From Coastal to Montane Forest Ecosystems, Using Drones for Multi-Species Research in the Tropics
Abstract
:1. Introduction
2. Materials and Methods
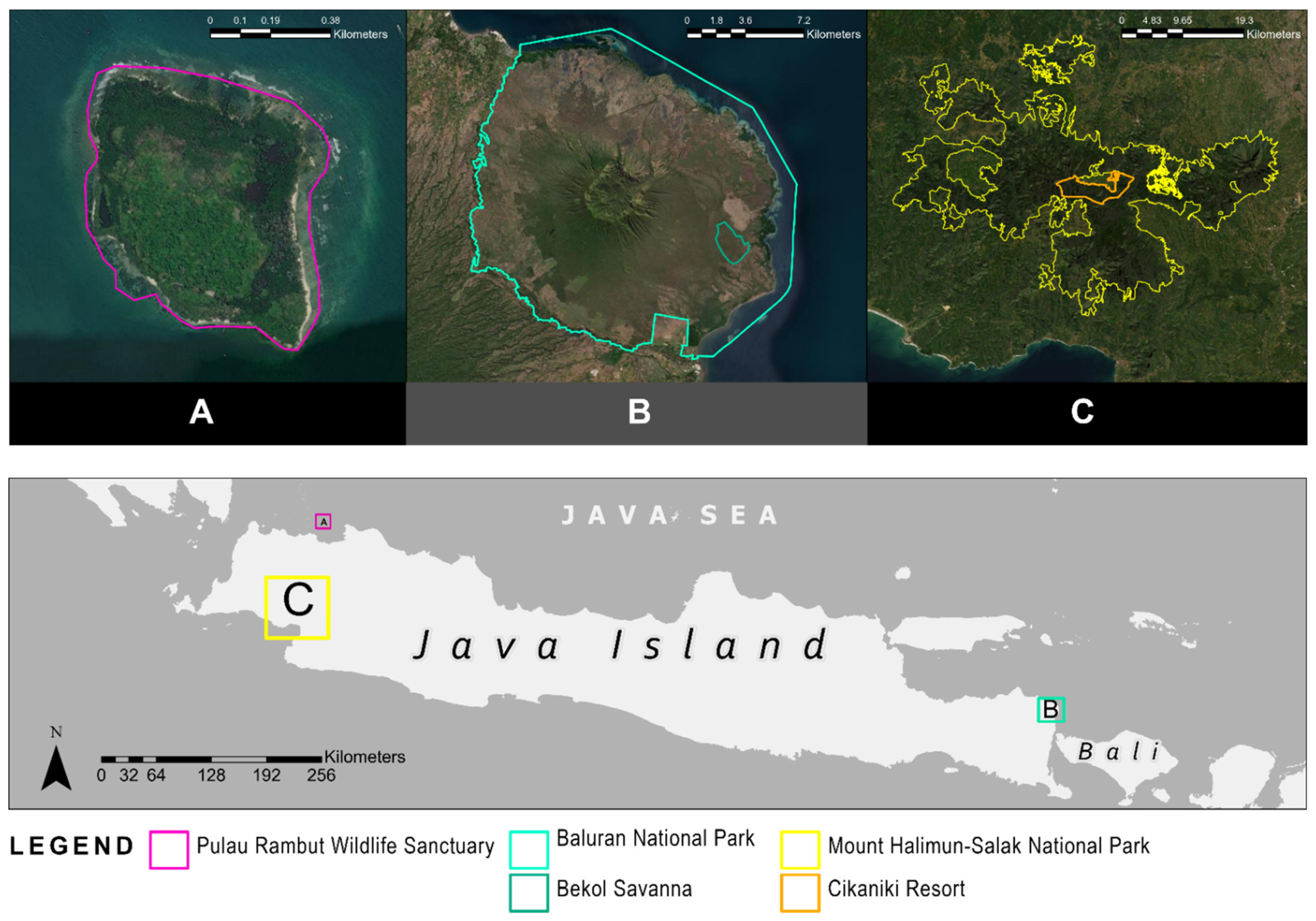
2.1. Study Areas
2.1.1. Pulau Rambut Wildlife Sanctuary (PRWS)
2.1.2. Baluran National Park (BNP)
2.1.3. Gunung Halimun Salak National Park (GHSNP)
2.2. Mission Planning Considerations
2.3. Survey Comparison Study
2.3.1. Drone Surveys
2.3.2. Ground Surveys
2.4. Data Analysis
2.4.1. Nest Counts and Observed Nesting Material and Breeding of Waterbirds in PRWS
2.4.2. Rapid Assessment of Species Diversity, Species Detection and Model Evaluation in BNP
2.4.3. Drone Flight and Behavioral Response of Primate in GHSNP
3. Results
3.1. Nest Counts and Observed Nesting Material and Breeding of Waterbirds in PRWS
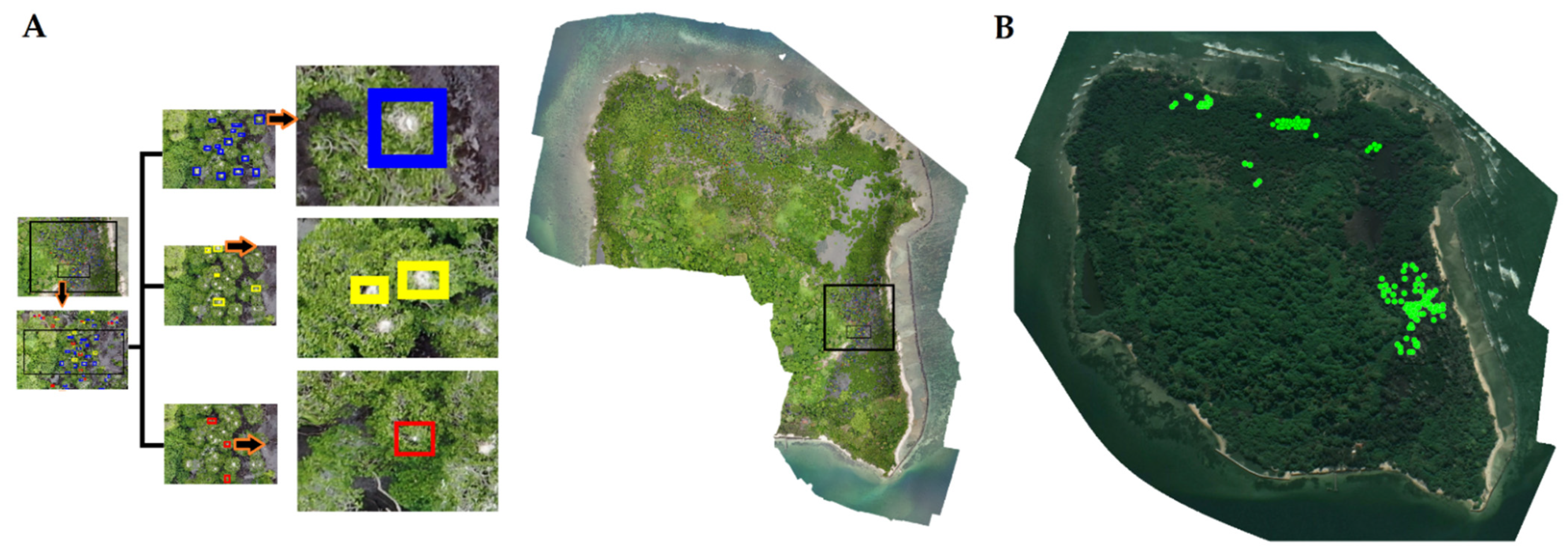
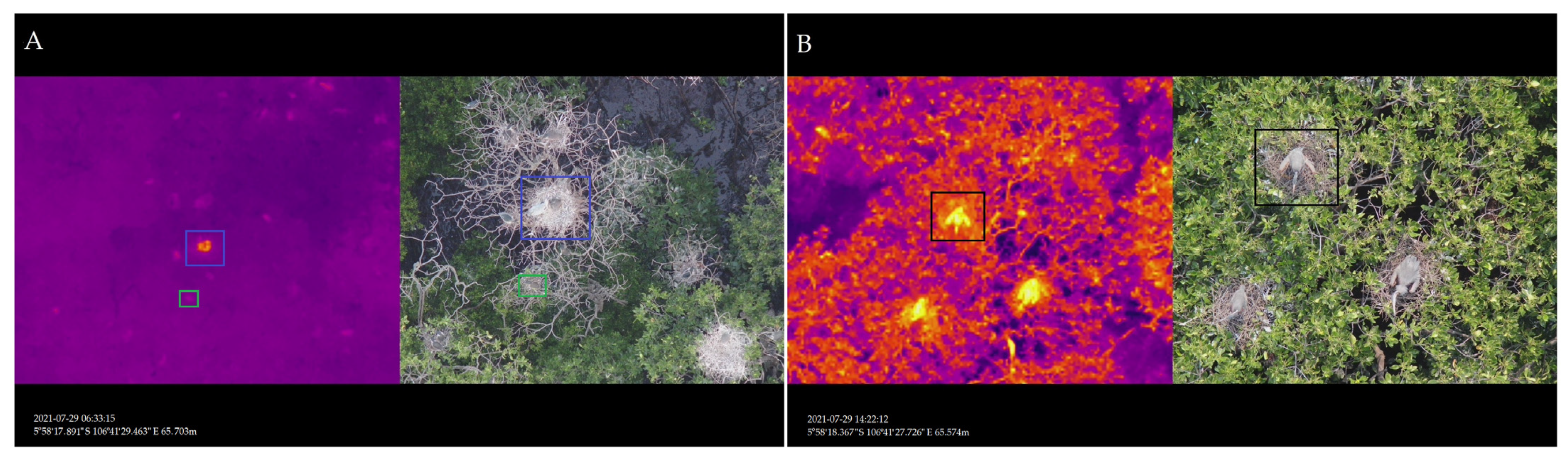
3.1.1. Interpretation of the Imagery
3.1.2. Nest Number, Nesting Material and Waterbird Breeding Monitoring
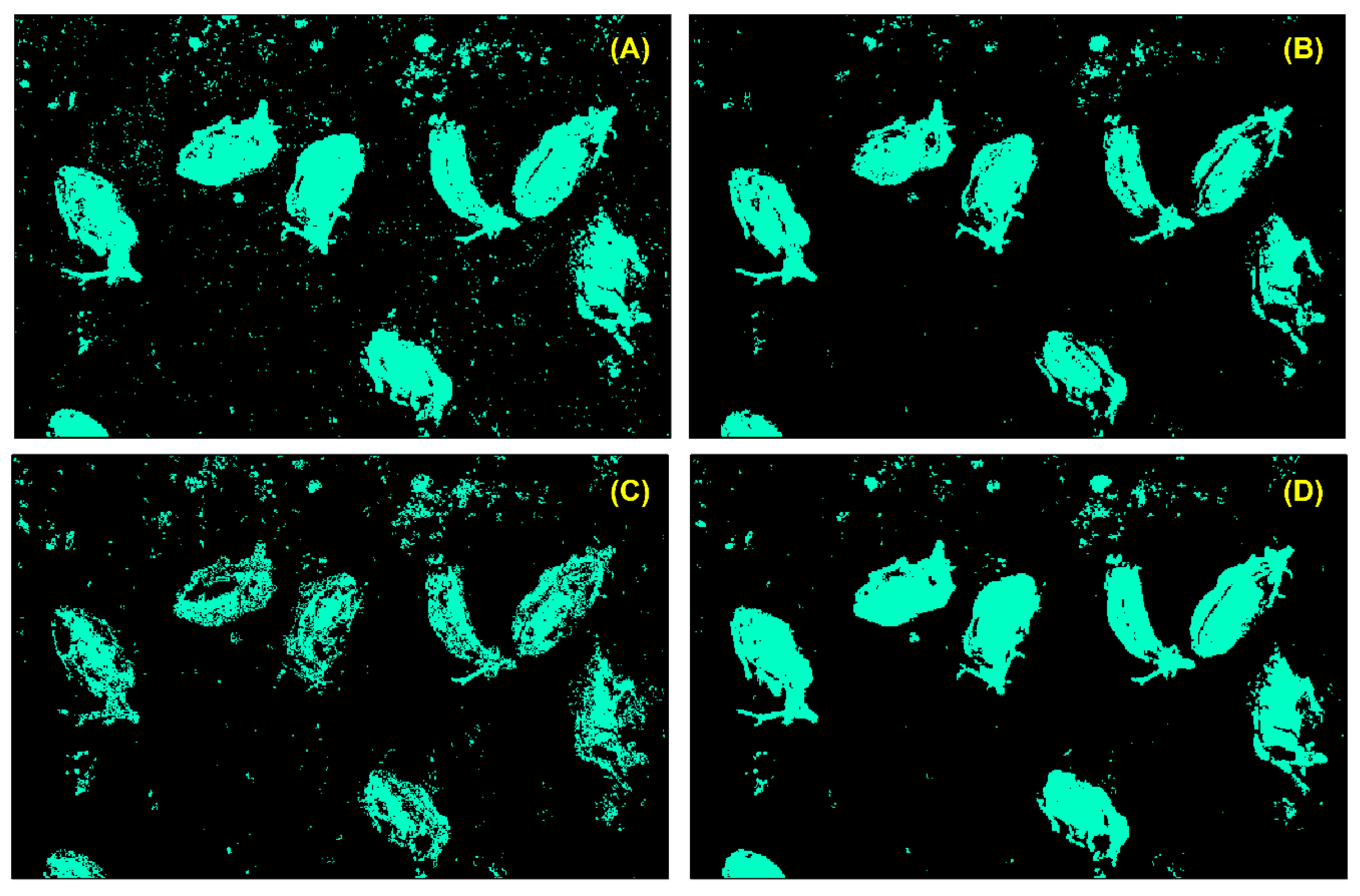
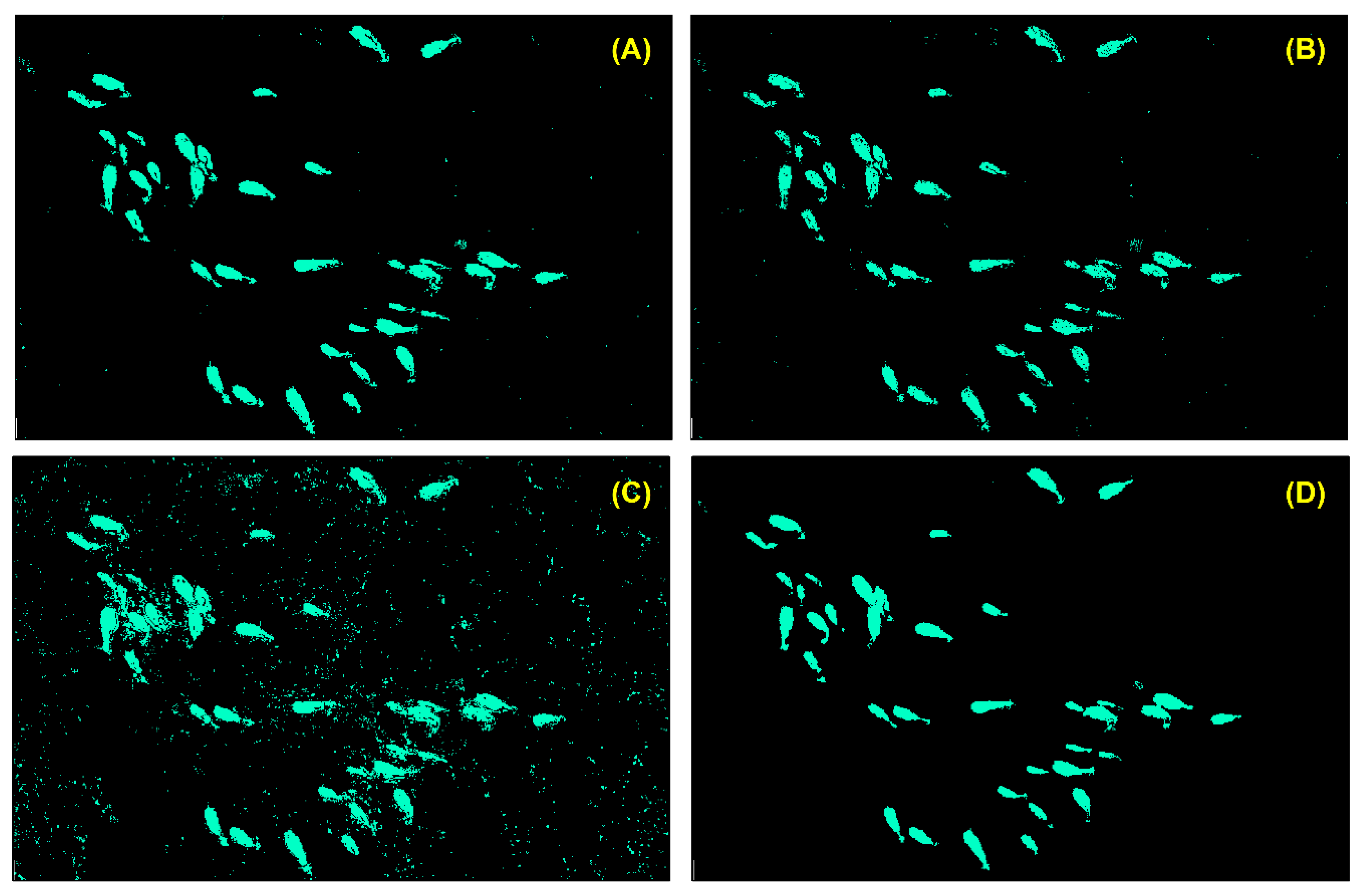
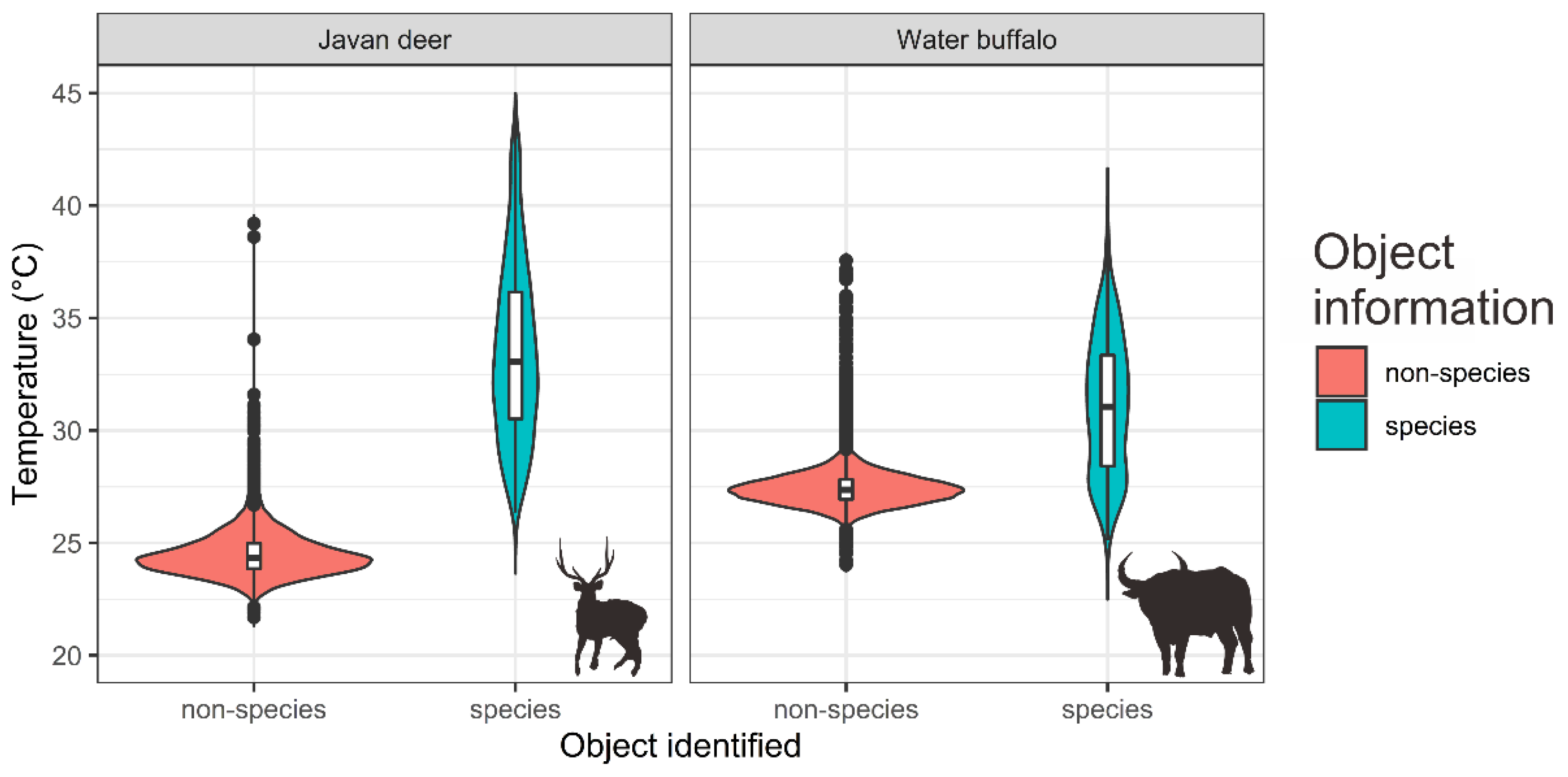
3.2. Rapid Assessment of Species Diversity, Species Detection and Model Evaluation in BNP
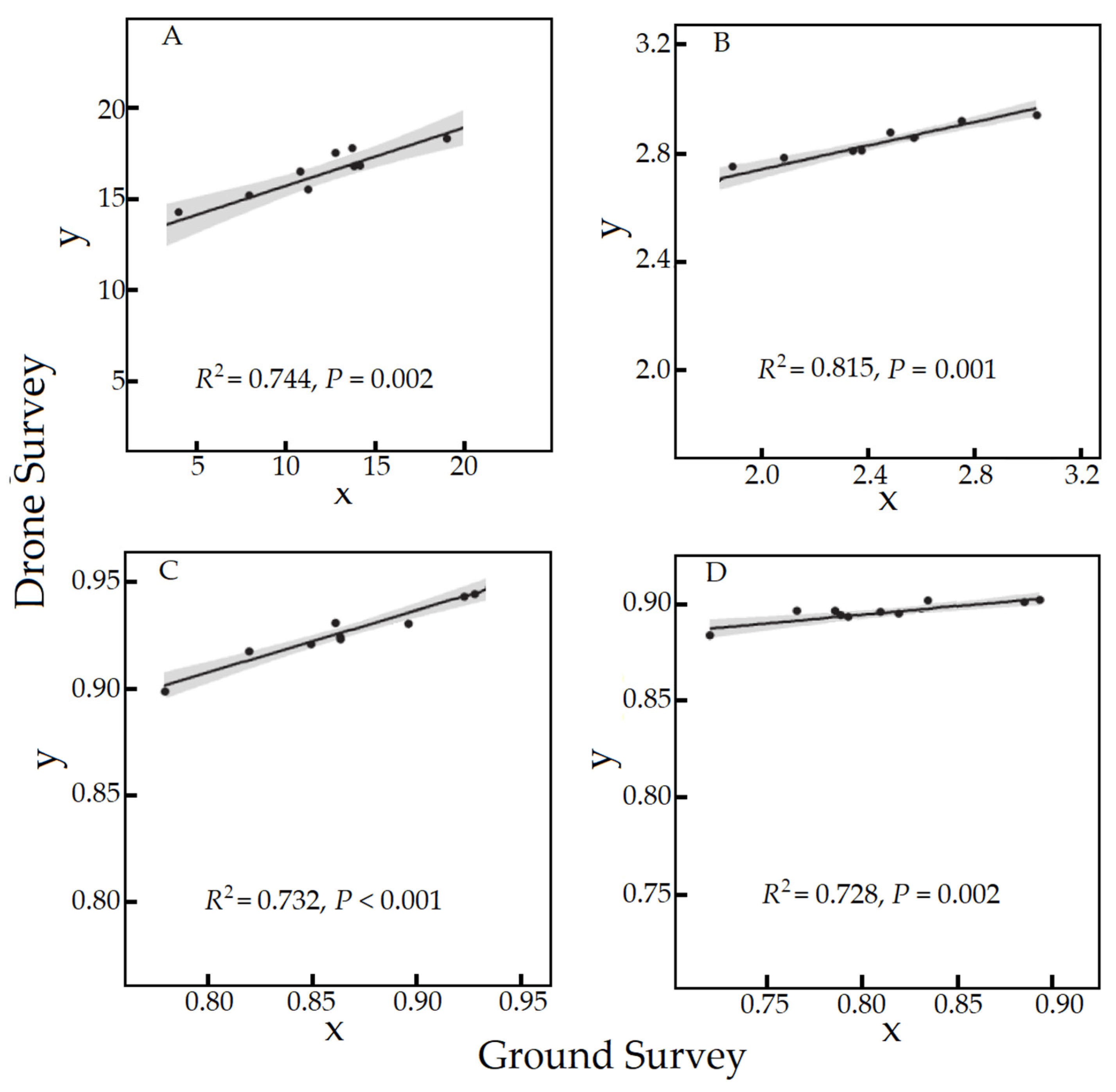
3.2.1. Relationship between Indices Estimated by the Ground and Drone Surveys
3.2.2. Species Detection and Model Evaluation
3.3. Behavioral Scores of Three Primates in GHSNP
3.3.1. Survey Comparison Study
3.3.2. Altitude Comparison Study
4. Discussion
4.1. Nest Counts and Observed Nesting Material and Breeding of Waterbirds in PRWS
4.2. Species Diversity and Detection Techniques from Thermal Photographs of Two Large-Sized Mammals in Baluran National Park (BNP)
4.3. Behavioral Response of the Three Primate Species in Gunung Halimun Salak National Park (GHSNP) to the Flight of UAVs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Project Overview
Appendix B
Appendix B.1. Drone Matrice 300 RTK Specification
| Hardware | Part of Drone | Detailed Information |
|---|---|---|
| Aircraft | Weight with one gimbal on the bottom | Approximately 3.6 kg (without battery) |
| Approximately 6.3 kg (with 2 batteries TB60) | ||
| Dimension | 430 × 420 × 430 mm (folded) | |
| 810 × 670 × 430 mm (takeoff) | ||
| Diagonal length | 895 mm | |
| Maximum load | 2.7 kg | |
| Maximum takeoff weight | 9 kg | |
| Operating frequency | 24,000–24,835 GHz 5725–5850 GHz | |
| RTK positioning accuracy (when on and installed) | 1.5 cm + 1 ppm (vertical) 1 cm + 1 ppm (horizontal) | |
| Maximum speed up | S mode: 6 m/s P mode: 5 m/s | |
| Maximum speed down (vertical) | S mode: 5 m/s P mode: 4 m/s | |
| Maximum speed down (tilt) | S mode: 7 m/s | |
| Maximum speed | S mode: 23 m/s P mode: 17 m/s | |
| Maximum wind obstacle | 15 m/s | |
| Maximum flight duration | 55 min | |
| Navigation system | GPS + GLONASS + BeiDou + Galileo | |
| Operating temperature | −20 °C to +50 °C | |
| Thermal Camera | Sensor | Uncooled VOx Microbolometer |
| Lens | DFOV: 40.6° Focal length: 13.5 mm (equivalent 58 mm) | |
| Aperture: f/1.0 Focus: 5 m to ∞ | ||
| Sensor resolution | 640 × 512 | |
| Pixel pitch | 12 µm | |
| Spectral band | 8–14 µm | |
| Image size | 640 × 480 (4:3) | |
| 640 × 360 (16:9) | ||
| Scene range | High gain: −40° to +150 °C | |
| Low gain: −40° to +550 °C | ||
| Image format | R-JPEG | |
| Video format | MP4 | |
| Visual Camera (Zoom Camera) | Sensor | Zoom camera: 1/1.7” CMOS Sensor, 20 MP Wide camera: 1/2.3” CMOS Sensor |
| Lens | Zoom camera DFOV: approximately 66.6°–4° Focal length: 6.83–119.94 mm Aperture: f/2.8–f/11 (normal); f/1.6–f/11 (mode malam) Focus: 1 m to ∞ (wide); 8 m to ∞ (telephoto) | |
| ISO range | Video: 100–25,600 (auto) Gambar: 100–25,600 (auto) | |
| Visual Camera (Wide Camera) | Sensor | Wide camera: 1/2.3” CMOS Sensor, 12 MP |
| Lens | DFOV: approximately 82.9° Focal length: 4.5 mm Aperture: f/2.8 Focus: 1 m to ∞ | |
| ISO range | Video: 100–25,600 (auto) Image: 100–25,600 (auto) |
Appendix B.2. Drone Mavic 2 Enterprise Dual Specification
| Hardware | Part of Drone | Detailed Information |
|---|---|---|
| Aircraft | Weight without accessories | 899 g |
| Maximum weight with accessories | 1100 g | |
| Dimension | 214 × 91 × 84 mm (folded) | |
| 322 × 242 × 84 mm (takeoff) | ||
| Diagonal length | 354 mm | |
| Maximum speed up | 4–5 m/s | |
| Maximum speed down | 3 m/s | |
| Maximum speed without wind disturbance | 72 km/hour | |
| Maximum flight duration | 31 min (constant speed 25 km/hour) | |
| Navigation system | GPS + GLONASS | |
| Operating frequency | 2400–2835 GHz | |
| 5725–5850 GHz | ||
| Internal storage | 24 GB | |
| Thermal Camera | Sensor | Uncooled VOx Microbolometer |
| Lens | HFOV: 57° | |
| Aperture: f/1.1 | ||
| Sensor resolution | 160 × 120 | |
| Pixel pitch | 12 µm | |
| Spectral band | 8–14 µm | |
| Image size | 640 × 480 (4:3) | |
| 640 × 360 (16:9) | ||
| Accuracy | High gain: Max ±5% (typical) | |
| Low gain: Max ±10% (typical) | ||
| Scene range | High gain: −10° to +140 °C | |
| Low gain: −10° to +400 °C | ||
| Image format | JPEG | |
| Video format | MP4, MOV MPEG-4 AVC/H.264) | |
| Visual Camera | Sensor | 1/2.3” CMOS; Effective pixels: 12 M FOV: approximately 85° 35 mm format equivalent: 24 mm |
| Lens | Aperture: f/2.8 Focus: 0.5 m to ∞ | |
| ISO range | Video: 100–12,800 (auto) Image: 100–1600 (auto) |
Appendix C
Appendix C.1. Drone Flight Plan
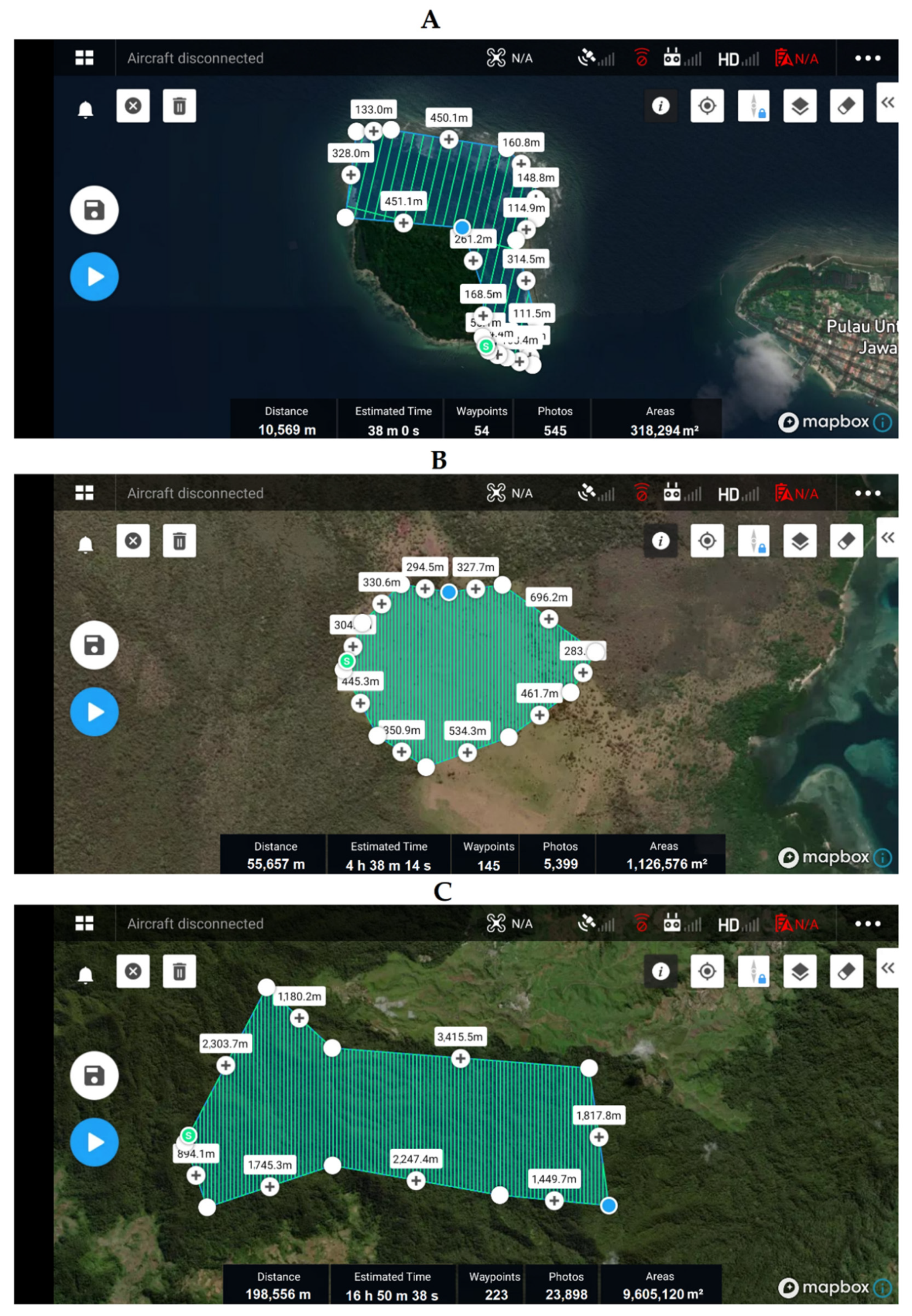
Appendix C.2. Drone System and Operation Details
| Location | Sensor | Time | Altitude (m) | GSD (cm/pixel) | Side Overlap | Frontal Overlap (%) |
|---|---|---|---|---|---|---|
| Pulau Rambut Wildlife Sanctuary | Zenmuse H20T Wide | 4 h 34 m 12 s | 120 | 4.14 | 70 | 80 |
| 4 h 47 m 18 s | 110 | 3.79 | 70 | 80 | ||
| 5 h 15 m 16 s | 100 | 3.45 | 70 | 80 | ||
| 5 h 57 m 54 s | 90 | 3.10 | 70 | 80 | ||
| 6 h 30 m 13 s | 80 | 2.76 | 70 | 80 | ||
| 7 h 24 m 33 s | 70 | 2.41 | 70 | 80 | ||
| 8 h 45 m 51 s | 60 | 2.07 | 70 | 80 | ||
| 10 h 13 m 37 s | 50 | 1.72 | 70 | 80 | ||
| 12 h 56 m 29 s | 40 | 1.38 | 70 | 80 | ||
| 16 h 58 m 9 s | 30 | 1.03 | 70 | 80 | ||
| 25 h 12 m 17 s | 20 | 0.69 | 70 | 80 | ||
| 33 h 34 m 22 s | 10 | 0.52 | 70 | 80 | ||
| Optimum detection | 7 h 55 m 13 s | 65 | 2.24 | 70 | 80 | |
| Baluran National Park | Zenmuse H20 T Wide | 1 h 22 m 49 s | 120 | 4.14 | 70 | 80 |
| 1 h 29 m 51 s | 110 | 3.79 | 70 | 80 | ||
| 1 h 38 m 34 s | 100 | 3.45 | 70 | 80 | ||
| 1 h 49 m 1 s | 90 | 3.10 | 70 | 80 | ||
| 2 h 1 m 45 s | 80 | 2.76 | 70 | 80 | ||
| 2 h 24 m 0 s | 70 | 2.41 | 70 | 80 | ||
| 3 h 13 m 24 s | 60 | 2.07 | 70 | 80 | ||
| 4 h 35 m 43 s | 50 | 1.72 | 70 | 80 | ||
| 7 h 8 m 29 s | 40 | 1.38 | 70 | 80 | ||
| 12 h 42 m 25 s | 30 | 1.03 | 70 | 80 | ||
| 28 h 59 m 1 s | 20 | 0.69 | 70 | 80 | ||
| 49 h 59 m 8 s | 10 | 0.52 | 70 | 80 | ||
| Optimum detection | 4 h 35 m 43 s | 50 | 1.72 | 70 | 80 | |
| Gunung Halimun Salak National Park | Mavic 2 Enterprise Dual | 19 h 55 m 45 s | 120 | 3.67 | 10 | 80 |
| 21 h 32 m 46 s | 110 | 3.36 | 10 | 80 | ||
| 23 h 32 m 30 s | 100 | 3.06 | 10 | 80 | ||
| 25 h 55 m 30 s | 90 | 2.75 | 10 | 80 | ||
| 28 h 56 m 12 s | 80 | 2.45 | 10 | 80 | ||
| 32 h 53 m 28 s | 70 | 2.14 | 10 | 80 | ||
| 38 h 6 m 23 s | 60 | 1.84 | 10 | 80 | ||
| 45 h 20 m 10 s | 50 | 1.53 | 10 | 80 | ||
| 56 h 15 m 15 s | 40 | 1.22 | 10 | 80 | ||
| 74 h 23 m 49 s | 30 | 0.92 | 10 | 80 | ||
| 110 h 38 m 39 s | 20 | 0.61 | 10 | 80 | ||
| 146 h 53 m 50 s | 10 | 0.46 | 10 | 80 | ||
| Optimum detection | 49 h 16 m 47 s | 45 | 1.38 | 70 | 80 | |
References
- Foody, G.M.; Cutler, M.E.J. Tree biodiversity in protected and logged Bornean tropical rain forests and its measurement by satellite remote sensing. J. Biogeogr. 2003, 30, 1053–1066. [Google Scholar] [CrossRef]
- Dent, D.H.; Wright, S.J. The future of tropical species in secondary forests: A quantitative review. Biol. Conserv. 2009, 142, 2833–2843. [Google Scholar] [CrossRef]
- Berry, N.J.; Phillip, S.L.; Lewis, S.L.; Hill, J.K.; Edwards, D.P.; Tawatao, N.B.; Ahmed, N.; Magintan, D.; Ken, C.V.; Maryati, M.; et al. The high value of logged tropical forests: Lessons from northern Borneo. Biodivers. Conserv. 2010, 19, 985–997. [Google Scholar] [CrossRef]
- Whitmore, T.C. Tropical Rain Forests of the Far East; Clarendon Press: Oxford, UK, 1984. [Google Scholar]
- Philips, O.L.; Gentry, A.H. Increasing turnover through time in tropical forests. Science 1994, 263, 954–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakaria, M.; Rajpar, M.N.; Ozdemir, I.; Rosli, Z. Fauna diversity in tropical rainforest: Threats from land-use change. In Tropical Forests—The Challenges of Maintaining Ecosystem Services While Managing the Landscape; Intech: London, UK, 2016; pp. 11–49. [Google Scholar] [CrossRef] [Green Version]
- Parikesit, P.; Okubo, S.; Husodo, T.; Takeuchi, K.; Muhamad, D. Biodiversity issues in Indonesia with special reference to biodiversity in human-dominated landscapes. In The Biodiversity Observation Network in the Asia-Pasific Region: Toward Further of Monitoring; Nakano, S., Yahara, T., Nakashizuka, T., Eds.; Springer: Tokyo, Japan, 2012; pp. 93–110. [Google Scholar]
- Ministry of Environment and Forestry Republic of Indonesia. The State of Indonesia’s Forests 2020; MoEF: Jakarta, Indonesia, 2020; p. 189.
- The National Development Agency. Indonesian Biodiversity Strategy and Action Plan (IBSAP) 2015–2020; The National Agency (BAPPENAS): Jakarta, Indonesia, 2016; p. 289.
- MacKinnon, K. Biological Diversity in Indonesia: A Resource Inventory; WWF Indonesia: Bogor, Indonesia, 1990. [Google Scholar]
- Collen, B.; Ram, M.; Zamin, T.; McRae, L. The tropical biodiversity data gap: Addressing disparity in global monitoring. Trop. Conserv. Sci. 2008, 1, 75–88. [Google Scholar] [CrossRef]
- Geldmann, J.; Barnes, M.; Coad, L.; Craigie, I.D.; Hockings, M.; Burgess, N.D. Effectiveness of terrestrial protected areas in reducing habitat loss and population declines. Biol. Conserv. 2013, 161, 230–238. [Google Scholar] [CrossRef]
- Feeley, K.J.; Silman, M.R. The data void in modeling current and future distributions of tropical species. Glob. Chang. Biol. 2011, 17, 626–630. [Google Scholar] [CrossRef]
- Brooks, T.M.; Wright, S.J.; Sheil, D. Evaluating the success of conservation actions in safegaurding tropical forest biodiversity. Conserv. Biol. 2009, 23, 1448–1457. [Google Scholar] [CrossRef]
- Pereira, H.M.; Ferrier, S.; Walters, M.; Geller, G.N.; Jongman, R.H.G.; Scholes, R.J.; Bruford, M.W.; Brummitt, N.; Butchart, S.H.M.; Cardoso, A.C.; et al. Essential Biodiversity Variables. Science 2013, 339, 277–278. [Google Scholar] [CrossRef] [Green Version]
- Danielsen, F.; Mendoza, M.; Tagtag, A.; Alviola, P.; Balete, D.; Jensen, A. Increasing conservation management action by involving local people in natural resource monitoring. Ambio 2007, 36, 566–570. [Google Scholar] [CrossRef]
- Rahman, D.A.; Gonzalez, G.; Aulagnier, S. Benefit of camera trapping for surveying the critically endangered Bawean deer Axis kuhlii (Temminck, 1836). Trop. Zool. 2016, 29, 155–172. [Google Scholar] [CrossRef]
- Buckland, S.; Magurran, A.; Green, R.; Fewster, R. Monitoring change in biodiversity through composite indices. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Bider, J.R. Animal activity in uncontrolled terrestrial communities as determined by a sand transect technique. Ecol. Monogr. 1968, 38, 269–308. [Google Scholar] [CrossRef]
- Plumptre, A. Monitoring mammal populations with line transect techniques in African forests. J. Appl. Ecol. 2000, 37, 356–368. [Google Scholar] [CrossRef]
- Stephens, P.A.; Zaumyslova, O.Y.; Miquelle, D.G.; Myslenkov, A.I.; Hayward, G.D. Estimating population density from indirect sign: Track counts and the Formozov-Malyshev-Pereleshin formula. Anim. Conserv. 2006, 9, 339–348. [Google Scholar] [CrossRef]
- Keeping, D.; Pelletier, R. Animal density and track counts: Understanding the nature of observations based on animal movements. PLoS ONE 2014, 9, e96598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckland, S.T.; Anderson, D.R.; Burnham, K.P.; Laake, J.L. Distance Sampling: Estimating Abundance of Biological Populations; Chapman and Hall: London, UK, 1993. [Google Scholar]
- Walsh, P.D.; White, L.J.T. What will it take to monitor forest elephant populations? Conserv. Biol. 1999, 13, 1194–1202. [Google Scholar] [CrossRef]
- Bennun, L.; Davies, G.; Howell, K.; Newing, H.; Linkie, M. African Forest Biodiversity: A Field Survey Manual for Vertebrates, 2nd ed.; Earthwatch Institute: Cambridge, UK, 2004. [Google Scholar]
- Shahbazi, M.; Théau, J.; Ménard, P. Recent applications of unmanned aerial imagery in natural resource management. GIScience Remote Sens. 2014, 51, 339–365. [Google Scholar] [CrossRef]
- Gallego, D.; Sarasola, J.H. Using drones to reduce human disturbance while monitoring breeding status of an endangered raptor. Remote Sens. Ecol. Conserv. 2021, 7, 550–561. [Google Scholar] [CrossRef]
- Brunton, E.; Bolin, J.; Leon, J.; Burnett, S. Fright or flight? Behavioural responses of kangaroos to drone-based monitoring. Drones 2019, 3, 41. [Google Scholar] [CrossRef] [Green Version]
- Lethbridge, M.; Stead, M.; Wells, C. Estimating kangaroo density by aerial survey: A comparison of thermal cameras with human observers. Wildl. Res. 2019, 46, 639–648. [Google Scholar] [CrossRef]
- Brunton, E.A.; Leon, J.X.; Burnett, S.C. Evaluating the efficacy and optimal deployment of thermal infrared and true-colour imaging when using drones for monitoring kangaroos. Drones 2020, 4, 20. [Google Scholar] [CrossRef]
- Witczuk, J.; Pagacz, S.; Zmarz, A.; Cypel, M. Exploring the feasibility of unmanned aerial vehicles and thermal imaging for ungulate surveys in forests-preliminary results. Int. J. Remote Sens. 2017, 39, 5504–5521. [Google Scholar] [CrossRef]
- Beaver, J.T.; Baldwin, R.W.; Messinger, M.; Newbolt, C.H.; Ditchkoff, S.S.; Silman, M.R. Evaluating the use of drones equipped with thermal sensors as an effective method for estimating wildlife. Wildl. Soc. Bull. 2020, 44, 434–443. [Google Scholar] [CrossRef]
- Ezat, M.A.; Fritsch, C.J.; Downs, C.T. Use of an unmanned aerial vehicle (drone) to survey Nile crocodile populations: A case study at Lake Nyamithi, Ndumo game reserve, South Africa. Biol. Conserv. 2018, 223, 76–81. [Google Scholar] [CrossRef]
- Chabot, D.; Bird, D.M. Evaluation of an off-the-shelf unmanned aircraft system for surveying flocks of geese. Waterbirds 2012, 35, 170–174. [Google Scholar] [CrossRef]
- Burke, C.; Rashman, M.; Wich, S.; Symons, A.; Theron, C.; Longmore, S. Optimising observing strategies for monitoring animals using drone-mounted thermal infrared cameras. Int. J. Remote Sens. 2019, 40, 439–467. [Google Scholar] [CrossRef] [Green Version]
- Shewring, M.P.; Vafidis, J.O. Using UAV-mounted thermal cameras to detect the presence of nesting nightjar in upland clear-fell: A case study in South Wales, UK. Ecol. Solut. Evid. 2021, 2, e12052. [Google Scholar] [CrossRef]
- Mardiastuti, A. Habitat and Nest-Site Characteristics of Waterbirds in Pulau Rambut Nature Reserve, Jakarta Bay, Indonesia. Ph.D. Thesis, Michigan State University, East Lansing, MI, USA, 1992. [Google Scholar]
- Tirtaningtyas, F.N.; Yordan, K. Updating the sea bird fauna of Jakarta Bay, Indonesia. Mar. Ornithol. 2017, 45, 11–16. [Google Scholar]
- Firdausy, M.S.; Mardiastuti, A.; Mulyani, Y.A. Abundance waterbirds and the distribution of trees nesting in Pulau Rambut (Rambut Island) Wildlife Sanctuary, Jakarta Bay, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 771, 012028. [Google Scholar] [CrossRef]
- Köppen, W. Das Geographische System der Klimate; Handbuch der Klimatologie, I., Teil, C., Eds.; Gebrüder Borntraeger: Berlin, Germany, 1936. [Google Scholar]
- Pudyatmoko, S. Free-ranging livestock influence species richness, occupancy, and daily behaviour of wild mammalian species in Baluran National Park, Indonesia. Mamm. Biol. 2017, 86, 33–41. [Google Scholar] [CrossRef]
- Rahman, D.A.; Setiawan, Y.; Rahman, A.A.A.F.; Martiyani, T.R. Javan langur responses to the repeated exposure of ground survey and novel stimulus, unmanned aerial vehicles. IOP Conf. Ser. Earth Environ. Sci. 2021, 948, 012006. [Google Scholar] [CrossRef]
- Koh, L.P.; Wich, S. Dawn of drone ecology: Low-cost autonomous aerial vehicles for conservation. Trop. Conserv. Sci. 2012, 5, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Karen, R.H.; Hartnett, D.C.; Robert, C.C.; Owensby, C.E. Grazing management effects on plant species diversity in tallgrass prairie. J. Range Manag. 2004, 57, 58–65. [Google Scholar] [CrossRef]
- Spellerberg, I.F.; Fedor, P.J. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’ Index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef] [Green Version]
- Bonham, C.D. Measurements for Terrestrial Vegetation, 2nd ed.; John Wiley & Sons: Chichester, UK, 2013. [Google Scholar]
- Bello, F.D.; Leps, J.; Sebastià, M.T. Variations in species and functional plant diversity along climatic and grazing gradients. Ecography 2006, 29, 801–810. [Google Scholar] [CrossRef]
- Wesuls, D.; Pellowski, M.; Suchrow, S.; Oldeland, J.; Jansen, F.; Dengler, J. The grazing fingerprint: Modelling species responses and trait patterns along grazing gradients in semi-arid Namibian rangelands. Ecol. Indic. 2013, 27, 61–70. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Karatzoglou, A.; Hornik, K.; Smola, A.; Zeileis, A. kernlab—An S4 package for kernel methods in R. J. Stat. Softw. 2004, 11, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Elith, J.; Leathwick, J.R.; Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-Vector Networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python Fabian. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar] [CrossRef]
- Thuiller, W. Patterns and uncertainties of species’ range shifts under climate change. Glob. Chang. Biol. 2004, 10, 2020–2027. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Leroy, B.; Delsol, R.; Hugueny, B.; Meynard, C.N.; Barhoumi, C.; Barbet-Massin, M.; Bellard, C. Without quality presence–absence data, discrimination metrics such as TSS can be misleading measures of model performance. J. Biogeogr. 2018, 45, 1994–2002. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple comparisons using rank sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Ogle, D. Introductory Fisheries Analyses with R; Chapman and Hall/CRC: New York, NY, USA, 2016. [Google Scholar]
- Christensen, R. Ordinal—Regression Models for Ordinal Data. R Package Version 2019.12-10. 2018. Available online: http://www.cran.r-project.org/package=ordinal/ (accessed on 20 September 2021).
- Valle, R.G.; Scarton, F. Drones improve effectiveness and reduce disturbance of censusing common redshanks Tringa totanus breeding on salt marshes. Ardea 2020, 107, 275–282. [Google Scholar] [CrossRef]
- Hodgson, J.C.; Baylis, S.M.; Mott, R.; Herrod, A.; Clarke, R.H. Precision wildlife monitoring using unmanned aerial vehicles. Sci. Rep. 2016, 6, 22574. [Google Scholar] [CrossRef] [Green Version]
- Kingsford, R.T.; Porter, J.L. Monitoring waterbird populations with aerial surveys what have we learnt? Wildl. Res. 2009, 36, 29–40. [Google Scholar] [CrossRef]
- Sardà-Palomera, F.; Bota, G.; Padilla, N.; Brotons, L.; Sardà, F. Unmanned aircraft systems to unravel spatial and temporal factors affecting dynamics of colony formation and nesting success in birds. J. Avian Biol. 2017, 48, 1273–1280. [Google Scholar] [CrossRef]
- Linchant, J.; Lisein, J.; Semeki, J.; Lejeune, P.; Vermeulen, C. Are unmanned aircraft systems (UASs) the future of wildlife monitoring? A review of accomplishments and challenges. Mammal. Rev. 2015, 45, 239–252. [Google Scholar] [CrossRef]
- Van Bijsterveldt, C.E.J.; van Wesenbeeck, B.K.; Ramadhani, S.; Raven, O.V.; van Gool, F.E.; Pribadi, R.; Bouma, T.J. Does plastic waste kill mangroves? A field experiment to assess the impact of macro plastics on mangrove growth, stress response and survival. Sci. Total Environ. 2021, 756, 143826. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.; van Sebille, E.; Hardesty, B.D. Threat of plastic pollution to seabirds is global, pervasive, annepd increasing. Proc. Natl. Acad. Sci. USA 2015, 112, 11899–11904. [Google Scholar] [CrossRef] [Green Version]
- Savoca, M.S.; Tyson, C.W.; McGill, M.; Slager, C.J. Odours from marine plastic debris induce food search behaviours in a forage fish. Proc. R. Soc. B 2017, 284, 1–8. [Google Scholar] [CrossRef]
- Acampora, A.; Lyashevska, O.; Van Franeker, J.A.; O’Connor, A. The use of beached bird surveys for marine plastic litter monitoring in Ireland. Mar. Environ. Res. 2016, 120, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.E.; Shephard, J.M. Semi-automated detection of eagle nests: An application of very high-resolution image data and advanced image analyses to wildlife surveys. Remote Sens. Ecol. Conserv. 2017, 3, 66–80. [Google Scholar] [CrossRef] [Green Version]
- Mulero-Pázmány, M.; Jenni-Eiermann, S.; Strebel, N.; Sattler, T.; Negro, J.J.; Tablado, Z. Unmanned aircraft systems as a new source of disturbance for wildlife: A systematic review. PLoS ONE 2017, 12, e0178448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ditmer, M.A.; Vincent, J.B.; Werden, L.K.; Tanner, J.C.; Laske, T.G.; Iaizzo, P.A.; Garshelis, D.L.; Fieberg, J.R. Bears show a physiological but limited behavioral response to unmanned aerial vehicles. Curr. Biol. 2015, 25, 2278–2283. [Google Scholar] [CrossRef] [Green Version]
- Pecchi, M.; Marchi, M.; Burton, V.; Giannetti, F.; Moriondo, M.; Bernetti, I.; Bindi, M.; Chirici, G. Species distribution modelling to support forest management. A literature review. Ecol. Model. 2019, 411, 108817. [Google Scholar] [CrossRef]
- Fernandes, A.C.M.; Gonzalez, R.Q.; Lenihan-Clarke, M.A.; Trotter, E.F.L.; Arsanjani, J.J. Machine learning for conservation planning in a changing climate. Sustainability 2020, 12, 7657. [Google Scholar] [CrossRef]
- Gobeyn, S.; Mouton, A.M.; Cord, A.F.; Kaim, A.; Volk, M.; Goethals, P.L.M. Evolutionary algorithms for species distribution modelling: A review in the context of machine learning. Ecol. Modell. 2019, 392, 179–195. [Google Scholar] [CrossRef]
- Rahman, D.A.; Setiawan, Y. Possibility of applying unmanned aerial vehicle and thermal imaging in several canopy cover class for wildlife monitoring—Preliminary results. E3S Web Conf. 2020, 211, 04007. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, C.; Turvey, S.T.; Sun, Z.; Tan, Z.; Yang, Q.; Long, W.; Wu, X.; Yang, D. Thermal infrared imaging from drones can detect individuals and nocturnal behavior of the world’s rarest primate. Glob. Ecol. Conserv. 2020, 23, e01101. [Google Scholar] [CrossRef]
- Mangewa, L.J.; Ndakidemi, P.A.; Munishi, L.K. Integrating UAV technology in an ecological monitoring system for community wildlife management areas in Tanzania. Sustainability 2019, 11, 6116. [Google Scholar] [CrossRef] [Green Version]
- Reisland, M.A.; Lambert, J.E. Sympatric apes in sacred forests: Shared space and habitat use by humans and endangered Javan gibbons (Hylobates moloch). PLoS ONE 2016, 11, e0146891. [Google Scholar] [CrossRef] [Green Version]
- Napier, J.R.; Napier, P.H. The Natural History of the Primates; British Meseum (Natural History) Cromwell Road: London, UK, 1985. [Google Scholar]
- Van Schaik, C.P. The socioecology of fission-fusion sociality in orangutans. Primates 1999, 40, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, F.; Rojano-Doñate, L.; Madsen, P.T.; Bejder, L. Noise levels of multi-rotor unmanned aerial vehicles with implications for potential underwater impacts on marine mammals. Front. Mar. Sci. 2016, 26, 277. [Google Scholar] [CrossRef] [Green Version]
- Weissensteiner, M.H.; Poelstra, J.W.; Wolf, J.W.B. Low-budget ready-to-fly unmanned aerial vehicles: An effective tool for evaluating the nesting status of canopy-breeding bird species. J. Avian Biol. 2015, 46, 425–430. [Google Scholar] [CrossRef]
- Wich, S.; Dellatore, D.; Houghton, M.; Ardi, R.; Koh, L.P. A preliminary assessment of using conservation drones for Sumatran orang-utan (Pongo abelii) distribution and density. J. Unmanned Veh. Syst. 2016, 4, 45–52. [Google Scholar] [CrossRef]
- Bonnin, N.; Van Andel, A.C.; Kerby, J.T.; Piel, A.K.; Pintea, L.; Wich, S.A. Assessment of chimpanzee nest detectability in drone-acquired images. Drones 2018, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Vas, E.; Lescroël, A.; Duriez, O.; Boguszewski, G.; Grémillet, D. Approaching birds with drones: First experiments and ethical guidelines. Biol. Lett. 2015, 11, 20140754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semel, B.P.; Karpanty, S.M.; Vololonirina, F.F.; Rakotonanahary, A.N. Eyes in the Sky: Assessing the Feasibility of Low-Cost, Ready-to-Use Unmanned Aerial Vehicles to Monitor Primate Populations Directly. Folia Primatol. 2020, 91, 69–82. [Google Scholar] [CrossRef]
- Elmore, J.A.; Curran, M.F.; Evans, K.O.; Samiappan, S.; Zhou, M.; Pfeiffer, M.B.; Blackwell, B.F.; Iglay, R.B. Evidence on the effectiveness of small unmanned aircraft systems (sUAS) as a survey tool for North American terrestrial, vertebrate animals: A systematic map protocol. Environ. Evid. 2021, 10, 1–6. [Google Scholar] [CrossRef]








| Score | Category | Description |
|---|---|---|
| 0 | Resting | Behaviors include inactive (not moving), sleeping (e.g., closing eyes), and other behaviors such as grooming, playing, and breeding. |
| 1 | Vigilant | Noting around and alert with regular lateral movement of the head and eyes open. |
| 2 | Looking | Specifically spotting, tracking objects, and visually following sources of disturbance (an observer on the ground or drone flying in the vicinity) with the head possibly turned up or down or sideways left and right. |
| 3 | Agonistic | Aggressive behavior is shown to observers on the ground or drone flying in the vicinity, including alarm calls. |
| 4 | Escape | Move away from the source of the disturbance (an observer on the ground or drone flying in the vicinity), including hiding so that they are not detected again. |
| Nest Category | Ground | Drone |
|---|---|---|
| Nest filled | 124 | 590 |
| Empty nest | 93 |
| Species | Algorithm | AUC | Kappa | TSS | Jaccard | Sorensen |
|---|---|---|---|---|---|---|
| Javan deer | BRT | 0.901 | 0.827 | 0.827 | 0.852 | 0.920 |
| SVM | 0.895 | 0.827 | 0.827 | 0.852 | 0.920 | |
| Random Forest | 0.893 | 0.827 | 0.827 | 0.852 | 0.920 | |
| Ensemble | 0.936 | 0.827 | 0.827 | 0.852 | 0.920 | |
| Water buffalo | BRT | 0.979 | 0.944 | 0.944 | 0.956 | 0.975 |
| SVM | 0.958 | 0.944 | 0.944 | 0.956 | 0.975 | |
| Random Forest | 0.965 | 0.944 | 0.944 | 0.956 | 0.975 | |
| Ensemble | 0.993 | 0.972 | 0.972 | 0.978 | 0.988 |
| Species | Comparison Study | Number of Individuals | Number Drone Approaches | Number Ground Survey | Number of Behavioural Scores per Observer | Total Number of Behavioural Scores |
|---|---|---|---|---|---|---|
| Javan gibbon | Survey | 7 | 6 | 6 | 46 | 138 |
| Altitude | 12 | 8 | - | 102 | 306 | |
| Javan langur | Survey | 10 | 16 | 18 | 144 | 432 |
| Altitude | 25 | 38 | - | 268 | 804 | |
| Javan surili | Survey | 7 | 6 | 6 | 66 | 198 |
| Altitude | 23 | 10 | - | 236 | 708 |
| Species | n | Treatment | df | χ2 | p |
|---|---|---|---|---|---|
| Javan gibbon | 46 | All surveys | 2 | 4.58 | <0.050 |
| 34 | Ground-drone | <0.050 | |||
| Javan langur | 144 | All surveys | 2 | 38.50 | <<0.001 |
| 100 | Ground-drone | <<0.001 | |||
| Javan surili | 66 | All surveys | 2 | 24.78 | <<0.001 |
| 40 | Ground-drone | <<0.001 |
| Model | Significant Coefficients | AIC | Δ AIC | Likelihood Ratio Test | |||
|---|---|---|---|---|---|---|---|
| β | Pr(>|z|) | (Df) χ2 | p | ||||
| Javan gibbon | |||||||
| Response~1 + (1|Observer) | - | - | - | 964.20 | 88.80 | - | - |
| Response~Altitude + (1|Observer) | 10 m | 1.690 | <<0.001 | 910.70 | 44.40 | (1) 35.44 | <<0.0001 |
| 20 m | 0.978 | <<0.001 | |||||
| Response~Altitude + Sex + Exposure + (1|Observer) | 10 m | 1.554 | <<0.001 | 840.50 | - | (2) 1.50 | <<0.0001 |
| 20 m | 1.158 | <<0.001 | |||||
| Up | 1.088 | <0.001 | |||||
| Male | 0.645 | 0.001 | |||||
| Low | 0.630 | <0.001 | |||||
| Javan langur | |||||||
| Response~1 + (1|Observer) | - | - | - | 5268.20 | - | - | - |
| Response~Altitude + (1|Observer) | 10 m | 2.764 | <<0.001 | 3445.10 | 924.50 | (4) 158.22 | <<0.0001 |
| 20 m | 1.890 | <<0.001 | |||||
| Response~Altitude + Social Group + (1|Observer) | 10 m | 2.893 | <<0.001 | 2914.80 | 278.90 | (2) 102.40 | <<0.0001 |
| 20 m | 1.797 | <<0.001 | |||||
| Harem | 0.814 | <0.001 | |||||
| Response~Altitude ∗ Social Group + Sex + (1|Observer) | 10 m | 0.690 | <<0.001 | 2544.70 | - | (1) 27.50 | <<0.0001 |
| 20 m | 0.758 | <<0.001 | |||||
| 10 m: Harem | 2.427 | <0.001 | |||||
| 20 m: Harem | 1.564 | <0.001 | |||||
| Male | 0.261 | <0.001 | |||||
| Javan surili | |||||||
| Response~1 + (1|Observer) | - | - | - | 7540.80 | - | - | - |
| Response~Altitude + (1|Observer) | 10 m | 1.664 | <0.001 | 7288.10 | 1556.40 | (4) 358.10 | <<0.0001 |
| 20 m | 0.981 | <0.001 | |||||
| Response~Altitude + Social Group + Sex + Exposure + (1|Observer) | 10 m | 1.603 | <<0.001 | 5580.60 | - | (1) 14.90 | <<0.0001 |
| 20 m | 0.965 | <<0.001 | |||||
| Harem | 0.885 | <0.001 | |||||
| Up | 0.253 | 0.001 | |||||
| Male | 0.500 | <<0.001 | |||||
| Low | 1.610 | <0.001 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, D.A.; Sitorus, A.B.Y.; Condro, A.A. From Coastal to Montane Forest Ecosystems, Using Drones for Multi-Species Research in the Tropics. Drones 2022, 6, 6. https://doi.org/10.3390/drones6010006
Rahman DA, Sitorus ABY, Condro AA. From Coastal to Montane Forest Ecosystems, Using Drones for Multi-Species Research in the Tropics. Drones. 2022; 6(1):6. https://doi.org/10.3390/drones6010006
Chicago/Turabian StyleRahman, Dede Aulia, Andre Bonardo Yonathan Sitorus, and Aryo Adhi Condro. 2022. "From Coastal to Montane Forest Ecosystems, Using Drones for Multi-Species Research in the Tropics" Drones 6, no. 1: 6. https://doi.org/10.3390/drones6010006
APA StyleRahman, D. A., Sitorus, A. B. Y., & Condro, A. A. (2022). From Coastal to Montane Forest Ecosystems, Using Drones for Multi-Species Research in the Tropics. Drones, 6(1), 6. https://doi.org/10.3390/drones6010006








