Quantifying the Effects of Vibration on Medicines in Transit Caused by Fixed-Wing and Multi-Copter Drones
Abstract
1. Introduction
1.1. The Effects of Vibration on Medical Cargoes during Transit
1.2. Packaging and Carriage Requirements Regarding Medical Cargoes during Transit
1.3. Sources of Vibration Emanating from Drones and Road Transportation
2. Methodology
2.1. Drones Used in the Trials
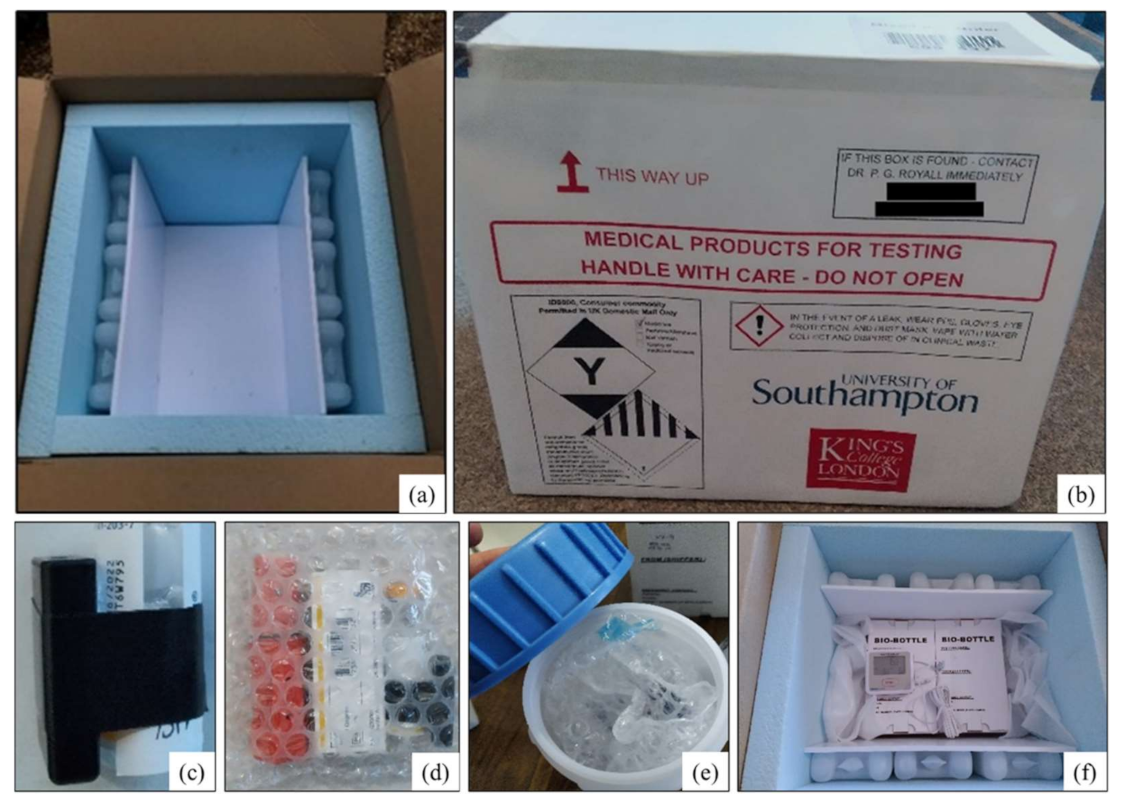
2.2. Containment Packaging Used and Cargo Monitoring Equipment
2.3. Experimental Design
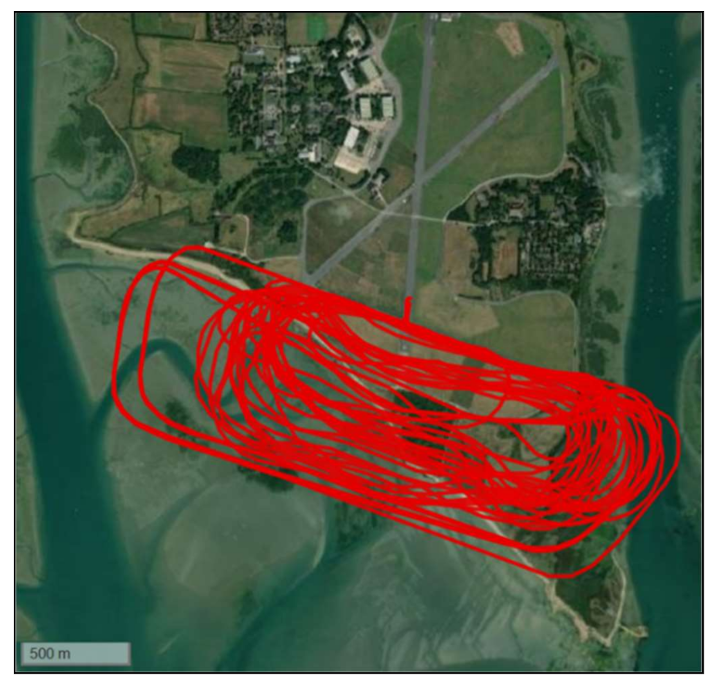
2.3.1. Trial 1: Fixed-Wing Mixed Manoeuvres (12 November 2020)
2.3.2. Trial 2: Multi-Copter Evasive Manoeuvres (10 December 2020)
2.3.3. Trial 3: Fixed-Wing Long Distance Flight (15 December 2020)
2.3.4. Trial Management and Analysis Process
3. Results and Discussions
3.1. Temperature Analysis (Trials 1, 2, 3)
3.2. Vibration Analysis—Fixed-Wing (Trial 3)
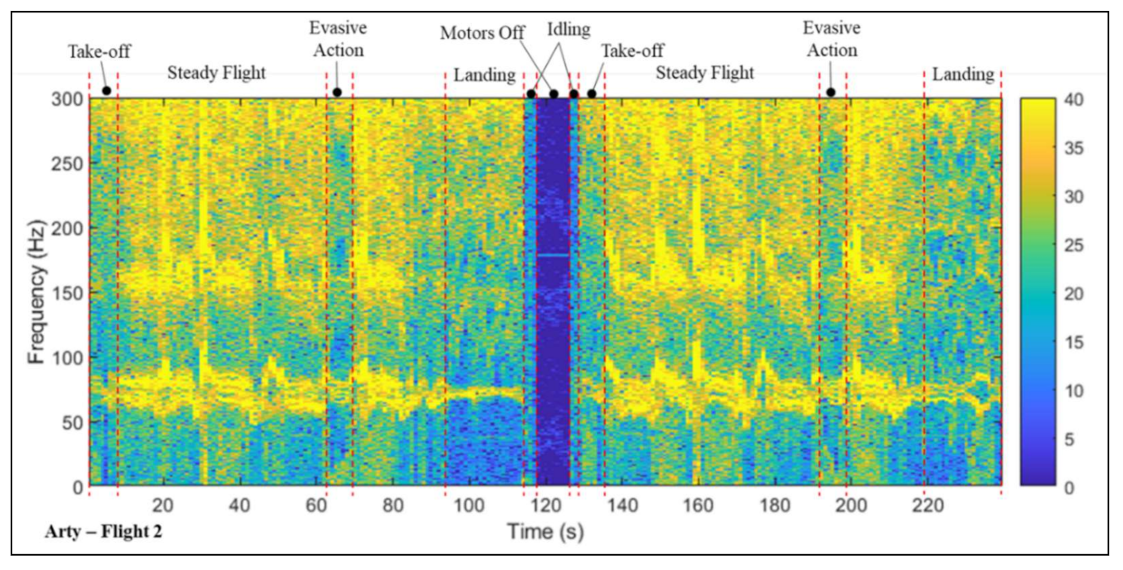
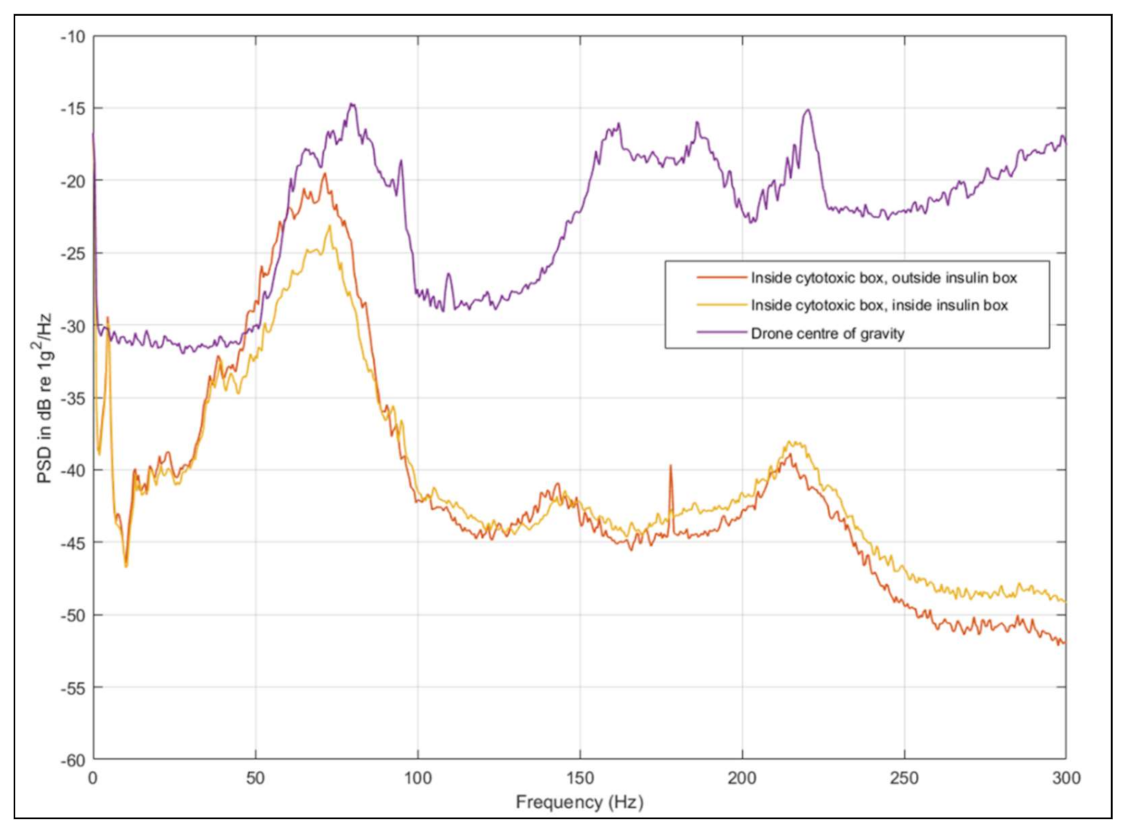
3.3. Vibration Analysis—Multi-Copter (Trial 2)
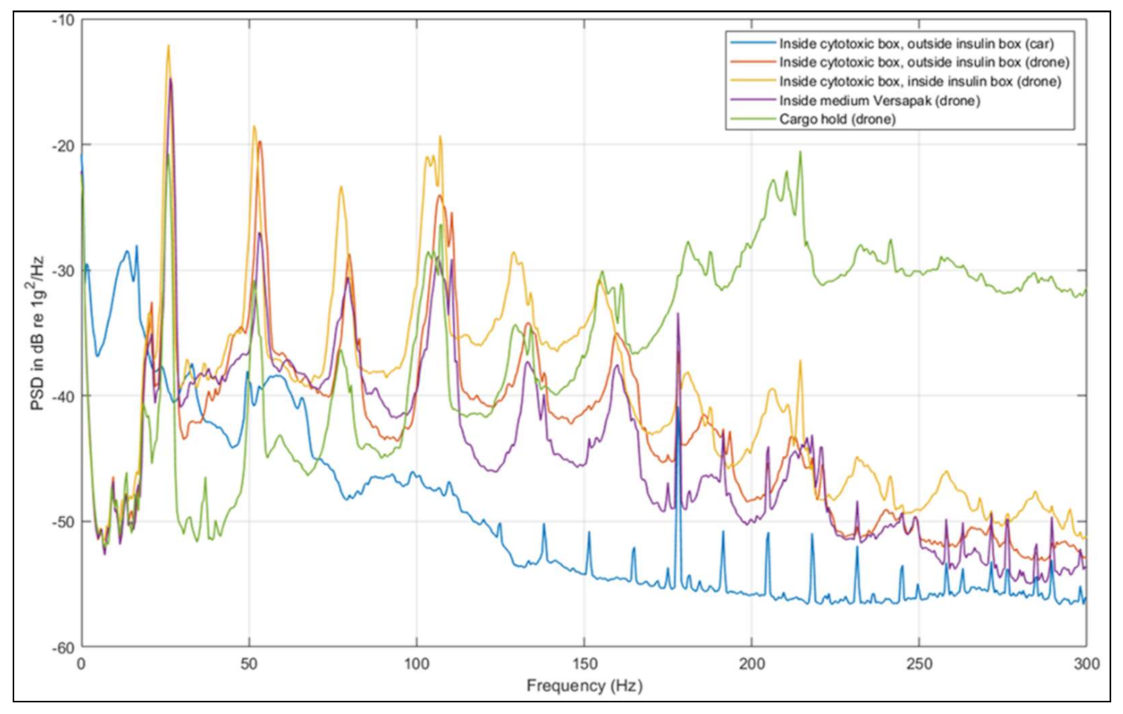
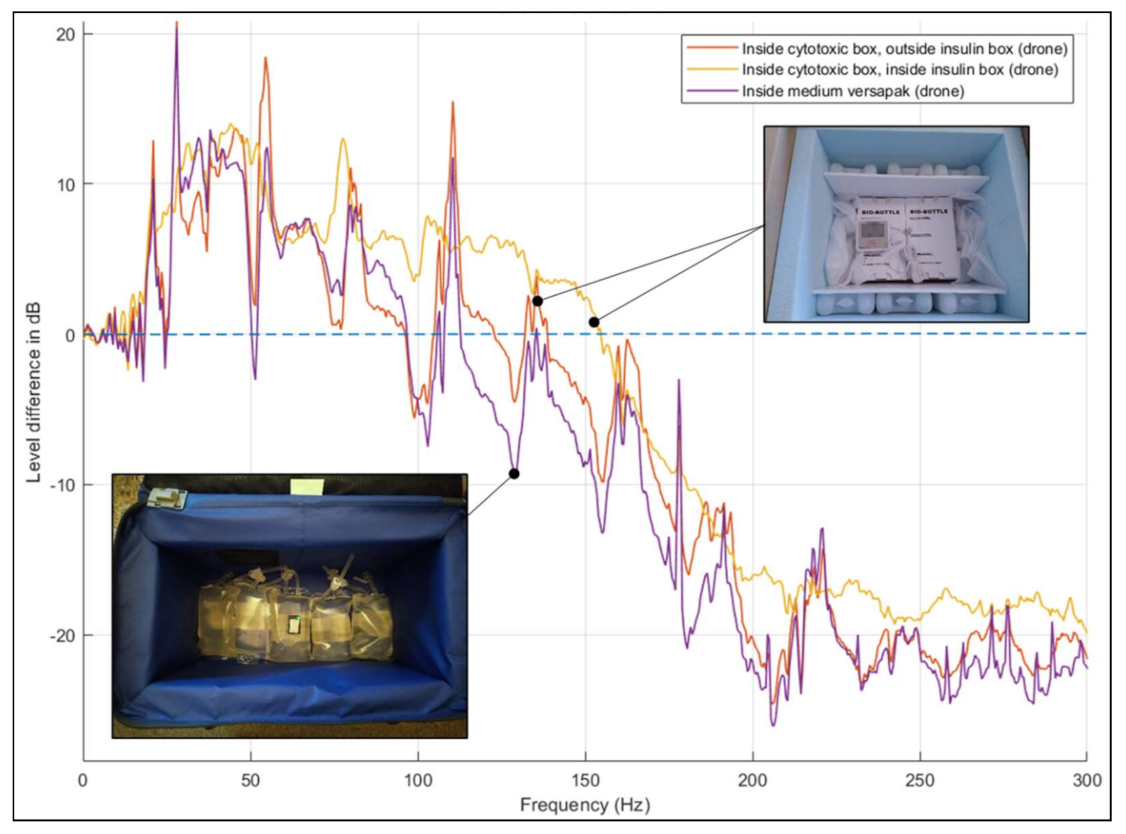
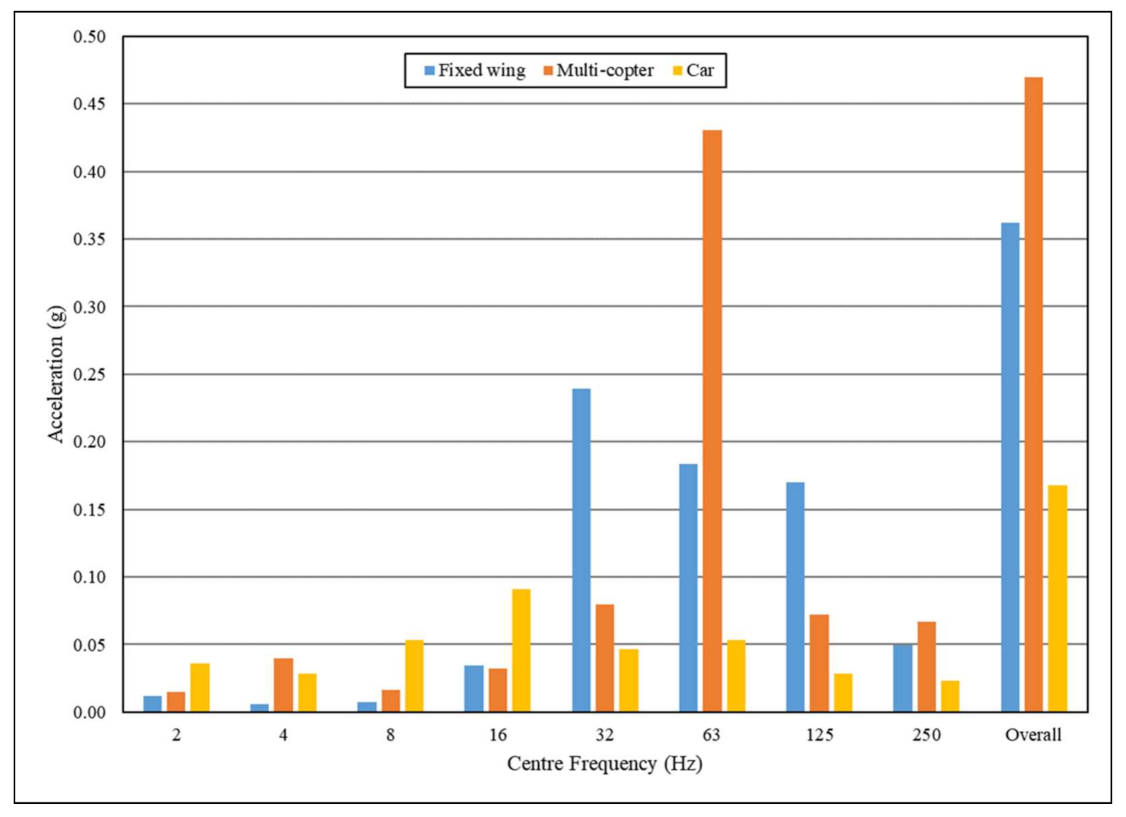
3.4. Comparison of Vibration Effects between Transport Modes
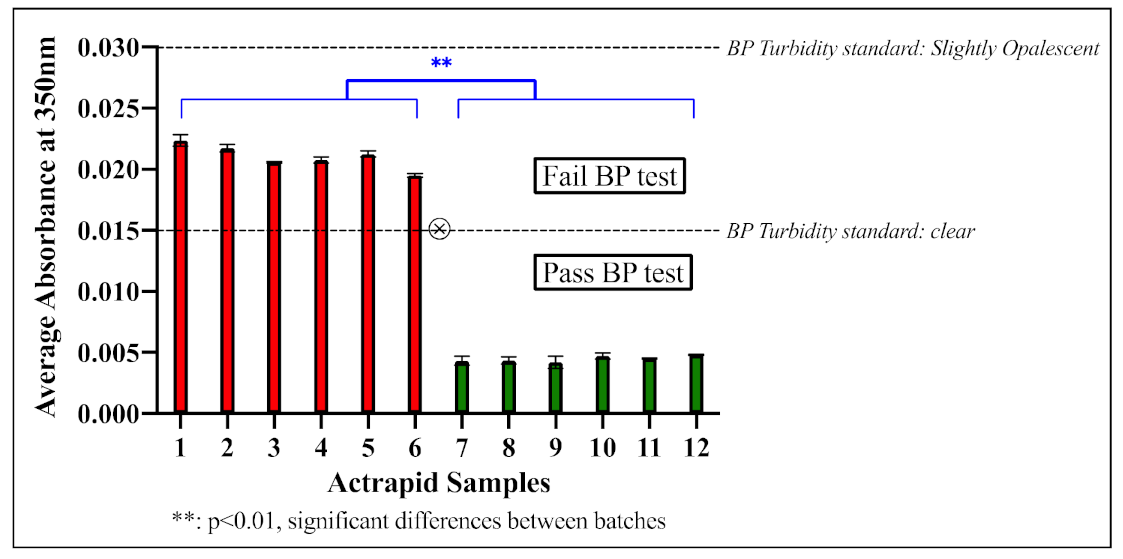
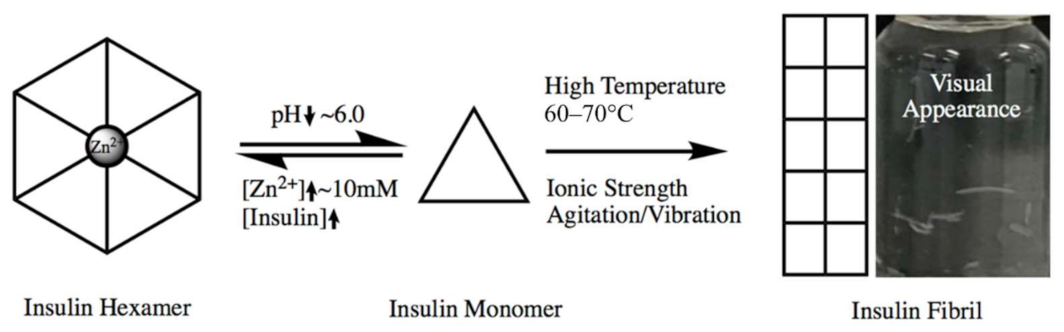
3.5. Insulin Analysis (Trials 1, 2, 3)—Understanding the Effects of Vibration
4. Conclusions
5. Recommendations
- 1.
- This paper identified that current medical grade packaging amplified the vibration of drone transport significantly more than car transport in specific frequency ranges. Further work to characterise the vibration isolation of medical packaging through bench tests should be undertaken. Subsequent assessment of the potential to optimise the packaging for multiple modes of transport should then be made.
- 2.
- In the review of existing work, it was identified that there is limited research which quantifies the effects of vibration frequency, amplitude, and duration on sensitive liquid healthcare products. Controlled bench tests to identify these effects should be undertaken.
- 3.
- Many drones are likely to operate delivery systems which connect with land-based transport, and as such further work investigating other possible modes should take place to enable a comparison of their detrimental effects on sensitive cargoes. Part of this research may take the form of a transport simulation study [59].
- 4.
- This study was focused on vibration, however future work should include an investigation of permitted temperature deviations on the quality of insulin, modelling the small temperature spikes which may occur during combined air and road transportation.
- 1.
- Identify the product’s regulatory packaging restrictions based on ICAO (air transport), IMRG (maritime transport), RID (rail transport), and ADR (road transport) guidance. Where classed as dangerous by these documents, the regulations must be followed.
- 2.
- Select appropriate packaging to protect the proposed product and any parties involved in its carriage. Label as needed.
- 3.
- Test the packaging in the proposed craft/vehicle using 3-axis (or greater) accelerometers to quantify the vibration transferred to potential products.
- 4.
- Make adjustments to the packaging and/or craft/vehicle and repeat step 3, as needed, to ensure that cargoes are not exposed to damaging levels of vibration.
- 5.
- In line with the EU GDP, where uncertainties may be present, flight tests matching the intended operations (including cargoes) are required, with associated analysis of the product post-flight to ensure that the quality and integrity of the medicine has not been affected by vibration in-flight.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Reference Suspension | Opalescent Values (NTU) | Component (mL) | Degree of Opalescence | |
|---|---|---|---|---|
| Standard of Opalescence | Ultra-Pure Water | |||
| I | 3 | 5 | 95 | Clear (≤Ref I) |
| II | 6 | 10 | 90 | Slightly Opalescent (≤Ref II) |
| III | 18 | 30 | 70 | Opalescent (≤Ref III) |
| IV | 30 | 50 | 50 | Very Opalescent (≤Ref IV) |

References
- Hiebert, B.; Nouvet, E.; Jeyabalan, V.; Donelle, L. The Application of drones in healthcare and health-related services in north america: A scoping review. Drones 2020, 4, 30. [Google Scholar] [CrossRef]
- O’Keeffe, D.T.; Johnson, K.; Maraka, S. Provision of bidirectional remote patient care with an unmanned aerial vehicle. Mayo Clin. Proc. 2020, 95, 830. [Google Scholar] [CrossRef] [PubMed]
- Mateen, F.J.; Leung, K.H.B.; Vogel, A.C.; Cissé, A.F.; Chan, T.C.Y. A drone delivery network for antiepileptic drugs: A framework and modelling case study in a low-income country. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Desloovere, W. An Evaluation of the Potential to Use Drone Deliveries as Last-Mile Logistics. Master’s Thesis, Mid Sweden University, Sundsvall, Sweden, 2020. [Google Scholar]
- Ilancheran, M. COVID-19, Medical Drones, & The Last Mile of The Pharma Supply Chain. 2020. Available online: https://www.pharmaceuticalonline.com/doc/covid-medical-drones-the-last-mile-of-the-pharma-supply-chain-0001 (accessed on 15 January 2021).
- Skorup, B.; Haaland, C. How drones can help fight the coronavirus. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Bishara, R.H. Cold Chain Management—An Essential Component of the Global Pharmaceutical Supply Chain. Am. Pharm. Outsourcing 2006, 9, 14–22. [Google Scholar]
- Cerba Healthcare and The Drone Office. Drones in Medical Logistics; Cerba Healthcare: Issy-les-Moulineaux, France, 2019. [Google Scholar]
- Choi-Fitzpatrick, A.; Chavarria, D.; Chchosz, E.; Dingers, J.P.; Duffey, M.; Koebel, K.; Siriphanh, S.; Tulen, M.Y.; Watanabe, H.; Juskaukas, T.; et al. Up in the Air A Global Estimate of Non-Violent Drone Use 2009–2015; University of San Diego: San Diego, CA, USA, 2016. [Google Scholar] [CrossRef]
- Amukele, T.; Ness, P.M.; Tobian, A.A.; Boyd, J.; Street, J. Drone transportation of blood products. Transfusion 2017, 57, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Güner, S.; Rathnayake, D.; Ahmadi, N.B.; Kim, B. Using Unmanned Aerial Vehicles—Drones—As a Logistic Method in Pharmaceutical Industry in Germany; Heilbronn University of Applied Sciences: Heilbronn, Germany, 2017. [Google Scholar]
- Haidari, L.A.; Brown, S.T.; Ferguson, M.; Bancroft, E.; Spiker, M.; Wilcox, A.; Ambikapathi, R.; Sampath, V.; Connor, D.L.; Lee, B.Y. The economic and operational value of using drones to transport vaccines. Vaccine 2016, 34, 4062–4067. [Google Scholar] [CrossRef] [PubMed]
- Direct Relief. Long-Range Drone Delivers Cold-Chain Medicines, Vaccines Between Islands in Caribbean. 2019. Available online: https://www.directrelief.org/2019/07/long-range-drone-delivers-cold-chain-medicines-vaccines-between-islands-in-caribbean/ (accessed on 15 January 2021).
- CNN Business. CVS just Delivered its First Prescriptions via Drone—CNN Video. 2019. Available online: https://edition.cnn.com/videos/business/2019/11/06/ups-cvs-drone-delivery-prescription-orig.cnn-business (accessed on 15 January 2021).
- Cohen, J.K. WakeMed Health and Hospitals joins forces with UPS, FAA for drone pilot. Modern Healthcare, 26 March 2019. Available online: https://www.modernhealthcare.com/care-delivery/wakemed-health-hospitals-joins-forces-ups-faa-drone-pilot (accessed on 15 January 2021).
- Brown, A. Skyports and thales partner to conduct drone delivery trial for NHS in Scotland to support UK COVID-19 Response. Skyports, 25 May 2020. Available online: https://skyports.net/2020/05/skyports-and-thales-partner-to-conduct-drone-delivery-trial-for-nhs-in-scotland-to-support-uk-covid-19-response/ (accessed on 15 January 2021).
- Pressroom UPS. UPS partners with matternet to transport medical samples via drone across hospital system in Raleigh, N.C. UPS Pressroom, 26 March 2019. Available online: https://pressroom.ups.com/pressroom/ContentDetailsViewer.page?ConceptType=PressReleases&id=1553546776652-986 (accessed on 15 January 2021).
- sUAS News. Matternet launches drone delivery operations at labor berlin in Germany. sUAS News, 08 December 2020. Available online: https://www.suasnews.com/2020/12/matternet-launches-drone-delivery-operations-at-labor-berlin-in-germany-2/ (accessed on 15 January 2021).
- Volansi. Volansi Launches Commercial Drone Delivery Program to Deliver Cold Chain Medicines in Rural North Carolina. Volansi, 20 October 2020. Available online: https://volansi.com/volansi-launches-commercial-drone-delivery-program-to-deliver-cold-chain-medicines-in-rural-north-carolina/ (accessed on 15 January 2021).
- Zipline. Zipline—Vital, On-Demand Delivery for the World. 2020. Available online: https://flyzipline.com/ (accessed on 10 September 2020).
- Pressroom UPS. UPS flight forward, CVS To Launch residential drone delivery service in Florida retirement community to assist in coronavirus response. UPS Pressroom, 27 April 2020. Available online: https://www.pressroom.ups.com/pressroom/ContentDetailsViewer.page?ConceptType=PressReleases&id=1587995241555-272 (accessed on 15 January 2021).
- Stradling, R. WakeMed in Raleigh is the spot as UPS makes its first deliveries with drones in the U.S. Raleigh News & Observer, 26 March 2019. Available online: https://www.newsobserver.com/news/local/article228373214.html (accessed on 15 January 2021).
- Kesteloo, H. Matternet drones to deliver COVID-19 tests in Berlin. DroneXL.co, 25 November 2020. Available online: https://dronexl.co/2020/11/25/matternet-drones-to-deliver-covid-19-tests-berlin/ (accessed on 27 January 2021).
- Badurina, G.; Majić, Z.; Pavlin, S. Evaluation of air transportation under controlled room temperature for pharmaceuticals. Promet Traffic Transp. 2011, 23, 121–130. [Google Scholar] [CrossRef]
- ICH. ICH harmonised tripartite guideline—Stability testing of new drug substances and products. Q1a (R2) Curr. Step. 2003, 4, 1–24. [Google Scholar]
- Rignall, A. ICHQ1A(R2) stability testing of new drug substance and product and ICHQ1C stability testing of new dosage forms. In ICH Quality Guidelines; Teasdale, A., Elder, D., Nims, R.W., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 3–44. [Google Scholar]
- Kommanaboyina, B.; Rhodes, C.T. Trends in stability testing, with emphasis on stability during distribution and storage. Drug Dev. Ind. Pharm. 1999, 25, 857–868. [Google Scholar] [CrossRef]
- Mahler, H.-C.; Friess, W.; Grauschopf, U.; Kiese, S. Protein aggregation: Pathways, induction factors and analysis. J. Pharm. Sci. 2009, 98, 2909–2934. [Google Scholar] [CrossRef] [PubMed]
- Sluzky, V.; Tamada, J.A.; Klibanov, A.M.; Langer, R. Kinetics of insulin aggregation in aqueous solutions upon agitation in the presence of hydrophobic surfaces. Proc. Natl. Acad. Sci. USA 1991, 88, 9377–9381. [Google Scholar] [CrossRef] [PubMed]
- International Civil Aviation Organization. Technical Instructions for the Safe Transport of Dangerous Goods by Air; International Civil Aviation Organization: Montreal, QC, Canada, 2019. [Google Scholar]
- Hii, M.S.Y.; Courtney, P.; Royall, P.G. An evaluation of the delivery of medicines using drones. Drones 2019, 3, 52. [Google Scholar] [CrossRef]
- Akbarian, M.; Yousefi, R. Human αB-crystallin as fusion protein and molecular chaperone increases the expression and folding efficiency of recombinant insulin. PLoS ONE 2018, 13, e0206169. [Google Scholar] [CrossRef]
- Kiese, S.; Papppenberger, A.; Friess, W.; Mahler, H.-C. Shaken, Not stirred: Mechanical stress testing of an IgG1 antibody. J. Pharm. Sci. 2008, 97, 4347–4366. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Seki, T.; Kojima, Y.; Miki, R.; Egawa, Y.; Hosoya, O.; Kasono, K.; Seki, T. Investigation of factors that cause insulin precipitation and/or amyloid formation in insulin formulations. J. Pharm. Health Care Sci. 2019, 5, 1–11. [Google Scholar] [CrossRef]
- Kerr, D.; Wizemann, E.; Senstius, J.; Zacho, M.; Ampudia-Blasco, F.J. Stability and performance of rapid-acting insulin analogs used for continuous subcutaneous insulin infusion: A systematic review. J. Diabetes Sci. Technol. 2013, 7, 1595–1606. [Google Scholar] [CrossRef]
- European Commission. Guidelines of 5 November 2013 on Good Distribution Practice of Medicinal Products for Human Use; European Commission: Brussels, Belgium, 2013; Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:C:2013:343:0001:0014:EN:PDF (accessed on 15 January 2021).
- PCI Services. EU-GDP-Implications-for-Shipping-Clinical-Materials-European-Market; PCI Services: Erie, PA, USA, 2019; Available online: https://pciservices.com/wp-content/uploads/2019/10/EU-GDP-Implications-for-Shipping-Clinical-Materials-European-Market.pdf (accessed on 15 January 2020).
- Department of Health. Safe Management of Healthcare Waste: Environment and Sustainability; Department of Health: London, UK, 2007. [Google Scholar]
- Murff, S.J. Safety and Health Handbook for Cytotoxic Drugs; Government Institutes: Lanham, MD, USA, 2012. [Google Scholar]
- Beck, S.; Bui, T.T.; Davies, A.; Courtney, P.; Brown, A.; Geudens, J.; Royall, P.G. An Evaluation of the Drone Delivery of Adrenaline Auto-Injectors for Anaphylaxis: Pharmacists’ Perceptions, Acceptance, and Concerns. Drones 2020, 4, 66. [Google Scholar] [CrossRef]
- Verbeke, J.; Debruyne, S. Vibration analysis of a UAV multirotor frame. In Proceedings of the of ISMA 2016 International Conference on Noise and Vibration Engineering, Leuven, Belgium, 9–21 September; pp. 2329–2337.
- Li, Z.; Lao, M.; Phang, S.K.; Hamid, M.R.A.; Tang, K.Z.; Lin, F. Development and Design Methodology of an Anti-Vibration System on Micro-UAVs. In Proceedings of the International Micro Air Vehicle Conference and Flight Competition (IMAV), Toulouse, France, 18–21 September 2017; pp. 223–228. [Google Scholar]
- Chonhenchob, V.; Singh, S.P.; Singh, J.J.; Stallings, J.; Grewal, G. Measurement and analysis of vehicle vibration for delivering packages in small-sized and medium-sized trucks and automobiles. Packag. Technol. Sci. 2012, 25, 31–38. [Google Scholar] [CrossRef]
- Ranathunga, C.L.; Jayaweera, H.H.E.; Suraweera, S.K.K.; Wattage, S.C.; Ruvinda, K.K.D.L.; Ariyaratne, T.R. Vibration Effects in Vehicular Road Transportation. Vidyodaya Sci. J. 2013, 18, 13–25. [Google Scholar]
- Axivity. AX3 3-Axis Logging Accelerometer. 2020. Available online: http://www.axivity.com/product/1 (accessed on 15 January 2021).
- RS online. RS PRO PRO-USB-1 Data Logger for Temperature Measurement. 2020. Available online: https://uk.rs-online.com/web/p/data-loggers/1799535/ (accessed on 15 January 2021).
- Lascar Electronics. EL-USB-2-LCD Temperature & Humidity USB Data Logger—Lascar. 2020. Available online: https://www.lascarelectronics.com/easylog-el-usb-2-lcd (accessed on 27 January 2021).
- Welch, P. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 1967, 15, 70–73. [Google Scholar] [CrossRef]
- Harris, F.J. On the use of windows for harmonic analysis with the discrete Fourier transform. Proc. IEEE 1978, 66, 51–83. [Google Scholar] [CrossRef]
- Esri. “Imagery Hybrid” [basemap]. Scale Not Given. World Imagery Hybrid Map. Jan. 06, 2020. Available online: https://www.arcgis.com/home/item.html?id=28f49811a6974659988fd279de5ce39f (accessed on 6 January 2021).
- Medicines and Healthcare products Regulatory Agency. British Pharmacopoeia—Appendix IV: A. Clarity of Solution; British Pharmacopoeia Commission: London, UK, 2020. [Google Scholar]
- Desai, K.G.; Colandene, J.D.; Adams, M. Comprehensive temperature excursion management program for the commercial distribution of biopharmaceutical drug products. J. Pharm. Sci. 2020, 109, 2131–2144. [Google Scholar] [CrossRef] [PubMed]
- Weather Underground. Emsworth, United Kingdom Weather History | Weather Underground. 2020. Available online: https://www.wunderground.com/history/daily/gb/west-thorney/IWESTT6/date/2020-11-11 (accessed on 16 January 2021).
- Weather Underground. Southampton, United Kingdom Weather History | Weather Underground. 2020. Available online: https://www.wunderground.com/history/daily/EGHI/date/2020-12-10 (accessed on 15 January 2021).
- Weather Underground. Penzance, United Kingdom Weather History | Weather Underground. 2020. Available online: https://www.wunderground.com/history/daily/ISENNEN2/date/2020-12-15 (accessed on 15 January 2021).
- Novo Nordisk Ltd. Actrapid 100 international units/ml, solution for injection in a vial—Summary of product characteristics (SmPC)—(emc). Medicines, 09 October 2020. Available online: https://www.medicines.org.uk/emc/product/3849/smpc (accessed on 27 January 2021).
- Sayers, M.W. The Little Book of Profiling: Basic Information About Measuring and Interpreting Road Profiles; University of Michigan Transportation Research Institute: Ann Arbor, MI, USA, 1998. [Google Scholar]
- Burgess, C.; Hammond, J.P. Specifying Accuracy and Precision Criteria for Ultraviolet Spectrometers; Spectroscopy Europe: Florence, Italy, 2019. [Google Scholar]
- Hutchinson, G.; Littlefield, D.J. Safe Transportation of COVID-19 Vaccines: Challenges and Solutions 2020. Available online: http://www.pharmoutsourcing.com/Featured-Articles/571355-Safe-Transportation-of-COVID-19-Vaccines-Challenges-and-Solutions/?ctid=1&cid=25400 (accessed on 26 January 2021).
- Eichleay, M.; Evens, E.; Stankevitz, K.; Parker, C. Using the unmanned aerial vehicle delivery decision tool to consider transporting medical supplies via drone. Glob. Health Sci. Pract. 2019, 7, 500–506. [Google Scholar] [CrossRef]





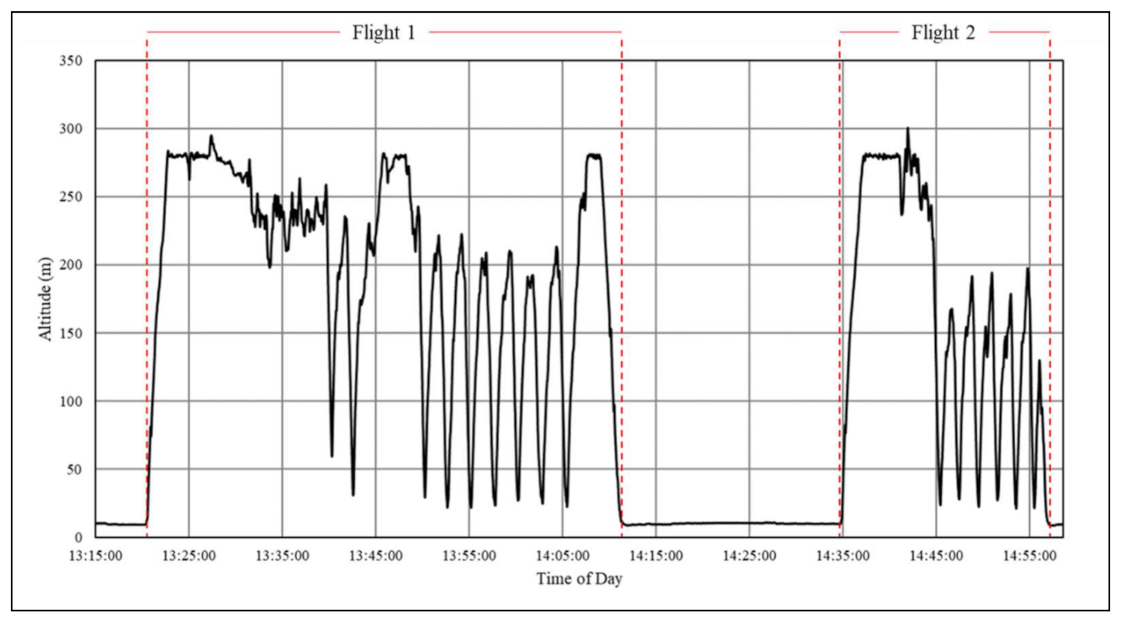
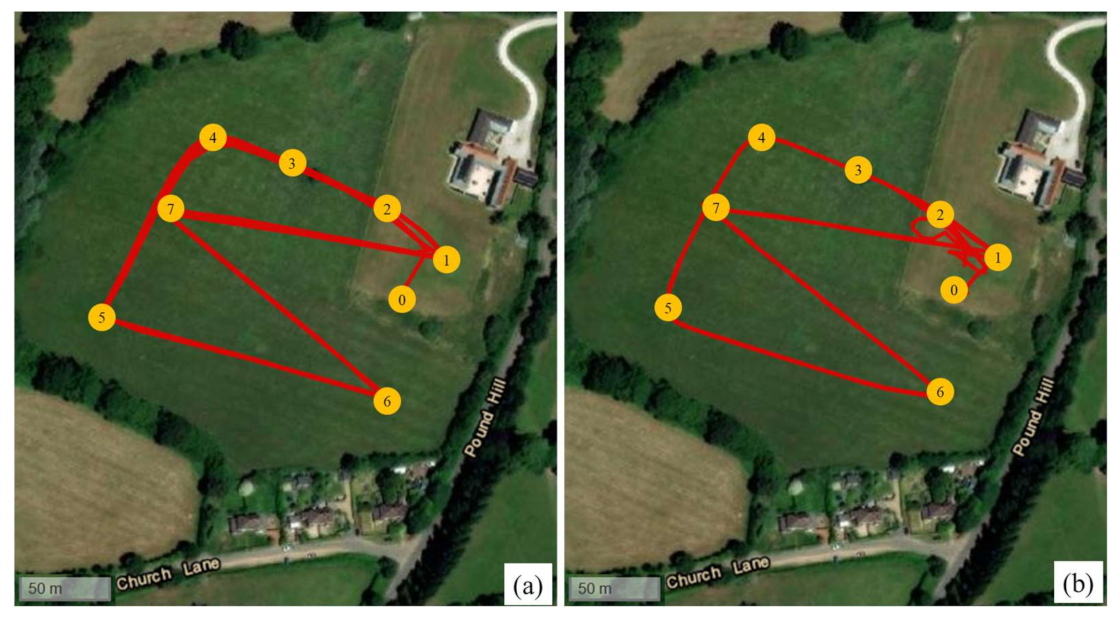
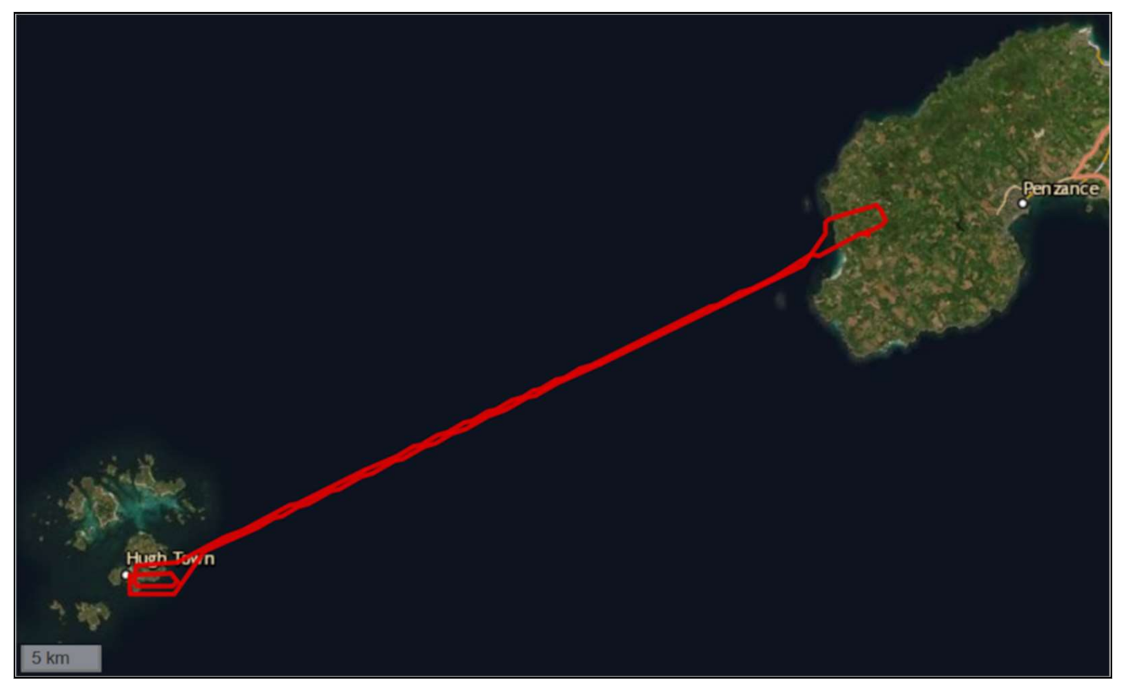
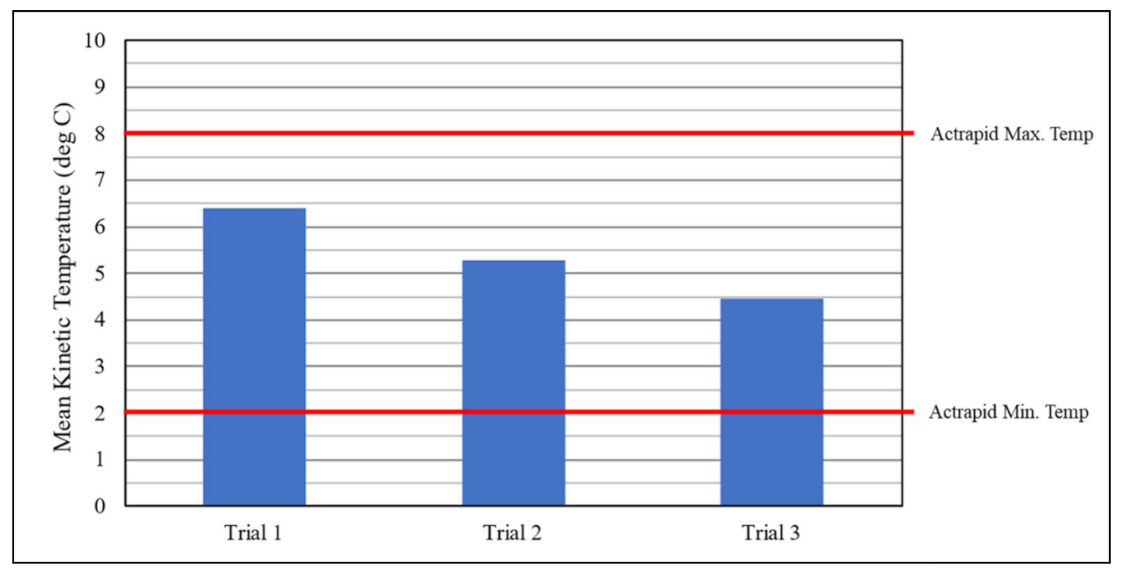




| Location | Start Date | Company Name (s) | Drone Type | Item Type | Reported Flight Duration * | Reported Distance (One-Way) * | Reported Max. Payload * | Cargo Protection (s) |
|---|---|---|---|---|---|---|---|---|
| Rural Virginia | July 2015 | Flirtey | Multi-copter Electric | Drug | 3 min | Not Mentioned | 5.5 kg | Anti-drone collision system (NASA), parachute landing |
| Rural Madagascar | July 2016 | Vayu | VTOL Fixed-wing Electric | Blood transfusion units and patient sample test specimens | Not Mentioned | Up to 60 km | 2 kg | Lock |
| Rwanda and Ghana | October 2016 | Zipline | Fixed-wing Electric | Blood transfusion units | 30 min | 80 km | 1.8 kg (3 units) | Redundant systems: parachute landing, communication and navigation |
| Urban Switzerland | March 2017 | Swiss Post and Matternet | Multi-copter Electric | Blood, test specimens | 10 min | 3 km | 2 kg | QR code for opening the case, parachute landing |
| Tanzania | October 2018 | DHL, GIZ GIZ and Wingcopter | VTOL Fixed-wing Electric | Snake venom antiserum, etc. | 40 min | 60 km | 6 kg | Thermally insulated box |
| Vanuatu | Dececember 2018 | Swoop Aero and Wingcopter | VTOL Fixed-wing Electric | Vaccines | 30 min | 45 km | 6 kg | Teardrop shape, remote control dropping, ice, temperature sensor |
| North Carolina, USA | December 2019 | UPS and Matternet (WakeMed) | Multi-copter Electric | Specimens | 5–10 min | Up to 12.5 miles (~20 km)—within hospital site | Up to 5 lb. (~2 kg) | Cable-controlled (20 ft/6 m) dropping, Authentication system (e.g., ID badge) |
| North Carolina, USA | October 2019 | UPS and Matternet (CVS Pharmacy) | Multi-copter Electric | Prescription only medicine | 5–10 min | Up to 12.5 miles (~20 km)—Pharmacy to patient’s home | Up to 5 lb. (~2 kg) | Cable-controlled (20 ft/6 m) dropping, Authentication system (e.g., ID badge) |
| Bahamas, Puerto Rico, USA | July 2020 | Volansi and Merck | VTOL Fixed-wing Electric | Temperature sensitive medicines | Not Mentioned | Up to 50 miles (80 km) | 4.5 kg | Temperature control devices (Details Not Mentioned) |
| Isle of Mull, Rural Scotland | December 2020 | Skyports, Wingcopter and Soarizon | VTOL Fixed-wing Electric | Medicines, Pathology samples | 15 min | 16 km, Over the sea | Not Mentioned | Not Mentioned |
| Berlin, Urban Germany (planned) | February 2021 | Matternet | Multi-copter Electric | Pathology samples | Varies by site, Approx. 10 min | About 7 miles (up to 20 km), From hospitals to laboratory | 2 kg | Authentication system (e.g., ID badge), temperature controlled payload boxes, parachute |
| Thorney Island, England Cornwall-Isles of Scilly, England | November 2020 December 2020 | Windracers (ULTRA TD1 prototype) | Fixed-wing Petrol | Actrapid (Medical Insulin) | ~12 min 38 min out, 16 min back | Manoeuvres only (no delivery taking place) ~30 miles one-way 1000 km max | 10–20 kg in tests 100 kg max | Insulated polystyrene and cardboard based packaging/Versapak Cargo net |
| West Wellow, New Forest, England | December 2020 | Motion Robotics (Arty) | Multi-copter Electric | Actrapid (Medical Insulin) | ~20 min total | ~7 km in tests 20 km max | ~7 kg in tests 11 kg max | Insulated polystyrene and cardboard based packaging |
| Regulation: | Details/Description | Illustrative Examples: |
|---|---|---|
| ID8000–Y963 | Non-toxic medicines which are treated as consumer commodities. | Aspirin tablets; Adrenaline and Insulin ampoules containing aqueous solutions for injection. |
| UN3248–PI352 Y341 | Liquid medicines which are toxic and flammable and within Class 3 dangerous goods carriage regulations. | Topical sprays—Pain Relief Heat Spray—containing ethyl nicotinate, methyl salicylate, racemic camphor—in denatured ethanol, butane, isobutane, propane—a liquid formulation in a pressurised container. |
| UN1851–PI654 Y641 | Liquid medicines which are toxic and within Class 6.1 dangerous goods carriage regulations. | Hydroxyurea solution for injection. |
| UN3249–PI669 Y644 | Solid medicine which are toxic and come under Class 6.1 dangerous goods carriage regulations. | Azacitidine lyophilized powder and Pentamidine Isetionate powder for solution for injection. |
| Battery 1 | Battery 2 |
|---|---|
|
|
| Packaging Layer 1 | Packaging Layer 2 | Packaging Layer 3 | Trial 1 * | Trial 2 | Trial 3 |
|---|---|---|---|---|---|
| Cytotoxic Drug Cool Box | Bio-bottle 1 | 3 × 10 mL Vial Actrapid (in manufacturer’s box) | ✓ (vials 7, 8, 9) | ✓ (vials 15, 16, 17) | ✓ (vials 21, 22, 23) |
| 1 × RS Pro Temperature Logger | ✓ | ✓ | ✓ | ||
| Bio-bottle 2 | 1 × 10 mL Vial Actrapid (unboxed) | ✓ (vial 18) | ✓ (vial 24) | ||
| 1 × 10 mL Vial Box | ✓ (from vial 18) | ✓ (from vial 24) | |||
| 2 × Axivity AX3 Vibration Sensor (1 attached to unboxed vial, 1 in vial box) | ✓ | ✓ | |||
| 1 × RS Pro Temperature Logger | ✓ | ✓ | |||
| Medium Versapak | 3 × 10 mL Vial Actrapid (in manufacturer’s box) | ✓ (vials 10, 11, 12) | |||
| 1 × RS Pro Temperature Logger | ✓ | ||||
| 5 × Dummy blood bags | ✓ | ||||
| 1 × Axivity AX3 (attached to one of the blood bags) | ✓ | ||||
| Small Versapak (Control—travels to site—not flown) | 1 × 10 mL Vial Actrapid (in manufacturer’s box) | ✓ (vial 2 *) | ✓ (vial 14) | ✓ (vial 20) | |
| 1 × Lascar USB-2-LCD Temperature Logger | ✓ | ✓ | ✓ | ||
| No packing (Control—remains in fridge at lab site) | ✓ (vial 1 *) | ✓ (vial 13) | ✓ (vial 19) | ||
| Mode | Packaging Box | Notes | |
|---|---|---|---|
| 1 | Car | Cytotoxic Drug Box | Alongside vials in boxes, within the Bio bottle |
| 2 | Drone | Cytotoxic Drug Box | Attached to a vial removed from its manufacturer’s box, outside the insulin box, within the Bio bottle |
| 3 | Drone | Cytotoxic Drug Box | Inside manufacturer’s box from vial used for sensor position 2, within the Bio bottle |
| 4 | Drone | Versapak (medium) | Attached to a dummy blood bag |
| 5 | Drone | - | Cargo hold floor (near front, port side) |
| Mode | Packaging Box | Notes | |
|---|---|---|---|
| 1 | Drone | Cytotoxic Drug Box | Attached to a vial removed from its manufacturer’s box, outside insulin box, within Bio bottle |
| 2 | Drone | Cytotoxic Drug Box | Inside manufacturer’s box from vial used for sensor position 1/2, within the Bio bottle |
| 3 | Drone | - | Mounted at drone’s (X–Y) centre of gravity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oakey, A.; Waters, T.; Zhu, W.; Royall, P.G.; Cherrett, T.; Courtney, P.; Majoe, D.; Jelev, N. Quantifying the Effects of Vibration on Medicines in Transit Caused by Fixed-Wing and Multi-Copter Drones. Drones 2021, 5, 22. https://doi.org/10.3390/drones5010022
Oakey A, Waters T, Zhu W, Royall PG, Cherrett T, Courtney P, Majoe D, Jelev N. Quantifying the Effects of Vibration on Medicines in Transit Caused by Fixed-Wing and Multi-Copter Drones. Drones. 2021; 5(1):22. https://doi.org/10.3390/drones5010022
Chicago/Turabian StyleOakey, Andrew, Tim Waters, Wanqing Zhu, Paul G. Royall, Tom Cherrett, Patrick Courtney, Dennis Majoe, and Nickolay Jelev. 2021. "Quantifying the Effects of Vibration on Medicines in Transit Caused by Fixed-Wing and Multi-Copter Drones" Drones 5, no. 1: 22. https://doi.org/10.3390/drones5010022
APA StyleOakey, A., Waters, T., Zhu, W., Royall, P. G., Cherrett, T., Courtney, P., Majoe, D., & Jelev, N. (2021). Quantifying the Effects of Vibration on Medicines in Transit Caused by Fixed-Wing and Multi-Copter Drones. Drones, 5(1), 22. https://doi.org/10.3390/drones5010022










