Using Drones to Assess Volitional Swimming Kinematics of Manta Ray Behaviors in the Wild
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Data Collection
2.3. Kinematic Analyses
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colefax, A.P.; Butcher, P.A.; Kelaher, B.P. The Potential for Unmanned Aerial Vehicles (UAVs) to Conduct Marine Fauna Surveys in Place of Manned Aircraft. ICES J. Mar. Sci. 2018, 75, 1–8. [Google Scholar] [CrossRef]
- Porter, M.E.; Ruddy, B.T.; Kajiura, S.M. Volitional Swimming Kinematics of Blacktip Sharks, Carcharhinus limbatus, in the Wild. Drones 2020, 4, 78. [Google Scholar] [CrossRef]
- Tapilatu, R.F.; Bonka, A.N.; Iwanggin, W.G.; Wona, H.; Woisiri, S.; Sembor, E.; Rumbiak, R.; Ampnir, T.; Bawole, R.; Wibbels, T. Utilizing Drone Technology to Assess Leatherback Sea Turtle (Dermochelys coriacea) Hatchling Fitness. In Proceedings of the Connections through Shallow Seas, Papua Barat, Indonesia, 6 July 2017. [Google Scholar]
- Tapilatu, R.F.; Bonka, A.N.; Iwanggin, W.G.; Wona, H.; Ampnir, T.; Rumbiak, R.; Bawole, R.; Wibbels, T. Unmanned Aerial Vehicle (UAV) Use as a Tool to Assess Crawling and Swimming Speeds in Hatchling Sea Turtles. Herpetol. Rev. 2019, 50, 722–726. [Google Scholar]
- Gray, P.C.; Fleishman, A.B.; Klein, D.J.; McKown, M.W.; Bézy, V.S.; Lohmann, K.J.; Johnston, D.W. A Convolutional Neural Network for Detecting Sea Turtles in Drone Imagery. Methods Ecol. Evol. 2019, 10, 345–355. [Google Scholar] [CrossRef]
- Schofield, G.; Esteban, N.; Katselidis, K.A.; Hays, G.C. Drones for Research on Sea Turtles and Other Marine Vertebrates–A Review. Biol. Conserv. 2019, 238, 108214. [Google Scholar] [CrossRef]
- Odzer, M.N.; Brooks, A.M.L.; Heithaus, M.R.; Whitman, E.R. Effects of Environmental Factors on the Detection of Subsurface Green Turtles in Aerial Drone Surveys. Wildl. Res. 2022, 49, 79–88. [Google Scholar] [CrossRef]
- Koski, W.R.; Gamage, G.; Davis, A.R.; Mathews, T.; LeBlanc, B.; Ferguson, S.H. Evaluation of UAS for Photographic Re-Identification of Bowhead Whales, Balaena mysticetus. J. Unmanned Veh. Syst. 2015, 3, 22–29. [Google Scholar] [CrossRef]
- Durban, J.W.; Fearnbach, H.; Barrett-Lennard, L.G.; Perryman, W.L.; Leroi, D.J. Photogrammetry of Killer Whales Using a Small Hexacopter Launched at Sea. J. Unmanned Veh. Syst. 2015, 3, 131–135. [Google Scholar] [CrossRef]
- Gough, W.T.; Segre, P.S.; Bierlich, K.C.; Cade, D.E.; Potvin, J.; Fish, F.E.; Dale, J.; di Clemente, J.; Friedlaender, A.S.; Johnston, D.W.; et al. Scaling of Swimming Performance in Baleen Whales. J. Exp. Biol. 2019, 222, jeb204172. [Google Scholar] [CrossRef] [PubMed]
- Fettermann, T.; Fiori, L.; Gillman, L.; Stockin, K.A.; Bollard, B. Drone Surveys Are More Accurate Than Boat-Based Surveys of Bottlenose Dolphins (Tursiops Truncatus). Drones 2022, 6, 82. [Google Scholar] [CrossRef]
- Butcher, P.A.; Colefax, A.P.; Gorkin, R.A., III; Kajiura, S.M.; López, N.A.; Mourier, J.; Purcell, C.R.; Skomal, G.B.; Tucker, J.P.; Walsh, A.J.; et al. The Drone Revolution of Shark Science: A Review. Drones 2021, 5, 8. [Google Scholar] [CrossRef]
- Oleksyn, S.; Tosetto, L.; Raoult, V.; Joyce, K.E.; Williamson, J.E. Going Batty: The Challenges and Opportunities of Using Drones to Monitor the Behaviour and Habitat Use of Rays. Drones 2021, 5, 12. [Google Scholar] [CrossRef]
- Kiszka, J.J.; Mourier, J.; Gastrich, K.; Heithaus, M.R. Using Unmanned Aerial Vehicles (UAVs) to Investigate Shark and Ray Densities in a Shallow Coral Lagoon. Mar. Ecol. Prog. Ser. 2016, 560, 237–242. [Google Scholar] [CrossRef]
- Bevan, E.; Whiting, S.; Tucker, T.; Guinea, M.; Raith, A.; Douglas, R. Measuring Behavioral Responses of Sea Turtles, Saltwater Crocodiles, and Crested Terns to Drone Disturbance to Define Ethical Operating Thresholds. PLoS ONE 2018, 13, e0194460. [Google Scholar] [CrossRef] [PubMed]
- Hensel, E.; Wenclawski, S.; Layman, C. Using a Small, Consumer Grade Drone to Identify and Count Marine Megafauna in Shallow Habitats. Lat. Am. J. Aquat. Res. 2018, 46, 1025–1033. [Google Scholar] [CrossRef]
- Gallagher, A.J.; Papastamatiou, Y.P.; Barnett, A. Apex Predatory Sharks and Crocodiles Simultaneously Scavenge a Whale Carcass. J. Ethol. 2018, 36, 205–209. [Google Scholar] [CrossRef]
- Colefax, A.P.; Butcher, P.A.; Pagendam, D.E.; Kelaher, B.P. Reliability of Marine Faunal Detections in Drone-Based Monitoring. Ocean. Coast. Manag. 2019, 174, 108–115. [Google Scholar] [CrossRef]
- Kelaher, B.P.; Peddemors, V.M.; Hoade, B.; Colefax, A.P.; Butcher, P.A. Comparison of Sampling Precision for Nearshore Marine Wildlife Using Unmanned and Manned Aerial Surveys. J. Unmanned Veh. Syst. 2019, 8, 30–43. [Google Scholar] [CrossRef]
- Frixione, M.G.; Gómez García Gauger, M.d.J.; Gauger, M.F.W. Drone Imaging of Elasmobranchs: Whale Sharks and Golden Cownose Rays Co-Occurrence in a Zooplankton Hot-Spot in Southwestern Sea of Cortez. Food Webs 2020, 24, e00155. [Google Scholar] [CrossRef]
- Barreto, J.; Cajaíba, L.; Teixeira, J.B.; Nascimento, L.; Giacomo, A.; Barcelos, N.; Fettermann, T.; Martins, A. Drone-Monitoring: Improving the Detectability of Threatened Marine Megafauna. Drones 2021, 5, 14. [Google Scholar] [CrossRef]
- Pirotta, V.; Hocking, D.P.; Iggleden, J.; Harcourt, R. Drone Observations of Marine Life and Human–Wildlife Interactions off Sydney, Australia. Drones 2022, 6, 75. [Google Scholar] [CrossRef]
- Marshall, A.D.; Compagno, L.J.V.; Bennett, M.B. Redescription of the Genus Manta with Resurrection of Manta Alfredi (Krefft, 1868) (Chondrichthyes; Myliobatoidei; Mobulidae). Zootaxa 2009, 2301, 1–28. [Google Scholar] [CrossRef]
- Stevens, G.; Fernando, D.; Di Sciara, G.N. Guide to the Manta and Devil Rays of the World; Princeton University Press: Princeton, NJ, USA, 2018; ISBN 9780691183329. [Google Scholar]
- Fish, F.E.; Schreiber, C.M.; Moored, K.W.; Liu, G.; Dong, H.; Bart-Smith, H. Hydrodynamic Performance of Aquatic Flapping: Efficiency of Underwater Flight in the Manta. Aerospace 2016, 3, 20. [Google Scholar] [CrossRef] [Green Version]
- Jaine, F.R.A.; Rohner, C.A.; Weeks, S.J.; Couturier, L.I.E.; Bennett, M.B.; Townsend, K.A.; Richardson, A.J. Movements and Habitat Use of Reef Manta Rays off Eastern Australia: Offshore Excursions, Deep Diving and Eddy Affinity Revealed by Satellite Telemetry. Mar. Ecol. Prog. Ser. 2014, 510, 73–86. [Google Scholar] [CrossRef] [Green Version]
- Germanov, E.S.; Marshall, A.D. Running the Gauntlet: Regional Movement Patterns of Manta Alfredi through a Complex of Parks and Fisheries. PLoS ONE 2014, 9, e110071. [Google Scholar]
- Armstrong, A.O.; Armstrong, A.J.; Bennett, M.B.; Richardson, A.J.; Townsend, K.A.; Dudgeon, C.L. Photographic Identification and Citizen Science Combine to Reveal Long Distance Movements of Individual Reef Manta Rays Mobula Alfredi along Australia’s East Coast. Mar. Biodivers. Rec. 2019, 12, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.L.; McGregor, P.K.; Oates, Y.; Stevens, G.M.W. Gone with the Wind: Seasonal Distribution and Habitat Use by the Reef Manta Ray (Mobula alfredi) in the Maldives, Implications for Conservation. Aquat. Conserv. 2020, 30, 1649–1664. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Armstrong, A.O.; McGregor, F.; Richardson, A.J.; Bennett, M.B.; Townsend, K.A.; Hays, G.C.; van Keulen, M.; Smith, J.; Dudgeon, C.L. Satellite Tagging and Photographic Identification Reveal Connectivity Between Two UNESCO World Heritage Areas for Reef Manta Rays. Front. Mar. Sci. 2020, 7, 725. [Google Scholar] [CrossRef]
- Dewar, H.; Mous, P.; Domeier, M.; Muljadi, A.; Pet, J.; Whitty, J. Movements and Site Fidelity of the Giant Manta Ray, Manta birostris, in the Komodo Marine Park, Indonesia. Mar. Biol. 2008, 155, 121–133. [Google Scholar] [CrossRef]
- Couturier, L.I.E.; Jaine, F.R.A.; Townsend, K.A.; Weeks, S.J.; Richardson, A.J.; Bennett, M.B. Distribution, Site Affinity and Regional Movements of the Manta Ray, Manta alfredi (Krefft, 1868), along the East Coast of Australia. Mar. Freshw. Res. 2011, 62, 628–637. [Google Scholar] [CrossRef] [Green Version]
- Deakos, M.H.; Baker, J.D.; Bejder, L. Characteristics of a Manta Ray (Manta alfredi) population off Maui, Hawaii, and Implications for Management. Mar. Ecol. Prog. Ser. 2011, 429, 245–260. [Google Scholar] [CrossRef] [Green Version]
- Marshall, A.D.; Dudgeon, C.L.; Bennett, M.B. Size and Structure of a Photographically Identified Population of Manta Rays (Manta alfredi) in Southern Mozambique. Mar. Biol. 2011, 158, 1111–1124. [Google Scholar] [CrossRef]
- Couturier, L.I.E.; Dudgeon, C.L.; Pollock, K.H.; Jaine, F.R.A.; Bennett, M.B.; Townsend, K.A.; Weeks, S.J.; Richardson, A.J. Population Dynamics of the Reef Manta Ray (Manta alfredi) in Eastern Australia. Coral Reefs 2014, 33, 329–342. [Google Scholar] [CrossRef] [Green Version]
- Braun, C.D.; Skomal, G.B.; Thorrold, S.R.; Berumen, M.L. Movements of the Reef Manta Ray (Manta alfredi) in the Red Sea Using Satellite and Acoustic Telemetry. Mar. Biol. 2015, 162, 2351–2362. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.D.; Beale, C.S.; Fernando, D.; Sianipar, A.B.; Burton, R.S.; Semmens, B.X.; Aburto-Oropeza, O. Spatial Ecology and Conservation of Manta birostris in the Indo-Pacific. Biol. Conserv. 2016, 200, 178–183. [Google Scholar] [CrossRef] [Green Version]
- Setyawan, E.; Sianipar, A.B.; Erdmann, M.V.; Fischer, A.M.; Haddy, J.A.; Beale, C.S.; Lewis, S.; Mambrasar, R. Site Fidelity and Movement Patterns of Reef Manta Rays (Mobula alfredi: Mobulidae) Using Passive Acoustic Telemetry in Northern Raja Ampat, Indonesia. Nat. Conserv. Res. 2018, 3, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Germanov, E.S.; Bejder, L.; Chabanne, D.B.H.; Dharmadi, D.; Hendrawan, I.G.; Marshall, A.D.; Pierce, S.J.; van Keulen, M.; Loneragan, N.R. Contrasting Habitat Use and Population Dynamics of Reef Manta Rays within the Nusa Penida Marine Protected Area, Indonesia. Front. Mar. Sci. 2019, 6, 215. [Google Scholar] [CrossRef]
- Peel, L.R.; Stevens, G.M.W.; Daly, R.; Keating Daly, C.A.; Lea, J.S.E.; Clarke, C.R.; Collin, S.P.; Meekan, M.G. Movement and Residency Patterns of Reef Manta Rays (Mobula alfredi) in the Amirante Islands, Seychelles. Mar. Ecol. Prog. Ser. 2019, 621, 169–184. [Google Scholar] [CrossRef]
- Andrzejaczek, S.; Chapple, T.K.; Curnick, D.J.; Carlisle, A.B.; Castleton, M.; Jacoby, D.M.P.; Peel, L.R.; Schallert, R.J.; Tickler, D.M.; Block, B.A. Individual Variation in Residency and Regional Movements of Reef Manta Rays (Mobula alfredi) in a Large Marine Protected Area. Mar. Ecol. Prog. Ser. 2020, 639, 137–153. [Google Scholar]
- Pate, J.H.; Marshall, A.D. Urban Manta Rays: Potential Manta Ray Nursery Habitat along a Highly Developed Florida Coastline. Endanger. Species Res. 2020, 43, 51–64. [Google Scholar]
- Venables, S.K.; van Duinkerken, D.I.; Rohner, C.A.; Marshall, A.D. Habitat Use and Movement Patterns of Reef Manta Rays Mobula alfredi in Southern Mozambique. Mar. Ecol. Prog. Ser. 2020, 634, 99–114. [Google Scholar] [CrossRef]
- Marshall, A.; Barreto, R.; Carlson, J.; Fernando, D.; Fordham, S.; Francis, M.P.; Herman, K.; Jabado, R.W.; Liu, K.M.; Pacoureau, N.; et al. Mobula alfredi; The IUCN Red List of Threatened Species, 2019: E.T195459A68632178. Available online: https://dx.doi.org/10.2305/IUCN.UK.2019-3.RLTS.T195459A68632178.en (accessed on 24 February 2022).
- Marshall, A.; Barreto, R.; Carlson, J.; Fernando, D.; Fordham, S.; Francis, M.P.; Derrick, D.; Herman, K.; Jabado, R.W.; Liu, K.M.; et al. Mobula alfredi; The IUCN Red List of Threatened Species, 2020: E.T198921A68632946. Available online: https://dx.doi.org/10.2305/IUCN.UK.2020-3.RLTS.T198921A68632946.en (accessed on 24 February 2022).
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davidson, L.N.; Fordham, S.V.; Francis, M.P.; et al. Extinction Risk and Conservation of the World’s Sharks and Rays. Elife 2014, 3, e00590. [Google Scholar] [CrossRef] [Green Version]
- Croll, D.A.; Dewar, H.; Dulvy, N.K.; Fernando, D.; Francis, M.P.; Galván-Magaña, F.; Hall, M.; Heinrichs, S.; Marshall, A.; Mccauley, D.; et al. Vulnerabilities and Fisheries Impacts: The Uncertain Future of Manta and Devil Rays. Aquat. Conserv. 2016, 26, 562–575. [Google Scholar] [CrossRef] [Green Version]
- Lawson, J.M.; Fordham, S.V.; O’Malley, M.P.; Davidson, L.N.K.; Walls, R.H.L.; Heupel, M.R.; Stevens, G.; Fernando, D.; Budziak, A.; Simpfendorfer, C.A.; et al. Sympathy for the Devil: A Conservation Strategy for Devil and Manta Rays. PeerJ 2017, 5, e3027. [Google Scholar] [CrossRef]
- Couturier, L.I.E.; Marshall, A.D.; Jaine, F.R.A.; Kashiwagi, T.; Pierce, S.J.; Townsend, K.A.; Weeks, S.J.; Bennett, M.B.; Richardson, A.J. Biology, Ecology and Conservation of the Mobulidae. J. Fish Biol. 2012, 80, 1075–1119. [Google Scholar] [CrossRef]
- Stewart, J.D.; Jaine, F.R.A.; Armstrong, A.J.; Armstrong, A.O.; Bennett, M.B.; Burgess, K.B.; Couturier, L.I.E.; Croll, D.A.; Cronin, M.R.; Deakos, M.H.; et al. Research Priorities to Support Effective Manta and Devil Ray Conservation. Front. Mar. Sci. 2018, 5, 314. [Google Scholar] [CrossRef] [Green Version]
- Perryman, R.J.Y. Social Organisation, Social Behaviour and Collective Movements in Reef Manta Rays. Ph.D. Thesis, Macquarie University, Sydney, Australia, 2020. [Google Scholar]
- Setyawan, E.; Erdmann, M.V.; Lewis, S.A.; Mambrasar, R.; Hasan, A.W.; Templeton, S.; Beale, C.S.; Sianipar, A.B.; Shidqi, R.; Heuschkel, H.; et al. Natural History of Manta Rays in the Bird’s Head Seascape, Indonesia, with an Analysis of the Demography and Spatial Ecology of Mobula alfredi (Elasmobranchii: Mobulidae). J. Ocean Sci. Found. 2020, 36, 49–83. [Google Scholar]
- Setyawan, E.; Stevenson, B.C.; Izuan, M.; Constantine, R.; Erdmann, M.V. How Big Is That Manta Ray? A Novel and Non-Invasive Method for Measuring Reef Manta Rays Using Small Drones. Drones 2022, 6, 63. [Google Scholar] [CrossRef]
- Graham, R.T.; Witt, M.J.; Castellanos, D.W.; Remolina, F.; Maxwell, S.; Godley, B.J.; Hawkes, L.A. Satellite Tracking of Manta Rays Highlights Challenges to Their Conservation. PLoS ONE 2012, 7, e36834. [Google Scholar]
- Armstrong, A.O.; Armstrong, A.J.; Jaine, F.R.A.; Couturier, L.I.E.; Fiora, K.; Uribe-Palomino, J.; Weeks, S.J.; Townsend, K.A.; Bennett, M.B.; Richardson, A.J. Prey Density Threshold and Tidal Influence on Reef Manta Ray Foraging at an Aggregation Site on the Great Barrier Reef. PLoS ONE 2016, 11, e0153393. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, A.O.; Stevens, G.M.W.; Townsend, K.A.; Murray, A.; Bennett, M.B.; Armstrong, A.J.; Uribe-Palomino, J.; Hosegood, P.; Dudgeon, C.L.; Richardson, A.J. Reef Manta Rays Forage on Tidally Driven, High Density Zooplankton Patches in Hanifaru Bay, Maldives. PeerJ 2021, 9, e11992. [Google Scholar] [CrossRef]
- Fish, F.E.; Kolpas, A.; Crossett, A.; Dudas, M.A.; Moored, K.W.; Bart-Smith, H. Kinematics of Swimming of the Manta Ray: Three-Dimensional Analysis of Open-Water Maneuverability. J. Exp. Biol. 2018, 221, jeb166041. [Google Scholar] [CrossRef] [Green Version]
- Hinojosa-Alvarez, S.; Walter, R.P.; Diaz-Jaimes, P.; Galván-Magaña, F.; Paig-Tran, E.M. A Potential Third Manta Ray Species near the Yucatán Peninsula? Evidence for a Recently Diverged and Novel Genetic Manta Group from the Gulf of Mexico. PeerJ 2016, 4, e2586. [Google Scholar] [CrossRef] [Green Version]
- Hosegood, J.; Humble, E.; Ogden, R.; de Bruyn, M.; Creer, S.; Stevens, G.M.W.; Abudaya, M.; Bassos-Hull, K.; Bonfil, R.; Fernando, D.; et al. Phylogenomics and Species Delimitation for Effective Conservation of Manta and Devil Rays. Mol. Ecol. 2020, 29, 4783–4796. [Google Scholar] [CrossRef]
- Marshall, A.D.; Pierce, S.J. The Use and Abuse of Photographic Identification in Sharks and Rays. J. Fish Biol. 2012, 80, 1361–1379. [Google Scholar] [CrossRef]
- Deakos, M.H. Paired-Laser Photogrammetry as a Simple and Accurate System for Measuring the Body Size of Free-Ranging Manta Rays (Manta alfredi). Aquat. Biol. 2010, 10, 1–10. [Google Scholar] [CrossRef]
- Kinovea. Available online: http://www.kinovea.org (accessed on 1 July 2021).
- Vogel, S. Comparative Biomechanics: Life’s Physical World-Second Edition; Princeton University Press: Princeton, NJ, USA, 2013; ISBN 9780691155661. [Google Scholar]
- Jodice, P.G.R.; Roby, D.D.; Suryan, R.M.; Irons, D.B.; Kaufman, A.M.; Turco, K.R.; Visser, G.H. Variation in Energy Expenditure among Black-Legged Kittiwakes: Effects of Activity-Specific Metabolic Rates and Activity Budgets. Physiol. Biochem. Zool. 2003, 76, 375–388. [Google Scholar] [CrossRef]
- Paig-Tran, E.W.M.; Bizzarro, J.J.; Strother, J.A.; Summers, A.P. Bottles as Models: Predicting the Effects of Varying Swimming Speed and Morphology on Size Selectivity and Filtering Efficiency in Fishes. J. Exp. Biol. 2011, 214, 1643–1654. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, S.L.; Porter, M.E. Body and Pectoral Fin Kinematics During Routine Yaw Turning in Bonnethead Sharks (Sphyrna tiburo). Integr. Comp. Biol. 2019, 1, 1–15. [Google Scholar]
- Papastamatiou, Y.P.; DeSalles, P.A.; McCauley, D.J. Area-Restricted Searching by Manta Rays and Their Response to Spatial Scale in Lagoon Habitats. Mar. Ecol. Prog. Ser. 2012, 456, 233–244. [Google Scholar] [CrossRef]
- Germanov, E.S.; Pierce, S.J.; Marshall, A.D.; Hendrawan, G.; Kefi, A.; Bejder, L.; Loneragan, N.R. Residency, movement patterns, behavior and demographics of reef manta rays in Komodo National Park. PeerJ 2022, submitted.
- Papastamatiou, Y.P.; Iosilevskii, G.; Di Santo, V.; Huveneers, C.; Hattab, T.; Planes, S.; Ballesta, L.; Mourier, J. Sharks Surf the Slope: Current Updrafts Reduce Energy Expenditure for Aggregating Marine Predators. J. Anim. Ecol. 2021, 90, 10. [Google Scholar] [CrossRef]
- Pate, J.H.; (Marine Megafauna Foundation, West Palm Beach, FL, USA). Unpublished work on Nortek ECO ADCP deployment. 2020.
- Venables, S. Short-Term Behavioural Responses of Manta Rays, Manta alfredi, to Tourism Interactions in Coral Bay, Western Australia. Bachelor’s Thesis, Murdoch University, Murdoch, Australia, 2013. [Google Scholar]
- Venables, S.; McGregor, F.; Brain, L.; van Keulen, M. Manta Ray Tourism Management, Precautionary Strategies for a Growing Industry: A Case Study from the Ningaloo Marine Park, Western Australia. Pac. Conserv. Biol. 2016, 22, 295–300. [Google Scholar] [CrossRef]
- Noren, S.R.; Biedenbach, G.; Edwards, E.F. Ontogeny of Swim Performance and Mechanics in Bottlenose Dolphins (Tursiops truncatus). J. Exp. Biol. 2006, 209, 4724–4731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, A.D.; Bennett, M.B. Reproductive Ecology of the Reef Manta Ray Manta alfredi in Southern Mozambique. J. Fish Biol. 2010, 77, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.M.W.; Hawkins, J.P.; Roberts, C.M. Courtship and Mating Behaviour of Manta Rays Mobula alfredi and M. Birostris in the Maldives. J. Fish Biol. 2018, 93, 344–359. [Google Scholar] [CrossRef] [Green Version]
- Leos-Barajas, V.; Photopoulou, T.; Langrock, R.; Patterson, T.A.; Watanabe, Y.Y.; Murgatroyd, M.; Papastamatiou, Y.P. Analysis of Animal Accelerometer Data Using Hidden Markov Models. Methods Ecol. Evol. 2017, 8, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Whoriskey, K.; Auger-Méthé, M.; Albertsen, C.M.; Whoriskey, F.G.; Binder, T.R.; Krueger, C.C.; Mills Flemming, J. A Hidden Markov Movement Model for Rapidly Identifying Behavioral States from Animal Tracks. Ecol. Evol. 2017, 7, 2112–2121. [Google Scholar] [CrossRef] [Green Version]
- Brewster, L.R.; Dale, J.J.; Guttridge, T.L.; Gruber, S.H.; Hansell, A.C.; Elliott, M.; Cowx, I.G.; Whitney, N.M.; Gleiss, A.C. Development and Application of a Machine Learning Algorithm for Classification of Elasmobranch Behaviour from Accelerometry Data. Mar. Biol. 2018, 165, 62. [Google Scholar] [CrossRef] [Green Version]
- Divi, R.V.; Strother, J.A.; Paig-Tran, E.W.M. Manta Rays Feed Using Ricochet Separation, a Novel Nonclogging Filtration Mechanism. Sci. Adv. 2018, 4, eaat9533. [Google Scholar] [CrossRef] [Green Version]
- Lawson, C.L.; Halsey, L.G.; Hays, G.C.; Dudgeon, C.L.; Payne, N.L.; Bennett, M.B.; White, C.R.; Richardson, A.J. Powering Ocean Giants: The Energetics of Shark and Ray Megafauna. Trends Ecol. Evol. 2019, 34, 1009–1021. [Google Scholar] [CrossRef]
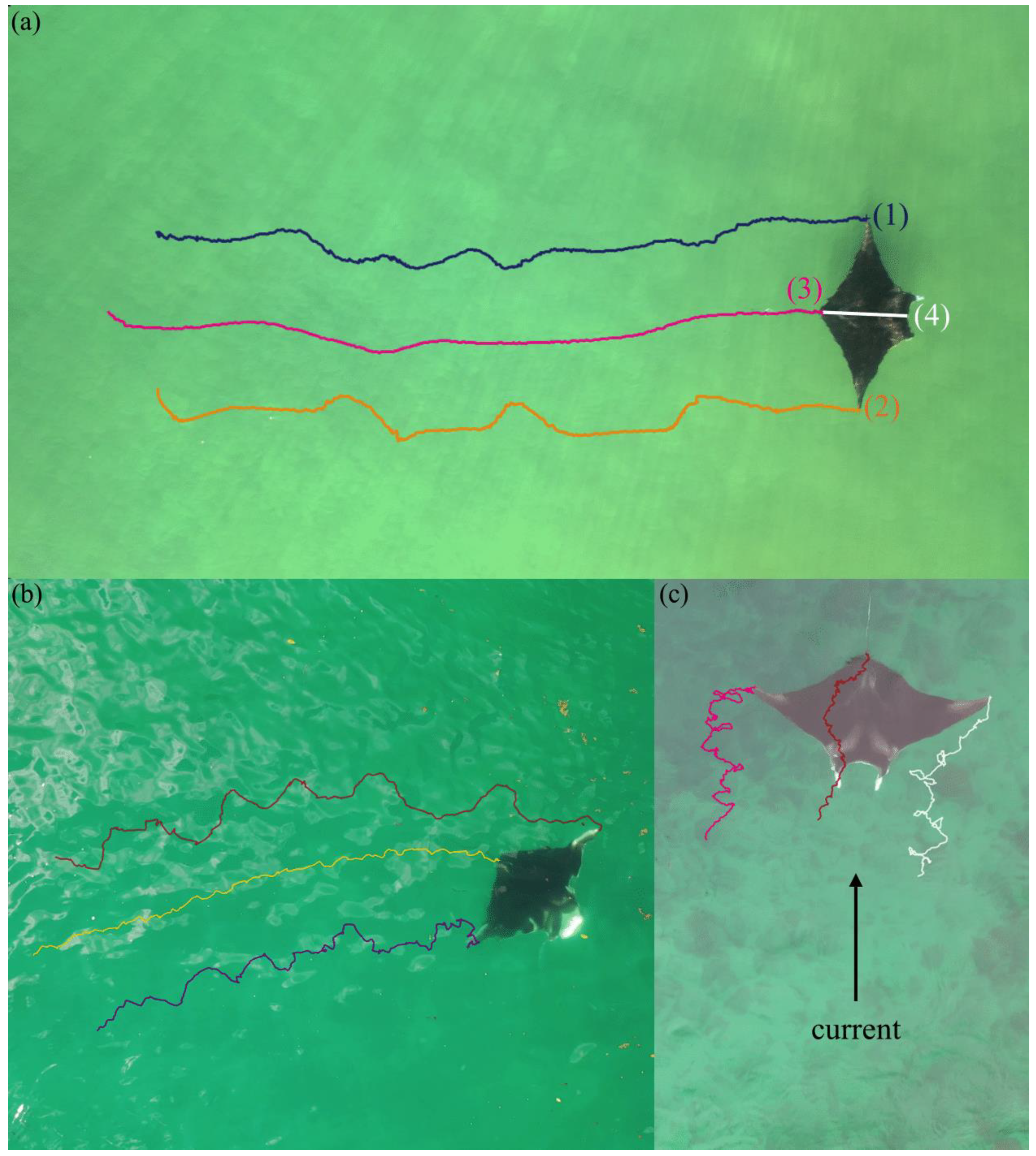
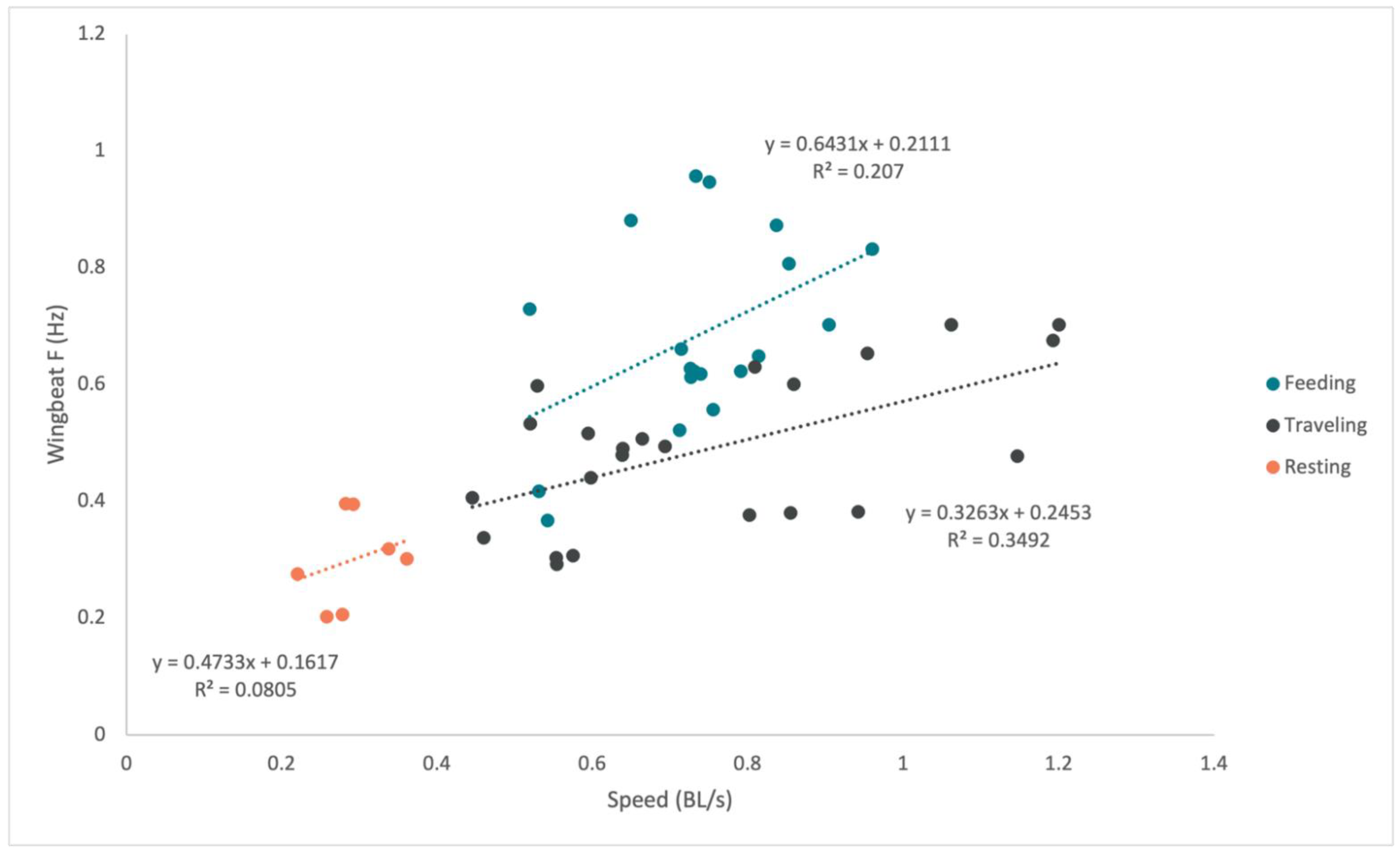
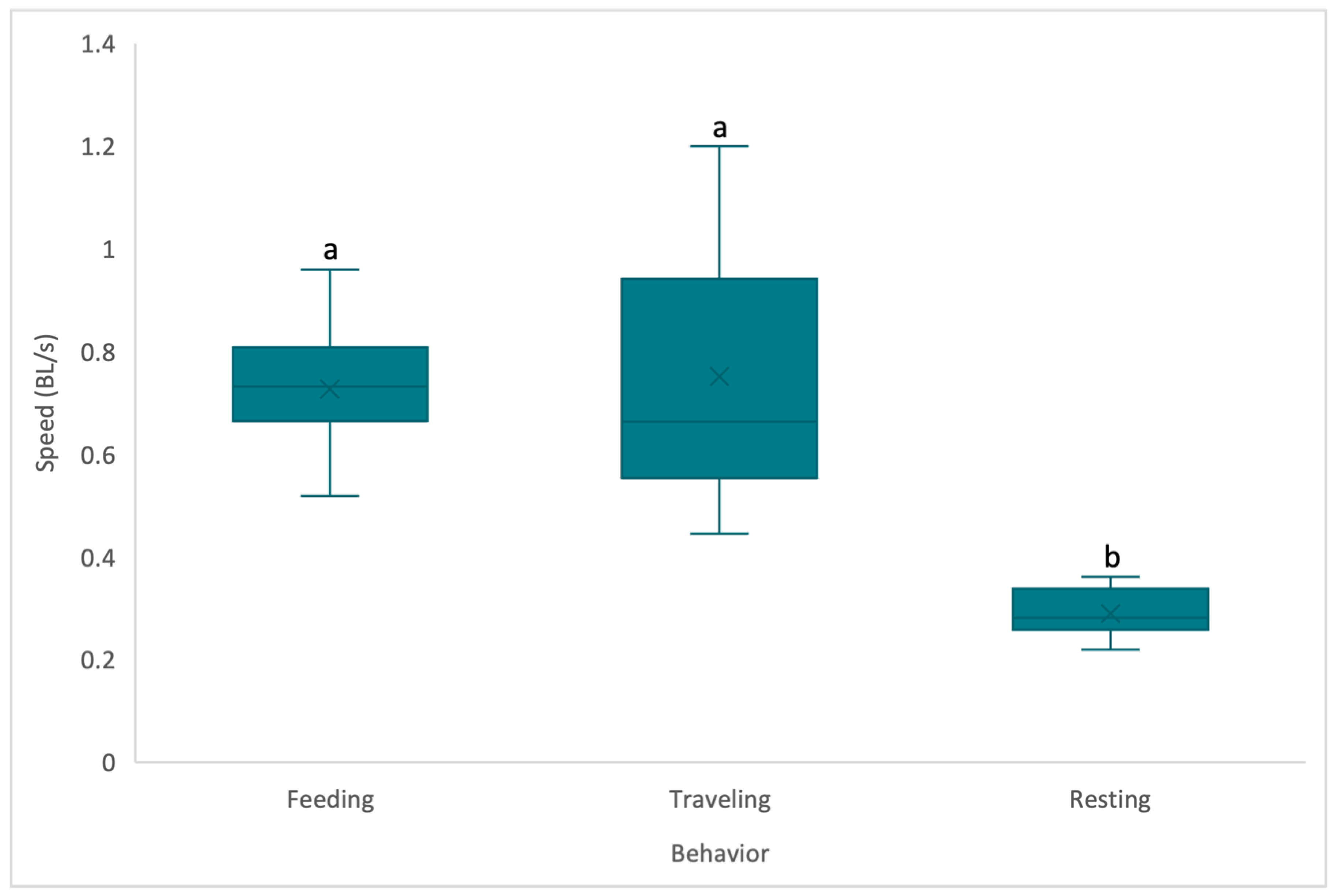
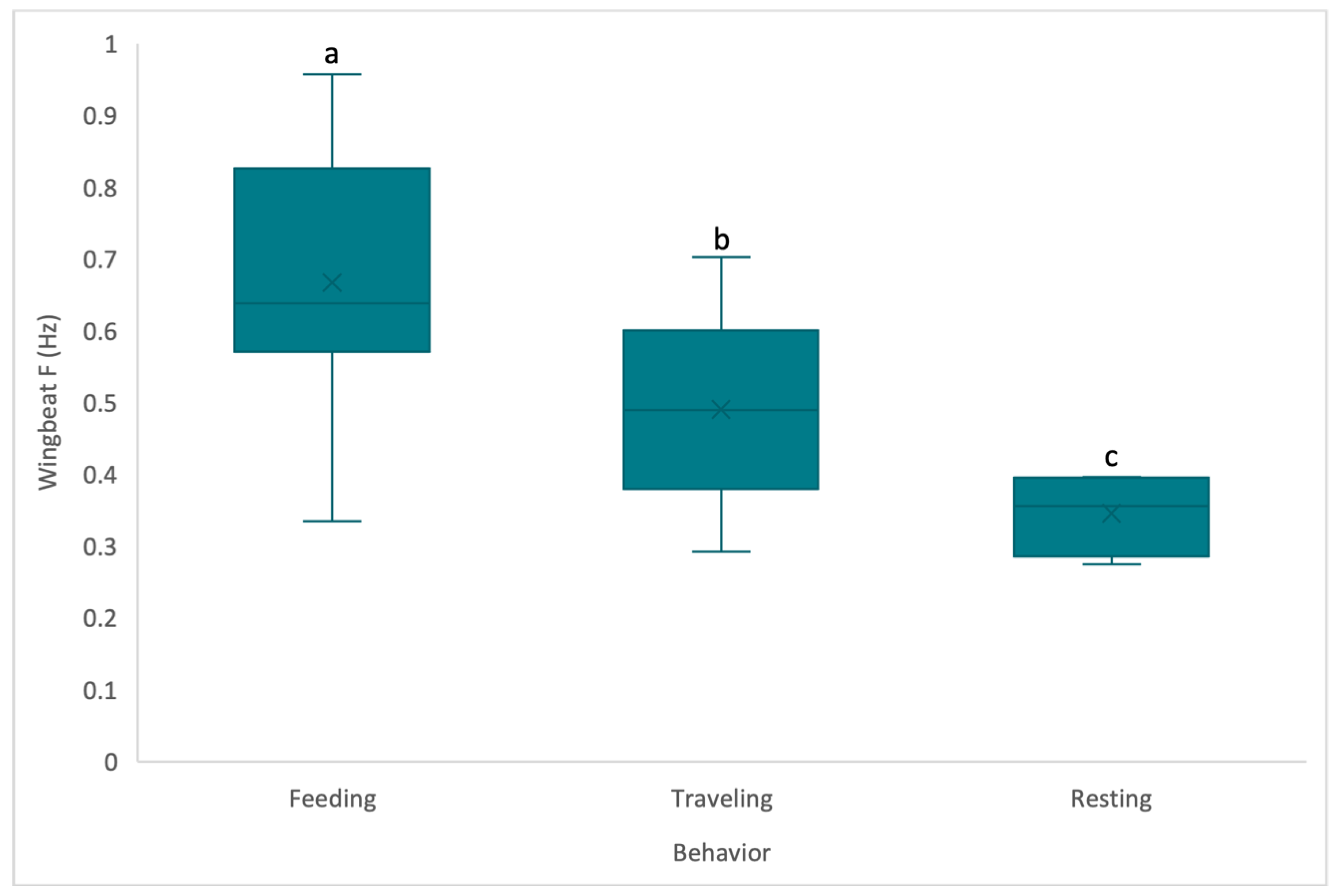
| Behavior | Description |
|---|---|
| Traveling | Cephalic fins rolled, mouth closed (if visible), maintaining directional heading while swimming |
| Feeding | Cephalic fins unrolled with tips often touching, mouth open (if visible), changing directions while swimming |
| Resting | Facing into strong current to maintain stationary position, located inside inlet |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fong, V.; Hoffmann, S.L.; Pate, J.H. Using Drones to Assess Volitional Swimming Kinematics of Manta Ray Behaviors in the Wild. Drones 2022, 6, 111. https://doi.org/10.3390/drones6050111
Fong V, Hoffmann SL, Pate JH. Using Drones to Assess Volitional Swimming Kinematics of Manta Ray Behaviors in the Wild. Drones. 2022; 6(5):111. https://doi.org/10.3390/drones6050111
Chicago/Turabian StyleFong, Vicky, Sarah L. Hoffmann, and Jessica H. Pate. 2022. "Using Drones to Assess Volitional Swimming Kinematics of Manta Ray Behaviors in the Wild" Drones 6, no. 5: 111. https://doi.org/10.3390/drones6050111
APA StyleFong, V., Hoffmann, S. L., & Pate, J. H. (2022). Using Drones to Assess Volitional Swimming Kinematics of Manta Ray Behaviors in the Wild. Drones, 6(5), 111. https://doi.org/10.3390/drones6050111







