Assessing the Temporal and Spatial Variability of Coffee Plantation Using RPA-Based RGB Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. RPA Flight and Image Acquisition
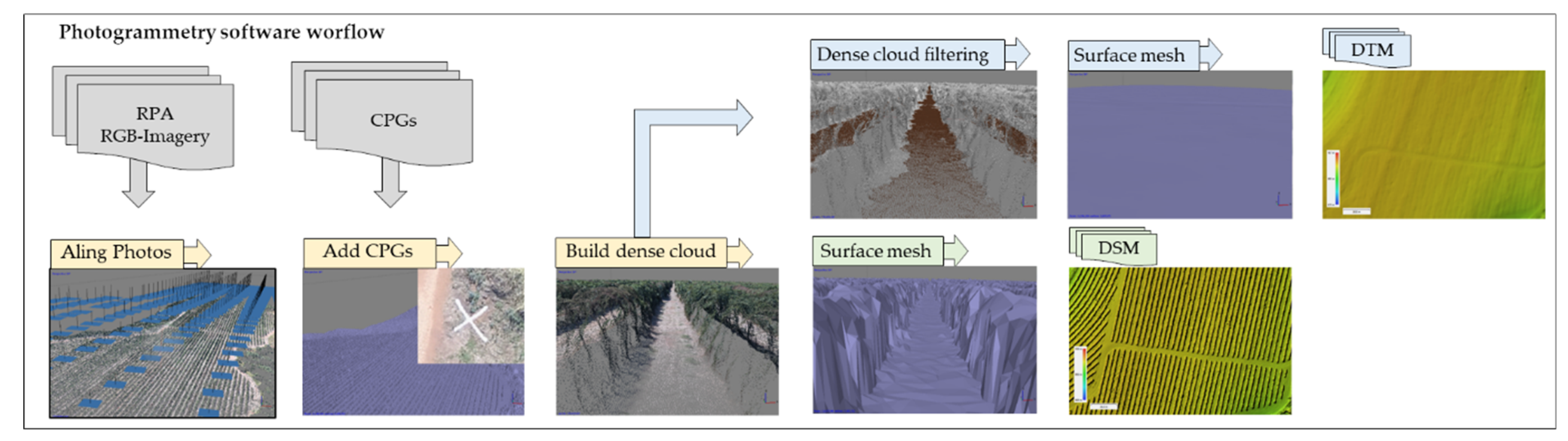
2.3. Canopy Surface Model Generation
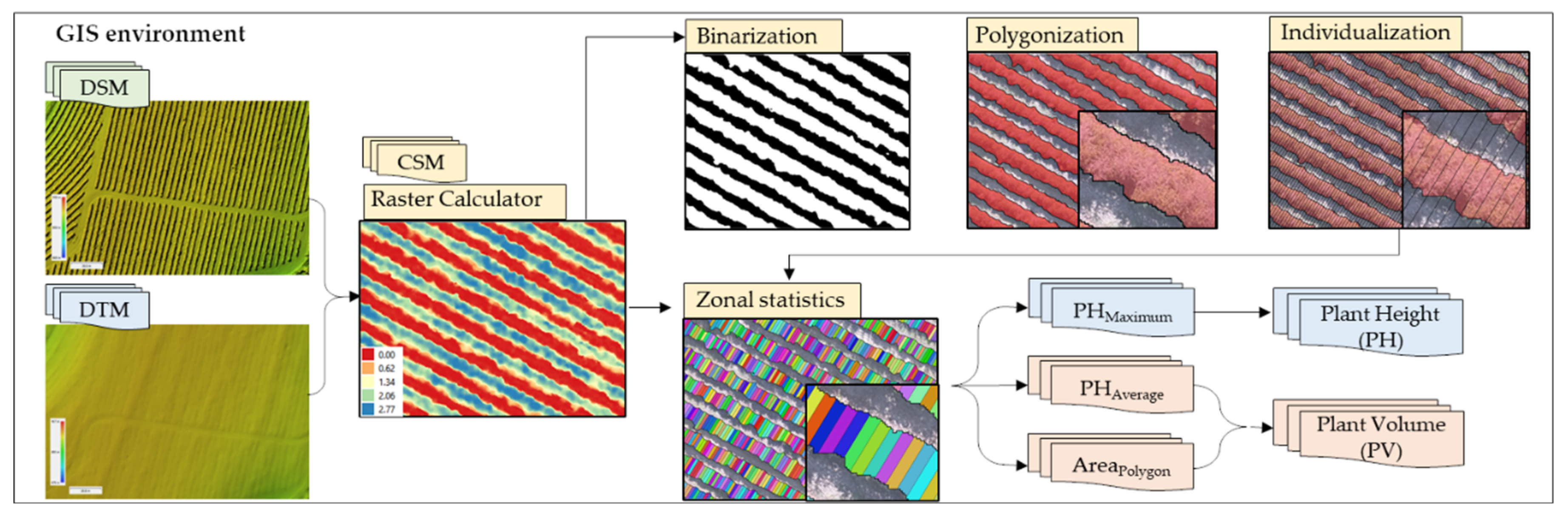
2.4. Extraction of Plant Height and Plant Volume Information
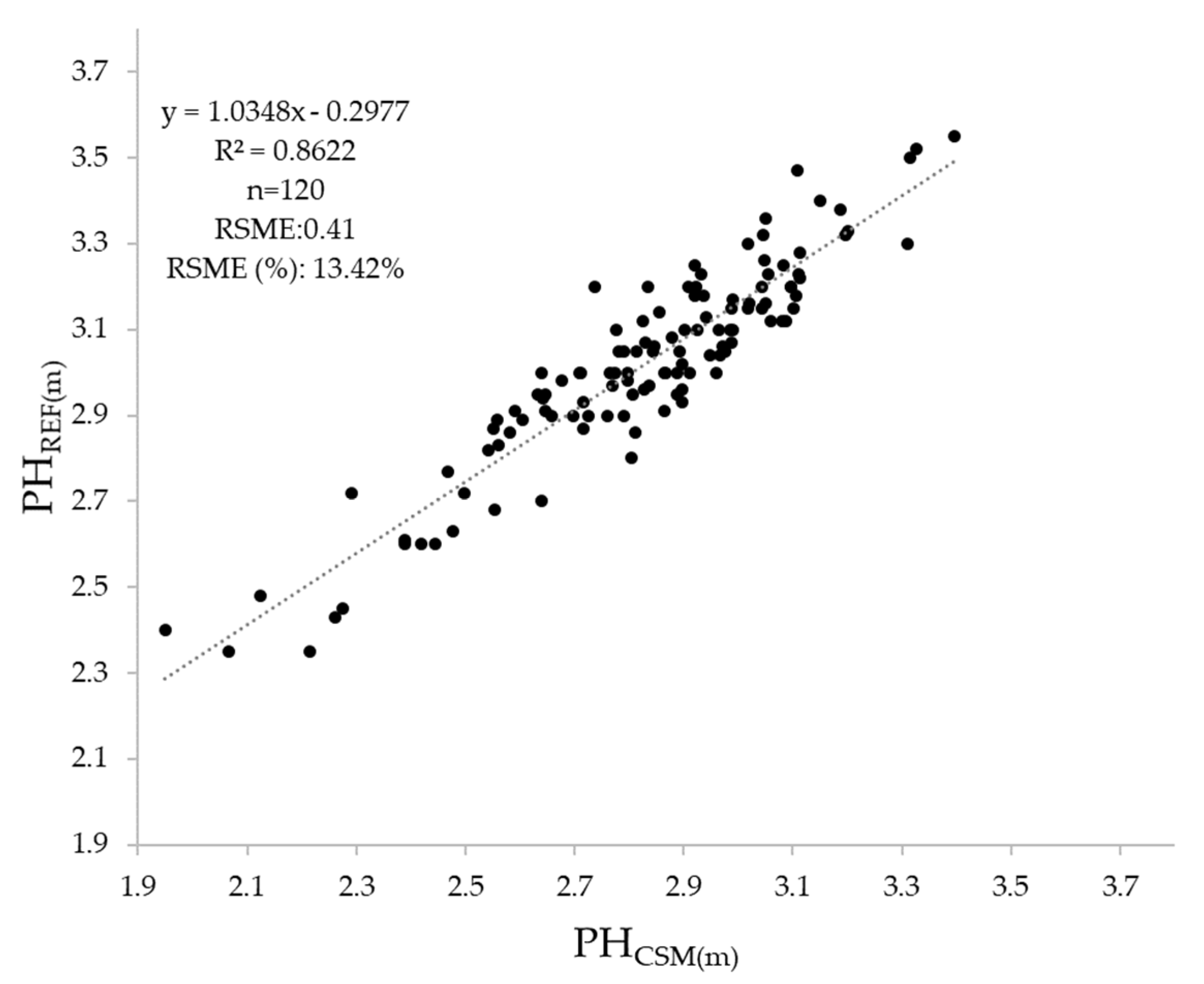
2.5. Validation of Plant Height Derived from CSMs
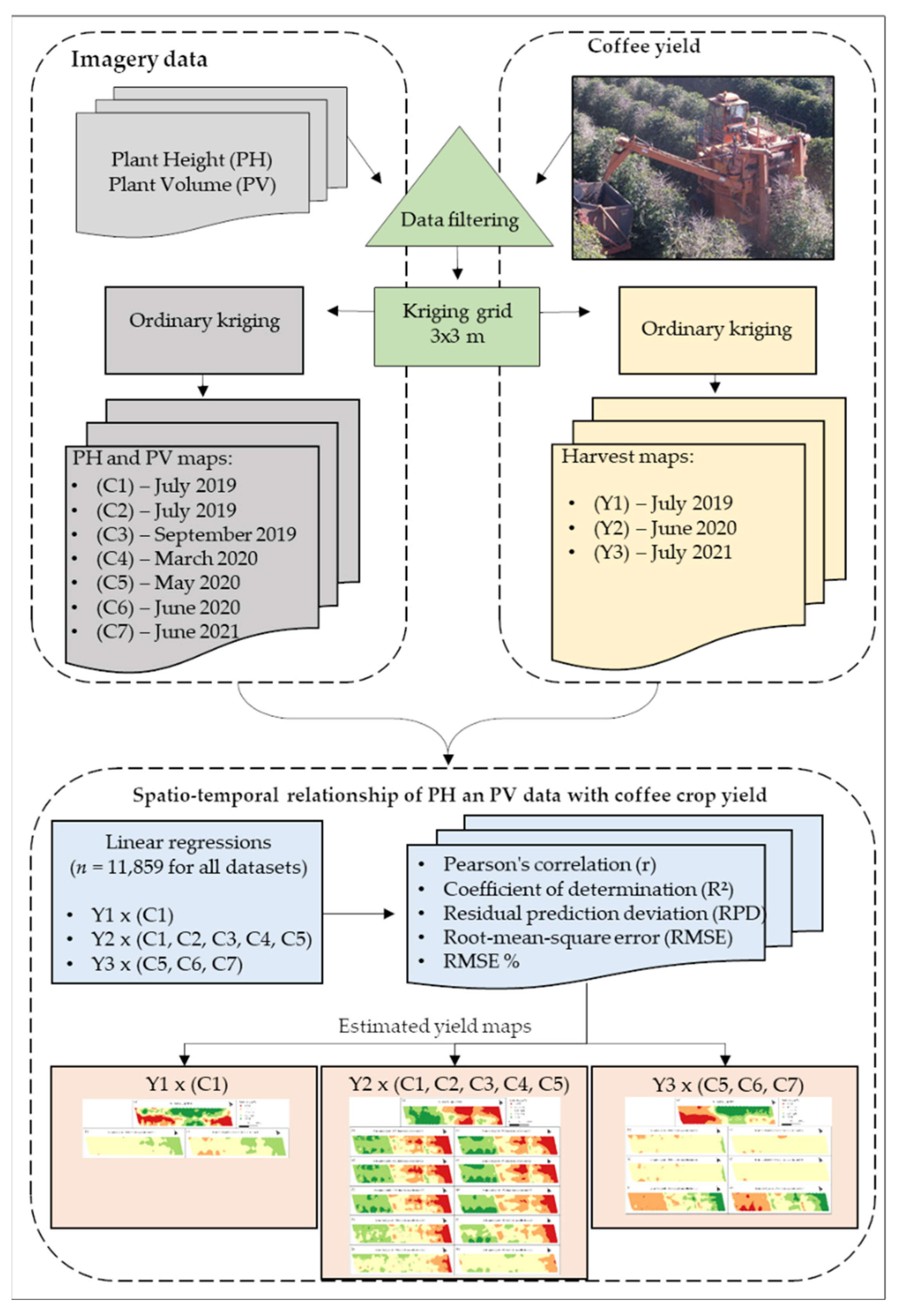
2.6. Spatio-Temporal Relationship between Image Data and Coffee Crop Yield
3. Results and Discussion
3.1. Plant Height Validation
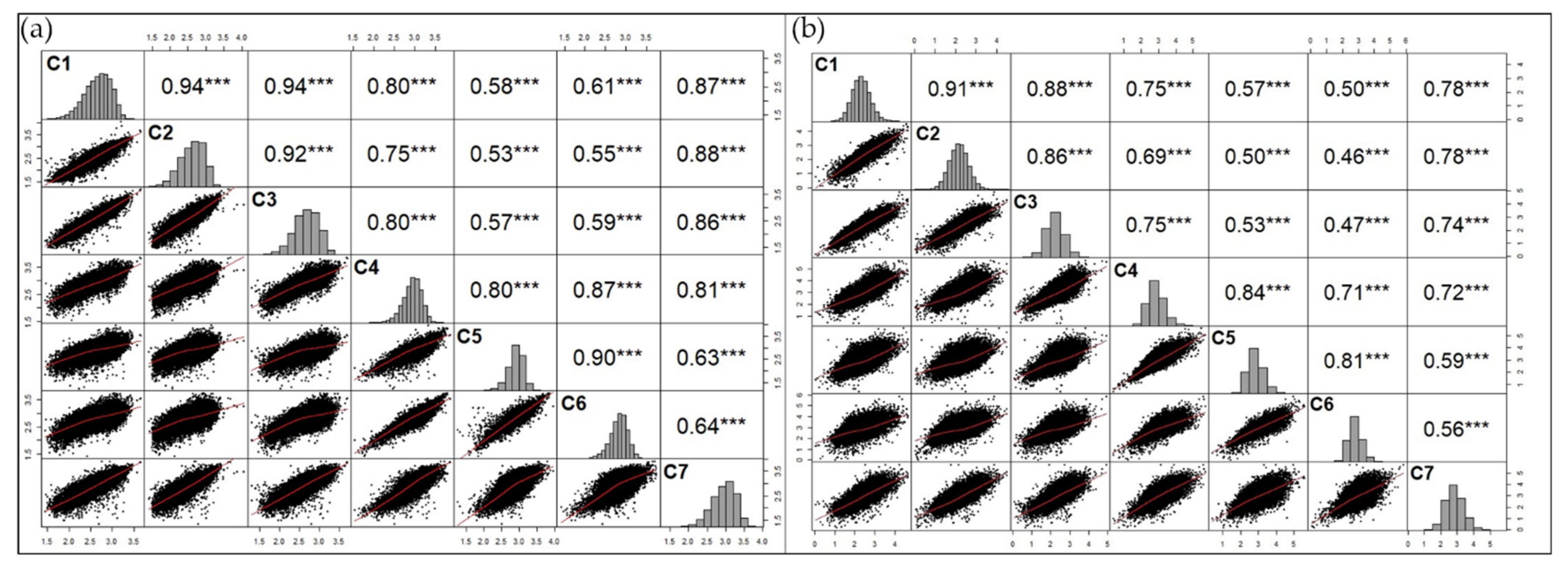
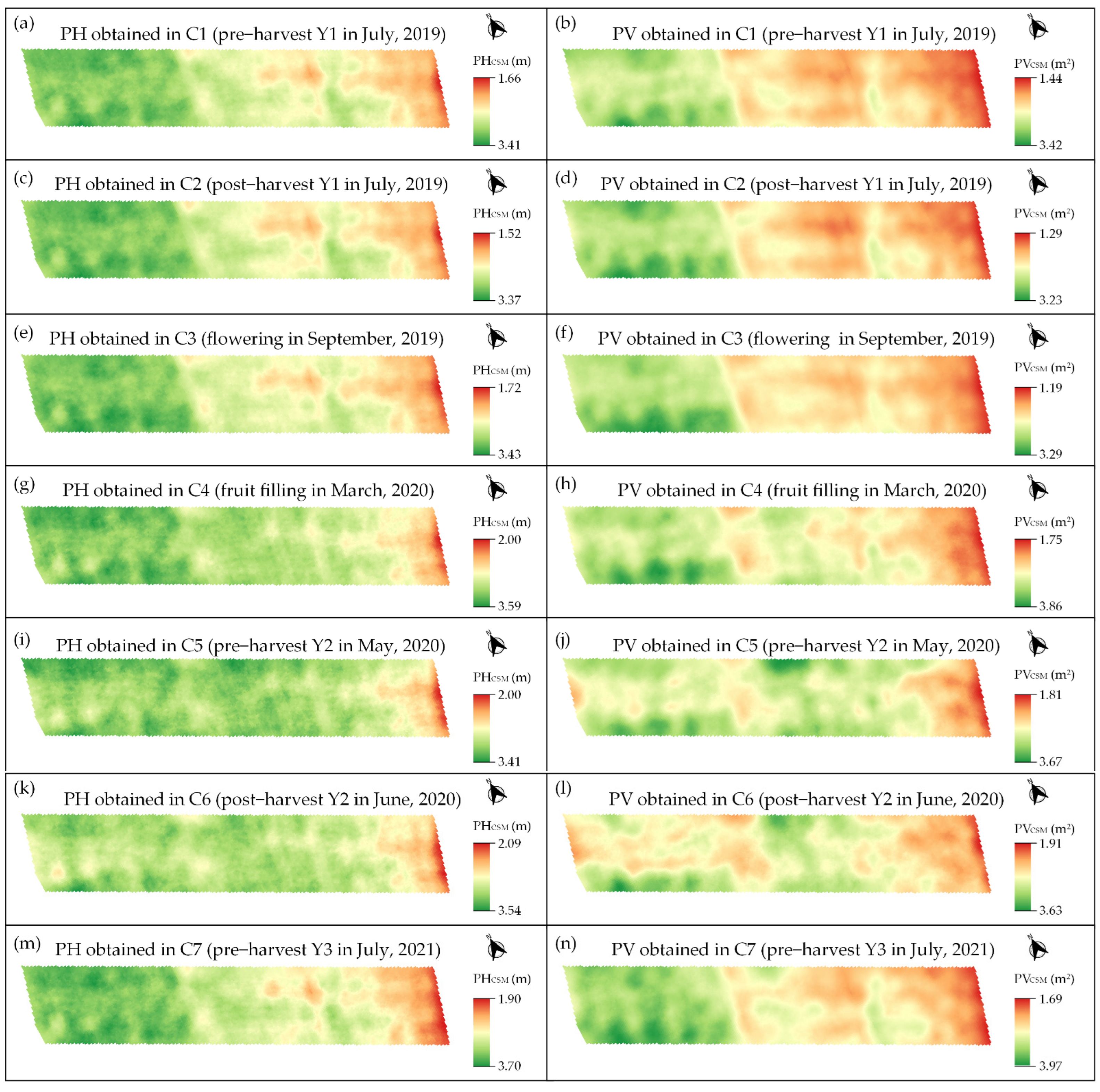
3.2. Exploratoy Analysis
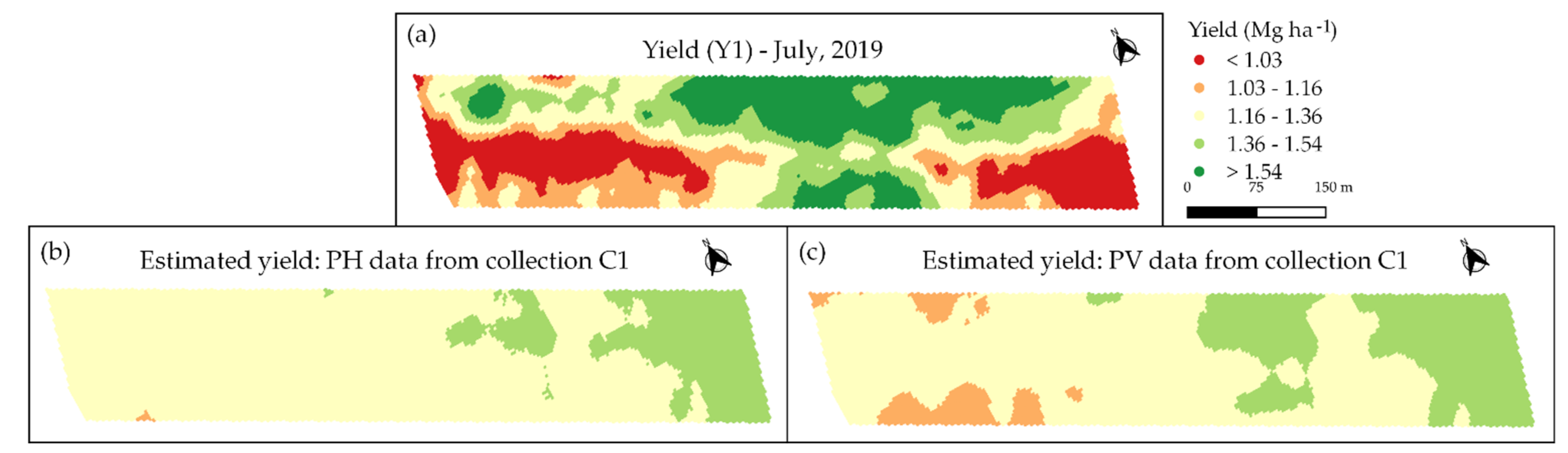
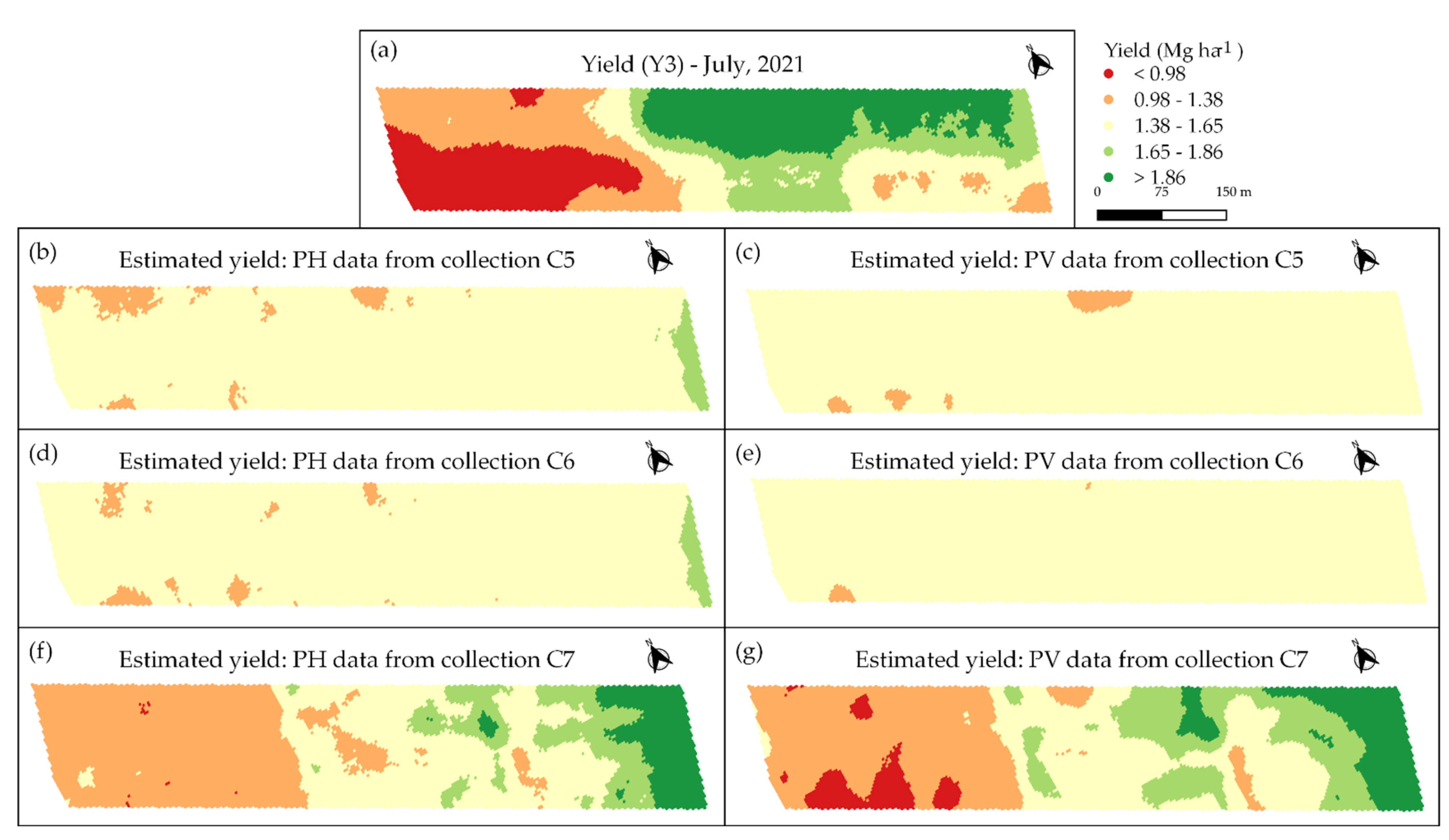
3.3. Spatio-Temporal Relationship between Image Data and Coffee Crop Yield
3.4. Limitations and Future Aspects
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mulla, D.J. Twenty five years of remote sensing in precision agriculture: Key advances and remaining knowledge gaps. Biosyst. Eng. 2013, 114, 358–371. [Google Scholar] [CrossRef]
- Florenzano, T.G. Imagens de Satélites para Estudos Ambientais; Oficina de Textos: São Paulo, Brazil, 2002. [Google Scholar]
- Gómez-Candón, D.; De Castro, A.I.; López-Granados, F. Assessing the accuracy of mosaics from unmanned aerial vehicle (UAV) imagery for precision agriculture purposes in wheat. Precis. Agric. 2014, 15, 44–56. [Google Scholar] [CrossRef]
- Sankaran, S.; Khot, L.R.; Espinoza, C.Z.; Jarolmasjed, S.; Sathuvalli, V.R.; Vandemark, G.J.; Miklas, P.N.; Carter, A.H.; PumphreY, M.O.; Knowles, R.R.N.; et al. Low-altitude, high-resolution aerial imaging systems for row and field crop phenotyping: A review. Eur. J. Agron. 2015, 70, 112–123. [Google Scholar] [CrossRef]
- Colomina, I.; Molina, P. Unmanned aerial systems for photogrammetry and remote sensing: A review. ISPRS J. Photogramm. Remote Sens. 2014, 92, 79–97. [Google Scholar] [CrossRef]
- Schirrmann, M.; Giebel, A.; Gleiniger, F.; Pflanz, M.; Lentschke, J.; Dammer, K.-H. Monitoring agronomic parameters of winter wheat crops with low-cost UAV imagery. Remote Sens. 2016, 8, 706. [Google Scholar] [CrossRef]
- Tilly, N.; Aasen, H.; Bareth, G. Fusion of plant height and vegetation indices for the estimation of barley biomass. Remote Sens. 2015, 7, 11449–11480. [Google Scholar] [CrossRef]
- Possoch, M.; Bieker, S.; Hoffmeister, D.; Bolten, A.; Schellberg, J.; Bareth, G. Multi-temporal crop surface models combined with the RGB vegetation index from UAV-based images for forage monitoring in grassland. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2016, 991–998. [Google Scholar] [CrossRef]
- Berni, J.; Zarco-Tejada, P.; Suarez, L.; Fererez, E. Thermal and narrow-band multispectral remote sensing for vegetation monitoring from an unmanned aerial vehicle. IEEE Trans. Geosci. Remote Sens. 2009, 47, 722–738. [Google Scholar] [CrossRef]
- Ballesteros, R.; Ortega, J.F.; Hernandez, D.; Moreno, M.A. Onion biomass monitoring using UAV-based RGB imaging. Precis. Agric. 2018, 19, 840–857. [Google Scholar] [CrossRef]
- Johnson, L.; Herwitz, S. Collection of ultra high spatial and spectral resolution image data over California vineyards with a small UAV. In Proceedings of the 30th International Symposium on Remote Sensing of Environment (ISRSE), Honolulu, HI, USA, 10–14 November 2003. [Google Scholar]
- Díaz-Varela, R.; De La Rosa, R.; León, L.; Zarco-Tejada, P. High-Resolution Airborne UAV Imagery to Assess Olive Tree Crown Parameters Using 3D Photo Reconstruction: Application in Breeding Trials. Remote Sens. 2015, 7, 4213–4232. [Google Scholar] [CrossRef]
- Bareth, G.; Aasen, H.; Bendig, J.; Gnyp, M.L.; Bolten, A.; Jung, A.; Michels, R.; Soukkamäki, J. Low-weight and UAV-based Hyperspectral Full-frame Cameras for Monitoring Crops: Spectral Comparison with Portable Spectroradiometer Measurements. Photogramm.—Fernerkund.—Geoinf. 2015, 1, 69–79. [Google Scholar] [CrossRef]
- Bendig, J.; Willkomm, M.; Tilly, N.; Gnyp, M.L.; Bennertz, S.; Qiang, C.; Miao, Y.; Bareth, G. Very high resolution crop surface models (CSMs) from UAV-based stereo images for rice growth monitoring in Northeast China. In Proceedings of the International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences, Rostock, Germany, 4–6 September 2013; pp. 4–6. [Google Scholar]
- Santesteban, L.G.; DI Gennaro, S.F.; Herrero-Langreo, A.; Miranda, C.; Royo, J.B.; Matese, A. High-resolution UAV-based thermal imaging to estimate the instantaneous and seasonal variability of plant water status within a vineyard. Agric. Water Manag. 2017, 183, 49–59. [Google Scholar] [CrossRef]
- Li, W.; Niu, Z.; Chen, H.; Li, D.; Wu, M.; Zhao, W. Remote estimation of canopy height and aboveground biomass of maize using high-resolution stereo images from a low-cost unmanned aerial vehicle system. Ecol. Indic. 2016, 67, 637–648. [Google Scholar] [CrossRef]
- Verhoeven, G. Taking computer vision aloft Archaeological three-dimensional reconstructions from aerialphotographs with photoscan. Archaeol. Prospect. 2011, 18, 67–73. [Google Scholar] [CrossRef]
- Bendig, J.; Bolten, A.; Bennertz, S.; Broscheit, J.; Eichfuss, S.; Bareth, G. Estimating Biomass of Barley Using Crop Surface Models (CSMs) Derived from UAV-Based RGB Imaging. Remote Sens. 2014, 6, 10395–10412. [Google Scholar] [CrossRef]
- Bendig, J.; Yu, K.; Aasen, H.; Bolten, A.; Bennertz, S.; Broscheit, J.; Gnyp, M.L.; Bareth, G. Combining UAV-based plant height from crop surface models, visible, and near infrared vegetation indices for biomass monitoring in barley. Int. J. Appl. Earth Obs. 2015, 39, 79–87. [Google Scholar] [CrossRef]
- Hoffmeister, D.; Bolten, A.; Curdt, C.; Waldhoff, G.; Bareth, G. High-resolution Crop Surface Models (CSM) and Crop Volume Models (CVM) on field level by terrestrial laser scanning. Proc. SPIE 2010, 7840, 78400E. [Google Scholar] [CrossRef]
- Da Cunha, J.P.A.R.; Sirqueira, M.A.; Hurtado, S.M.C. Estimating vegetation volume of coffee crops using images from unmanned aerial vehicles. Eng. Agrícola 2019, 39, 41–47. [Google Scholar] [CrossRef]
- Santos, L.M.; Barbosa, B.D.D.S.; Diotto, A.V.; MacieL, D.T.; Xavier, L.A.G. Biophysical parameters of coffee crop estimated by UAV RGB images. Precis. Agric. 2020, 21, 1227–1241. [Google Scholar] [CrossRef]
- Bento, N.L.; Ferraz, G.A.S.; Barata, R.A.P.; Soares, D.V.; Santos, L.M.D.; Santana, L.S.; Ferraz, P.F.P.; Conti, L.; Palchetti, E. Characterization of Recently Planted Coffee Cultivars from Vegetation Indices Obtained by a Remotely Piloted Aircraft System. Sustainability 2022, 14, 1446. [Google Scholar] [CrossRef]
- Furfaro, R.; Ganapol, B.D.; Johnson, L.F.; Herwitz, S.R. Neural network algorithm for coffee ripeness evaluation using airborne images. Appl. Eng. Agric. 2007, 23, 379–387. [Google Scholar] [CrossRef]
- Rosas, J.T.F.; de Carvalho Pinto Fd de Queiroz, D.M.; de Melo Villar, F.M.; Valente, D.S.M.; Martins, R.N. Monitoramento da maturação do café usando uma câmera multiespectral de baixo custo montada em VANT. Precisão Agric. 2022, 23, 300–318. [Google Scholar] [CrossRef]
- Johnson, L.F.; Herwitz, S.R.; Lobitz, B.M.; Dunagan, S.E. Feasibility of monitoring coffee field ripeness with airborne multispectral imagery. Appl. Eng. Agric. 2004, 20, 845–849. [Google Scholar] [CrossRef]
- Martello, M.; Molin, J.P.; Bazame, H.C. Obtaining and Validating High-Density Coffee Yield Data. Horticulturae 2022, 8, 421. [Google Scholar] [CrossRef]
- Molin, J.P.; do Amaral, L.R.; Colaço, A. Agricultura de Precisão, 1st ed.; Oficina de Textos: São Paulo, Brazil, 2015; p. 224. [Google Scholar]
- Miranda, J.M.; Reinato, R.A.O.; da Silva, A.B. Modelo matemático para previsão da produtividade do cafeeiro. Rev. Bras. De Eng. Agrícola E Ambient. Camp. Gd. 2014, 18, 353–361. [Google Scholar] [CrossRef]
- Rocha, H.G.; Da Silva, A.B.; Nogueira, D.A.; Miranda, J.M.; Mantovani, J.R. Coffee productivity mapping from mathematical models for prediction of harvest. Coffee Sci. 2016, 11, 108–116. [Google Scholar]
- Alvares, A.C.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Koppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Malambo, L.; Popescu, S.C.; Murray, S.C.; Putman, E.; Pughna Horne, D.W.; Bishop, M. Multitemporal field-based plant height estimation using 3D point cloudsgenerated from small unmanned aerial systems high-resolution imagery. Int. J. Appl. EarthObservation Geoinf. 2018, 64, 31–42. [Google Scholar] [CrossRef]
- Maldaner, L.F.; Molin, J.P. Data processing within rows for sugarcane yield mapping. Sci. Agric. 2020, 77, e20180391. [Google Scholar] [CrossRef]
- Minasny, B.; Mcbratney, A.B.; Whelan, B.M. Vesper, Version 1.62. Australian Centre for Precision Agriculture, McMillan Building A05. The University of Sydney: Sydney, Australia, 2005.
- Chang, C.W.; Laird, D.A.; Mausbach, M.J.; Hurburgh, C.R. Near-infrared reflectance spectroscopy–principal components regression analyses of soil properties. Soil Sci. Soc. Am. J. 2011, 65, 480–490. [Google Scholar] [CrossRef]
- Barbosa, B.; Ferraz, G.A.S.; Dos Santos, L.M.; Santana, L.; Marin, D.B.; Rossi, G.; Conti, L. Application of RGB images obtained by uav in coffee farming. Remote Sens. 2021, 13, 2397. [Google Scholar] [CrossRef]
- Acorsi, M.G.; Miranda, F.D.A.; Martello, M.; Smaniotto, D.A.; Sartor, L.R. Estimating biomass of black oat using UAV-based RGB imaging. Agronomy 2019, 9, 344. [Google Scholar] [CrossRef]
- Grüner, E.; Astor, T.; Wachendor, F.M. Biomass prediction of heterogeneous temperate grasslands using an SfM approach based on UAV imaging. Agronomy 2019, 9, 54. [Google Scholar] [CrossRef]
- Panagiotidis, D.; Abdollahnejad, A.; Surový, P.; Chiteculo, V. Determining tree height and crown diameter from high-resolution UAV imagery. Int. J. Remote Sens. 2016, 38, 2392–2410. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Diaz-Varela, R.; Angileri, V.; Loudjani, P. Tree Height Quantification Using Very High Resolution Imagery Acquired from an Unmanned Aerial Vehicle (UAV) and Automatic 3D Photo-Reconstruction Methods. Eur. J. Agron. 2014, 55, 89–99. [Google Scholar] [CrossRef]
- Hobart, M.; Pflanz, M.; Weltzien, C.; Schirrmann, M. Growth Height Determination of Tree Walls for Precise Monitoring in Apple Fruit Production Using UAV Photogrammetry. Remote Sens. 2020, 12, 1656. [Google Scholar] [CrossRef]
- Santinato, F. Inovações Tecnológicas para Cafeicultura de Precisão. Ph.D. Thesis, Faculdade de Ciências Agrárias e Veterinárias—UNESP, Jaboticabal, SP, Brazil, 2016; 125p. [Google Scholar]
- Cannell, M.G.R. Crop physiological aspects of coffee bean yield—A review. Kenya Coffee 1976, 41, 245–253. [Google Scholar]
- Pereira, S.O.; Bartholo, G.F.; Baliza, D.P.; Sogreira, F.M.; Guimarães, R.J. Productivity and coffee biannuality depending on the crop spacing. Pesqui. Agropecuária Bras. 2011, 46, 152–160. [Google Scholar] [CrossRef]
- Valadares, S.V.; Neves, J.C.L.; Rosa, G.N.G.P.; Martinez, H.E.P.; Venegas, V.H.A.; Lima, P.C. Productivity and biennial production of dense coffee plantations under different doses of N and K. Pesqui. Agropecuária Bras. 2013, 13, 96–303. [Google Scholar]
- Gil, E.; Escolà, A.; Rosell, J.R.; Planas, S.; Val, L. Variable rate application of plant protection products in vineyard using ultrasonic sensors. Crop Prot. 2007, 26, 1287–1297. [Google Scholar] [CrossRef]
- Siegfried, W.; Viret, O.; Huber, B.; Wohlhauser, R. Dosage of plant protection products adapted to leaf area index in viticulture. Crop Prot. 2007, 26, 73–82. [Google Scholar] [CrossRef]
- Rena, A.B.; Maestri, M. Fisiologia do cafeeiro. Inf. Agropecuário 1985, 11, 26–40. [Google Scholar]
- Colaço, A.F.; Molin, J.P.; Rosell-Polo, J.R.; Escolà, A. Spatial variability in commercial orange groves. Part 2: Relating canopy geometry to soil attributes and historical yield. Precis. Agric. 2019, 20, 805–822. [Google Scholar] [CrossRef]
- Peloso, A.F.; Tatagiba, S.D.; Amaral, J.F.T. Limitations of vegetative growth in arabica coffee plants promoted by water deficit. Rev. Eng. Na Agric. 2017, 25, 139–147. [Google Scholar]
- Pilau, F.G.; Angelocci, L.R. Patterns of interception of solar radiation by coffee trees as a function of leaf area. Coffee Sci. 2016, 11, 127–136. [Google Scholar]
- De Camargo, A.P.; de Camargo, M.B.P. Definition and outline for the phenological phases of arabic coffee under Brazilian tropical conditions. Bragantia 2001, 60, 65–68. [Google Scholar]
- Fialho, C.M.T.; Silva, G.R.; Freitas, M.A.M.; França, A.C.; Mello, C.A.D.; Silva, A.A. Competition of weeds with coffee plants, in two times of infestation. Planta Daninha 2011, 28, 969–978. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Mendes, A.N.; Botelho, C.E.; Oliveira, A.C.; Rezende, J.C.; Rezende, R.M. Agronomic performance of coffee cultivars resistant to coffee rust in Minas Gerais state, Brazil. Bragantia 2012, 71, 481–487. [Google Scholar] [CrossRef]
- Lopes, P.R.; Araújo, K.C.S.; Ferraz, J.M.G.; Lopes, I.M.; Fernandes, L.G. Producing agroecological coffee in Southern Minas Gerais: Alternative systems forintensive production of agrochemicals. Rev. Bras. Agroecol. 2012, 7, 25–38. [Google Scholar]
- Wadt, P.G.S.; Dias, J.R.M. Regional and inter-regional DRIS norms for nutritional evaluation of Conilon coffee. Pesqui. Ag-Ropecuária Bras. 2012, 47, 822–830. [Google Scholar] [CrossRef]
- Scalco, M.S.; Alvarenga, L.A.; Guimarães, R.J.; Dominghetti, A.W.; Colombo, A.; Assis, G.A.; Abreu, G.F. Leaf contents of phosphorus and zinc, productivity, and growth of irrigated coffee. Pesqui. Agropecuária Bras. 2014, 49, 95–101. [Google Scholar] [CrossRef]


| Collection | Stage | Date | Local Time | Images |
|---|---|---|---|---|
| C1 | Pre-harvest | 9 July 2019 | 11:57 | 407 |
| C2 | Post-harvest | 13 July 2019 | 11:10 | 406 |
| C3 | Flowering | 29 September 2019 | 11:02 | 401 |
| C4 | Fruit filling | 20 March 2020 | 13:47 | 401 |
| C5 | Pre-harvest | 29 May 2020 | 13:15 | 402 |
| C6 | Post-harvest | 3 June 2020 | 12:15 | 404 |
| C7 | Pre-harvest | 24 June 2021 | 12:49 | 405 |
| Variable * | Unit | n | Mean | Min | Max | SD | CV (%) | n | Mean | Min | Max | SD | CV (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw Data | Filtered Data | ||||||||||||
| PV-C1 | m3 | 51,196 | 2.30 | 0.04 | 4.64 | 0.48 | 21.10 | 27,329 | 2.31 | 1.44 | 3.42 | 0.31 | 13.50 |
| PV-C2 | m3 | 51,196 | 2.16 | 0.02 | 4.50 | 0.49 | 22.59 | 26,205 | 2.20 | 1.29 | 3.23 | 0.32 | 14.59 |
| PV-C3 | m3 | 51,196 | 2.21 | 0.02 | 4.91 | 0.49 | 22.23 | 29,441 | 2.25 | 1.19 | 3.29 | 0.36 | 15.79 |
| PV-C4 | m3 | 51,196 | 2.86 | 0.10 | 5.71 | 0.51 | 17.96 | 29,099 | 2.80 | 1.75 | 3.86 | 0.30 | 10.86 |
| PV-C5 | m3 | 51,196 | 2.84 | 0.18 | 5.58 | 0.49 | 17.42 | 28,143 | 2.79 | 1.81 | 3.67 | 0.26 | 9.31 |
| PV-C6 | m3 | 51,196 | 2.79 | 0.04 | 5.84 | 0.47 | 16.89 | 29,002 | 2.75 | 1.91 | 3.63 | 0.23 | 8.50 |
| PV-C7 | m3 | 51,196 | 2.83 | 0.02 | 5.77 | 0.58 | 20.58 | 25,495 | 2.81 | 1.69 | 3.97 | 0.36 | 12.92 |
| PH-C1 | m | 51,196 | 2.66 | 0.44 | 3.66 | 0.32 | 12.13 | 42,364 | 2.71 | 1.66 | 3.41 | 0.26 | 9.55 |
| PH-C2 | m | 51,196 | 2.60 | 0.36 | 4.06 | 0.35 | 13.47 | 40,196 | 2.67 | 1.52 | 3.37 | 0.28 | 10.53 |
| PH-C3 | m | 51,196 | 2.66 | 0.32 | 3.70 | 0.33 | 12.52 | 43,160 | 2.71 | 1.72 | 3.43 | 0.27 | 9.98 |
| PH-C4 | m | 51,196 | 2.93 | 0.65 | 3.86 | 0.25 | 8.52 | 46,971 | 2.95 | 2.00 | 3.59 | 0.20 | 6.78 |
| PH-C5 | m | 51,196 | 2.82 | 0.48 | 3.75 | 0.24 | 8.54 | 45,982 | 2.85 | 2.00 | 3.41 | 0.18 | 6.47 |
| PH-C6 | m | 51,196 | 2.89 | 0.61 | 3.97 | 0.25 | 8.59 | 45,703 | 2.93 | 2.09 | 3.54 | 0.18 | 6.17 |
| PH-C7 | m | 51,196 | 2.94 | 0.33 | 3.90 | 0.33 | 11.29 | 42,838 | 2.99 | 1.9 | 3.7 | 0.27 | 9.11 |
| In Comparison with Y1 | In Comparison with Y2 | In Comparison with Y3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C1 | C1 | C2 | C3 | C4 | C5 | C5 | C6 | C7 | |
| ----------------------------------------------PHCSM--------------------------------------------- | |||||||||
| r | −0.25 | 0.88 | 0.90 | 0.86 | 0.67 | 0.43 | −0.15 | −0.15 | −0.65 |
| R2 | 0.06 | 0.77 | 0.81 | 0.75 | 0.45 | 0.18 | 0.02 | 0.02 | 0.42 |
| RMSE | 0.28 | 0.24 | 0.22 | 0.25 | 0.37 | 0.45 | 0.43 | 0.43 | 0.33 |
| RMSE% | 21.38 | 11.88 | 10.72 | 12.37 | 18.18 | 22.27 | 29.10 | 29.13 | 22.38 |
| RPD | 1.03 | 2.07 | 2.30 | 1.99 | 1.35 | 1.11 | 1.01 | 1.01 | 1.32 |
| ----------------------------------------------PVCSM--------------------------------------------- | |||||||||
| r | −0.32 | 0.90 | 0.92 | 0.86 | 0.69 | 0.28 | −0.10 | −0.07 | −0.77 |
| R2 | 0.10 | 0.80 | 0.84 | 0.73 | 0.48 | 0.08 | 0.01 | 0.00 | 0.59 |
| RMSE | 0.27 | 0.22 | 0.20 | 0.26 | 0.36 | 0.48 | 0.43 | 0.43 | 0.28 |
| RMSE% | 20.92 | 10.93 | 9.77 | 12.75 | 17.77 | 23.62 | 29.30 | 29.38 | 18.84 |
| RPD | 1.05 | 2.25 | 2.52 | 1.93 | 1.39 | 1.04 | 1.01 | 1.00 | 1.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martello, M.; Molin, J.P.; Angnes, G.; Acorsi, M.G. Assessing the Temporal and Spatial Variability of Coffee Plantation Using RPA-Based RGB Imaging. Drones 2022, 6, 267. https://doi.org/10.3390/drones6100267
Martello M, Molin JP, Angnes G, Acorsi MG. Assessing the Temporal and Spatial Variability of Coffee Plantation Using RPA-Based RGB Imaging. Drones. 2022; 6(10):267. https://doi.org/10.3390/drones6100267
Chicago/Turabian StyleMartello, Maurício, José Paulo Molin, Graciele Angnes, and Matheus Gabriel Acorsi. 2022. "Assessing the Temporal and Spatial Variability of Coffee Plantation Using RPA-Based RGB Imaging" Drones 6, no. 10: 267. https://doi.org/10.3390/drones6100267
APA StyleMartello, M., Molin, J. P., Angnes, G., & Acorsi, M. G. (2022). Assessing the Temporal and Spatial Variability of Coffee Plantation Using RPA-Based RGB Imaging. Drones, 6(10), 267. https://doi.org/10.3390/drones6100267









