, 63–75. [Google Scholar] [CrossRef]
- Jagielski, P.M.; Dey, C.J.; Gilchrist, H.G.; Richardson, E.S.; Love, O.P.; Semeniuk, C.A.D. Polar bears are inefficient predators of seabird eggs. R. Soc. Open Sci. 2021, 8, 210391. [Google Scholar] [CrossRef] [PubMed]
- Barnas, A.F.; Geldart, E.A.; Love, O.P.; Jagielski, P.M.; Harris, C.M.; Gilchrist, H.G.; Hennin, H.L.; Richardson, E.S.; Dey, C.J.; Semeniuk, C.A.D. Predatory cue use in flush responses of a colonial nesting seabird during polar bear foraging. Anim. Behav. 2022, 193, 75–90. [Google Scholar] [CrossRef]
- Wood, S.A.; Robinson, P.W.; Costa, D.P.; Beltran, R.S. Accuracy and precision of citizen scientist animal counts from drone imagery. PLoS ONE 2021, 16, e0244040. [Google Scholar] [CrossRef]
- Adame, K.; Pardo, M.A.; Salvadeo, C.; Beier, E.; Elorriaga-Verplancken, F.R. Detectability and categorization of California sea lions using an unmanned aerial vehicle. Mar. Mamm. Sci. 2017, 33, 913–925. [Google Scholar] [CrossRef]
- Colefax, A.P.; Butcher, P.A.; Pagendam, D.E.; Kelaher, B.P. Reliability of marine faunal detections in drone-based monitoring. Ocean Coast Manag. 2019, 174, 108–115. [Google Scholar] [CrossRef]
- Kelaher, B.P.; Colefax, A.P.; Tagliafico, A.; Bishop, M.J.; Giles, A.; Butcher, P.A. Assessing variation in assemblages of large marine fauna off ocean beaches using drones. Mar. Freshw. Res. 2019, 71, 68–77. [Google Scholar] [CrossRef]
- McIntosh, R.R.; Holmberg, R.; Dann, P. Looking without landing-using remote piloted aircraft to monitor fur seal populations without disturbance. Front. Mar. Sci. 2018, 5, 1–13. [Google Scholar] [CrossRef]
- Goto, Y.; Isono, T.; Ikuta, S.; Burkanov, V. Origin and abundance of Steller sea lions (Eumetopias jubatus) in winter haulout at Benten-Jima Rock off Cape Soya, Hokkaido, Japan between 2012-2017. Mamm. Study. 2022, 47, 87–101. [Google Scholar] [CrossRef]
- Koski, W.R.; Young, B.G. A new scoring system for use in capture–recapture studies for bowhead whales photographed with drones. Drone Syst. Appl. 2022, 10, 15–25. [Google Scholar] [CrossRef]
- Ratsimbazafindranahaka, M.; Razafimahatratra, E.; Mathevet, R.; Adam, O.; Huetz, C.; Charrier, I.; Saloma, A. Morphometric study of humpback whale mother-calf pairs in the Sainte Marie channel, Madagascar, using a simple drone-based photogrammetric method. West. Indian Ocean J. Mar. Sci. 2022, 20, 95–107. [Google Scholar] [CrossRef]
- Aoki, K.; Isojunno, S.; Bellot, C.; Iwata, T.; Kershaw, J.; Akiyama, Y.; Martín López, L.M.; Ramp, C.; Biuw, M.; Swift, R.; et al. Aerial photogrammetry and tag-derived tissue density reveal patterns of lipid-store body condition of humpback whales on their feeding grounds. Proc. R. Soc. B 2021, 288, 20202307. [Google Scholar] [CrossRef] [PubMed]
- Fiori, L.; Martinez, E.; Orams, M.B.; Bollard, B. Using unmanned aerial vehicles (UAVs) to assess humpback whale behavioral responses to swim-with interactions in Vava’u, Kingdom of Tonga. J. Sustain. Tour. 2020, 28, 1743–1761. [Google Scholar] [CrossRef]
- Jones, L.S.; Stephenson, T.A.; Zoidis, A.M.; Todd, S.K. Drone observations of a mother–calf humpback whale (Megaptera novaeangliae) pair synchronous feeding in the Bay of Fundy, Canada. Aquat. Mamm. 2022, 48, 716–719. [Google Scholar] [CrossRef]
- Herr, H.; Viquerat, S.; Devas, F.; Lees, A.; Wells, L.; Gregory, B.; Giffords, T.; Beecham, D.; Meyer, B. Return of large fin whale feeding aggregations to historical whaling grounds in the Southern Ocean. Sci. Rep. 2022, 12, 9458. [Google Scholar] [CrossRef]
- Hartman, K.; van der Harst, P.; Vilela, R. Continuous focal group follows operated by a drone enable analysis of the relation between sociality and position in a group of male Risso’s dolphins (Grampus griseus). Front. Mar. Sci. 2020, 7, 283. [Google Scholar] [CrossRef]
- Fettermann, T.; Fiori, L.; Gillman, L.; Stockin, K.A.; Bollard, B. Drone surveys are more accurate than boat-based surveys of bottlenose dolphins (Tursiops truncatus). Drones 2022, 6, 82. [Google Scholar] [CrossRef]
- Orbach, D.N.; Eaton, J.; Fiori, L.; Piwetz, S.; Weir, J.S.; Würsig, M.; Würsig, B. Mating patterns of dusky dolphins (Lagenorhynchus obscurus) explored using an unmanned aerial vehicle. Mar. Mamm. Sci. 2020, 36, 1097–1110. [Google Scholar] [CrossRef]
- Weir, J.S.; Fiori, L.; Orbach, D.N.; Piwetz, S.; Protheroe, C.; Würsig, B. Dusky dolphin (Lagenorhynchus obscurus) mother-calf pairs: An aerial perspective. Aquat. Mamm. 2018, 44, 603–607. [Google Scholar] [CrossRef]
- Chung, T.Y.; Ho, H.H.; Tsui, H.C.; Kot, B.C. First unmanned aerial vehicle observation of epimeletic behavior in Indo-Pacific humpback dolphins. Animals. 2022, 12, 1463. [Google Scholar] [CrossRef] [PubMed]
- Pegus, C.; Atkinson, S.; Quinn, T.; Pyare, S. Evaluating the accuracy of unmanned aerial systems to quantify glacial ice habitats of harbor seals in Alaska. Ecosphere 2022, 13, e4287. [Google Scholar] [CrossRef]
- Cleguer, C.; Kelly, N.; Tyne, J.; Wieser, M.; Peel, D.; Hodgson, A. A novel method for using small unoccupied aerial vehicles to survey wildlife species and model their density distribution. Front. Mar. Sci. 2021, 8, 640338. [Google Scholar] [CrossRef]
- Infantes, E.; Carroll, D.; Silva, W.T.A.F.; Härkönen, T.; Edwards, S.V.; Harding, K.C. An automated work-flow for pinniped surveys: A new tool for monitoring population dynamics. Front. Ecol. Evol. 2022, 10, 905309. [Google Scholar] [CrossRef]
- Dujon, A.M.; Ierodiaconou, D.; Geeson, J.J.; Arnould, J.P.Y.; Allan, B.M.; Katselidis, K.A.; Schofield, G. Machine learning to detect marine animals in UAV imagery: Effect of morphology, spacing, behaviour and habitat. Remote Sens. Ecol. Conserv. 2021, 7, 341–354. [Google Scholar] [CrossRef]
- Sorrell, K.J.; Clarke, R.H.; Holmberg, R.; McIntosh, R.R. Remotely piloted aircraft improve precision of capture–mark–resight population estimates of Australian fur seals. Ecosphere 2019, 10, e02812. [Google Scholar] [CrossRef]
- Brown, A.M.; Allen, S.J.; Kelly, N.; Hodgson, A.J. Using unoccupied aerial vehicles to estimate availability and group size error for aerial surveys of coastal dolphins. Remote Sens. Ecol. Conserv. 2022, 9, 340–353. [Google Scholar] [CrossRef]
- Ramp, C.; Gaspard, D.; Gavrilchuk, K.; Unger, M.; Schleimer, A.; Delarue, J.; Landry, S.; Sears, R. Up in the air: Drone images reveal underestimation of entanglement rates in large rorqual whales. Endang. Species Res. 2021, 44, 33–44. [Google Scholar] [CrossRef]
- Russell, G.; Colefax, A.; Christiansen, F.; Russell, G.; Fowler, Z.; Cagnazzi, D. Body condition and migration timing of east Australian humpback whales. Mar. Ecol. Prog. Ser. 2022, 692, 169–183. [Google Scholar] [CrossRef]
- Glarou, M.; Gero, S.; Frantzis, A.; Brotons, J.M.; Vivier, F.; Alexiadou, P.; Cerdà, M.; Pirotta, E.; Christiansen, F. Estimating body mass of sperm whales from aerial photographs. Mar. Mamm. Sci. 2022, 37, 251–273. [Google Scholar] [CrossRef]
- Arranz, P.; Christiansen, F.; Glarou, M.; Gero, S.; Visser, F.; Oudejans, M.G.; Aguilar de Soto, N.; Sprogis, K. Body condition and allometry of free-ranging short-finned pilot whales in the North Atlantic. Sustainability 2022, 14, 14787. [Google Scholar] [CrossRef]
- Christie, A.I.; Colefax, A.P.; Cagnazzi, D. Feasibility of using small UAVs to derive morphometric measurements of Australian snubfin (Orcaella heinsohni) and humpback (Sousa sahulensis) dolphins. Remote Sens. 2022, 14, 21. [Google Scholar] [CrossRef]
- Ramos, E.A.; Landeo-Yauri, S.; Castelblanco-Martínez, N.; Arreola, M.R.; Quade, A.H.; Rieucau, G. Drone-based photogrammetry assessments of body size and body condition of Antillean manatees. Mamm. Biol. 2022, 102, 765–779. [Google Scholar] [CrossRef]
- Ortiz, S.T.; Stedt, J.; Midtiby, H.S.; Egemose, H.D.; Wahlberg, M. Group hunting in harbour porpoises (Phocoena phocoena). Can. J. Zool. 2021, 99, 511–520. [Google Scholar] [CrossRef]
- Edwards, H.H.; Hostetler, J.A.; Stith, B.M.; Martin, J. Monitoring abundance of aggregated animals (Florida manatees) using an unmanned aerial system (UAS). Sci. Rep. 2021, 11, 12920. [Google Scholar] [CrossRef]
- Weiss, M.N.; Franks, D.W.; Giles, D.A.; Youngstrom, S.; Wasser, S.K.; Balcomb, K.C.; Ellifrit, D.K.; Domenici, P.; Cant, M.A.; Ellis, S.; et al. Age and sex influence social interactions, but not associations, within a killer whale pod. Proc. R. Soc. B 2021, 288, 20210617. [Google Scholar] [CrossRef]
- Martin, M.J.; Torres Ortiz, S.; Reyes Reyes, M.V.; Marino, A.; Iñíguez Bessega, M.; Wahlberg, M. Commerson’s dolphins (Cephalorhynchus commersonii) can relax acoustic crypsis. Behav. Ecol. Sociobiol. 2021, 75, 100. [Google Scholar] [CrossRef]
- Brennecke, D.; Siebert, U.; Kindt-Larsen, L.; Midtiby, H.S.; Egemose, H.D.; Ortiz, S.T.; Knickmeier, K.; Wahlberg, M. The fine-scale behavior of harbor porpoises towards pingers. Fish. Res. 2022, 255, 106437. [Google Scholar] [CrossRef]
- Yamato, C.; Ichikawa, K.; Arai, N.; Tanaka, K.; Nishiyama, T.; Kittiwattanawong, K. Deep neural networks based automated extraction of dugong feeding trails from UAV images in the intertidal seagrass beds. PLoS ONE. 2021, 16, e0255586. [Google Scholar] [CrossRef]
- Mamaev, E.G. A new method of counting Phoca vitulina ssp. stejnegeri (Phocidae, Carnivora) on the Commander Islands (Russia). Nat. Conserv. Res. 2018, 3, 44–58. [Google Scholar] [CrossRef]
- Hirtle, N.O.; Stepanuk, J.E.F.; Heywood, E.I.; Christiansen, F.; Thorne, L.H. Integrating 3D models with morphometric measurements to improve volumetric estimates in marine mammals. Methods Ecol. Evol. 2022, 13, 2478–2490. [Google Scholar] [CrossRef]
- King, S.L.; Connor, R.C.; Krützen, M.; Allen, S.J. Cooperation-based concept formation in male bottlenose dolphins. Nat. Commun. 2021, 12, 2373. [Google Scholar] [CrossRef] [PubMed]
- Ejrnæs, D.D.; Sprogis, K.R. Ontogenetic changes in energy expenditure and resting behaviour of humpback whale mother-calf pairs examined using unmanned aerial vehicles. Wildl. Res. 2021, 49, 34–45. [Google Scholar] [CrossRef]
- Harkness, P.; Sprogis, K.R. Silver gull harassment of humpback whales in Exmouth Gulf, Western Australia. Mar. Freshw. Res. 2021, 72, 584–592. [Google Scholar] [CrossRef]
- Torres, L.G.; Barlow, D.R.; Chandler, T.E.; Burnett, J.D. Insight into the kinematics of blue whale surface foraging through drone observations and prey data. PeerJ 2020, 8, e8906. [Google Scholar] [CrossRef]
- Frouin-Mouy, H.; Tenorio-Hallé, L.; Thode, A.; Swartz, S.; Urbán, J. Using two drones to simultaneously monitor visual and acoustic behaviour of gray whales (Eschrichtius robustus) in Baja California, Mexico. J. Exp. Mar. Biol. Ecol. 2020, 525, 151321. [Google Scholar] [CrossRef]
- Kelaher, B.P.; Peddemors, V.M.; Hoade, B.; Colefax, A.P.; Butcher, P.A. Comparison of sampling precision for nearshore marine wildlife using unmanned and manned aerial surveys. J. Unmanned Veh. Syst. 2020, 8, 30–43. [Google Scholar] [CrossRef]
- Raudino, H.C.; Tyne, J.A.; Smith, A.; Ottewell, K.; McArthur, S.; Kopps, A.M.; Chabanne, D.; Harcourt, R.G.; Pirotta, V.; Waples, K. Challenges of collecting blow from small cetaceans. Ecosphere 2019, 10, e02901. [Google Scholar] [CrossRef]
- Lonati, G.L.; Zitterbart, D.P.; Miller, C.A.; Corkeron, P.; Murphy, C.T.; Moore, M.J. Investigating the thermal physiology of critically endangered North Atlantic right whales Eubalaena glacialis via aerial infrared thermography. Endang. Species. Res. 2022, 48, 139–154. [Google Scholar] [CrossRef]
- Bierlich, K.C.; Hewitt, J.; Schick, R.S.; Pallin, L.; Dale, J.; Friedlaender, A.S.; Christiansen, F.; Sprogis, K.R.; Dawn, A.H.; Bird, C.N.; et al. Seasonal gain in body condition of foraging humpback whales along the Western Antarctic Peninsula. Front. Mar. Sci. 2022, 9, 1036860. [Google Scholar] [CrossRef]
- Christiansen, F.; Sprogis, K.R.; Gross, J.; Castrillon, J.; Warick, H.A.; Leunissen, E.; Nash, S.B. Variation in outer blubber lipid concentration does not reflect morphological body condition in humpback whales. J. Exp. Biol. 2020, 223, jeb213769. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.R.; Rayment, W.; Dawson, S.M. Morphometrics and body condition of southern right whales on the calving grounds at Port Ross, Auckland Islands. Mamm. Biol. 2022, 102, 1525–1536. [Google Scholar] [CrossRef]
- Dawson, S.M.; Bowman, M.H.; Leunissen, E.; Sirguey, P. Inexpensive aerial photogrammetry for studies of whales and large marine animals. Front. Mar. Sci. 2017, 4, 1–7. [Google Scholar] [CrossRef]
- Christiansen, F.; Vivier, F.; Charlton, C.; Ward, R.; Amerson, A.; Burnell, S.; Bejder, L. Maternal body size and condition determine calf growth rates in southern right whales. Mar. Ecol. Prog. Ser. 2018, 592, 267–282. [Google Scholar] [CrossRef]
- Christiansen, F.; Dawson, S.M.; Durban, J.W.; Fearnbach, H.; Miller, C.A.; Bejder, L.; Uhart, M.; Sironi, M.; Corkeron, P.; Rayment, W.; et al. Population comparison of right whale body condition reveals poor state of the North Atlantic right whale. Mar. Ecol. Prog. Ser. 2020, 640, 1–16. [Google Scholar] [CrossRef]
- Christiansen, F.; Bejder, L.; Burnell, S.; Ward, R.; Charlton, C. Estimating the cost of growth in southern right whales from drone photogrammetry data and long-term sighting histories. Mar. Ecol. Prog. Ser. 2022, 687, 173–194. [Google Scholar] [CrossRef]
- Christiansen, F.; Uhart, M.M.; Bejder, L.; Clapham, P.; Ivashchenko, Y.; Tormosov, D.; Lewin, N.; Sironi, M. Fetal growth, birth size and energetic cost of gestation in southern right whales. J. Physiol. 2022, 600, 2245–2266. [Google Scholar] [CrossRef]
- Christiansen, F.; Sironi, M.; Moore, M.J.; Di Martino, M.; Ricciardi, M.; Warick, H.A.; Irschick, D.J.; Gutierrez, R.; Uhart, M.M. Estimating body mass of free-living whales using aerial photogrammetry and 3D volumetrics. Methods Ecol. Evol. 2019, 10, 2034–2044. [Google Scholar] [CrossRef]
- Christiansen, F.; Rodríguez-González, F.; Martínez-Aguilar, S.; Urbán, J.; Swartz, S.; Warick, H.; Vivier, F.; Bejder, L. Poor body condition associated with an unusual mortality event in gray whales. Mar. Ecol. Prog. Ser. 2021, 658, 237–252. [Google Scholar] [CrossRef]
- Dickson, T.; Rayment, W.; Dawson, S. Drone photogrammetry allows refinement of acoustically derived length estimation for male sperm whales. Mar. Mamm. Sci. 2021, 37, 1150–1158. [Google Scholar] [CrossRef]
- Irschick, D.J.; Martin, J.; Siebert, U.; Kristensen, J.H.; Madsen, P.T.; Christiansen, F. Creation of accurate 3D models of harbor porpoises (Phocoena phocoena) using 3D photogrammetry. Mar. Mamm. Sci. 2021, 37, 482–491. [Google Scholar] [CrossRef]
- Martins, M.C.I.; Miller, C.; Hamilton, P.; Robbins, J.; Zitterbart, D.P.; Moore, M. Respiration cycle duration and seawater flux through open blowholes of humpback (Megaptera novaeangliae) and North Atlantic right (Eubalaena glacialis) whales. Mar. Mamm. Sci. 2020, 36, 1160–1179. [Google Scholar] [CrossRef]
- Fudala, K.; Bialik, R.J. Breeding colony dynamics of southern elephant seals at Patelnia Point, King George Island, Antarctica. Remote Sens. 2020, 12, 2964. [Google Scholar] [CrossRef]
- Fudala, K.; Bialik, R.J. Seals from outer space—Population census of southern elephant seals using VHR satellite imagery. Remote Sens. Appl. Soc. Environ. 2022, 28, 100836. [Google Scholar] [CrossRef]
- Currie, J.J.; van Aswegen, M.; Stack, S.H.; West, K.L.; Vivier, F.; Bejder, L. Rapid weight loss in free ranging pygmy killer whales (Feresa attenuata) and the implications for anthropogenic disturbance of odontocetes. Sci. Rep. 2021, 11, 8181. [Google Scholar] [CrossRef]
- Hodgson, J.C.; Holman, D.; Terauds, A.; Koh, L.P.; Goldsworthy, S.D. Rapid condition monitoring of an endangered marine vertebrate using precise, non-invasive morphometrics. Biol. Conserv. 2020, 242, 108402. [Google Scholar] [CrossRef]
- Atkinson, S.; Rogan, A.; Baker, C.S.; Dagdag, R.; Redlinger, M.; Polinski, J.; Urban, J.; Sremba, A.; Branson, M.; Mashburn, K.; et al. Genetic, endocrine, and microbiological assessments of blue, humpback and killer whale health using unoccupied aerial systems. Wildl. Soc. Bull. 2021, 45, 654–669. [Google Scholar] [CrossRef]
- Baylis, A.M.M.; Orben, R.A.; Arkhipkin, A.A.; Barton, J.; Brownell, R.L.; Staniland, I.J.; Brickle, P. Re-evaluating the population size of South American fur seals and conservation implications. Aquat. Conserv. 2019, 29, 1988–1995. [Google Scholar] [CrossRef]
- Morimura, N.; Mori, Y. Social responses of travelling finless porpoises to boat traffic risk in Misumi West Port, Ariake Sound, Japan. PLoS ONE 2019, 14, e0208754. [Google Scholar] [CrossRef]
- Dickens, J.; Hollyman, P.R.; Hart, T.; Clucas, G.V.; Murphy, E.J.; Poncet, S.; Trathan, P.N.; Collins, M.A. Developing UAV monitoring of South Georgia and the South Sandwich Islands’ iconic land-based marine predators. Front. Mar. Sci. 2021, 8, 654215. [Google Scholar] [CrossRef]
- Baird, R.W.; Mahaffy, S.D.; Lerma, J.K. Site fidelity, spatial use, and behavior of dwarf sperm whales in Hawaiian waters: Using small-boat surveys, photo-identification, and unmanned aerial systems to study a difficult-to-study species. Mar. Mam. Sci. 2021, 38, 326–348. [Google Scholar] [CrossRef]
- Chenoweth, E.M.; Houston, J.; Huntington, J.B.; Straley, J.M. A virtual necropsy: Applications of 3D scanning for marine mammal pathology and education. Animals. 2022, 12, 527. [Google Scholar] [CrossRef] [PubMed]
- Baird, R.W.; Lerma, J.K.; Cornforth, C.J.; Wood, K.A. An unexpected benefit from drone-assisted fecal sample collection: Picking up subsurface poop after it floats to the surface. Aquat. Mamm. 2022, 48, 565–567. [Google Scholar] [CrossRef]
- Barreto, J.; Cajaíba, L.; Teixeira, J.B.; Nascimento, L.; Giacomo, A.; Barcelos, N.; Fettermann, T.; Martins, A. Drone-monitoring: Improving the detectability of threatened marine megafauna. Drones 2021, 5, 14. [Google Scholar] [CrossRef]
- Giacomo, A.B.D.; Barreto, J.; Teixeira, J.B.; Oliveira, L.; Cajaíba, L.; Joyeux, J.C.; Barcelos, N.; Martins, A.S. Using drones and ROV to assess the vulnerability of marine megafauna to the Fundão tailings dam collapse. Sci. Total Environ. 2021, 800, 149302. [Google Scholar] [CrossRef]
- Babatunde, D.; Pomeroy, S.; Lepper, P.; Clark, B.; Walker, R. Autonomous deployment of underwater acoustic monitoring devices using an unmanned aerial vehicle: The flying hydrophone. Sensors 2020, 20, 6064. [Google Scholar] [CrossRef]
- Infantes, E.; Cossa, D.; Stankovic, M.; Panyawai, J.; Tuntiprapas, P.; Daochai, C.; Prathep, A. Dugong (Dugong dugon) reproductive behaviour in Koh Libong, Thailand: Observations using drones. Aquat. Mamm. 2020, 46, 603–608. [Google Scholar] [CrossRef]
- Horton, T.W.; Hauser, N.; Cassel, S.; Klaus, K.F.; de Oliveira, T.F.; Key, N. Doctor drone: Non-invasive measurement of humpback whale vital signs using unoccupied aerial system infrared thermography. Front. Mar. Sci. 2019, 6, 466. [Google Scholar] [CrossRef]
- Murakami, R.; Toyoshima, T.; Furusawa, D.; Suzuki, M.; Masumoto, K.; Owada, S.; Tsumaki, Y.; Mori, K. Logger attaching system for sperm whales using a drone. J. Robot. Mechatron. 2021, 33, 475–483. [Google Scholar] [CrossRef]
- Harasyn, M.L.; Chan, W.S.; Ausen, E.L.; Barber, D.G. Detection and tracking of belugas, kayaks and motorized boats in drone video using deep learning. Drone Syst. Appl. 2022, 10, 77–96. [Google Scholar] [CrossRef]
- Christiansen, F.; Dujon, A.M.; Sprogis, K.R.; Arnould, J.P.Y.; Bejder, L. Noninvasive unmanned aerial vehicle provides estimates of the energetic cost of reproduction in humpback whales. Ecosphere 2016, 7, e01468. [Google Scholar] [CrossRef]
- Centelleghe, C.; Carraro, L.; Gonzalvo, J.; Rosso, M.; Esposti, E.; Gili, C.; Bonato, M.; Pedrotti, D.; Cardazzo, B.; Povinelli, M.; et al. The use of unmanned aerial vehicles (UAVs) to sample the blow microbiome of small cetaceans. PLoS ONE 2020, 15, e0235537. [Google Scholar] [CrossRef] [PubMed]
- Gooday, O.J.; Key, N.; Goldstien, S.; Zawar-Reza, P. An assessment of thermal-image acquisition with an unmanned aerial vehicle (UAV) for direct counts of coastal marine mammals ashore. J. Unmanned Veh. Syst. 2018, 6, 100–108. [Google Scholar] [CrossRef]
- Goebel, M.E.; Perryman, W.L.; Hinke, J.T.; Krause, D.J.; Hann, N.A.; Gardner, S.; LeRoi, D.J. A small unmanned aerial system for estimating abundance and size of antarctic predators. Polar Biol. 2015, 38, 619–630. [Google Scholar] [CrossRef]
- Leslie, M.S.; Perkins-Taylor, C.M.; Durban, J.W.; Moore, M.J.; Miller, C.A.; Chanarat, P.; Bahamonde, P.; Chiang, G.; Apprill, A. Body size data collected non-invasively from drone images indicate a morphologically distinct Chilean blue whale (Balaenoptera musculus) taxon. Endang. Species Res. 2020, 43, 291–304. [Google Scholar] [CrossRef]
- Stewart, J.D.; Durban, J.W.; Knowlton, A.R.; Lynn, M.S.; Fearnbach, H.; Barbaro, J.; Perryman, W.L.; Miller, C.A.; Moore, M.J. Decreasing body lengths in North Atlantic right whales. Curr. Biol. 2021, 31, 3174–3179. [Google Scholar] [CrossRef]
- Pallin, L.; Bierlich, K.C.; Durban, J.; Fearnbach, H.; Savenko, O.; Baker, C.S.; Bell, E.; Double, M.C.; de la Mare, W.; Goldbogen, J.; et al. Demography of an ice-obligate mysticete in a region of rapid environmental change. R. Soc. Open Sci. 2022, 9, 220724. [Google Scholar] [CrossRef]
- Groskreutz, M.J.; Durban, J.W.; Fearnbach, H.; Barrett-Lennard, L.G.; Towers, J.R.; Ford, J.K.B. Decadal changes in adult size of salmon-eating killer whales in the eastern North Pacific. Endang. Species Res. 2019, 40, 183–188. [Google Scholar] [CrossRef]
- Durban, J.W.; Fearnbach, H.; Paredes, A.; Hickmott, L.S.; LeRoi, D.J. Size and body condition of sympatric killer whale ecotypes around the Antarctic Peninsula. Mar. Ecol. Prog. Ser. 2021, 677, 209–217. [Google Scholar] [CrossRef]
- Stewart, J.D.; Durban, J.W.; Fearnbach, H.; Barrett-Lennard, L.G.; Casler, P.K.; Ward, E.J.; Dapp, D.R. Survival of the fattest: Linking body condition to prey availability and survivorship of killer whales. Ecosphere 2021, 12, e03660. [Google Scholar] [CrossRef]
- Kotik, C.; Durban, J.W.; Fearnbach, H.; Barrett-Lennard, L.G. Morphometrics of mammal-eating killer whales from drone photogrammetry, with comparison to sympatric fish-eating killer whales in the eastern North Pacific. Mar. Mamm. Sci. 2022, 39, 42–58. [Google Scholar] [CrossRef]
- Krause, D.J.; Hinke, J.T.; Perryman, W.L.; Goebel, M.E.; LeRoi, D.J. An accurate and adaptable photogrammetric approach for estimating the mass and body condition of pinnipeds using an unmanned aerial system. PLoS ONE 2017, 12, e0187465. [Google Scholar] [CrossRef] [PubMed]
- Apprill, A.; Miller, C.A.; Moore, M.J.; Durban, J.W.; Fearnbach, H.; Barrett-Lennard, L.G. Extensive core microbiome in drone-captured whale blow supports a framework for health monitoring. mSystems 2017, 2, e00119-17. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.J.; Hinke, J.T. Finally within reach: A drone census of an important, but practically inaccessible, Antarctic fur seal colony. Aquat. Mamm. 2021, 47, 349–354. [Google Scholar] [CrossRef]
- Cheney, B.J.; Dale, J.; Thompson, P.M.; Quick, N.J. Spy in the sky: A method to identify pregnant small cetaceans. Remote Sens. Ecol. Conserv. 2022, 8, 492–505. [Google Scholar] [CrossRef]
- Fiori, L.; Martinez, E.; Bader, M.K.F.; Orams, M.B.; Bollard, B. Insights into the use of an unmanned aerial vehicle (UAV) to investigate the behavior of humpback whales (Megaptera novaeangliae) in Vava’u, Kingdom of Tonga. Mar. Mamm. Sci. 2020, 36, 209–223. [Google Scholar] [CrossRef]
- Colefax, A.P.; Kelaher, B.P.; Walsh, A.J.; Purcell, C.R.; Pagendam, D.E.; Cagnazzi, D.; Butcher, P.A. Identifying optimal wavelengths to maximise the detection rates of marine fauna from aerial surveys. Biol. Conserv. 2021, 257, 109102. [Google Scholar] [CrossRef]
- Shero, M.R.; Dale, J.; Seymour, A.C.; Hammill, M.O.; Mosnier, A.; Mongrain, S.; Johnston, D.W. Tracking wildlife energy dynamics with unoccupied aircraft systems and three-dimensional photogrammetry. Methods Ecol. Evol. 2021, 12, 2458–2472. [Google Scholar] [CrossRef]
- Pedersen, N.J.; Brinkman, T.J.; Shideler, R.T.; Perham, C.J. Effects of environmental conditions on the use of forward-looking infrared for bear den detection in the Alaska Arctic. Conserv. Sci. Pract. 2020, 2, e215. [Google Scholar] [CrossRef]
- Boulil, Z.L.; Durban, J.W.; Fearnbach, H.; Joyce, T.W.; Leander, S.G.M.; Scharf, H.R. Detecting changes in dynamic social networks using multiply-labeled movement data. J. Agric. Biol. Environ. Stat. 2022, 28, 243–259. [Google Scholar] [CrossRef]
- Durban, J.W.; Southall, B.L.; Calambokidis, J.; Casey, C.; Fearnbach, H.; Joyce, T.W.; Fahlbusch, J.A.; Oudejans, M.G.; Fregosi, S.; Friedlaender, A.S.; et al. Integrating remote sensing methods during controlled exposure experiments to quantify group responses of dolphins to navy sonar. Mar. Pollut. Bull. 2022, 174, 113194. [Google Scholar] [CrossRef] [PubMed]
- Bigal, E.; Galili, O.; van Rijn, I.; Rosso, M.; Cleguer, C.; Hodgson, A.; Scheinin, A.; Tchernov, D. Reduction of species identification errors in surveys of marine wildlife abundance utilising unoccupied aerial vehicles (UAVs). Remote Sens. 2022, 14, 4118. [Google Scholar] [CrossRef]
- Krause, D.J.; Hinke, J.T.; Goebel, M.E.; Perryman, W.L. Drones minimize Antarctic predator responses relative to ground survey methods: An appeal for context in policy advice. Front. Mar. Sci. 2021, 8, 648772. [Google Scholar] [CrossRef]
- Fettermann, T.; Fiori, L.; Bader, M.; Doshi, A.; Breen, D.; Stockin, K.A.; Bollard, B. Behaviour reactions of bottlenose dolphins (Tursiops truncatus) to multirotor unmanned aerial vehicles (UAVs). Sci. Rep. 2019, 9, 8558. [Google Scholar] [CrossRef]
- Giles, A.B.; Butcher, P.A.; Colefax, A.P.; Pagendam, D.E.; Mayjor, M.; Kelaher, B.P. Responses of bottlenose dolphins (Tursiops spp.) to small drones. Aquat. Conserv. 2021, 31, 677–684. [Google Scholar] [CrossRef]
- Ramos, E.A.; Maloney, B.; Magnasco, M.O.; Reiss, D. Bottlenose dolphins and Antillean manatees respond to small multi-rotor unmanned aerial systems. Front. Mar. Sci. 2018, 5, 316. [Google Scholar] [CrossRef]
- Pomeroy, P.; O’Connor, L.; Davies, P. Assessing use of and reaction to unmanned aerial systems in gray and harbor seals during breeding and molt in the UK. J. Unmanned Veh. Syst. 2015, 3, 102–113. [Google Scholar] [CrossRef]
- Young, B.G.; Koski, W.R.; Kilabuk, R.; Watt, C.A.; Ryan, K.P.; Ferguson, S.H. Collaborative field research using drones for whale photo-identification studies in Cumberland Sound, Nunavut. Drone Syst. Appl. 2022, 10, 156–265. [Google Scholar] [CrossRef]
- Gray, P.C.; Bierlich, K.C.; Mantell, S.A.; Friedlaender, A.S.; Goldbogen, J.A.; Johnston, D.W. Drones and convolutional neural networks facilitate automated and accurate cetacean species identification and photogrammetry. Methods Ecol. Evol. 2019, 10, 1490–1500. [Google Scholar] [CrossRef]
- Bierlich, K.C.; Hewitt, J.; Bird, C.N.; Schick, R.S.; Friedlaender, A.; Torres, L.G.; Dale, J.; Goldbogen, J.; Read, A.J.; Calambokidis, J.; et al. Comparing uncertainty associated with 1-, 2-, and 3D aerial photogrammetry-based body condition measurements of baleen whales. Front. Mar. Sci. 2021, 8, 749943. [Google Scholar] [CrossRef]
- Allan, B.M.; Ierodiaconou, D.; Hoskins, A.J.; Arnould, J.P.Y. A rapid UAV method for assessing body condition in fur seals. Drones. 2019, 3, 24. [Google Scholar] [CrossRef]
- Gough, W.T.; Cade, D.E.; Czapanskiy, M.F.; Potvin, J.; Fish, F.E.; Kahane-Rapport, S.R.; Savoca, M.S.; Bierlich, K.C.; Johnston, D.W.; Friedlaender, A.S.; et al. Fast and furious: Energetic tradeoffs and scaling of high-speed foraging in rorqual whales. Integr. Org. Biol. 2022, 4, obac038. [Google Scholar] [CrossRef] [PubMed]
- Pirotta, V.; Smith, A.; Ostrowski, M.; Russell, D.; Jonsen, I.D.; Grech, A.; Harcourt, R. An economical custom-built drone for assessing whale health. Front. Mar. Sci. 2017, 4, 425. [Google Scholar] [CrossRef]
- Geoghegan, J.L.; Pirotta, V.; Harvey, E.; Smith, A.; Buchmann, J.P.; Ostrowski, M.; Eden, J.-S.; Harcourt, R.; Holmes, E.C. Virological sampling of inaccessible wildlife with drones. Viruses. 2018, 10, 300. [Google Scholar] [CrossRef] [PubMed]
- Hague, E.L.; McCaffrey, N.; Stockin, K.A.; Orbach, D.N. Previously undocumented long-finned pilot whale (Globicephala melas) placental expulsion in coastal waters of Shetland, United Kingdom. Aquat. Mamm. 2022, 48, 610–616. [Google Scholar] [CrossRef]
- Pontalti, M.; Barreto, A.S. Use of unnamed aerial vehicles (UAVs) to monitor marine megafauna strandings in beach monitoring programs. J. Coast Conserv. 2022, 26, 80. [Google Scholar] [CrossRef]
- Ten, S.; Konishi, K.; Raga, J.A.; Pastene, L.A.; Aznar, F.J. Epibiotic fauna of the Antarctic minke whale as a reliable indicator of seasonal movements. Sci. Rep. 2022, 12, 22214. [Google Scholar] [CrossRef]
- Fiori, L.; Doshi, A.; Martinez, E.; Orams, M.B.; Bollard-Breen, B. The use of unmanned aerial systems in marine mammal research. Remote Sens. 2017, 9, 543. [Google Scholar] [CrossRef]
- Bröker, K.C.A.; Hansen, R.G.; Leonard, K.E.; Koski, W.R.; Heide-Jørgensen, M.P. A Comparison of image and observer based aerial surveys of narwhal. Mar. Mamm. Sci. 2019, 35, 1253–1279. [Google Scholar] [CrossRef]
- Stevenson, B.C.; Borchers, D.L.; Fewster, R.M. Cluster capture-recapture to account for identification uncertainty on aerial surveys of animal populations. Biometrics 2019, 75, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, T.; Oliveira, P.; Silvestre, C. Model-based filters for 3-D positioning of marine mammals using AHRS- and GPS-equipped UAVs. IEEE Trans. Aerosp. Electron. Syst. 2015, 51, 3307–3320. [Google Scholar] [CrossRef]
- Williams, P.J.; Hooten, M.B.; Womble, J.N.; Bower, M.R. Estimating occupancy and abundance using aerial images with imperfect detection. Methods Ecol. Evol. 2017, 8, 1679–1689. [Google Scholar] [CrossRef]
- Chabot, D.; Stapleton, S.; Francis, C.M. Measuring the spectral signature of polar bears from a drone to improve their detection from space. Biol. Consev. 2019, 237, 125–132. [Google Scholar] [CrossRef]
- Hinke, J.T.; Giuseffi, L.M.; Hermanson, V.R.; Woodman, S.M.; Krause, D.J. Evaluating thermal and color sensors for automating detection of penguins and pinnipeds in images collected with an unoccupied aerial system. Drones 2022, 6, 255. [Google Scholar] [CrossRef]
- Boyd, C.; Hobbs, R.C.; Punt, A.E.; Shelden, K.E.W.; Sims, C.L.; Wade, P.R. Bayesian estimation of group sizes for a coastal cetacean using aerial survey data. Mar. Mamm. Sci. 2019, 35, 1322–1346. [Google Scholar] [CrossRef]
- Quiñonez, Y.; Lizarraga, C.; Peraza, J.; Zatarain, O. Image recognition in UAV videos using convolutional neural networks. IET Software 2020, 14, 176–181. [Google Scholar] [CrossRef]
- Erbe, C.; Parsons, M.; Duncan, A.; Osterrieder, S.K.; Allen, K. Aerial and underwater sound of unmanned aerial vehicles (UAV). J. Unmanned. Veh. Syst. 2017, 5, 92–101. [Google Scholar] [CrossRef]
- Christiansen, F.; Rojano-Doñate, L.; Madsen, P.T.; Bejder, L. Noise levels of multi-rotor unmanned aerial vehicles with implications for potential underwater impacts on marine mammals. Front. Mar. Sci. 2016, 3, 277. [Google Scholar] [CrossRef]
- Palomino-González, A.; Kovacs, K.M.; Lydersen, C.; Ims, R.A.; Lowther, A.D. Drones and marine mammals in Svalbard, Norway. Mar. Mamm. Sci. 2021, 37, 1212–1229. [Google Scholar] [CrossRef]
- Duporge, I.; Spiegel, M.P.; Thomson, E.R.; Chapman, T.; Lamberth, C.; Pond, C.; Macdonald, D.W.; Wang, T.; Klinck, H. Determination of optimal flight altitude to minimise acoustic drone disturbance to wildlife using species audiograms. Methods Ecol. Evol. 2021, 12, 2196–2207. [Google Scholar] [CrossRef]
- Thirtyacre, D.; Brookshire, G.; Callan, S.; Arvizu, B.; Sherman, P. Small unmanned aircraft systems acoustic analysis for noninvasive marine mammal response: An exploratory field study. Int. J. Aviat. Aeronaut. Aerosp. 2021, 8, 11. [Google Scholar] [CrossRef]
- Domínguez-Sánchez, C.A.; Acevedo-Whitehouse, K.A.; Gendron, D. Effect of drone-based blow sampling on blue whale (Balaenoptera musculus) behavior. Mar. Mamm. Sci. 2018, 34, 841–850. [Google Scholar] [CrossRef]
- Christiansen, F.; Nielsen, M.L.K.; Charlton, C.; Bejder, L.; Madsen, P.T. Southern right whales show no behavioral response to low noise levels from a nearby unmanned aerial vehicle. Mar. Mamm. Sci. 2020, 36, 953–963. [Google Scholar] [CrossRef]
- Castro, J.; Borges, F.O.; Cid, A.; Laborde, M.I.; Rosa, R.; Pearson, H.C. Assessing the behavioural responses of small cetaceans to unmanned aerial vehicles. Remote Sens. 2021, 13, 156. [Google Scholar] [CrossRef]
- Landeo-Yauri, S.S.; Castelblanco-Martínez, D.N.; Hénaut, Y.; Arreola, M.R.; Ramos, E.A. Behavioural and physiological responses of captive Antillean manatees to small aerial drones. Wildl. Res. 2022, 49, 24–33. [Google Scholar] [CrossRef]
- Laborie, J.; Christiansen, F.; Beedholm, K.; Madsen, P.T.; Heerah, K. Behavioural impact assessment of unmanned aerial vehicles on Weddell seals (Leptonychotes weddellii). J. Exp. Mar. Biol. Ecol. 2021, 536, 151509. [Google Scholar] [CrossRef]
- Rebolo-Ifrán, N.; Grilli, M.G.; Lambertucci, S.A. Drones as a threat to wildlife: YouTube complements science in providing evidence about their effect. Environ. Conserv. 2019, 46, 205–210. [Google Scholar] [CrossRef]
- Bierlich, K.C.; Schick, R.S.; Hewitt, J.; Dale, J.; Goldbogen, J.A.; Friedlaender, A.S.; Johnston, D.W. Bayesian approach for predicting photogrammetric uncertainty in morphometric measurements derived from drones. Mar. Ecol. Prog. Ser. 2021, 673, 193–210. [Google Scholar] [CrossRef]
- Suarez-Bregua, P.; Álvarez-González, M.; Parsons, K.M.; Rotllant, J.; Pierce, G.J.; Saavedra, C. Environmental DNA (eDNA) for monitoring marine mammals: Challenges and opportunities. Front. Mar. Sci. 2022, 9, 987774. [Google Scholar] [CrossRef]
| Type of UAV | UAV Model | Use | Target Species | References |
|---|---|---|---|---|
| Launch and recovery system, fixed-wing | General Atomics MQ-9 Predator B | Line-transect survey | Megaptera novaeangliae, Pseudorca crassidens | [1] |
| Brican Systems TD100E | Photo-ID | Balaena mysticetus, Eschrichtius robustus, Delphinapterus leucas | [2] | |
| PW-ZOOM | Pinniped aggregation census | Mirounga leonina, Arctocephalus gazella, Leptonychotes weddelli | [3,4] | |
| Boeing Insitu ScanEagle | Line-transect survey | Balaena mysticetus, Delphinapterus leucas, Eschrichtius robustus | [5,6] | |
| Megaptera novaeangliae | [7] | |||
| Dugong dugon | [8] | |||
| Histriophoca fasciata, Phoca largha | [9] | |||
| Boeing Insitu Insight A-20 | Line-transect survey | Balaena mysticetus, Eschrichtius robustus, Delphinapterus leucas | [10] | |
| CryoWing Micro RPAS | Line-transect survey | Megaptera novaeangliae, Orcinus orca, Phocoena phocoena | [11] | |
| CryoWing Scout RPAS | ||||
| Trimble UX5 | Line-transect survey | Ursus maritimus | [12] | |
| Hand-launched, fixed-wing | Puma All-Environment | Pinniped aggregation census | Neomonachus schauinslandi | [1] |
| Eumetopias jubatus | [13] | |||
| SenseFly eBee | Pinniped aggregation census | Halychoerus grypus | [14,15,16] | |
| SenseFly eBee Plus | Pinniped aggregation census | Phoca vitulina richardii, Callorhinus ursinus, Eumetopias jubatus | [17] |
| Type of UAV | UAV Model | Use | Target Species | References |
|---|---|---|---|---|
| Quadcopter rotary-wing | DJI Phantom 3 | Line-transect survey | Sotalia fluviatilis, Inia geoffrensis | [18] |
| Group size estimation | Delphinapterus leucas | [19] | ||
| Photo-ID | Delphinapterus leucas | [20] | ||
| DJI Phantom 3 Pro | Line transect survey | Cetaceans | [21] | |
| Photo-ID | Trichechus manatus | [22] | ||
| Photogrammetry | Balaenoptera musculus, Eschrichtius robustus | [23] | ||
| Megaptera novaeangliae, Balaenoptera musculus, B. physalus, B. edeni, B. bonaerensis, B. borealis | [24,25] | |||
| Eschrichtius robustus | [26,27] | |||
| Behavioural study | Megaptera novaeangliae | [28] | ||
| Eubalaena australis | [29,30] | |||
| Eschrichtius robustus | [31] | |||
| Balaenoptera edeni | [32] | |||
| Ursus maritimus | [33,34,35] | |||
| DJI Phantom 3 Advanced | Pinniped aggregation census | Zalophus californianus | [36,37] | |
| Eumetopias jubatus | [36] | |||
| DJI Phantom 4 | Line-transect survey | Tursiops spp. | [38,39] | |
| Pinniped aggregation census | Arctocephalus pusillus | [40] | ||
| Eumetopias jubatus | [41] | |||
| Photo-ID | Balaena mysticetus | [42] | ||
| Delphinapterus leucas | [20] | |||
| Trichechus manatus | [22] | |||
| Photogrammetry | Megaptera novaeangliae | [43,44] | ||
| Balaenoptera musculus, Eschrichtius robustus | [23] | |||
| Eschrichtius robustus | [26,27] | |||
| Behavioural study | Megaptera novaeangliae | [45,46] | ||
| Balaenoptera physalus | [47] | |||
| Grampus griseus | [48] | |||
| Tursiops truncatus | [49] | |||
| Lagenorhynchus obscurus | [50,51] | |||
| Sousa chinensis | [52] | |||
| Habitat study | Phoca vitulina | [53] | ||
| DJI Phantom 4 Pro | Line-transect survey | Dugong dugon | [54] | |
| Pinniped aggregation census | Phoca vitulina | [55] | ||
| Arctocephalus pusillus | [40,56,57] | |||
| Group size estimation | Sousa sahulensis | [58] | ||
| Scarring assessment | Megaptera novaeangliae, Balaenoptera musculus, Balaenoptera physalus | [59] | ||
| Photogrammetry | Megaptera novaeangliae | [60] | ||
| Megaptera novaeangliae, Balaenoptera musculus, B. physalus, B. edeni, B. bonaerensis, B. borealis | [24,25] | |||
| Eschrichtius robustus | [26,27] | |||
| Physeter macrocephalus | [61] | |||
| Globicephala macrorhynchus | [62] | |||
| Orcaella heinsohni, Sousa sahulensis | [63] | |||
| Trichechus manatus | [64] | |||
| Behavioural study | Phocoena phocoena | [65] | ||
| Ursus maritimus | [33,34,35] | |||
| DJI Phantom 4 Pro V2.0 | Abundance study | Trichechus manatus latirostris | [66] | |
| Behavioural study | Orcinus orca | [67] | ||
| Cephalorhynchus commersonii | [68] | |||
| Phocoena phocoena | [69] | |||
| Habitat study | Dugong dugon | [70] | ||
| DJI Phantom 4 Pro+ | Pinniped aggregation census | Phoca vitulina | [71] | |
| Photogrammetry | Megaptera novaeangliae | [72] | ||
| Behavioural study | Tursiops aduncus | [73] | ||
| DJI Phantom 4 Advanced | Behavioural study | Megaptera novaeangliae | [74,75] | |
| Balaenoptera musculus | [76] | |||
| Eschrichtius robustus | [31] | |||
| DJI Phantom 4 Advanced+ | Behavioural study | Eschrichtius robustus | [77] | |
| DJI Inspire 1 | Line-transect survey | Tursiops spp. | [78] | |
| Blow sample collection | Tursiops aduncus, Sousa sahulensis | [79] | ||
| Thermography | Eubalaena glacialis | [80] | ||
| DJI Inspire 1 Pro/Raw | Pinniped aggregation census | Eumetopias jubatus | [41] | |
| Photogrammetry | Megaptera novaeangliae | [81,82] | ||
| Eubalaena australis | [83,84,85,86,87,88,89] | |||
| Eschrichtius robustus | [90] | |||
| Physeter macrocephalus | [61,91] | |||
| Globicephala macrorhynchus | [62] | |||
| Phocoena phocoena | [92] | |||
| Behavioural study | Eubalaena glacialis, Megaptera novaeangliae | [93] | ||
| DJI Inspire 2 | Pinniped aggregation census | Mirounga leonina | [94,95] | |
| Scarring assessment | Megaptera novaeangliae, Balaenoptera musculus, Balaenoptera physalus | [59] | ||
| Photogrammetry | Feresa attenuata | [96] | ||
| Neophoca cinerea | [97] | |||
| Blow sample collection | Megaptera novaeangliae, Balaenoptera musculus, Orcinus orca | [98] | ||
| Behavioural study | Balaenoptera physalus | [47] | ||
| DJI Mavic Pro | Pinniped aggregation census | Arctocephalus australis | [99] | |
| Eumetopias jubatus | [41] | |||
| Blow sample collection | Megaptera novaeangliae, Balaenoptera musculus, Orcinus orca | [98] | ||
| Behavioural study | Neophocaena asiaeorientalis | [100] | ||
| DJI Mavic Pro Platinum | Behavioural study | Eubalaena australis | [30] | |
| DJI Mavic 2 Pro | Pinniped aggregation census | Mirounga leonina | [101] | |
| Photo-ID and behavioural study | Kogia sima | [102] | ||
| Photogrammetry | Megaptera novaeangliae | [103] | ||
| Trichechus manatus | [64] | |||
| Searching faecal plumes | Globicephala macrorhynchus | [104] | ||
| DJI Mavic 2 Zoom | Line-transect survey | Steno bredanensis, Sotalia guianensis, Pontoporia blainvillei | [105,106] | |
| Pinniped aggregation census | Zalophus californianus, Eumetopias jubatus | [36] | ||
| DJI Matrice 100 | Hydrophone attachment | Phocoena phocoena | [107] | |
| DJI Matrice 200 | Pinniped aggregation census | Phoca vituluna | [55] | |
| Behavioural study | Dugong dugon | [108] | ||
| Thermography | Megaptera novaeangliae | [109] | ||
| Logger attachment | Physeter macrocephalus | [110] | ||
| DJI Matrice 210 RTK | Abundance study | Delphinapterus leucas | [111] | |
| SwellPro SplashDrone | Photogrammetry | Megaptera novaeangliae | [112] | |
| Blow sample collection | Tursiops truncatus | [113] | ||
| Tursiops aduncus, Sousa sahulensis | [79] | |||
| SwellPro SplashDrone 3+ | Hydrophone attachment | Eschrichtius robustus | [77] | |
| Draganflyer X4-P | Pinniped aggregation census | Arctocephalus forsteri | [114] | |
| Microdrones MD4-1000 | Pinniped aggregation census | Arctocephalus gazella, Hyrdurga leptonyx | [115] | |
| APQ-18 | Pinniped aggregation census | Arctocephalus gazella, Hyrdurga leptonyx | [115] | |
| Hexacopter rotary-wing | APH-22 | Pinniped aggregation census | Arctocephalus gazella, Hyrdurga leptonyx | [115] |
| Halychoerus grypus | [14] | |||
| Eumetopias jubatus | [13] | |||
| Photogrammetry | Balaenoptera musculus | [116] | ||
| Eubalaena glacialis | [86,117] | |||
| Balaenoptera bonaerensis | [118] | |||
| Orcinus orca | [119,120,121,122] | |||
| Hydrurga leptonyx | [123] | |||
| Blow sample collection | Megaptera novaeangliae | [124] | ||
| APH-28 | Pinniped aggregation census | Arctocephalus gazella | [125] | |
| LemHex-44 | Photogrammetry | Megaptera novaeangliae | [81] | |
| Megaptera novaeangliae, Balaenoptera musculus, B. physalus, B. edeni, B. bonaerensis, B. borealis | [24,25] | |||
| Balaenoptera bonaerensis | [118] | |||
| Tursiops truncatus | [126] | |||
| Hex H2O TM | Behavioural study | Megaptera noveangliae | [45,127] | |
| Tursiops truncatus | [49] | |||
| DJI Matrice 600 | Line-transect survey | Tursiops aduncus | [128] | |
| Behavioural study | Orcinus orca | [67] | ||
| FreeFly Alta 6 | Photogrammetry | Megaptera novaeangliae | [81] | |
| Megaptera novaeangliae, Balaenoptera musculus, B. physalus, B. edeni, B. bonaerensis, B. borealis | [24,25] | |||
| Balaenoptera bonaerensis | [118] | |||
| Halychoerus grypus | [129] | |||
| Ptarmigan | Habitat study | Ursus maritimus | [130] | |
| Octocopter rotary-wing | APO-42 | Photogrammetry | Orcinus orca | [122] |
| Behavioural study | Grampus griseus | [131] | ||
| Delphinus delphis | [132] | |||
| Gryphon Dynamics X8-1400 | Pinniped aggregation census | Arctocephalus pusillus | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-González, M.; Suarez-Bregua, P.; Pierce, G.J.; Saavedra, C. Unmanned Aerial Vehicles (UAVs) in Marine Mammal Research: A Review of Current Applications and Challenges. Drones 2023, 7, 667. https://doi.org/10.3390/drones7110667
Álvarez-González M, Suarez-Bregua P, Pierce GJ, Saavedra C. Unmanned Aerial Vehicles (UAVs) in Marine Mammal Research: A Review of Current Applications and Challenges. Drones. 2023; 7(11):667. https://doi.org/10.3390/drones7110667
Chicago/Turabian StyleÁlvarez-González, Miguel, Paula Suarez-Bregua, Graham J. Pierce, and Camilo Saavedra. 2023. "Unmanned Aerial Vehicles (UAVs) in Marine Mammal Research: A Review of Current Applications and Challenges" Drones 7, no. 11: 667. https://doi.org/10.3390/drones7110667
APA StyleÁlvarez-González, M., Suarez-Bregua, P., Pierce, G. J., & Saavedra, C. (2023). Unmanned Aerial Vehicles (UAVs) in Marine Mammal Research: A Review of Current Applications and Challenges. Drones, 7(11), 667. https://doi.org/10.3390/drones7110667






